Abstract
How changes in biodiversity alter the transmission of infectious diseases is presently under debate. Epidemiologists and ecologists have put a lot of effort to understand the mechanism behind biodiversity–disease relationship. Two important mechanisms, i.e. dilution and amplification theories have in some manner made it clear that biodiversity and disease outcome have an intimate relationship. The dilution effect theory seems to answer some overarching questions, but paucity of information about many disease systems is a real obstacle for its acceptance. Also, there is hardly any agreement on host population threshold and critical community size vis-à-vis wild life diseases. We suggest a multidimensional approach whereby the same disease system needs to be studied in different ecological zones and then the effect of biodiversity on disease outcome needs to be ascertained. Nonetheless, caution is to be taken while jumping to any conclusion as biodiversity–disease relationship is a multifactorial process.
Abbreviations: CEM, Classical Epidemiological Model; DEH, dilution effect; MNV, Murine Norovirus
Keywords: Biodiversity, Dilution effect, Amplification, Disease outcome, Host population threshold, Critical community size
1. Introduction
It has been a topic of keen interest for ecologists and epidemiologists to understand alterations in the biodiversity and how they bear on the disease occurrence. The Classical Epidemiological Model (CEM) explained the relationship between host abundance and infection occurrence, but it did not describe the interaction between biodiversity and disease outcomes. In recent years, researchers have taken up this important aspect primarily because a shift in biodiversity has an intimate relationship with the transmission of disease. Perusal of the literature shows both positive and negative correlations between biodiversity and disease outbreak, though it is early to speculate which phenomenon explains this relationship in a more balanced way.
It is also evident from the recent studies on some disease systems that biodiversity does not have any influence on the outcome of a disease. Stalkeld et al. (2013), based on his meta-analysis data on zoonotic diseases concluded that disease risk is more of a local trait mainly depending on the composition of reservoir hosts and vectors. However, such studies cannot be validated for other disease systems. Also, zoonotic pathogens have different transmission patterns (i.e. it is multi-species phenomenon; see Salkeld et al., 2013) which operate under specific conditions.
Knowing the ambiguous nature of the biodiversity–disease relationship, here we critically review recent theories which have been proposed by different epidemiologists, especially in the last decade. We further look at how these theories are different from the Classical Epidemiological Model and provide suggestions for a better understanding of this relationship.
2. How is biodiversity related to disease occurrence?
Alterations in biological diversity have the potency to affect the disease occurrence both in plants and animals (Keesing et al., 2010). It has been suggested that biodiversity plays a dual role in the propagation of disease; it can on the one hand become a safe haven for novel pathogens, but at the same time helps to reduce the disease risk (Keesing et al., 2010). However, more evidence favors the mechanism in which biodiversity loss actually can increase the transmission rate (Keesing et al., 2010). Keesing et al. (2010) further stated that a reduction in biodiversity can reduce disease transmission if the lost species is a host of the infectious organisms. The paucity of empirical data does not allow us to confirm the above mentioned mechanism.
There is an intimate relationship between host competence (the ability to maintain and transmit infections) and species richness. Johnson et al. (2013) found that biodiversity decreases the disease outcome through an alteration in host competence. It has been predicted that there is a strong association between species richness, community competence and the individual characters of host species (Johnson et al., 2013). The importance of ‘Community Competence’ with reference to the biodiversity loss and outbreak of disease risk was also supported by Keesing et al. (2010). These studies indicated that the loss of biodiversity can affect the disease risk by altering the abundance, behavior and condition of the host or vector (Keesing et al., 2010). Moreover, they recognized that multiple mechanisms could also occur in different disease systems.
It is also important that ecologists need to understand the causes which are responsible for low and high host competence. As ecologists expect that the disease pattern may change due to global warming (Zargar, 2011), it is also essential to understand host competence under different ecological conditions. It is pertinent to understand the possible outcomes of the impact of increased host diversity on the infection pattern at different latitudes.
3. ‘Dilution effect’ hypothesis vs ‘amplification effect’ hypothesis
Biodiversity–disease relationships have been studied in a variety of ways by using different hypotheses. The most important hypothesis which has been mostly discussed and debated is the ‘dilution effect’ hypothesis. The ‘dilution effect’ hypothesis (DEH) stresses the fact that increased diversity will actually decrease the disease transmission (Fig. 1). DEH is supported by various studies (LoGiudice et al., 2003; Keesing et al., 2006). The Lyme and West Nile Virus diseases show an indirect relationship with the biodiversity. It has been opined that the expression of the Lyme disease is reduced when the diversity of hosts for ticks increases (LoGiudice et al., 2003). Similarly, higher avian biodiversity has been suggested to reduce Murine Norovirus (MNV; Ezenwa et al., 2006; Swaddle and Calos, 2008; Allan et al., 2009). Schmidt and Ostfeld (2001) in their model study of the Lyme disease found that with the increase in species richness there was a considerable decrease in the disease outcome, however no change was observed in evenness. There are some questions which are to be answered before we fully accept the ‘dilution effect’ mechanism vis-à-vis the biodiversity–disease relationship. For example, it is not well known how this mechanism operates across different climatic zones and how climatic change is related to this relationship.
Figure 1.
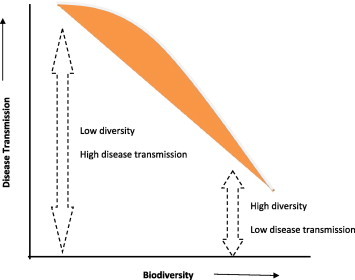
Diagrammatic representation of ‘dilution effect’ theory (based on LoGiudice et al., 2003 and Keesing et al., 2006).
The universality of dilution effect is under deep scrutiny. Critics differ in their opinion by suggesting that this mechanism has many loopholes (Randolph and Dobson, 2012). Dilution effect was found to be weak by Salkeld et al. (2013) while carrying out a meta-analysis of biodiversity–disease relationship. Out of 18 studies, they found that in 13 studies, disease occurrence shows negative correlation of size effect, while only 5 studies showed a positive correlation of the size effect (Fig. 2). However, average negative correlation with respect to size effect was very weak (r = −0.447; Fig. 3), thus suggesting that the dilution effect hypothesis cannot be generalized for all disease systems.
Figure 2.
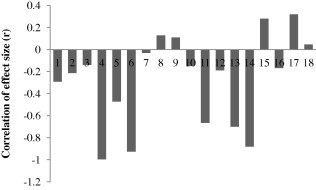
Correlation of effect size (r) of the biodiversity–disease relationship (Numbers 1, 2, 3…. indicate different studies which were carried out between 2005 and 2011; this figure is based on table 1 of Salkeld et al., 2013).
Figure 3.
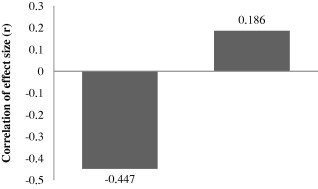
Average correlation of effect size (r) of the biodiversity–disease relationship (This figure is based on table 1 of Salkeld et al., 2013).
In recent years, the dilution and the amplification effect mechanisms of biodiversity–disease interaction have been checked under different conditions. In their study, Miller and Hupport (2013) developed a model by which they analyzed both mechanisms of biodiversity–disease interaction and also investigated the conditions under which these mechanisms occur. It was interesting to see that the diversity amplification effect takes place under conditions where the host has the highest transmission ability, while the dilution effect was seen where the vector showed hardly any preference (Miller and Hupport, 2013).
Amplification theory – that is there exists a positive correlation between biodiversity and disease risk, can be seen as very close to the classical epidemiological theory. Although the Classical Epidemiological Model does not link host–parasite association with the biodiversity, itpredicts that a reduction in the number of host species should also reduce rates of parasitization of the hosts (Lafferty and Kuris, 1999). However, it is pertinent to mention that this model has been applied to the fish–parasite disease system. According to Kermack and Mckendrick (1927) there is a minimum density of hosts, or a ‘‘host threshold’’, below which a disease would not invade. So, low density populations may have too few host interactions (Figs. 4 and 5). Persistence of disease in the host population is possible only if the host density is high enough to uphold transmission (Hamer, 1906; Black, 1966). These models in the later part of 1980’s were used for the management of fishery resources.
Figure 4.
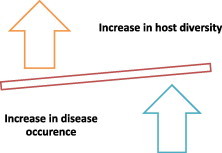
Increase in host diversity can increase the occurrence of disease (based on Kermack and Mckendrick, 1927).
Figure 5.
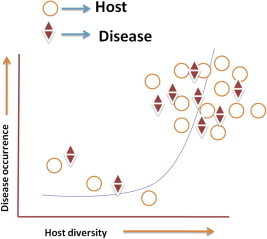
Disease occurrence increases with the increase in host diversity (based on Kermack and Mckendrick, 1927).
Ecologists and epidemiologists recognize the importance of host population threshold and critical community size (Bartlett, 1957) for the transmission of infectious disease (Grenfell and Dobson, 1995; Hudson et al., 2002; Deredec and Courchamp, 2003). There are, however, less empirical evidences which support the importance of thresholds in wildlife diseases (Lloyd-Smith et al., 2005). Moreover, evidences support that small sample size and confounding ecological factors are the major hurdles which diminish the possibilities for measuring the host thresholds (Lloyd-Smith et al., 2005). Top down and bottom up mechanistic approaches have been suggested for future investigation to find out a way to obtain best empirical evidence in favour of the host threshold (Lloyd-Smith et al., 2005). The understanding of the relationship between host abundance and disease transmission by taking the above measures will help to unravel dilution effect and amplification mechanisms in a better way. This will also carry forward the advances in understanding the biodiversity–disease relationships in an innovative way.
4. Generalist vs. specialist debate
Another important aspect which needs much attention is how diversity of specialist or generalist species alters disease propagation. There are evidences which show that loss of generalist species will favor more disease encounters (Keesing et al., 2010). In general, loss of host species will decrease the encounter between the host and the pathogen. The encounter rate will increase if lost species is not the host of a pathogen or the host species is a generalist one (Keesing et al., 2010). It is also due to the fact that generalist species have high adaptability to varied types of habitat and food resources and their immune competence is also low (Keesing et al., 2010). It is further speculated that specialist host species have narrow habitat and food resources and have advanced immune competence. So, loss of any specialist host species will actually decrease the disease transmission. However, arguments may differ among ecologists about such mechanisms operating under different climatic conditions because multiple factors are making their influence.
5. What are new issues related to Biodiversity–diseases relationship?
Biodiversity–disease relationship needs to be verified vis-à-vis climatic alteration. The response to climate change at small spatial scales as well as at the global scale is still unknown. It is interesting to see how climatic alteration affects biodiversity and then how change in biodiversity affects the disease outcome. It has been argued that ecologists should analyze the infection pattern in the context of biodiversity and ecological interaction (Lafferty, 2009).
Global warming is said to affect the disease pattern, and it is essential for epidemiologists to understand such patterns in relation to biodiversity. Such an approach can have a dramatic impact on the public health strategies for disease prevention and control. Climate change may have variable effects on different diseases; some diseases may be sensitive to climatic changes, while others may be less responsive (Zargar, 2011). Climate change may actually expand the range of vector borne diseases from the tropical zone, where the species diversity of hosts is comparatively high in contrast to the temperate climatic zone, where species diversity is very low (Dobson and Carper, 1992; Harvell et al., 2002). It is, however, early to predict the impact of biodiversity and global warming on the propagation of vector borne disease as the vector behavior and transmission mechanisms of the host differ (Miller and Huppert, 2013). It is also necessary to initiate innovative research and systematic monitoring programs to obtain first-hand information about the patterns of disease occurrence and relate it with biodiversity.
6. Conclusion
In sum, it can be concluded that biodiversity plays an important role in the transmission of diseases. However, the mechanism by which the biodiversity–disease relationship is controlled is still ambiguous, as biodiversity and disease pattern show varied degrees of complexities which need to be addressed in future studies. Although, dilution effect hypothesis is a far more accepted hypothesis among epidemiologists and ecologists, but it also has its shortcomings. It is early to imagine whether the biodiversity–disease relationship is influenced by the amplification effect as we have not understood well enough the different disease patterns in natural ecosystems. We suggest extensive studies on biodiversity–disease relationships in different ecological zones in order to unravel the hidden enigma behind this relationship. However, the task is not easy for ecologists as the dynamic nature of ecosystems poses difficulties in understanding various eco-based relationships.
Acknowledgements
The article published in the Nature, entitled “Biodiversity decreases disease through predictable changes in host community competence” actually moved us to write this recap. The 1st author viz., U.R. Zargar is highly obliged to Dr. Omar M. Amin, Professor at the Scottsdale University, USA for his constant encouragement. The authors are also thankful to the Director CORD, Prof. Bashir Ahmed Ganai for his support at the research centre.
Footnotes
Peer review under responsibility of King Saud University.
References
- Allan B.F., Langerhans R.B., Ryberg W.A., Landesman W.J., Griffin N.W., Katz R.S. Ecological correlates of risk and incidence of West Nile virus in the United States. Oecologia. 2009;158:699–708. doi: 10.1007/s00442-008-1169-9. [DOI] [PubMed] [Google Scholar]
- Bartlett M.S. Measles periodicity and community size. J. R. Stat. Soc. Ser. A. 1957;120:48–71. [Google Scholar]
- Black F.L. Measles endemicity in insular populations: critical community size and its evolutionary implication. J. Theor. Biol. 1966;11:207–211. doi: 10.1016/0022-5193(66)90161-5. [DOI] [PubMed] [Google Scholar]
- Deredec A., Courchamp F. Extinction thresholds in host–parasite dynamics. Ann. Zool. Fenn. 2003;40:115–130. [Google Scholar]
- Dobson A., Carper R. Yale University Press; Connecticut: 1992. Global Warming and Potential Changes in Host–Parasite and Disease–Vector Relationship; pp. 201–217. [Google Scholar]
- Ezenwa V.O., Godsey M.S., King R.J., Guptill S.C. Avian diversity and West Nile virus: testing associations between biodiversity and infectious disease risk. Proc. R. Soc. B. 2006;273:109–117. doi: 10.1098/rspb.2005.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenfell B.T., Dobson A.P., editors. Ecology of Infectious Diseases in Natural Populations. Cambridge University Press; 1995. [Google Scholar]
- Hamer W.H. Epidemic diseases in England. Lancet. 1906;1:733–739. [Google Scholar]
- Harvell C.D., Mitchell C.E., Ward J.R., Altizer S., Dobson A.P. Ecology – climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- Hudson P.J., editor. The Ecology of Wildlife Diseases. Oxford University Press; 2002. [Google Scholar]
- Johnson R.T.J., Preston D.L., Hoverman J.T., Richgels K.L.D. Biodiversity decreases disease through predictable changes in host community competence. Nature. 2013;494:230–233. doi: 10.1038/nature11883. [DOI] [PubMed] [Google Scholar]
- Keesing F., Holt R.D., Ostfeld R.S. Effects of species diversity on disease risk. Ecol. Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Keesing F., Belden L.K., Daszak P., Dobson A., Harvell D.C., Holt R.D., Hudson P., Jolles A., Jones K.E., Mitchell C.E., Myers S.S., Bogich T., Ostfeld R.S. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermack W.O., Mckendrick A.G. A contribution to the mathematical theory of epidemics. Part 3. In further studies of the problem of endemicity. Proc. R. Soc. Lond. A: Mat. 1927;141:92–122. [Google Scholar]
- Lafferty K.D. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- Lafferty K.D., Kuris A.M. How environmental stress affects the impacts of parasites. Limnol. Oceanogr. 1999;44:925–931. [Google Scholar]
- Lloyd-Smith J.O., Cross P.C., Briggs C.J., Daugherty M., Getz W.M., Latto J., Sanchez M.S., Smith A.B., Swei A. Should we expect population thresholds for wildlife disease? Trends Ecol. Evol. 2005;20:511–519. doi: 10.1016/j.tree.2005.07.004. [DOI] [PubMed] [Google Scholar]
- LoGiudice K., Ostfeld R.S., Schmidt K.A., Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. U.S.A. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E., Huppert A. The effects of host diversity on vector-borne disease: the conditions under which diversity will amplify or dilute the disease risk. PLoS ONE. 2013;8:e80279. doi: 10.1371/journal.pone.0080279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph S.E., Dobson A.D.M. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology. 2012;139:847–863. doi: 10.1017/S0031182012000200. [DOI] [PubMed] [Google Scholar]
- Salkeld D.J., Padgett K.A., Jones J.H. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol. Lett. 2013;16:679–686. doi: 10.1111/ele.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K.A., Ostfeld R.S. Biodiversity and the dilution effect in disease ecology. Ecology. 2001;82:609–619. [Google Scholar]
- Swaddle J.P., Calos S.E. Increased avian diversity is associated with lower incidence of human West Nile infection: observation of the dilution effect. PLoS ONE. 2008;3:e2488. doi: 10.1371/journal.pone.0002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargar, U.R., 2011. Proceed with caution on disease eradication. Science and Development Network. http://www.scidev.net/en/health/health-policy/editor-letters/proceed-with-caution-on-diseaseeradication-1.html (accessed 03.09.12).



