Abstract
Gastroretentive levofloxacin (LVF) floating mini-tablets for the eradication of Helicobacter pylori (H. pylori) were prepared using the matrix forming polymer hydroxypropyl methylcellulose (HPMC K100M), alone or with Carbopol 940P in different ratios by wet granulation technique. Buoyancy of mini-tablets was achieved by an addition of an effervescent mixture consisting of sodium bicarbonate and anhydrous citric acid to some formulations. The prepared mini-tablets were evaluated for weight variation, thickness, friability, hardness, drug content, in vitro buoyancy, water uptake and in vitro release. The optimized formula was subjected to further studies: FT-IR, DSC analysis and in vivo examination in healthy volunteers. The prepared mini-tablets exhibited satisfactory physicochemical characteristics. Incorporation of gas-generating agent improved the floating parameters. HPMC K100M mini-tablet formulation (F1) offered the best controlled drug release (>8 h) along with floating lag time <1 s and total floating time >24 h. The obtained DSC thermograms and FT-IR charts indicated that there is no positive evidence for the interaction between LVF and ingredients of the optimized formula. The in vivo test confirmed the success of the optimized formula F1 in being retained in the stomach of the volunteers for more than 4 h. LVF floating mini-tablets based on HPMC K100M is a promising formulation for eradication of H. pylori.
Abbreviations: LVF, levofloxacin; H. pylori, Helicobacter pylori; HPMC, hydroxypropyl methylcellulose; FT-IR, Fourier transform infrared spectroscopy; DSC, differential scanning calorimetry; PVP, polyvinyl pyrrolidone; SI, swelling index
Keywords: Gastroretentive, Levofloxacin, Helicobacter pylori, Floating, Mini-tablets, Fluoroquinolones
1. Introduction
Helicobacter pylori (H. pylori) infection is the causative organism in chronic active gastritis, duodenal ulcers and gastric adenocarcinoma (Khalifa et al., 2010). This bacterium is highly adapted for colonization in the human stomach, the majority of these bacteria are free living in the gastric mucus layer although about 20% is in close contact with epithelial cells (Hessey et al., 1990). Antimicrobial resistance, patient’s poor compliance with the antibiotic regimen, and drug-related side effects are said to be the major problems with eradication of H. pylori (Vakil, 2005). Levofloxacin (LVF) is a broad spectrum third-generation fluoroquinolone antibiotic (Kassab et al., 2010). Some studies have demonstrated that LVF has a remarkable in vitro activity against H. pylori when its strains are resistant to clarithromycin and metronidazole (Antos et al., 2006). These favorable results have been confirmed in vivo, indicating that most of the patients with both metronidazole and clarithromycin resistance are cured with the LVF-based regimen. LVF has shown promising results in different first-line triple regimens in several countries, with an eradication rate ranging from 72% to 96% (Gisbert and Pajares, 2010).
Complete eradication of H. pylori requires high concentrations of antibiotics to be maintained within gastric mucosa for prolonged period of time (Nayak et al., 2010). This approach can be achieved by preparing gastroretentive dosage forms that ensure prolonged drug release near the ecological niche of the bacterium (Bardonnet et al., 2006).
Floating drug delivery systems are those systems having a bulk density less than that of the gastric fluids and thus these systems remain buoyant for a prolonged period of time in the stomach without being affected by the gastric emptying rate. The drug is released slowly at the desired rate from the system and after release of the drug; the residual system is emptied from the stomach (Sharma et al., 2011). Most of the floating systems previously reported are single unit systems such as tablets and capsules. Multiple unit floating drug delivery systems, such as pellets or mini-tablets, show several advantages over monolithic ones, which include avoiding all or nothing emptying, more predictable drug release kinetics, less chance of localized mucosal damage and administration of units with different release profiles or containing incompatible substances (Ishak et al., 2007, Christian et al., 2011). Mini-tablets offer an alternative for pellets because of their relative ease of manufacturing. In addition, they offer dosage forms of equal dimensions and weight with smooth regular surface that could be obtained in a reproducible and continuous way. Mini-tablets could be either filled into hard capsules or compacted into bigger tablets (Lopes et al., 2006). In the present study, gastroretentive floating LVF mini-tablets, for the eradication of H. pylori were prepared and evaluated.
2. Materials and methods
2.1. Materials
Levofloxacin hemihydrate antibiotic (Zhejiang Apeloa Kangyu Pharmaceutical Co., Ltd., China). Methocel™ K100M Premium DC (Hydroxypropyl methyl cellulose), with an apparent viscosity 75,000–140,000 mPa s for a 2% solution in water at 20 °C (The Dow Chemical Company and Colorcon, Inc., USA). Carbopol® 940 NF polymer, with viscosity 40,000–60,000 mPa s (0.5% mucilage at 25 °C) (Lubrizol Advanced Materials, Inc., Calvert City, KY, USA). Barium sulfate for oral and rectal use (Commercial Firm for Chemicals and Pharmacies Supply, Turkey). Levoxin® (Amoun Pharmaceutical Co., Egypt). All other chemicals and solvents were of analytical grade.
2.2. Preparation of mini-tablets by wet granulation technique
Composition of mini-tablet formulations is listed in Table 1. All ingredients were weighed, and mixed using the geometric dilution technique except magnesium stearate. The mixture was granulated using isopropyl alcohol. Polyvinyl pyrrolidone (PVP) was added as a binder to the granulating solvent by 5% whenever needed. The obtained dough mass was passed through 1.4 mm mesh sieve to prepare the granules. The granules were dried at 60 °C in the thermostatic hot air oven. Dried granules were ground in a mortar and then sieved. Granules that passed sieve No. 45 (355 μm) were used. Magnesium stearate was then added as 2%. Mini-tablets, weighing 50 mg, were obtained using a single punch tablet press fitted with a 4 mm diameter concave punch. Each dose comprised 14 mini-tablets which are equivalent to 250 mg LVF.
Table 1.
Composition of mini-tablet formulations.
| Formula | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| Ingredients | mg | ||||
| Levofloxacin | 250 | ||||
| Citric Acid | 65 | 65 | 65 | ||
| NaHCO3 | 130 | 130 | 130 | ||
| Carbopol 940P | 218 | 120.5 | 145.3 | 80.3 | |
| HPMC K100M | 241 | 218 | 120.5 | 290.6 | 160.6 |
| Magnesium stearate (2%) | 14 | ||||
| Total weight | 700 | ||||
2.3. Evaluation of the pre-compression parameters of powder mixtures
Pre-compression parameters: bulk density, tapped density, angle of repose, Carr’s index and Hausner’s ratio (Hadi et al., 2012), were measured.
2.4. Evaluation of the post-compression parameters of LVF mini-tablets
Compressed mini-tablets were characterized for weight variation, crushing strength, diameter, thickness and friability as follows:
2.4.1. Weight variation of mini-tablets
The weight variation test was conducted by weighing 20 randomly selected mini-tablets individually (Rao et al., 2012). The average weight and standard deviation were calculated.
2.4.2. Diameter and thickness for mini-tablets
The diameter and thickness of ten randomly selected mini-tablets from each formulation were measured with a Vernier caliper scale. The average and standard deviation were reported.
2.4.3. Mini-tablet crushing strength/ hardness test
Crushing strength of the mini-tablets was determined using the tablet hardness tester (Dr. Schleuniger Pharmatron AG, USA). Hardness was determined using six mini-tablets from each formulation and crushing strength that just caused the mini-tablet to break was recorded (Al Remawia et al., 2013). The average of 6 records expressed in Newton was used.
2.4.4. Friability test for mini-tablets
Friability test was carried out by using Copley friability tester FRV2000 (Copley® Nottingham, UK) (Sathali and Ganesan, 2012).
2.4.5. Content uniformity test
Ten mini-tablets of the chosen formula (F1) were randomly selected and weighed. Each one was crushed individually and dissolved in 50 ml 0.1 N HCl. The volume was adjusted to 50 ml using 0.1 N HCl and then filtered. Samples were assayed for LVF content spectrophotometrically at predetermined λmax 294 nm and the drug concentration in each mini-tablet was calculated, after suitable dilution. The drug content in each mini-tablet was compared to the label claim.
The drug content for the other formulations was calculated from their corresponding absorbance values at 24 h during the release study.
Drug content for the selected formulation was also tested by crushing 20 mini-tablets and the blend equivalent to 250 mg of LVF was weighed, dissolved in 0.1 N HCl, filtered and suitably diluted. The drug content was analyzed spectrophotometrically at λmax 294 nm.
2.4.6. In vitro buoyancy test
In vitro buoyancy was determined by measuring buoyancy lag time and total floating duration (Jagdale et al., 2009). The buoyancy test was performed using the USP dissolution apparatus II containing 900 ml of 0.1 N HCl as the dissolution medium was maintained at 37 ± 0.5 °C. Fourteen mini-tablets were spread over the surface and the medium was rotated at 75 rpm for 24 h. The measurements of both floating lag time and total floating time were carried out for each formulation. The floating and the settled mini-tablets were also observed visually and the results were presented as % floating after 4 h. Matrix integrity was also observed throughout the in vitro buoyancy studies.
2.4.7. In vitro drug release test and modeling of drug release profiles
Fourteen mini-tablets containing 250 mg LVF were placed in 900 ml 0.1 N HCl as a dissolution medium was maintained at 37 ± 0.5 °C. Drug release was performed using a USP type II apparatus at 75 rpm for 24 h. Aliquots of 5 ml were withdrawn at specified intervals of time, filtered and replenished with 5 ml fresh dissolution medium. Samples’ absorbance was measured at λmax 294 nm after suitable dilution (Mouzam et al., 2011). The studies were performed in triplicate. The cumulative % of LVF released was calculated at each time interval.
Drug release data were analyzed according to zero order, first order, Higuchi, Hixon-Crowell, Peppas and Weibull kinetic equations (Dash et al., 2010). DDSolver, an add-in program for Microsoft Excel, for modeling and comparison of drug release profiles was used (Zhang et al., 2010).
The model with the highest coefficient of determination (R2) was considered to be the best fitting one.
2.5. Water-uptake study
Water-uptake was carried out for all mini-tablets according to the method adopted by Chinthala et al. (2012) with modifications. The study was performed in duplicates. Mini-tablets were individually weighed and each one was transferred into a beaker containing 25 ml 0.1 N HCl at 37 ± 0.5 °C and stirred at 25 rpm. At the specified intervals of time, each mini-tablet was gently wiped with a filter paper to remove surface water, and re-weighed. The degree of swelling was calculated according to the following equation:
where: S = the degree of swelling/swelling index or water-uptake (%); Ws the weight of swollen mini-tablet and Wd the weight of dry mini-tablet.
2.6. Viscosity measurement
Gels (2%) of HPMC K100M and HPMC K100M: Carbopol 1:1 and 2:1 were prepared in distilled water. Brookfield RV DV-II viscometer (Brookfield Engineering Laboratories, USA) equipped with spindle number 15 was used for viscosity measurements. All the rheological studies were carried out at 20 °C.
2.7. Fourier transform infrared (FT-IR) spectroscopy
FT-IR spectra were obtained for the pure drug, polymer and selected formula in the range of 4000–500 cm−1 using (Perkin-Elmer, USA).
2.8. Differential scanning calorimetry (DSC)
Samples (5 mg) of LVF, HPMC K100M, PVP, physical mixture of LVF with HPMC K100M and mixture prepared by wet granulation technique of LVF with HPMC K100M using PVP as binder were accurately weighed in aluminum pans and hermetically sealed. All samples were scanned at 10 °C/min under nitrogen gas purge over temperature range from 25–350 °C.
2.9. In vivo study in healthy volunteers
Three healthy volunteers participated in the study after giving informed written consent. The subjects’ ages ranged from 29 to 33 years and weights from 70 to 82 kg. Health status of the volunteers was confirmed by complete medical history and physical examination. The subjects were instructed to take no medicines for 1 week prior to and during the course of the study. The study was approved by the ethics committee of faculty of medicine, Alexandria University.
To make the chosen formula (F1) X-ray opaque, 140 mg of the drug was replaced with barium sulfate (BaSO4) and all other ingredients were kept constant. This amount was determined experimentally to allow X-ray visibility but not to hinder tablet buoyancy. After overnight fasting, the volunteers were fed with a low calorie food. The barium sulfate-labeled fourteen mini-tablets combined in capsules were then given to every subject with 200 ml of water. The volunteers were exposed to abdominal X-ray imaging in a standing position before administration and 2 and 4 h post-administration of mini-tablets.
2.10. Statistical analysis
The resulting data were analyzed by using the software SPSS 17.0 (SPSS Inc., Chicago, USA) applying a two-way ANOVA. Post hoc multiple comparisons were applied when necessary. Differences between formulations were considered to be significant at p ⩽ 0.05.
3. Results and discussion
3.1. Pre-compression parameters of powder mixtures
The angle of repose of the powder mixture for all formulations (F1–F5) ranged from 25.9° to 27.7° indicating excellent flow properties (Couto et al., 2013). Bulk and tapped density of the powder mixture for all formulations varied from 0.358 to 0.455 gm/cm3 and from 0.432 to 0.581 gm/cm3, respectively. Hausner’s ratios and compressibility indices ranged from 1.2 to 1.27 and 18.54% to 21.68%, respectively. The results of flow properties are acceptable for granules (Trivedi et al., 2008).The values of compressibility indices further confirmed the good compressibility of the prepared granules (Wilson et al., 2011).
3.2. Post-compression parameters for mini-tablets
Concerning appearance, the mini-tablets were light yellow, whitish-buff or white in color, all were round concave, with smooth surface in both sides and no visible cracks were observed.
The mean diameter of mini-tablets was 4.0 ± 0.0 mm while mean thickness ranged from 3.2 to 4.7 mm. Mean hardness was in the range of 31.7–54.7 N indicating that the mini-tablets are of sufficient strength to withstand physical abrasion (Manivannan and Chakole, 2011). The percentage friability for all formulations was less than 1% which is an indication of satisfactory mechanical resistance of the mini-tablets (Savic et al., 2013). The mini-tablets showed no evidence of capping, cracking, cleavage or breaking after being removed from the friabilator. The percentage of mean drug content (14 mini-tablets) ranged from 95.7–98.3% which met the standard pharmacopeial requirements (90–110%) (Garg and Gupta, 2013).Since the mixtures of powders used were free flowing, the obtained mini-tablets were of uniform weight due to uniform die fill. The mean weight of mini-tablets was 50 ± 0.0 mg, (n = 20). The USP specification is generally ±10% for tablets weighing 130 mg or less (Bano et al., 2011). This means that no difference was observed in the weight of individual mini-tablets from the labeled weight indicating uniformity of weight.
3.3. In vitro buoyancy test
In the present study the floating system employed sodium bicarbonate (NaHCO3) and citric acid in an optimized ratio (2:1) as gas forming mixture (Pare et al., 2008). This ratio was used in order to provide the shortest possible floating lag time and floating duration of up to 24 h. Sodium bicarbonate induced effervescence that leads to pore formation and consequently, rapid hydration of the mini-tablets’ matrices thus enhancing their floating ability (Tadros, 2010).
Table 2 shows the results of floating lag time and total floating time of different mini-tablet formulations (F1–F5).
Table 2.
Floating properties of LVF mini-tablets.
| Formula | Floating lag time | Total floating time (h) |
|---|---|---|
| HPMC K100M Efferv. (F1) | 1 s | >24 |
| HPMC K100M: Carbopol (1:1) (F2) | 30 min | 2 |
| HPMC K100M: Carbopol (1:1), Efferv. (F3) | 1 s | 8 (after 24 h half are below, half were suspended in the middle) |
| HPMC K100M: Carbopol (2:1) (F4) | 20 min | >24 (11 floated while 3 sank) |
| HPMC K100M: Carbopol (2:1), Efferv. (F5) | 1 s | >24 |
Efferv.: containing effervescent mixture.
Effervescent formulations containing HPMC K100M (F1, F3 and F5) showed good floating lag time and total floating time. This might be due to the rapid hydration and subsequent formation of viscous gelatinous layer when HPMC K100M was exposed to an aqueous medium (Basak and Jayakumar Reddy, 2006). This viscous gelatinous layer prevented escape of evolved carbon dioxide from the formed matrices leading to decreased density, consequently, short time was needed for floating (Prajapati et al., 2008). For effervescent (F3, F5) and non-effervescent (F2, F4) formulations, as the ratio of Carbopol in the polymer blend increased, the floating time decreased. This observation was in agreement with (Pare et al. (2008) who stated that Carbopol had a negative effect on floating behavior and it was used only for its drug release retardant characteristics. Another study by Li et al. (2003) showed the same negative effect of Carbopol on the floating behavior of the delivery system. This was explained on the basis that the moisture gain for Carbopol is significantly higher compared to moisture gain for HPMC K4M (55% weight gain for Carbopol vs. 33% for HPMC K4M at RH of 95%) which results in a dramatic increase in the density of the floating drug delivery system that, in turn, shows a corresponding decrease in its floating capacity (Li et al., 2003). HPMC K100M non-effervescent mini-tablet formulations, F2 and F4, showed a longer floating lag time of 30 and 20 min and a variable total floating time of 2 h and >24 h, respectively. Reduction in HPMC K100M level in F2 compared to F4 mini-tablet formulations, prolonged the floating lag time and shortened the total floating time, this was in agreement with the previously reported study by Shah et al. (2010).
3.4. In vitro drug release, modeling of drug release profiles and water uptake
The release profiles of LVF from HPMC K100M based mini-tablets are shown in Fig. 1.
Figure 1.
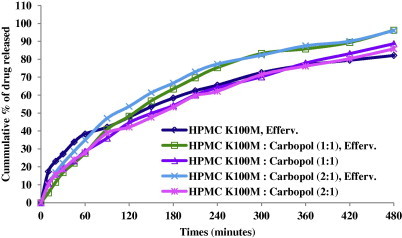
The release profiles for different HPMC K100M based mini-tablet formulations in 0.1 N HCl at 37 ± 0.5 °C (n = 3).
Concerning effervescent formulations F1, F3 and F5: Formula F1 containing HPMC K100M exhibited burst release since about 17% LVF released in 10 min. Whereas, formulae F3 and F5 containing mixture of HPMC K100M and Carbopol 940P in a ratio 1:1 and 2:1 released 5% and 10% LVF, respectively, in 10 min. The initial burst effect for F1 could be due to rapid dissolution of the drug from the surface while the HPMC K100M undergoes hydration to form a protective gel layer (Tiwari and Rajabi-Siahboomi, 2009). The addition of Carbopol as an anionic polymer in the matrix decreased the drug release in the acidic medium by forming an insoluble mass that acts as a barrier to drug diffusion (Tatavarti et al., 2004) and, consequently, the initial burst effect was decreased.
A two-way ANOVA of the percent drug released from various HPMC K100M mini-tablet formulations showed statistically significant difference (P ⩽ 0.0001) for the effect of the type of formulations on the percent drug released. The results of Duncan test (Table 3) showed that the release of LVF from the prepared formulations was in the following order F5 > F1 = F3 > F2 = F4.
Table 3.
Mean and standard error of the percent LVF released from various mini-tablet formulations (Duncan test).
| Formula | Mean % drug released ± SE |
|---|---|
| F1 | 49.76 ± 0.525b |
| F2 | 45.38 ± 0.525c |
| F3 | 49.39 ± 0.525b |
| F4 | 44.60 ± 0.525c |
| F5 | 52.96 ± 0.525a |
N.B. Means of similar symbols are statistically insignificant a > b > c.
The effervescent formulae containing HPMC K100M (F1) and HPMC K100M: Carbopol 940P (1:1) (F3) had the same overall mean drug released as shown in Table 3 (Duncan test). However, the rate of drug release from F1 and F3 was different. At 0.5 h around 27% and 17% LVF was released from F1 and F3, respectively. On the other hand, at 8 h about 80% and 96% LVF was released from F1 and F3 mini-tablets, respectively. This could be explained based on the difference in viscosities in each formulation. A mixture of HPMC K100M with Carbopol 940P increased the release rate due to the decreased amount of the more viscous polymer (HPMC K100M) that is replaced by a less viscous polymer (Carbopol 940P). These results were confirmed by viscosities’ measurements where HPMC K100M showed the high viscosity, while HPMC K100M: Carbopol (2:1) had an intermediate viscosity and finally the (1:1) ratio had the lowest viscosity. It is obvious that as much as the contribution of the higher viscosity polymer increased the drug release decreased. It was concluded that polymer viscosity had a major influence on the drug release from hydrophilic matrix tablets.
Fig. 2 shows the viscosities of 2% w/v aqueous solution of HPMC K100M either alone or in combination with Carbopol 940P in ratios (1:1) and (2:1).
Figure 2.
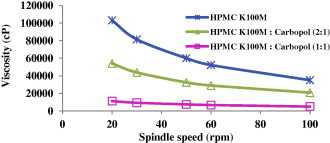
Viscosity of 2% w/v aqueous solution of polymer or polymers mixture at 20 °C vs. spindle speed.
Concerning F5, it showed a higher overall mean drug released than both F1 and F3 as revealed by statistical analysis (Duncan test). Besides the effect of viscosities of the used polymers, the presence of effervescent components that created uncontrollable numbers of pores along the formed matrices affects LVF release patterns from F1, F3 and F5 mini-tablets.
Although F5 contains a higher proportion of the viscous polymer (HPMC K100M) than F3, the release of LVF from F5 was higher than from F3. The presence of the effervescent component, sodium bicarbonate, formed a large number of pores along the matrices of the mini-tablets. It has been also documented that sodium bicarbonate increased the cummulative amount of drug released from HPMC but not that released from Carbopol. This was explained based on the difference in hydration characteristics of the two polymers; the addition of sodium bicarbonate caused an increase of the hydration volume that leads to matrix volume expansion in case of HPMC (Gutiérrez-Sánchez et al., 2008).
It is concluded that both the type of polymer used and the presence of effervescence components affect the % of LVF released from HPMC K100M mini-tablet formulations.
Concerning the non-effervescent formulae F2 and F4 mini-tablets containing HPMC K100M: Carbopol 940P in ratios (1:1) and (2:1), respectively, both resulted in the same overall mean drug released according to Duncan test. They offered the slowest release rate among formulations containing HPMC K100M. This could be explained based on the absence of gas-forming agents and consequently the absence of effervescence and pore formation. As a result there was slower hydration of the mini- matrices leading to a slower drug release rate (Tadros, 2010). This finding was in agreement with Tadros (2010) who found that there was an inverse relationship between the investigated concentration of the gas forming agent and the drug release rate.
Unfortunately, F2 and F4 mini-tablets showed 30 and 20 min floating lag time, respectively, as mentioned in Table 2. They represented the longest floating lag time therefore they could not be considered successful ones.
The swelling indices of different HPMC K100M mini-tablet formulations (F1–F5) after being immersed for 4 h in 0.1 N HCl are shown in Fig. 3.
Figure 3.
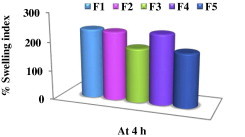
Swelling indices of HPMC K100M mini-tablet formulations after 4 h in 0.1 N HCl at 37 °C.
Concerning effervescent mini-tablet formulations F1, F3 and F5:
Results revealed that F1 showed the highest % SI (240.57%) after 4 h. This high degree of swelling is attributed to the presence of the highly hydrophilic hydroxypropoxyl groups in HPMC K100M (Vueba et al., 2004). The swelling indices of both F3 and F5 formulations were less than that of F1 at the same time. This resulted in a higher percent drug released from both F3 and F5 at 4 h compared to F1 as shown in Fig. 2. The differences in swelling indices for F1, F3 and F5 could be due to a lesser proportion of the hydrophilic polymer HPMC K100M in both F3 and F5 compared to F1.
Concerning non-effervescent mini-tablet formulations F2 and F4:
The % SI for F2 (237%) and F4 (240%) were nearly equal which supported the statistical insignificant difference in their overall mean drug released according to Duncan test (Table 3).
The swelling indices of the non-effervescent formulae (F2 and F4) were greater than their corresponding effervescent formulae (F3 and F5), respectively. This could be a reason for the lower mean drug released observed from F2 and F4 compared to that of F3 and F5 mini-tablets as previously mentioned in Duncan test (Table 3).
The carbon di-oxide bubbles produced by sodium bicarbonate expand the matrices facilitating the drug dissolution and their presence might also obstruct the diffusion path through the hydrated gel layer (Lara-Hernández et al., 2009). This explains the difference in release and swelling behaviors between the effervescent and non effervescent mini-tablet formulations.
All HPMC K100M mini-tablet formulations (F1–F5) kept their integrity throughout the swelling studies.
Over time, the matrix of the tablets was deformed because there is a slow diminution of the matrix thickness due to polymer dissolution. The polymer in the matrix undergoes simultaneous swelling, dissolution and diffusion into the bulk medium, resulting in erosion and reduction of the matrix strength. It is also considered that the gas bubbles dissipating from the inside to the outside of the matrix debilitate the matrix structure (Lara-Hernández et al., 2009).
To explain the release kinetics of LVF from mini-tablets, the data of in vitro release experiment were treated according to the model-dependent methods, zero order, first order, Higuchi model, Korsmeyer–Peppas model, Hixson–Crowell model and Weibull equation.
Criteria for selecting the most appropriate model was based on best fit indicated by the value of coefficient of determination (R2) nearer to 1 (Prabakaran et al., 2003).
Concerning F1, F2 and F4 the highest values of R2 were obtained after fitting the data into Peppas equation. The value of (n) allows the release to be characterized as either Fickian diffusion n ⩽ 0.5, anomalous diffusion (non-Fickian) (0.5 < n < 1) or zero-order release (n = 1) (Costa and Sousa Lobo, 2001). The n values for F2 and F4 were 0.53 and 0.56 respectively, that indicated anomalous diffusion (non-Fickian) which refers to a combination of both diffusion and erosion controlled-drug release (Sabar et al., 2011). Whereas, the value of (n) in case of F1 (n = 0.39) revealed a Fickian diffusion mechanism of LVF from these mini-tablets.
F3 had the same coefficient of determination, R2 = 0.999, for first order as well as for Weibull equation. The first order describes the release from system where release rate is concentration dependent therefore LVF released from F3 mini-tablets was proportional to the amount of LVF remaining in its interior, in such way, that the amount of LVF released diminished by unit of time (Costa and Sousa Lobo, 2001). Weibull model helped in describing the shape of the release curve of F3 deduced from its β value (1.02) which was found to be exponential (Costa and Sousa Lobo, 2001).
F5 followed Weibull’s equation (R2 = 0.999). F5 had β value (0.87) so the curve was supposed to be parabolic, with a higher initial slope and after that consistent with the exponential (Costa and Sousa Lobo, 2001).
Based on the above results, it concluded that the effervescent LVF mini-tablet formulation (F1) that contained HPMC K100M was optimum, with respect to its acceptable post-compression parameters, its excellent floating characteristics as well as its prolonged drug release. It could provide such controlled release profile using single polymer which is more beneficial as it decreases the liability to any drug–polymer interaction or any incompatibility between ingredients of the prepared mini-tablets. Therefore F1 was selected for further studies; FT-IR spectroscopy, DSC, comparison to the marketed LVF product (Levoxin®) and in vivo test.
3.5. FT-IR spectroscopy and DSC studies
Drug-excipients compatibility study to F1 formulation was conducted using FT-IR spectroscopy and DSC study. IR spectra were obtained for LVF, polymer used and a blend of them prepared by wet granulation technique.
The LVF FT-IR charts got characteristic absorption peaks for the –OH group of the –COOH moiety at around 3261.4 cm−1 and –C O peak near 1725.1 cm−1. The aromatic C–H peaks are also observed in the range 2900–3000 cm−1. Charts for mixture of LVF with HPMC K100M polymer used in mini-tablet formulations exhibited characteristic absorption peaks in the same range of pure drug peaks. Hence, it could be confirmed that there is no interaction between drug and excipients in this formulation (Doodipala et al., 2011).
DSC study for the selected formulation F1, was also conducted. The thermograms of drug, physical mixture of drug and HPMC K100M, and a mixture (LVF, HPMC K100M and PVP) prepared by wet granulation technique are shown in Fig. 4. The LVF DSC thermogram (Fig. 4a) showed an endothermic peak at ∼91 °C due to the dehydration of LVF hemihydrate. In addition an endothermic peak at ∼227 °C due to the melting of the γ form, an endothermic peak at ∼230 °C due to the melting of the β form and an endothermic peak at ∼231 °C due to the melting of the α form were also observed. Kitaoka et al. (1995) studied the effect of dehydration on the formation of LVF pseudopolymorphs and the effect of heating rate on DSC curves of LVF.
Figure 4.
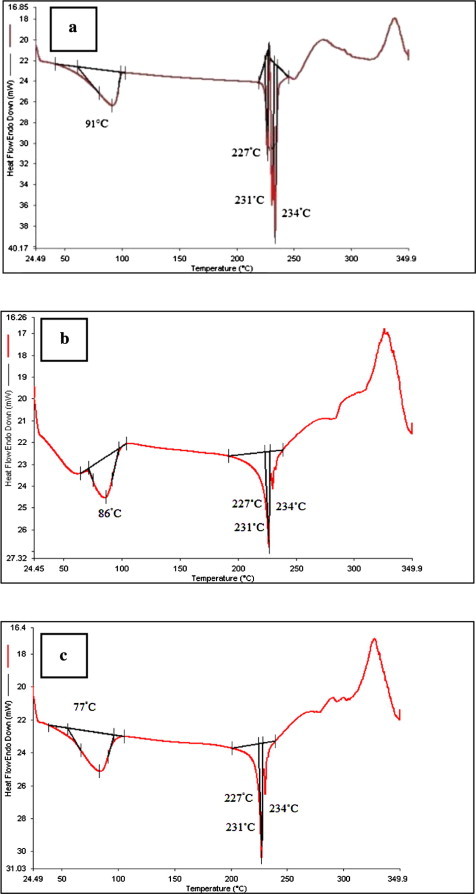
DSC thermograms for: (a) LVF hemihydrate, (b) the mixture of LVF and HPMC K100M prepared by wet granulation technique using isopropyl alcohol and PVP and (c) the physical mixture of LVF and HPMC K100M.
In the present study, the LVF peaks were in agreement with the documented DSC chart in the literature under the same heating rate 10 °C/min (Kitaoka et al., 1995). The observed exothermic peaks might be due to the crystallization of an amorphous form that resulted partially from LVF dehydration (Kitaoka et al., 1995). The DSC thermogram of the optimized formulation (F1) showed a slight change in the peak shape with little broadening (Fig. 4c) which could be attributed to the mixing process that lowers the purity of each component in the mixture (Verma and Garg, 2004). The obtained thermograms indicated that there is no positive evidence for the interaction between LVF and ingredients of the optimized formulation.
3.6. Comparison of the selected mini-tablet formulations with the marketed LVF product (Levoxin®)
The results of the comparative in vitro release study of optimized formula F1 with marketed LVF (Fig. 5) showed that F1 released 28.52% LVF in 30 min, whereas the marketed product released 97.25% LVF in the same duration. F1 released 82.1% LVF in 8 h with T50% of 126.8 min and floating lag time <1 s and total floating time >24 h.
Figure 5.
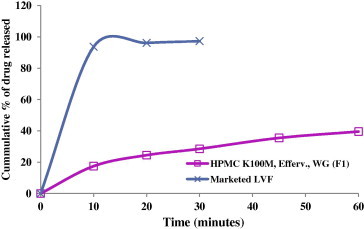
Comparison of the release profiles of optimized formula F1 and marketed LVF (Levoxin®) in 0.1 N HCl at 37 ± 0.5 °C.
3.7. In vivo test
It has to be pointed out that good in vitro floating behavior alone is not a sufficient proof for efficient gastric retention in vivo. The effects of simultaneous presence of food and of the complex motility of the stomach are difficult to estimate. Obviously in vivo studies can provide a definite proof that prolonged gastric residence could be obtained.
A radiological method was adopted to monitor the units in the gastric region of humans in feeding conditions. The obtained radiographs obtained at 0, 2 and 4 h are shown in Fig. 6. At zero time, the stomach was free from any radio-opaque materials. Almost all of the floating mini-tablets were still buoyant on the gastric content up to 4 h.
Figure 6.
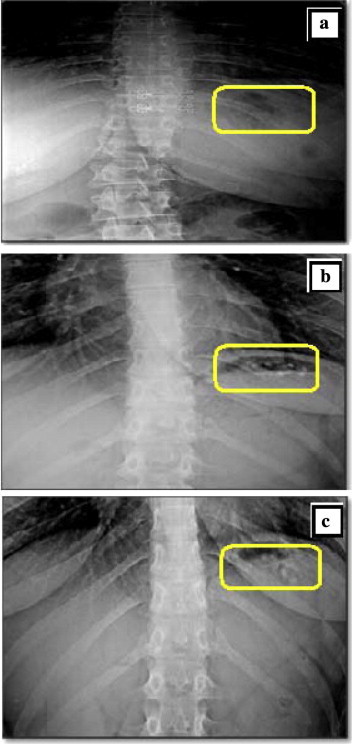
X-ray photographs of the gastric region of a healthy volunteer: (a) before administration of mini-tablets, (b) 2 h after dosing of mini-tablets-in-capsule system in the fed state and (c) 4 h after dosing in the same condition. N.B. b and c show LVF mini-tablets floating over the gastric content of a volunteer.
4. Conclusion
According to the above results, HPMC K100M mini-tablet formulation (F1) offered best LVF controlled release along with floating lag time <1 s and total floating time >24 h. The optimized formula (F1) showed the absence of interaction between LVF and the used polymer/additives which confirmed the compatibility among its ingredients. In vivo studies can provide a definite proof that prolonged gastric residence could be obtained. The in vivo test confirmed the success of the optimized formula (F1) in being retained in the stomach of the volunteer over the tested period providing localized LVF release.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al Remawia M., Al-akayleh F., Salem M., Al Shami M., Badwan A. Application of excipient made from Chitosan-Xanthan as a single component for the controlled release of ambroxol tablet. J. Excipients Food. Chem. 2013;4:48–57. [Google Scholar]
- Antos D., Schneider-Brachert W., Bästlein E., Hänel C., Haferland C., Buchner M., Meier E., Trump F., Stolte M., Lehn N. 7-Day triple therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and high-dose esomeprazole in patients with known antimicrobial sensitivity. Helicobacter. 2006;11:39–45. doi: 10.1111/j.0083-8703.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- Bano R., Gauhar S., Naqvi S.B.S., Mahmood S. Pharmaceutical evaluation of different brands of levofloxacin tablets (250 mg) available in local market of Karachi (Pakistan) Int. J. Curr. Pharm. Res. 2011;3:15–22. [Google Scholar]
- Bardonnet P., Faivre V., Pugh W., Piffaretti J., Falson F. Gastroretentive dosage forms: overview and special case of Helicobacter pylori. J. Control Release. 2006;111:1–18. doi: 10.1016/j.jconrel.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Basak S., Jayakumar Reddy B. Formulation and release behaviour of sustained release ambroxol hydrochloride HPMC matrix tablet. Indian J. Pharm. Sci. 2006;68:594–598. [Google Scholar]
- Chinthala C.S.K., Kota K.S.R., Hadassah M., Metilda E.H., Sridevi S. Formulation and evaluation of gastroretentive floating tablets of gabapentin using effervescent technology. Int. J. Pharm. Biomed. Res. 2012;3:202–208. [Google Scholar]
- Christian V., Ghedia T., Gajjar V. A review on floating drug delivery system as a part of GRDDS. Int. J. Pharm. Res. Dev. 2011;3:233–241. [Google Scholar]
- Costa P., Sousa Lobo J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001;13:123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- Couto R.O., Martins F.S., Chaul L.T., Conceição E.C., Freitas L.A.P., Bara M.T.F., Paula J.R. Spray drying of Eugenia dysenterica extract: effects of in-process parameters on product quality. Revista Brasileira de Farmacognosia. 2013;23:115–123. [Google Scholar]
- Dash S., Murthy P.N., Nath L., Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010;67:217–223. [PubMed] [Google Scholar]
- Doodipala N., Palem C., Reddy S., Rao Y. Pharmaceutical development and clinical pharmacokinetic evaluation of gastroretentive floating matrix tablets of levofloxacin. Int. J. Pharm. Sci. Nanotech. 2011;4:1463–1469. [Google Scholar]
- Garg A., Gupta M. Taste masking and formulation developement & evaluation of mouth dissolving tablets of levocetrizine dihydrochloride. J. Drug Deliv. Ther. 2013;3:123–130. [Google Scholar]
- Gisbert J.P., Pajares J.M. Treatment of Helicobacter pylori infection: the past and the future. Eur. J. Intern. Med. 2010;21:357–359. doi: 10.1016/j.ejim.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Sánchez P.E., Hernández-León A., Villafuerte-Robles L. Effect of sodium bicarbonate on the properties of metronidazole floating matrix tablets. Drug Dev. Ind. Pharm. 2008;34:171–180. doi: 10.1080/03639040701506478. [DOI] [PubMed] [Google Scholar]
- Hadi M.A., Babu V.L., Pal N. Formulation and evaluation of sustained release matrix tablets of glimepiride based on combination of hydrophilic and hydrophobic polymers. J. Appl. Pharm. Sci. 2012;2:101–107. [Google Scholar]
- Hessey S., Spencer J., Wyatt J., Sobala G., Rathbone B., Axon A., Dixon M. Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut. 1990;31:134–138. doi: 10.1136/gut.31.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak R.A.H., Awad G.A.S., Mortada N.D., Nour S.A.K. Preparation, in vitro and in vivo evaluation of stomach-specific metronidazole-loaded alginate beads as local anti-Helicobacter pylori therapy. J. Control Release. 2007;119:207–214. doi: 10.1016/j.jconrel.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Jagdale S.C., Agavekar A.J., Pandya S.V., Kuchekar B.S., Chabukswar A.R. Formulation and evaluation of gastroretentive drug delivery system of propranolol hydrochloride. AAPS PharmSciTech. 2009;10:1071–1079. doi: 10.1208/s12249-009-9300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassab N.M., Amaral M.S., Singh A.K., Santoro M.I.R.M. Development and validation of UV spectrophotometric method for determination of levofloxacin in pharmaceutical dosage forms. Quím Nova. 2010;33:968–971. [Google Scholar]
- Khalifa M.M., Sharaf R.R., Aziz R.K. Helicobacter pylori: a poor man’s gut pathogen. Gut Pathog. 2010;2:1–12. doi: 10.1186/1757-4749-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaoka H., Wada C., Moroi R., Hakusui H. Effect of dehydration on the formation of levofloxacin pseudopolymorphs. Chem. Pharm. Bull. 1995;43:649–653. [Google Scholar]
- Lara-Hernández B., Hernández-León A., Villafuerte-Robles L. Effect of stearic acid on the properties of metronidazole/methocel K4M floating matrices. Braz. J. Pharm. Sci. 2009;45:497–505. [Google Scholar]
- Li S., Lin S., Daggy B.P., Mirchandani H.L., Chien Y.W. Effect of HPMC and Carbopol on the release and floating properties of gastric floating drug delivery system using factorial design. Int. J. Pharm. 2003;253:13–22. doi: 10.1016/s0378-5173(02)00642-7. [DOI] [PubMed] [Google Scholar]
- Lopes C.M., Lobo J.M.S., Pinto J.F., Costa P. Compressed mini-tablets as a biphasic delivery system. Int. J. Pharm. 2006;323:93–100. doi: 10.1016/j.ijpharm.2006.05.063. [DOI] [PubMed] [Google Scholar]
- Manivannan R., Chakole V. Formulation and development of extended release floating tablet of atenolol. Int. J. Recent Adv. Pharm. Res. 2011;3:25–30. [Google Scholar]
- Mouzam M.I., Dehghan M., Asif S., Sahuji T., Chudiwal P. Preparation of a novel floating ring capsule-type dosage form for stomach specific delivery. Saudi Pharm. J. 2011;19:85–93. doi: 10.1016/j.jsps.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A.K., Maji R., Das B. Gastroretentive drug delivery systems: a review. Asian J. Pharm. Clin. Res. 2010;3:2–10. [Google Scholar]
- Pare A., Yadav S., Patil U. Formulation and evaluation of effervescent floating tablet of amlodipine besylate. Res. J. Pharm. Tech. 2008;1:526–530. [Google Scholar]
- Prabakaran D., Singh P., Kanaujia P., Vyas S.P. Effect of hydrophilic polymers on the release of diltiazem hydrochloride from elementary osmotic pumps. Int. J. Pharm. 2003;259:173–179. doi: 10.1016/s0378-5173(03)00230-8. [DOI] [PubMed] [Google Scholar]
- Prajapati S.T., Patel L.D., Patel D.M. Gastric floating matrix tablets: design and optimization using combination of polymers. Acta Pharm. 2008;58:221–229. doi: 10.2478/v10007-008-0006-3. [DOI] [PubMed] [Google Scholar]
- Rao N.G.R., Hadi M.A., Panchal H., Reddy B.M. Formulation and evaluation of biphasic drug delivery system of Montelukast sodium for chronotherapy. World J. Pharm. Res. 2012;1:757–775. [Google Scholar]
- Sabar M., Samein L., Sahib H.B. Some variables affecting the formulation of ketoprofen sustained release oral tablet using polyelectrolyte complex as a matrix former. J. Pharm. Allied Health Sci. 2011;1:1–15. [Google Scholar]
- Sathali A.A.H., Ganesan S. Formulation and evaluation of fast dissolving tablets of Desloratadine. Int. J. Pharm. World Res. 2012;3:1–31. [Google Scholar]
- Savic I.M., Nikolic K., Nikolic G., Savic I., Agbaba D., Cakic M. Application of mathematical modeling for the development and optimization formulation with bioactive copper complex. Drug Dev. Ind. Pharm. 2013;39:1–7. doi: 10.3109/03639045.2012.707208. [DOI] [PubMed] [Google Scholar]
- Shah S.H., Patel J.K., Patel N.V. Formulation and evaluation of effervescent floating tablet of levofloxacin against H. pylori infection. Der Pharm. Sin. 2010;1:232–244. [Google Scholar]
- Sharma S., Prashar M., Sahu R.K. Floating drug delivery system: Incredible revolution. Pharmacologyonline. 2011;3:1039–1054. [Google Scholar]
- Tadros M.I. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: development, optimization and in vitro–in vivo evaluation in healthy human volunteers. Eur. J. Pharm. Biopharm. 2010;74:332–339. doi: 10.1016/j.ejpb.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Tatavarti A., Mehta K., Augsburger L., Hoag S. Influence of methacrylic and acrylic acid polymers on the release performance of weakly basic drugs from sustained release hydrophilic matrices. J. Pharm. Sci. 2004;93:2319–2331. doi: 10.1002/jps.20129. [DOI] [PubMed] [Google Scholar]
- Tiwari S.B., Rajabi-Siahboomi A.R. Applications of complementary polymers in HPMC hydrophilic extended release matrices. Drug Deliv. Technol. 2009;9:20–27. [Google Scholar]
- Trivedi P., Verma A., Garud N. Preparation and characterization of aceclofenac microspheres. Asian J. Pharm. 2008;2:110–115. [Google Scholar]
- Vakil N. Primary and secondary treatment for Helicobacter pylori in the United States. Rev. Gastroenterol. Disord. 2005;5:67–72. [PubMed] [Google Scholar]
- Verma R.K., Garg S. Compatibility studies between isosorbide mononitrate and selected excipients used in the development of extended release formulations. J. Pharm. Biomed. Anal. 2004;35:449–458. doi: 10.1016/j.jpba.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Vueba M., Batista de Carvalho L., Veiga F., Sousa J., Pina M. Influence of cellulose ether polymers on ketoprofen release from hydrophilic matrix tablets. Eur. J. Pharm. Biopharm. 2004;58:51–59. doi: 10.1016/j.ejpb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Wilson B., Sitarambhai P.H., Sajeev M., Vinothapooshan G. Design and evaluation of sustained release matrix tablets of levofloxacin for effective treatment of microbial infections. Int. J. Drug Deliv. 2011;3:305–314. [Google Scholar]
- Zhang Y., Huo M., Zhou J., Zou A., Li W., Yao C., Xie S. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12:263–271. doi: 10.1208/s12248-010-9185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]



