Abstract
Objectives
The aim of this study was to examine the composition of metabolic syndrome (MetS) components among middle-aged and older Koreans.
Methods
A total of 263 participants (age 40 + years) in a lifestyle modification intervention program who met the MetS definition of National Cholesterol Education Program (NCEP) – Adult Treatment Panel (ATP) III criteria were included in the study. The frequent patterns and clustering of MetS components were investigated. Clustering of changes in individual components, through a lifestyle modification intervention, was also identified. All characteristics were stratified by and compared between sexes.
Results
Approximately 80% of the participants had three of five MetS risk factors at baseline. The prevalence of each risk differed by sex. MetS composition patterns that do not include low high-density lipoprotein (HDL) cholesterol were more noticeable in men because of the low prevalence of low HDL cholesterol. In women, with higher prevalence of low HDL cholesterol, more patterns that include low HDL cholesterol were observed. The most common combination was “elevated blood pressure + abdominal obesity + impaired fasting glucose” in both sexes. Clustering of MetS risks was also found with most of the frequent combinations of MetS components. Through the lifestyle intervention, the greatest change was observed in HDL cholesterol among men and blood pressure among women. Triglycerides and HDL cholesterol were likely to be improved with blood pressure in men and abdominal obesity in women.
Conclusion
Differences in the prevalent patterns of MetS compositions were observed prior to and after the intervention, along with during-intervention changes. It is recommended that intervention strategies and guidelines for MetS management consider the MetS composition patterns for effectiveness.
Keywords: clustering, CVD risk factors, lifestyle intervention, metabolic syndrome
1. Introduction
Metabolic syndrome (MetS) refers to a cluster of underlying, major, and emerging risk factors associated with increased incidence and mortality of cardiovascular diseases. The prevalence of MetS has been increasing globally, although its characteristics vary widely by demographic and environmental factors [1]. It also carries a high socioeconomic burden owing to the higher prevalence of comorbidities [2]. Because the rapid increase of MetS cases in Western countries has been a serious public health concern in the past decade, the epidemic of MetS has been considered an important public health problem in most Asian countries, with increasing prevalence [3,4]. Asians are more likely to have a high risk of type 2 diabetes and cardiovascular diseases at lower body weight or smaller waist circumference than Caucasians [5,6].
The prevalence of MetS has been increasing in Korea. The age-standardized prevalence of MetS was 31.3% in 2007, from 24.9% in 1998, and this figure is expected to increase significantly [3]. The percentage of Korean adults older than 30 years who were reported to have MetS is approximately 30% [7]. Among the five parameters of MetS, it is estimated that 73.7% of Koreans have at least one of them at an abnormal level. Over a 10-year period, the prevalence of low HDL cholesterol in Koreans increased the most, by 13.8%. This was followed by the prevalence of abdominal obesity (by 8.7%) and hypertriglyceridemia (by 4.9%).
Given the definitions of MetS, most etiological studies and intervention programs dealt with their data by considering MetS as a whole rather than exploring the contributions or effects of its individual components [8]. However, there were several previous studies conducted to identify the central features of MetS such as associations and clustering of components in MetS using the exploratory factor analysis.
From two to four factors were usually found with strong intercorrelations among the components of MetS, which were hyperinsulinemia loaded with one or more MetS components such as obesity, hyperglycemia, or dyslipidemia [9]. There were several trials whose aim was to identify a major factor that had a central role among MetS components. Insulin resistance has been known as a primary cause of MetS [10,11], but the results have not been consistent and are still controversial. Different patterns of clustering were found especially with different ethnic groups. A few Korean studies also attempted to identify the dominant components among MetS risk factors. It has been reported that insulin resistance is not the only contributor to MetS. Among Koreans, it was shown to have a strong intercorrelation with other MetS components [9,12]. However, grouping of contributing factors to MetS might differ by the variables entered in the analysis, and the analyses were limited to small sections of an urban district of Seoul.
The aim of this study was to examine the composition of MetS components from randomly selected nationwide samples in a lifestyle intervention program for Korean middle-aged and older adults. Changes and the remaining risk factors in MetS components after the intervention and clustering of changes were investigated as well to provide any suggestions to help develop the intervention guidelines for MetS management. All characteristics were stratified by and compared between sexes.
2. Material and methods
2.1. Study data
A total of 263 Korean participants aged 40 years and older in a lifestyle modification intervention program for MetS at Korea Association of Health Promotion (KAHP) in 2011 are included the study. The participants were diagnosed with MetS at one of the 16 regional branch health facilities of KAHP based on a definition of the National Cholesterol Education Program (NCEP) - Adult Treatment Panel (ATP) III criteria for MetS with the Asia Pacific standard for abdominal obesity [13]. That is, middle-aged and older adults are diagnosed to have MetS if they meet three or more of the following five criteria: (1) elevated blood pressure: systolic blood pressure ≥130 mmHg and diastolic blood pressure ≥85 mmHg; (2) abdominal obesity: waist circumference >90 cm for men or >80 cm for women; (3) impaired fasting glucose: fasting glucose ≥100 mg/dL; (4) elevated triglyceride: triglyceride ≥150 mg/dL; and (5) low high-density lipoprotein (HDL) cholesterol level: HDL cholesterol <40 mg/dL for men or <50 mg/dL for women. Intervention participants were not in treatment or on medication for MetS-related health conditions such as hypertension, dyslipidemia, and diabetes, and they signed consent forms for participating in the study.
This theory-based 12-week multicomponent intervention consisted of individual health counseling, health education classes, and a booklet and newsletters for self-management. One-on-one health counseling was provided every week by trained dietitians working at the health facilities where the participants had been screened. The participants set behavioral goals and strategies for their healthy lifestyle including dietary behaviors, physical activity, smoking, alcohol drinking, and stress management. Five clinical and anthropometric outcomes for MetS were measured by trained research assistants among the participants at baseline and after the intervention (12 weeks from the baseline). The study protocol was approved by the Institutional Review Board of the KAHP (IRB No. 10-B-01), and informed consent was obtained from all participants.
2.2. Study design
Baseline sociodemographic data and MetS characteristics of the study population were reported and compared between the men and women with Chi-square tests and Fisher's exact tests for discrete variables and t tests for continuous variables. The main analysis of the study comprised three parts (as described below).
First, as all participants have MetS when they were recruited, there are 16 possible combinations for MetS components at baseline. This study first describes all the possible combinations from baseline data, and then identifies the frequent patterns and cluster effects among the combinations. Clustering of multiple MetS components was examined based on the ratios of observed and expected prevalence of combinations simultaneously occurring MetS components [14,15]. A combination of MetS components was considered a cluster if the ratio of observed and expected prevalence (O/E) was >1.50. The expected proportion was calculated by multiplying the individual probabilities of each MetS component based on their prevalence in the study population for men and women separately. Sex differences of prevalence at baseline were examined with Chi-square tests and Fisher's exact tests.
Second, improvements in the prevalence of MetS risks after the 12-week intervention were investigated. Change in each MetS component after the intervention was calculated and compared between men and women with Chi-square tests. Clustering in each pair of two components was examined based on the ratios of observed and expected probabilities of changes in that particular pair. It represents a cluster effect of improvements in two or more components including the two components in the pair.
Finally, patterns of MetS risks that remain at abnormal levels after the improvements through the intervention were investigated with prevalence, cluster effect, and prevalence odds ratio (POR) from each pair of MetS component after the intervention. POR represents the estimation of the relative odds of having a certain component given the prevalence of another component, and indicates the degree of clustering of a pair of MetS components. The analysis was conducted separately for men and women, and POR was adjusted for the participants' age. All statistical analyses were performed using the SAS 9.3 statistical software package (SAS Institute, Cary, NC, USA).
3. Results
3.1. Participants' characteristics
Table 1 presents the baseline characteristics of the study population. The mean age of the participants was 57.7 years (standard deviation = 8.0). Sex differences were observed in several sociodemographic characteristics: education level, marital status, and working status (p < 0.01). Men had a higher education level than women, and the majority of the men were married and living with their spouses (92.5%) in comparison to women (78.8%). More than half of the men (64.5%) were employed, whereas more than half of the women (57.0%) were housewives.
Table 1.
Baseline characteristics and risk factors for metabolic syndrome.
| Men (n = 93) |
Women (n = 170) |
All (n = 263) |
|||
|---|---|---|---|---|---|
| Sociodemographic characteristics | n (%) | n (%) | n (%) | pa | |
| Age (y) | Mean (SD) | 56.9 (9.1) | 58.1 (7.4) | 57.7 (8.0) | 0.2566 |
| Education (6 missed) | Middle school | 18 (19.4) | 78 (47.6) | 96 (37.4) | <0.0001 |
| High school | 41 (44.1) | 52 (31.7) | 93 (36.2) | ||
| College | 34 (36.6) | 34 (20.7) | 68 (26.5) | ||
| Marital status (5 missed) | Live with spouse | 86 (92.5) | 130 (78.8) | 216 (83.7) | 0.0043 |
| Live without spouse | 7 (7.5) | 35 (21.2) | 42 (16.3) | ||
| Working (5 missed) | Employed | 60 (64.5) | 58 (35.2) | 118 (45.7) | <0.0001 |
| Housewives | 0 (0.0) | 94 (57.0) | 94 (36.4) | ||
| Others | 33 (35.5) | 13 (7.9) | 46 (17.8) | ||
| Monthly income (13 missed) | >US$1000 | 10 (11.4) | 37 (22.8) | 47 (18.8) | 0.0733 |
| US$1000–3000 | 40 (45.5) | 69 (42.6) | 109 (43.6) | ||
| ≥US$3000 | 38 (43.2) | 56 (34.6) | 94 (37.6) | ||
| Metabolic syndrome (MetS) characteristics | |||||
| No. of MetS components, mean (SD) | 3.3 (0.5) | 3.2 (0.4) | 3.2 (0.5) | 0.0949 | |
| 3 components | 68 (73.1) | 139 (81.8) | 207 (78.7) | 0.1725 | |
| 4 components | 22 (23.7) | 29 (17.1) | 51 (19.4) | ||
| 5 components | 3 (3.2) | 2 (1.2) | 5 (1.9) | ||
| Elevated blood pressure (EBP) | 79 (84.9) | 115 (67.6) | 194 (73.8) | 0.0023 | |
| Abdominal obesity (AO) | 72 (77.4) | 147 (86.5) | 219 (83.3) | 0.0601 | |
| Impaired fasting glucose (IFG) | 73 (78.5) | 98 (57.6) | 171 (65.0) | 0.0007 | |
| Elevated triglycerides (ETG) | 72 (77.4) | 108 (63.5) | 180 (68.4) | 0.0205 | |
| Low HDL cholesterol (HDL) | 11 (11.8) | 75 (44.1) | 86 (32.7) | <0.0001 | |
ap value was based on a t test for continuous variables and the Chi-square tests and Fisher's exact tests for categorical variables.
HDL cholesterol = high-density lipoprotein cholesterol; SD = standard deviation.
At baseline, participants in this study had 3.2 risks of MetS on average. Almost 80% of the participants were diagnosed with MetS for having three out of five risks of MetS. The most common MetS risk was elevated blood pressure for men (84.9%) and abdominal obesity for women (86.5%). Although the average number of MetS risks was similar among men and women, the distribution of the prevalence of MetS risks differed by sex. The prevalence rates of elevated blood pressure (84.9%), impaired fasting glucose (78.5%), and elevated triglycerides (77.4%) were significantly higher in men than in women (67.6%, 57.6%, and 63.5%, respectively; p < 0.05). Meanwhile, the prevalence of low HDL cholesterol was higher in women (44.1%) than in men (11.8%, p < 0.0001). The difference in the prevalence of abdominal obesity between women (86.5%) and men (77.4%) was marginal (p = 0.0601).
3.2. Combinations of MetS components at baseline
Table 2 contains the observed and expected prevalence of the combination of MetS components at baseline. All participants had MetS at baseline, with 16 types of combinations of MetS components.
Table 2.
Prevalence rates and cluster effects of MetS component combinations at baseline.
| Prevalence of MetS components |
Men (n = 93) |
Women (n = 170) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EBP | AO | IFG | ETG | HDL |
O |
E |
O/E |
O |
E |
O/E |
| n (%) | (%) | n (%) | (%) | |||||||
| + | + | + | + | + | 3 (3.2) | 4.7 | 0.68 | 2 (1.2) | 9.5 | 0.12 |
| + | + | + | + | − | 20 (21.5)*** | 35.2 | 0.61 | 7 (4.1) | 12.0 | 0.34 |
| + | + | + | − | + | 0 (0.0) | 1.4 | 0.00 | 4 (2.4) | 5.4 | 0.43 |
| + | + | − | + | + | 0 (0.0) | 1.3 | 0.00 | 10 (5.9) | 6.9 | 0.85 |
| + | − | + | + | + | 2 (2.2) | 1.4 | 1.56a | 0 (0.0) | 1.5 | 0.00 |
| − | + | + | + | + | 0 (0.0) | 0.8 | 0.00 | 8 (4.7) | 4.5 | 1.04 |
| + | + | + | − | − | 20 (21.5) | 10.3 | 2.09a | 42 (24.7) | 6.9 | 3.59a |
| + | + | − | + | − | 15 (16.1) | 9.7 | 1.67a | 19 (11.2) | 8.8 | 1.27 |
| + | + | − | − | + | 0 (0.0) | 0.4 | 0.00 | 13 (7.6) | 4.0 | 1.92a |
| + | − | + | + | − | 15 (16.1)** | 10.3 | 1.57a | 8 (4.7) | 1.9 | 2.51a |
| + | − | + | − | + | 0 (0.0) | 0.4 | 0.00 | 2 (1.2) | 0.9 | 1.39 |
| + | − | − | + | + | 4 (4.3) | 0.4 | 11.39a | 8 (4.7) | 1.1 | 4.33a |
| − | + | + | + | − | 12 (12.9) | 6.2 | 2.07a | 19 (11.2) | 5.7 | 1.95a |
| − | + | + | − | + | 1 (1.1) | 0.2 | 4.40a | 1 (0.6) | 2.6 | 0.23 |
| − | + | − | + | + | 1 (1.1) | 0.2 | 4.69a | 22 (12.9)** | 3.3 | 3.90a |
| − | − | + | + | + | 0 (0.0) | 0.2 | 0.00 | 5 (2.9) | 0.7 | 4.16a |
**p < 0.01 and ***p < 0.001 based on Chi-square tests and Fisher's exact tests for categorical variables.
AO = abdominal obesity; E = expected prevalence rates; EBP = elevated blood pressure; ETG = elevated triglycerides; HDL = low high-density lipoprotein cholesterol; IFG = impaired fasting glucose; MetS = metabolic syndrome; O = observed prevalence rates.
Ratio of observed and expected prevalence (O/E)≥1.50.
Supposing an equal distribution of combinations among participants, combinations that consisted of either six or more men (93 men/16 combinations = 5.8) or 11 or more women (170/16 = 10.6) were considered “frequent” patterns. In this manner, five types of frequent combinations were identified for men and women, respectively. The most common combination was “elevated blood pressure + abdominal obesity + impaired fasting glucose” for both men (21.5%) and women (24.7%). The ratio of observed prevalence over expected prevalence (O/E) of having this combination was 2.09 in men, and 3.59 in women. The combination of “elevated blood pressure + abdominal obesity + impaired fasting glucose + elevated triglycerides” was the most common four-component combination among men (21.5%) versus among women (4.1%, p < 0.0001) with no cluster effect (O/E ratio = 0.61).
The second most frequent type of combinations among men was “elevated blood pressure + elevated triglycerides + either abdominal obesity or impaired fasting glucose” (16.1%). Both of these combinations were clustered: O/E ratio = 1.67 and O/E ratio = 1.57, respectively. The combination of “abdominal obesity + elevated triglycerides + low HDL cholesterol” was the second most common and clustered among women (12.9%, O/E ratio = 3.90). The third most frequent combination was “abdominal obesity + impaired fasting glucose + elevated triglycerides” for both men (12.9%, O/E ratio = 2.07) and women (11.2%, O/E ratio = 1.95).
Because the prevalence of low HDL cholesterol was low among men (11.8%), the expected prevalence of combinations that contain the low HDL cholesterol was around 1%. However, the observed prevalence of such combinations was not as low as the expected prevalence. Therefore, caution should be exercised when interpreting the cluster effects of combinations including HDL cholesterol among men.
3.3. Improvements in MetS components in the intervention
Postintervention improvements in the prevalence of individual MetS components are shown in Figure 1. The greatest change was observed in low HDL cholesterol among men and blood pressure among women. More than half of the women (52.2%) who had elevated blood pressure at baseline lowered their blood pressure after the intervention, which was a significant improvement compared with men (30.4%). Almost half of the men with low HDL cholesterol (45.5%) and women with elevated triglycerides (48.2%) showed improvement in their respective MetS risk as well, although sex differences in such risks were not significant.
Figure 1.
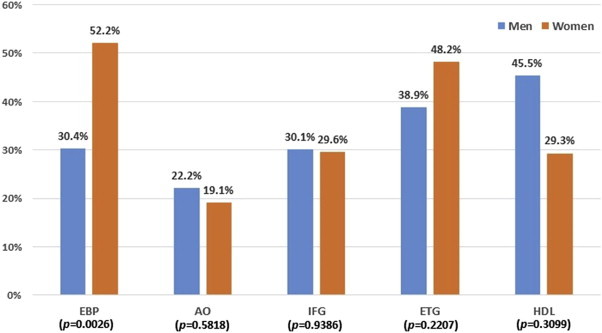
Improvements in the prevalence of MetS risks. The p value based on Chi-square tests for comparison of prevalence rates changes between men and women. AO = abdominal obesity; EBP = elevated blood pressure; ETG = elevated triglycerides; HDL = low high-density lipoprotein cholesterol; IFG = impaired fasting glucose.
The probability for men whose low HDL cholesterol improved with changes in either elevated blood pressure (O/E ratio = 3.22) or elevated triglycerides (O/E ratio = 2.26) was higher than the expected probability that each of these MetS components changes independently (Table 3). Abdominal obesity was the least changed element in women (19.1%). The probabilities of improvement in abdominal obesity were greater, however, in combination with triglyceride (O/E ratio = 1.63) and with HDL cholesterol (O/E ratio = 2.09). Improvements in compositions with three or more components were scarcely observed through the intervention (<5 cases), which made it difficult to calculate the O/E ratio.
Table 3.
Cluster effects of changes in MetS components' prevalence rates.
| Changes in MetS components | Men |
Women |
|||||
|---|---|---|---|---|---|---|---|
|
O |
E |
O/E |
O |
E |
O/E | ||
| (%) | (%) | (%) | (%) | ||||
| EBP | AO | 5.17 | 6.75 | 0.77 | 8.25 | 9.94 | 0.83 |
| EBP | IFG | 11.67 | 9.16 | 1.27 | 18.46 | 15.44 | 1.20 |
| EBP | ETG | 18.64 | 11.81 | 1.58a | 27.78 | 25.12 | 1.11 |
| EBP | HDL | 44.44 | 13.81 | 3.22a | 15.38 | 15.30 | 1.00 |
| AO | IFG | 7.14 | 6.70 | 1.07 | 6.02 | 5.64 | 1.07 |
| AO | ETG | 9.80 | 8.64 | 1.13 | 14.94 | 9.17 | 1.63a |
| AO | HDL | 20.00 | 10.10 | 1.98a | 11.67 | 5.59 | 2.09a |
| IFG | ETG | 13.46 | 11.72 | 1.15 | 12.24 | 14.25 | 0.86 |
| IFG | HDL | 16.67 | 13.70 | 1.22 | 4.55 | 8.68 | 0.52 |
| ETG | HDL | 40.00 | 17.68 | 2.26a | 18.18 | 14.12 | 1.29 |
AO = abdominal obesity; E = expected prevalence rates; EBP = elevated blood pressure; ETG = elevated triglycerides; HDL = low high-density lipoprotein cholesterol; IFG = impaired fasting glucose; MetS = metabolic syndrome; O = observed probabilities of changes.
Ratio of observed and expected prevalence (O/E) ≥1.50.
3.4. Prevalence of MetS components after the intervention
Despite the improvements of MetS components during the intervention, approximately half of the participants still had MetS (49.5% in men, 57.1% in women; data not shown). The prevalence, cluster effects, and POR of the remaining MetS risks are shown in Table 4. Cluster effects were less likely to be found after the intervention. However, POR was significant in two pairs of MetS components in both men and women. Men with impaired fasting glucose were 3.119 times more likely to have elevated triglycerides [95% confidence interval (CI) = 1.292–7.534]; and men with elevated triglycerides were 4.249 times more likely to have low HDL cholesterol as well (95% CI = 1.080–16.718). Women with impaired fasting glucose and elevated triglycerides had 0.505-fold odds (95% CI = 0.271–0.939) and 3.274-fold odds (95% CI = 1.721–6.229) of having low HDL cholesterol, respectively.
Table 4.
Prevalence rates, cluster effects and prevalence odds ratio of MetS components after the intervention.
| MetS components | Men (n = 93) |
Women (n = 170) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
O |
E |
O/E | POR |
O |
E |
O/E | POR | ||
| (%) | (%) | (%) | (%) | ||||||
| EBP | AO | 40.86 | 38.85 | 1.05 | 1.365 | 31.18 | 28.52 | 1.09 | 1.771 |
| EBP | IFG | 37.63 | 37.46 | 1.00 | 0.960 | 21.18 | 17.85 | 1.19 | 1.770 |
| EBP | ETG | 39.78 | 36.07 | 1.10 | 1.847 | 14.12 | 15.53 | 0.91 | 0.780 |
| EBP | HDL | 9.68 | 10.41 | 0.93 | 0.809 | 16.47 | 17.85 | 0.92 | 0.798 |
| AO | IFG | 35.48 | 34.96 | 1.01 | 0.935 | 34.71 | 32.77 | 1.06 | 1.504 |
| AO | ETG | 33.33 | 33.67 | 0.99 | 0.793 | 25.88 | 28.52 | 0.91 | 0.582 |
| AO | HDL | 6.45 | 9.71 | 0.66 | 0.377 | 31.18 | 32.77 | 0.95 | 0.737 |
| IFG | ETG | 39.78 | 32.47 | 1.23 | 3.119* | 17.06 | 17.85 | 0.96 | 0.873 |
| IFG | HDL | 7.53 | 9.37 | 0.80 | 0.592 | 16.47 | 20.52 | 0.80 | 0.505* |
| ETG | HDL | 12.90 | 9.02 | 1.43 | 4.249* | 24.71 | 17.85 | 1.38 | 3.274*** |
*p < 0.05, ***p < 0.001.
AO = abdominal obesity; E = expected prevalence rates; EBP = elevated blood pressure; ETG = elevated triglycerides; HDL = low high-density lipoprotein cholesterol; IFG = impaired fasting glucose; MetS = metabolic syndrome; O = observed prevalence rates; POR = prevalence odds ratio adjusted for ages.
4. Discussion
The effects of the 16 combinations of MetS components on cardiovascular risks might not be the same, but research to specify all the differences faces challenges that include, but are not limited to, feasibility [10]. In previous research, each component of MetS carries a different effect for predicting the prevalence and incidence, or mortality of cardiovascular diseases, and the results were inconsistent across studies [16–19]. MetS, by definition, is diagnosed as a cluster of risk factors related to the increased incidence and mortality of cardiovascular diseases. However, little is known about the composing patterns and changes in those patterns of MetS risks among people with MetS.
This study examined the patterns of MetS composition among middle-aged and older adults in a lifestyle modification program for MetS in 16 regional branches of a health institution in Korea. Women with MetS in this study tended to have three MetS risk factors at baseline. The common composition of MetS risks in women tended to include abdominal obesity. By contrast, the common patterns of MetS risk composition in men included all MetS parameters rather evenly except for low HDL cholesterol. The prevalence of low HDL cholesterol was low among men (11.8%), which led us to exclude HDL cholesterol from common MetS compositions among men. Instead, men in this study demonstrated fewer yet distinctive patterns of MetS composition. By comparison, more diverse types of MetS composition were observed in women, where HDL cholesterol was included in the mix.
MetS risks in common types of composition tended to be clustered. No specific MetS element appeared to lead the formation of clusters. Meanwhile, low HDL cholesterol, despite its low prevalence, tended to cluster with high blood pressure or triglyceride in both men and women as their O/E ratios were high. In other words, those with low HDL cholesterol had a higher likelihood of having abnormal blood pressure or triglyceride as well. This raises a point of monitoring for potential cardiovascular disease risks that are likely to codevelop.
Lifestyle modification is recommended as the main method of MetS treatment [11]. Almost 80% of adults with MetS in this study had three MetS risk factors prior to the lifestyle modification intervention. By showing improvement in one of these risk factors, they could be out of MetS. The one component improved by the intervention differed by the MetS composition and by sex. For instance, triglyceride and HDL cholesterol were likely to improve with the improvement of blood pressure in men. In women, improvement of triglyceride and HDL cholesterol were clustered with the improvement of abdominal obesity. Although clustering of MetS components was less noticeable after the intervention, significant PORs were found with some pairs. Both men and women who still had elevated triglycerides after the intervention were more likely to have low HDL cholesterol. Among those with impaired fasting glucose after the intervention, men were more likely to have elevated triglycerides, and women were less likely to have low HDL cholesterol.
The results of this study imply that the impact of a lifestyle modification on MetS may be reflected differently for the type of MetS risk compositions and for sex. Examining the postintervention MetS composition could be a way to monitor the long-term sustainability of intervention effects. General guidelines of lifestyle modification intervention programs for MerS management target healthy diet and regular physical activity. Such strategies can be effective for improving abdominal obesity and blood pressure [20]. By contrast, low HDL cholesterol, whose prevalence is increasing in Korea [21], is known as a less changeable component of MetS by lifestyle interventions [22,23], because such interventions to reduce fat intake may reversely affect the HDL cholesterol level. This study raises a point that improvements in abdominal obesity and blood pressure tend to accompany the improvement of HDL cholesterol level; thus improvement of HDL cholesterol level could be expected with lifestyle modification strategies among people with the MetS composition of abdominal obesity, elevated blood pressure, and low HDL cholesterol level.
Although the data in this study were collected from participants of a long-term nationwide MetS intervention program, the size of subgroups stratified by sex and each composition of MetS components were small. More stable and in-depth findings are expected with larger samples in future studies covering various combinations among the five MetS risks. Despite such limitations of small subsamples, the findings of this study reiterate the need for tailored interventions for varying MetS composition and clustering of MetS risks.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was supported by the National Research Foundation of Korea (Grant No. NRF-2010-0004016). This study is registered in the Clinical Research Information Service by Korea Centers for Diseases Control and Prevention, Republic of Korea (# KCT 0000446).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Kassi E., Pervanidou P., Kaltsas G. Metabolic syndrome: definitions and controversies. BMC Med. 2011 May;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitch K., Pyenson B., Iwasaki K. Metabolic syndrome and employer sponsored medical benefits: an actuarial analysis. Value Health. 2007 Jan/Feb;10(S1):S21–S28. [Google Scholar]
- 3.Lim S., Shin H., Song J.H. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care. 2011 Jun;34(6):1323–1328. doi: 10.2337/dc10-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001 May;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Palaniappan L.P., Wong E.C., Shin J.J. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes. 2011 Mar;35(3):393–400. doi: 10.1038/ijo.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan J. The metabolic syndrome: an Asian perspective. Diabetes Voice. 2006;51:18–20. [Google Scholar]
- 7.National Health Insurance Service . 2012. 2011 National Health Screening Statistical Yearbook; Seoul; p. 730. [Google Scholar]
- 8.Anderson P.J., Critchley J.A., Chan J.C. Factor analysis of the metabolic syndrome: obesity vs insulin resistance as the central abnormality. Int J Obes. 2001 Dec;25(12):1782–1788. doi: 10.1038/sj.ijo.0801837. [DOI] [PubMed] [Google Scholar]
- 9.Oh J.Y., Hong Y.S., Sung Y.A. Prevalence and factor analysis of metabolic syndrome in an urban Korean population. Diabetes Care. 2004 Aug;27(8):2027–2032. doi: 10.2337/diacare.27.8.2027. [DOI] [PubMed] [Google Scholar]
- 10.Kahn R., Buse J., Ferrannini E. The metabolic syndrome: time for a critical appraisal – Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005 Sep;28(9):2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 11.Grundy S.M., Brewer H.B., Cleeman J.I., National Heart, Lung and Blood Institute; American Heart Association Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004 Feb;109(2):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 12.Choi K.M., Lee J., Kim K.B. Factor analysis of the metabolic syndrome among elderly Koreans – the South-west Seoul Study. Diabet Med. 2003 Feb;20(2):99–104. doi: 10.1046/j.1464-5491.2003.00890.x. [DOI] [PubMed] [Google Scholar]
- 13.National Institutes of Health . U.S. Department of Health and Human Services; 2002. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. NHI Publication No. 02-5215. [PubMed] [Google Scholar]
- 14.Jeon J.Y., Yoo S., Kim H. Clustering patterns and correlates of multiple health behaviors in middle-aged Koreans with metabolic syndrome. Korean J Health Educ Promot. 2012;29(2):93–105. [Google Scholar]
- 15.Schuit A.J., van Loon A.J., Tijhuis M. Clustering of lifestyle risk factors in a general adult population. Prev Med. 2002 Sep;35(3):219–224. doi: 10.1006/pmed.2002.1064. [DOI] [PubMed] [Google Scholar]
- 16.McNeill A.M., Rosamond W.D., Girman C.J. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the Atherosclerosis Risk in Communities study. Diabetes Care. 2005 Feb;28(2):385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 17.Hunt K.J., Resendez, Williams K., San Antonio Heart Study National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004 Sep;110(10):1251–1257. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 18.Alexander C.M., Landsman P.B., Teutsch S.M. Third National Health and Nutrition Examination Survey (NHANES III), National Cholesterol Education Program (NCEP): NCEP defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003 May;52(5):1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 19.Golden S.H., Folsom A.R., Coresh J. Risk factor groupings related to insulin resistance and their synergistic effects on subclinical atherosclerosis: the Atherosclerosis Risk in Communities study. Diabetes. 2002 Oct;51(10):3069–3076. doi: 10.2337/diabetes.51.10.3069. [DOI] [PubMed] [Google Scholar]
- 20.Yoo S., Kim H., Cho H.I. Improvements in the metabolic syndrome and stages of change for lifestyle behaviors in Korean older adults. Osong Public Health Res Perspect. 2012 Jun;3(2):85–93. doi: 10.1016/j.phrp.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi S.J., Park S.H., Park H.Y. Increased prevalence of low high-density lipoprotein cholesterol (HDL-C) levels in Korean adults: analysis of the Three Korean National Health and Nutrition Examination Surveys (KNHANES 1998–2005) Osong Public Health Res Perspect. 2011 Sep;2(2):94–103. doi: 10.1016/j.phrp.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindstrom J., Louheranta A., Mannelin M. The Finnish Diabetes Preventive Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003 Dec;26(12):3230–3236. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 23.Yamaoka K., Tango T. Effects of lifestyle modification on metabolic syndrome: a systematic review and meta-analysis. BMC Med. 2012 Nov;10:138. doi: 10.1186/1741-7015-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]


