Abstract
Galectins are β-galactoside binding lectins with a potential hemolytic role on erythrocyte membrane integrity and permeability. In the present study, goat heart galectin-1 (GHG-1) was purified and investigated for its hemolytic actions on erythrocyte membrane. When exposed to various saccharides, lactose and sucrose provided maximum protection against hemolysis, while glucose and galactose provided lesser protection against hemolysis. GHG-1 agglutinated erythrocytes were found to be significantly hemolyzed in comparison with unagglutinated erythrocytes. A concentration dependent rise in the hemolysis of trypsinized rabbit erythrocytes was observed in the presence of GHG-1. Similarly, a temperature dependent gradual increase in percent hemolysis was observed in GHG-1 agglutinated erythrocytes as compared to negligible hemolysis in unagglutinated cells. The hemolysis of GHG-1 treated erythrocytes showed a sharp rise with the increasing pH up to 7.5 which became constant till pH 9.5. The extent of erythrocyte hemolysis increased with the increase in the incubation period, with maximum hemolysis after 5 h of incubation. The results of this study establish the ability of galectins as a potential hemolytic agent of erythrocyte membrane, which in turn opens an interesting avenue in the field of proteomics and glycobiology.
Keywords: Goat heart galectin-1, Agglutination, Erythrocyte, Membrane, Hemolysis
Abbreviations: GHG-1, goat heart galectin-1; PBS, phosphate buffered saline
1. Introduction
Galectins are β-galactoside specific binding lectins reported to be expressed ubiquitously in all taxa of living world and is involved in vital biological functions (Hasan et al., 2007). The ability of galectins to act as humoral factors in the defense mechanism against various pathogenic agents suggests important applications for these proteins (Hussain et al., 2013; Liu et al., 2014; Shi et al., 2014). Over the years, galectins have been reported to have potential hemolytic actions on membranes and other cell organelles (Armstrong et al., 1996; Unno et al., 2014). Lytic action of some galectins has been ascribed to enzymatic activity (Hittelet et al., 2003), perturbation of the activities of membrane associated enzymes (Lowe and Marth, 2003), and pore formation in the membranes (Yu et al., 2002). In the present study, the hemolytic actions of purified goat heart galectin-1 (GHG-1) (Ashraf et al., 2011) have been studied by monitoring their effect on erythrocyte membrane integrity and permeability to understand the possible role of galectins in hemolytic destruction of foreign cells in the mammalian nervous system. The effect of various physiological parameters like concentration, temperature, pH and incubation time on GHG-1 mediated erythrocyte membrane lysis was also investigated.
The galectin dependent association of glycans has been suggested to potentially affect the key membrane features such as permeability, fluidity and osmofragility, and enhances its impact on membrane characteristics by interacting with hydrophobic membrane patches (Gupta et al., 2006). Moreover, glycan residues attached to cell membranes play a crucial role in membrane lysis (Semrau et al., 2010; St-Pierre et al., 2011). Galectins are the lectins with specific attraction for β-galactoside moieties, and the presence of this glycan residue makes them a potent membrane lysis molecule (Yu et al., 2002). Hence, the effect of various saccharides on erythrocyte hemolysis was also investigated to assess the ability of non-electrolytes to protect GHG-1 induced lysis of erythrocytes, and also to identify the sugar molecule providing maximum protection against membrane lysis.
2. Materials and methods
2.1. Reagents
Sephadex G100 and G50, molecular weight markers (14.4–97.4 kDa), coomassie brilliant blue (CBB) G-250 and R-250, and sugars were purchased from Sigma Aldrich (St Louis, MO, USA). All other chemicals used were of analytical grade and purchased from Qualigens Fine Chemicals and Merck India Ltd, India. All the experiments were carried out in triplicates and mean value has been reported.
2.2. Isolation and purification of GHG-1
The GHG-1 was isolated and purified in essence according to our previously published studies on heart lectins (Ashraf et al., 2010a,b, 2011). Protein concentration was estimated by Lowry et al. (1951) and its activity was determined by hemagglutination activity using trypsinized rabbit erythrocytes by two fold serial dilutions on a microtiter V-shaped plate (Laxbro, India) (Raz and Lotan, 1981).
2.3. Isolation of erythrocytes and trypsinization
A fresh blood sample from the vein of a healthy rabbit was collected in a tube containing heparin. The erythrocytes were isolated by centrifuging the blood sample at 2000 rpm for 5 min at room temperature and washed three to four times with Phosphate buffered saline (PBS) ‘B’ (5 ml per ml packed cells). Washed erythrocytes were then made 4% (v/v) by adding PBS ‘B’. An 8% (v/v) suspension of washed erythrocytes was prepared in PBS ‘B’. A trypsin solution (100 mg%) was then added to the erythrocytes (0.1 ml of trypsin solution per ml of erythrocyte suspension) and incubated for 1 h at 37 °C. The trypsinized erythrocytes were washed four to five times with PBS ‘B’ and 4% (v/v) suspension was prepared for further use.
2.4. Effect of GHG-1 mediated agglutination on the osmofragility of trypsinized rabbit erythrocytes
The osmofragility of trypsinized rabbit erythrocytes in the presence of GHG-1 was determined according to a previously described method (Pande et al., 1998) with some modifications. An 8% trypsinized rabbit erythrocyte suspension (200 μl) was mixed with 100 μl GHG-1 (100 μg/ml in 50 μl of normal saline containing 5 mM β-ME) and incubated for one hour at room temperature to allow agglutination, followed by the addition of 1.5 ml hypotonic NaCl solution with concentrations ranging from 0.3% to 0.8%. This was then followed by incubation of cells for 4 h at room temperature with intermittent gentle shaking and centrifuged at 2000 rpm for 5 min. The supernatant was collected after centrifugation, the extent of hemolysis was measured at 540 nm and the results were reported as percent lysis. The lysis of erythrocytes with an equal volume of distilled water was taken as 100%.
2.5. Effect of varying concentrations of GHG-1 on erythrocyte hemolysis
An 8% trypsinized rabbit erythrocyte suspension (200 μl) treated with varying concentrations of 100 μl GHG-1 (10–100 μg/ml in 50 μl of normal saline containing 5 mM β-ME) was incubated for 4 h at 37° C. Samples were centrifuged and RBC lysates were analyzed at 540 nm.
2.6. Hemolysis of GHG-1 agglutinated erythrocytes as a function of increasing temperature
An 8% trypsinized rabbit erythrocyte suspension (200 μl) in the presence and absence of 100 μl GHG-1 (100 μg/ml in 50 μl of normal saline containing 5 mM β-ME) was incubated at increasing temperatures (10–50° C) for 4 h. Samples were centrifuged and RBC lysates were analyzed at 540 nm.
2.7. pH induced hemolysis of GHG-1 agglutinated erythrocytes
An 8% trypsinized rabbit erythrocyte suspension (200 μl) in the presence and absence of 100 μl GHG-1 (100 μg/ml in 50 μl of normal saline containing 5 mM β-ME) was treated at varying pH values (3.5–11.5) at 37° C for 4 h. The buffers used were, 0.1 M sodium acetate buffer (pH 3.5–5.5), 0.1 M sodium phosphate buffer (pH 6.5–7.5), 0.1 M Tris–HCl buffer (pH 8.5–9.5) and 0.1 M glycine–NaOH buffer (pH 10.5–11.5) for 24 h at 4 °C. Samples were centrifuged and RBC lysates were analyzed at 540 nm.
2.8. Hemolysis of GHG-1 agglutinated erythrocytes as a function of incubation period
An 8% trypsinized rabbit erythrocyte suspension (200 μl) in the presence and absence of 100 μl GHG-1 (100 μg/ml in 50 μl of normal saline containing 5 mM β-ME) was incubated for different time intervals (0.5–4.0 h) at 37° C. Samples were centrifuged and RBC lysates were analyzed at 540 nm.
2.8. Effect of saccharides on erythrocyte hemolysis in the presence and absence of GHG-1
The GHG-1 agglutinated erythrocyte suspensions (300 μl) were mixed with 1.5 ml PBS ‘B’ (75 mM sodium phosphate pH 7.2, containing 0.15 M NaCl, 5 mM β-ME and 0.02% (w/v) sodium azide) with 30 mM each of galactose, glucose, sucrose and lactose. The suspension was then incubated at room temperature for 6 h with gentle shaking and percent hemolysis was measured. Erythrocytes incubated with various saccharides in the absence of GHG-1 served as control.
3. Results and discussion
Proteins with hemolytic activities have been of much interest to membrane biologists, mainly because the study of the mode of their action has contributed considerably to the understanding of complexities of biomembrane organization and function. Interestingly, GHG-1 exhibited hemolytic activity toward rabbit and human erythrocytes. Thus, we examined the interaction of GHG-1 with erythrocyte membrane in order to elucidate the mechanism of hemolysis by GHG-1 using rabbit erythrocytes as a model system.
Extent of hemolysis of trypsinized rabbit erythrocytes in the presence and absence of GHG-1 is depicted in Fig. 1. Erythrocytes exhibited a variable hemolysis when varying concentrations (0.3–0.8%) of hypotonic NaCl solution were added. At 0.6% NaCl concentration, GHG-1 agglutinated erythrocytes showed a significant (P < 0.001) 74% hemolysis compared to 51% hemolysis in unagglutinated erythrocytes. The study showed that GHG-1 agglutinated erythrocytes were significantly hemolyzed in comparison with unagglutinated erythrocytes, suggesting that lectins by virtue of their glycan binding property create pores or leaks in the cellular membranes, thus disrupting its function as a selective cell barrier (Deuticke et al., 1986). Hence, due to an imbalanced colloid-osmotic pressure of intracellular solutes, uptake of water and salts rises, causing an increase in hemolysis in lectin agglutinated cells (Deuticke et al., 1986).
Figure 1.

Effect of GHG-1 on hemolysis of trypsinized erythrocytes at varying concentrations of NaCl solution. An 8% trypsinized rabbit erythrocyte suspension treated with varying concentrations of NaCl (0.3–0.8%) in the absence and presence of 100 μl GHG-1 (100 μg/ml) was incubated for 4 h at 37° C. Samples were centrifuged and RBC lysates were analyzed at 540 nm.
Some plant and animal lectins have been reported to disturb membrane integrity and fluidity, probably by interacting with membrane glycoconjugates and thus penetrating the lipid bilayer (Gupta et al., 2006). Lectins, upon contact with cell surface glycans like laminin, fibronectin and lysozyme associated proteins, oligomerize into dynamic complexes that interact with the lipid bilayer, resulting in substantial reorganization of membrane components (Surolia et al., 1997). Though, contact point of the lipid bilayer and membrane integrated proteins remain same, any displacement of membrane proteins due to the lectin binding may disturb the sealed interaction, thus forming an aqueous pore (Israelachvili, 1977). Moreover, aqueous trans-membrane channels in conjugation with hydrophobic protein domains have been reported to form a cluster of aggregated and displaced proteins (Gupta et al., 2006).
A concentration dependent rise in the hemolysis of trypsinized rabbit erythrocytes was observed in the presence of GHG-1. At 100 μg/ml concentration, GHG-1 showed significant (P < 0.001) 84% hemolysis as compared to negligible hemolysis in the absence of GHG-1 (control) (Fig. 2).
Figure 2.

Effect of varying concentrations of GHG-1 on erythrocyte hemolysis. An 8% trypsinized rabbit erythrocyte suspension (200 μl) treated with varying concentrations of 100 μl GHG-1 (10–100 μg/ml) was incubated for 4 h at 37° C. Samples were centrifuged and RBC lysates were analyzed at 540 nm.
A temperature dependent gradual increase in percent hemolysis was observed. At 50° C, GHG-1 treated erythrocytes displayed a significant (P < 0.001) 83% hemolysis, as compared to negligible hemolysis in unagglutinated cells (control) (Fig. 3).
Figure 3.

Hemolysis of GHG-1 agglutinated erythrocytes as a function of increasing temperature. An 8% trypsinized rabbit erythrocyte suspension (200 μl) in the presence and absence of 100 μl GHG-1 (100 μg/ml) was incubated at increasing temperatures (10–50° C) for 4 h. Samples were centrifuged and RBC lysates were analyzed at 540 nm.
Pre-incubation of erythrocytes with GHG-1 in various buffers of pH range (pH 3.5–11.5) revealed a sharp enhancement of percent hemolysis with a maximum of 79% for GHG-1 treated erythrocytes at pH 7.5 (Fig. 4). The hemolysis of the erythrocytes in the presence of GHG-1 showed a sharp rise with increasing pH up to 7.5 and became constant till pH 9.5. This might reflect the presence of some ionizable group of amino acid residues of the purified lectin with a pKa in the range of pH 7.5–9.5, which is involved in the hemolytic activity.
Figure 4.
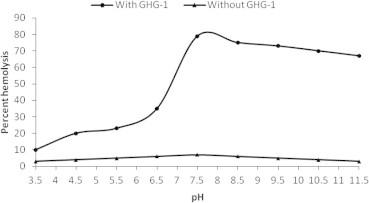
pH induced hemolysis of GHG-1 agglutinated erythrocytes. An 8% trypsinized rabbit erythrocyte suspension (200 μl) in the presence and absence of 100 μl GHG-1 (100 μg/ml) was treated at varying pH values (3.5–11.5) at 37° C for 4 h. Samples were centrifuged and RBC lysates were analyzed at 540 nm.
The extent of erythrocyte hemolysis increased with the increase in incubation period. After 5 h incubation, GHG-1 treated erythrocytes showed a significant (P < 0.001) 88% hemolysis, compared to negligible hemolysis for unagglutinated erythrocytes (control) (Fig. 5).
Figure 5.

Hemolysis of GHG-1 agglutinated erythrocytes as a function of incubation period. An 8% trypsinized rabbit erythrocyte suspension (200 μl) in the presence and absence of 100 μl GHG-1 (100 μg/ml) was incubated for different time intervals (0.5–4.0 h) at 37° C. Samples were centrifuged and RBC lysates were analyzed at 540 nm.
Variation in lectin concentration, pH, temperature and incubation time probably enhances the lectin glycan interaction, thus increasing the number and size of the erythrocyte membrane pores. This in turn results in an increased rate of electrolyte diffusion through the pores, thus justifying the reason for increase in percent hemolysis.
Presuming that the increased hemolysis of lectin agglutinated erythrocytes is due to the formation of ion permeable leaks formed in the membrane, as with some hemolytic proteins (Hatakeyama et al., 1995), attempts were made to prevent the colloid osmotic lyses by addition of non-electrolytes to the extra cellular medium, which may counterbalance the osmotic pressure exerted by intracellular macromolecules (Gupta et al., 2006). The ability of non-electrolytes to protect GHG-1 induced lysis of erythrocytes was assessed after incubation with different sugar solutions for 4 h. GHG-1 agglutinated erythrocytes exhibited 75% hemolysis (P < 0.001) in control samples, compared to 4.5% hemolysis of unagglutinated erythrocytes (Fig. 6). The presence of 30 mM lactose and sucrose provided maximum protection, with only 16% hemolysis of GHG-1 agglutinated erythrocytes. The increase in hemolysis of GHG-1 agglutinated erythrocytes in the presence of lactose and sucrose can be attributed to the fact that these disaccharides being large molecular weight carbohydrates could not pass easily through the ion permeable leaks, thus providing maximum protection against hemolysis. Moreover, lactose may have also competed with active sites of the lectin, thus resulting in decreased hemolysis of erythrocytes. On the other hand, glucose and galactose being low molecular weight molecules easily penetrated through the pores, thus providing lesser protection against hemolysis.
Figure 6.

Effect of saccharides on erythrocyte hemolysis in the presence and absence of GHG-1. Hemolysis of 8% trypsinized rabbit erythrocyte suspension (200 μl) was measured in the presence and absence of 100 μl GHG-1 (100 μg/ml) in PBS ‘B’ alone and in the presence of 30 mM each of galactose, glucose, sucrose and lactose. Samples were centrifuged and RBC lysates were analyzed at 540 nm.
4. Conclusion
The enhanced hemolysis efficiency in the presence of GHG-1 and under various physiological parameters at standard values establishes potential hemolytic action of the purified galectin on cell membranes. The purified galectin by the virtue of its hemolytic functions might well play a significant role in the defense mechanism of mammalian nervous system, not only neutralizing foreign substances by binding to their carbohydrate moieties, but also acting directly as a toxic protein to invading microorganisms.
Conflict of interest
None declared.
Acknowledgments
The authors are grateful to Aligarh Muslim University (Aligarh, India) for the facilities. Thanks are also due to King Fahd Medical Research Center, King Abdulaziz University (Jeddah, Saudi Arabia) for other facilities.
Footnotes
Peer review under responsibility of King Saud University.
References
- Armstrong P.B., Swarnakar S., Srimal S., Misquith S., Hahn E.A., Aimes R.T., Quigley J.P. A cytolytic function for a sialic acid-binding lectin that is a member of the pentraxin family of proteins. J. Biol. Chem. 1996;271:14717–14721. doi: 10.1074/jbc.271.25.14717. [DOI] [PubMed] [Google Scholar]
- Ashraf G.M., Banu N., Ahmad A., Singh L.P., Kumar R. Purification, characterization, sequencing and biological chemistry of galectin-1 purified from Capra hircus (goat) heart. Protein J. 2011;30:39–51. doi: 10.1007/s10930-010-9300-2. [DOI] [PubMed] [Google Scholar]
- Ashraf G.M., Bilal N., Suhail N., Hasan S., Banu N. Glycosylation of purified buffalo heart galectin-1 plays crucial role in maintaining its structural and functional integrity. Biochemistry. 2010;75:1450–1457. doi: 10.1134/s0006297910120059. [DOI] [PubMed] [Google Scholar]
- Ashraf G.M., Rizvi S., Naqvi S., Suhail N., Bilal N., Hasan S., Tabish M., Banu N. Purification, characterization, structural analysis and protein chemistry of a buffalo heart galectin-1. Amino Acids. 2010;39:1321–1332. doi: 10.1007/s00726-010-0574-7. [DOI] [PubMed] [Google Scholar]
- Deuticke B., Heller K.B., Haest C.W. Leak formation in human erythrocytes by the radical-forming oxidant t-butylhydroperoxide. Biochim. Biophys. Acta. 1986;854:169–183. doi: 10.1016/0005-2736(86)90108-2. [DOI] [PubMed] [Google Scholar]
- Gupta R.K., Pande A.H., Gulla K.C., Gabius H.-J., Hajela K. Carbohydrate-induced modulation of cell membrane. VIII. Agglutination with mammalian lectin galectin-1 increases osmofragility and membrane fluidity of trypsinized erythrocytes. FEBS Lett. 2006;580:1691–1695. doi: 10.1016/j.febslet.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Hasan S.S., Ashraf G.M., Banu N. Galectins – potential targets for cancer therapy. Cancer Lett. 2007;253:25–33. doi: 10.1016/j.canlet.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Hatakeyama T., Nagatomo H., Yamasaki N. Interaction of the hemolytic lectin CEL-III from the marine invertebrate Cucumaria echinata with the erythrocyte membrane. J. Biol. Chem. 1995;270:3560–3564. doi: 10.1074/jbc.270.8.3560. [DOI] [PubMed] [Google Scholar]
- Hittelet A., Legendre H., Nagy N., Bronckart Y., Pector J.-C., Salmon I., Yeaton P., Gabius H.-J., Kiss R., Camby I. Upregulation of galectins-1 and -3 in human colon cancer and their role in regulating cell migration. Int. J. Cancer. 2003;103:370–379. doi: 10.1002/ijc.10843. [DOI] [PubMed] [Google Scholar]
- Hussain A., Li Y.-F., Cheng Y., Liu Y., Chen C.-C., Wen S.-Y. Immune-related transcriptome of Coptotermes formosanus Shiraki workers: the defense mechanism. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili J.N. Refinement of the fluid-mosaic model of membrane structure. Biochim. Biophys. Acta. 1977;469:221–225. doi: 10.1016/0005-2736(77)90185-7. [DOI] [PubMed] [Google Scholar]
- Liu Y., Shen D., Zhou F., Wang G., An C. Identification of immunity-related genes in Ostrinia furnacalis against entomopathogenic fungi by RNA-seq analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J.B., Marth J.D. A genetic approach to mammalian glycan function. Annu. Rev. Biochem. 2003;72:643–691. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Pande A.H., Sumati, Hajela N., Hajela K. Carbohydrate induced modulation of cell membrane VII. Binding of exogenous lectin increases osmofragility of erythrocytes. FEBS Lett. 1998;427:21–24. doi: 10.1016/s0014-5793(98)00384-6. [DOI] [PubMed] [Google Scholar]
- Raz A., Lotan R. Lectin-like activities associated with human and murine neoplastic cells. Cancer Res. 1981;41:3642–3647. [PubMed] [Google Scholar]
- Semrau S., Monster M.W.L., van der Knaap M., Florea B.I., Schmidt T., Overhand M. Membrane lysis by gramicidin S visualized in red blood cells and giant vesicles. Biochim. Biophys. Acta BBA Biomembr. 2010;1798:2033–2039. doi: 10.1016/j.bbamem.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Shi X.-Z., Wang L., Xu S., Zhang X.-W., Zhao X.-F., Vasta G.R., Wang J.-X. A galectin from the kuruma shrimp (Marsupenaeus japonicus) functions as an opsonin and promotes bacterial clearance from hemolymph. PLoS ONE. 2014;9:12–15. doi: 10.1371/journal.pone.0091794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre C., Manya H., Ouellet M., Clark G.F., Endo T., Tremblay M.J., Sato S. Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J. Virol. 2011;85:11742–11751. doi: 10.1128/JVI.05351-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surolia A., Swaminathan C.P., Ramkumar R., Podder S.K. Unusual structural stability and ligand induced alterations in oligomerization of a galectin. FEBS Lett. 1997;409:417–420. doi: 10.1016/s0014-5793(97)00432-8. [DOI] [PubMed] [Google Scholar]
- Unno H., Goda S., Hatakeyama T. Hemolytic lectin CEL-III heptamerizes via a large structural transition from α-Helices to a β-barrel during the transmembrane pore formation process. J. Biol. Chem. 2014;289:12805–12812. doi: 10.1074/jbc.M113.541896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Finley R.L., Jr., Raz A., Kim H.-R.C. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J. Biol. Chem. 2002;277:15819–15827. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]


