Abstract
Galectins are mammalian lectins established to play a crucial role in the progression of various cancer types by the virtue of their differential expression in normal and cancerous cells. In the present study, goat heart galectin-1 (GHG-1) was purified and investigated for its potential role in the detection of post-malignant changes in glycosylation pattern. When exposed to superoxide radicals generated from a pyrogallol auto-oxidation system, GHG-1 treated erythrocyte suspension released higher amount of oxyhemoglobin than the unagglutinated erythrocytes. The extent of erythrocyte hemolysis was found to be directly proportional to concentrations of hypochlorous acid. GHG-1 was used to detect the change in the β-galactoside expression pattern in erythrocyte membrane from human donors suffering from prostate and breast cancer. No significant change was observed in the hemolysis of lectin agglutinated erythrocytes collected from pre-operated breast cancer patients, whereas significant increase was observed in normal healthy control and post-operated samples. Findings of this study proclaim GHG-1 as an important tool for the detection of post-malignant changes in glycosylation pattern.
Abbreviations: Gal-1, galectin-1; GHG-1, goat heart galectin-1; HOCl, hypochlorous acid; OxyHb, oxyhemoglobin
Keywords: Goat heart galectin-1, Pyrogallol, HOCl, Oxyhemoglobin, Glycosylation, Cancer
1. Introduction
Galectins are β-galactoside binding lectins established as potential target for cancer therapy (Hasan et al., 2007). Galectin-1 (Gal-1) has been reported to bind preferentially to ganglioside GM1 on neuroblastoma cells and facilitate growth control (Kopitz et al., 2003; Robert et al., 2012) and also provide hydrophobic tails as interaction site for oncogenic H-Ras (Rotblat et al., 2004). The underlying mechanism to carry out this role is its glycan binding property present on cell membranes, thereby causing lysis of cells. A number of glycoconjugates expressed on the erythrocyte membranes have been reported to be altered in primary cancerous and metastatic conditions (Pugalendhi et al., 2010). Some noticeable alterations in the serum glycoconjugates have been observed in patients with various cancer types (Shetty et al., 2013). These alterations in glycoconjugates can act as excellent indicators for diagnostics, staging, prognostics, therapeutics, and detection of early recurrence in cancer (Baxi et al., 1991; Hernández-Hernández et al., 2006; Shetty et al., 2013). Owing to their multivalent sugar binding property, lectins have been used as an excellent tool for the detection of aberrant glycosylation related to various carcinomas and may provide useful diagnostic or prognostic information, thus contributing directly to cancer biology (Hernández-Hernández et al., 2006). The remarkable role played by galectins ranging from cell signaling to apoptosis make them potent tumorigenic molecules, and have been reported to be over-expressed quite often in cancerous cells and cancer associated stromal cells (Lahm et al., 2004). This altered expression of galectins correlates with the acquisition of metastatic phenotype and tumor aggressiveness, indicating toward the potential ability of galectins in the modulation of tumor progression thus influencing the outcome of the disease (Greco et al., 2004).
In the present study, goat heart galectin-1 (GHG-1) was purified (Ashraf et al., 2011) and investigated for the effect of pyrogallol and hypochlorous acid (HOCl) on the hemolysis of GHG-1 agglutinated erythrocytes. In our earlier study, we also reported that glycosylation plays a crucial role in maintaining the structural and functional integrity of GHG-1 (Ashraf et al., 2010a). Since erythrocytes of various carcinoma cells have been reported to show distinct glycosylation patterns which become a diagnostic index to examine the presence and proliferation of well known cancers, we also investigated the varied expression pattern of β-galactoside sugar residues on erythrocyte membrane of breast and prostate cancer patients using GHG-1 as a diagnostic tool.
2. Materials and methods
2.1. Reagents
Sephadex G100 and G50, molecular weight markers (14.4–97.4 kDa), coomassie brilliant blue (CBB) G-250 and R-250, sugars, pyrogallol and HOCl were purchased from Sigma Aldrich (St Louis, MO, USA). All other chemicals used were of analytical grade and were purchased from Qualigens Fine Chemicals and Merck India Ltd., India.
2.2. Isolation and purification of GHG-1
GHG-1 was isolated and purified essentially according to the methods used in our earlier studies on heart galectins (Ashraf et al., 2010a,b, 2011). The standard method of Lowry was used to estimate the protein concentration (Lowry et al., 1951). The method of two fold serial dilutions was used to determine the protein activity (Raz and Lotan, 1981).
2.3. Effect of GHG-1 on pyrogallol induced free radical damage to erythrocyte membrane
GHG-1 agglutinated erythrocyte suspensions (300 μl) were exposed to superoxide radicals generated from a pyrogallol auto-oxidation system (by adding 10 μl of 0.02 M pyrogallol solution freshly prepared in hydrogen peroxide) and incubated at 37 °C for 20 min. Erythrocytes were recovered by centrifugation at 3000 rpm for 5 min. Cells were then washed thrice and centrifuged with PBS ‘B’ and all the washings were pooled for analyzing the oxyhemoglobin (OxyHb) released. The released OxyHb concentration in the supernatant was measured by the equation of Winterbourn (1985).
2.4. Effect of HOCl on GHG-1 induced hemolysis of trypsinized rabbit erythrocytes
GHG-1 agglutinated erythrocyte suspensions (300 μl) were treated with varying concentrations of HOCl (50–350 μM) in PBS ‘B’ at 22 °C for 20 min. Cells were then washed thrice with excess of cold PBS ‘B’ and suspended in PBS ‘B’ as 10% suspension. The susceptibility of erythrocytes to HOCl induced oxidative damage was measured in terms of percent hemolysis as discussed above.
2.5. Differential hemolytic action of GHG-1 toward erythrocytes of breast and prostate cancer patients
Pre-operated and post-operated (7 days after surgery) heparinized venous blood samples from breast and prostate cancer patients (age > 45 years) were procured from the surgery outpatient department and male surgical wards of the Department of Surgery, Jawaharlal Nehru Medical College, Aligarh, India. Plasma was separated by centrifuging the blood sample at 5000 rpm for 5 min, the pellet containing erythrocytes was washed thrice with cold PBS ‘B’ and 4% RBC suspension was prepared. A trypsin solution (100 mg%) was then added to erythrocytes (0.1 ml of trypsin solution per ml of erythrocyte suspension) and incubated for 1 h at 37 °C. The trypsinized erythrocytes were washed four to five times with PBS ‘B’ and finally 8% erythrocyte suspension was prepared and percent hemolysis was determined as discussed above.
3. Results and discussion
Oxidative stress has been reported to enhance endothelial binding of lectins and activate the lectin complement pathway (Collard et al., 2001; Laderach et al., 2013), thus proclaiming an interesting correlation between lectins and oxidative stress. We thus investigated the effect of GHG-1 on pyrogallol induced free radical damage to erythrocyte membrane. Trypsinized rabbit erythrocyte suspension (8%) when treated with GHG-1 for 1 and 4 h and then exposed to superoxide radicals generated from a pyrogallol auto-oxidation system, released 28 and 36 μM oxyhemoglobin, respectively, in comparison with 6 μM oxyhemoglobin released by unagglutinated erythrocytes (Fig. 1). However, no release of oxyhemoglobin was observed in erythrocytes, which were neither treated with lectin nor exposed to oxidative damage (control), or lectin treated cells but not exposed to oxyradical shock. Lectin mediated crosslinking of erythrocyte surface glycoprotein has been reported to significantly enhance the susceptibility to free radical induced membrane damage (Hajela et al., 1997). The effect of lectin induced agglutination on the rate of oxidative damage to the cell membrane revealed that lectin induced perturbations in cell membrane make it more vulnerable to oxidative attack. The leaks or pores formed in the lipid bilayer facilitate the superoxide ions to seep through the hydrophobic cellular membrane which was earlier impervious to these ions.
Figure 1.
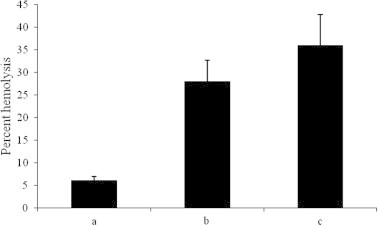
Effect of GHG-1 on pyrogallol induced free radical damage to erythrocyte membrane: OxyHb concentration was measured in the hemolysates of ‘a’: erythrocytes exposed to superoxide radicals in the absence of GHG-1, ‘b’: erythrocytes exposed to superoxide radicals in the presence of GHG-1 after 1 h, ‘c’: erythrocytes exposed to superoxide radicals in the presence of GHG-1 after 4 h. Samples were centrifuged and RBC lysates were analyzed at 540 nm. Values shown are the mean ± S.E.M obtained from three observations.
It is a well established fact that HOCl is an extremely toxic oxidant that can react with a variety of cellular components. It has also been confirmed that HOCl causes erythrocyte lysis through lipid modification, membrane cross linking, K+ leakage and cell swelling (Vissers and Winterbourn, 1991; Zavodnik et al., 2002). Therefore, the oxidizing action of HOCl was monitored in the presence of GHG-1 in terms of percent erythrocyte hemolysis. The extent of erythrocyte hemolysis was found to be directly proportional to HOCl concentrations (Fig. 2) Prior agglutination of erythrocytes with GHG-1 resulted in significant (P < 0.001) enhancement of hemolysis as compared to untreated cells in the presence of varying concentrations of HOCl. At the concentration of 350 μM HOCl, GHG-1 agglutinated erythrocytes showed 50% hemolysis, as compared to 26% in non-agglutinated erythrocytes.
Figure 2.
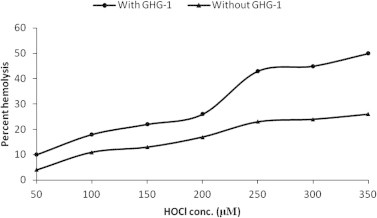
Effect of hypochlorous acid on GHG-1 induced hemolysis of trypsinized rabbit erythrocytes: an 8% trypsinized rabbit erythrocyte suspension (200 μl) treated with 100 μl GHG-1 (100 μg/ml) was incubated at varying concentrations of HOCl (50–350 μM). Samples were centrifuged and RBC lysates were analyzed at 540 nm.
An additive effect on the increase in percent hemolysis of erythrocytes is observed by lectin mediated HOCl induced cell damage, thus suggesting that together with hypochlorite, lectins may be an active pore forming agent, thereby making the membrane components more susceptible to oxidative assault. Heart lectin is a homodimeric protein with two binding sites which can cross-link identical ligands on the cell surface or to the extracellular matrix inducing conformational changes in membrane proteins and altering lipid fluidity, and thus may increase the accessibility of HOCl to RBC components. As the average number of pores formed by HOCl per cell is less than one (Zavodnik et al., 2002), prior agglutination with lectin possibly increases the number of short lived pores in plasma membrane. This suggests the possibility of the role of galectins in HOCl induced neutrophil mediated cellular damage, which may have direct implications in various inflammatory conditions where increased expression of galectin has been reported (Rorive et al., 2001). Thus, lectins exhibiting hemolytic and cytolytic functions might be involved in the defense mechanism of mammalian nervous system, not only neutralizing foreign substances by binding to their carbohydrate moieties, but also acting directly as a toxic protein to invading microorganisms. Hence, lectin interaction with membranes acts as a perturbing tool causing a change in membrane topology and assembly, which may play an important role under physiological demands or pathological conditions in mammalian nervous system. This is further strengthened by the fact that the antigenic determinants present on the exterior surface of erythrocytes and other cells are carried by both glycolipids and glycoproteins, and soluble blood group substances are strictly glycoprotein in nature (Toivanen et al., 2008).
Galectins have been reported to modulate functions crucial for cell survival, migration and metastasis, thus making them potential targets for cancer therapy (Vladoiu et al., 2014). The close involvement of galectins in various cancer types continues to be established (Huang et al., 2014; Song et al., 2014). GHG-1 mediated agglutination of erythrocytes collected from breast and prostate cancer patients showed a differential pattern of hemolytic activity when compared to healthy controls. In breast cancer patients, agglutination with GHG-1 displayed an increase in percent hemolysis in both pre-operated (29%) and post-operated (41%) erythrocytes with respect to unagglutinated pre-operated (24%) and post-operated (10%) erythrocytes (Fig. 3). In prostate cancer patients, agglutination with GHG-1 displayed an increase in percent hemolysis in both pre-operated (71%) and post-operated (47%) erythrocytes with respect to unagglutinated pre-operated (47%) and post-operated (13%) erythrocytes (Fig. 4).
Figure 3.
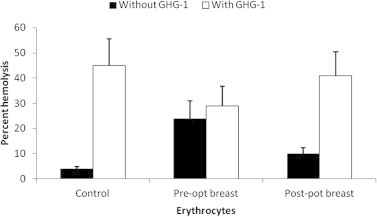
Hemolysis of human erythrocytes collected from normal, pre-operated and post-operated breast cancer patients in the absence and presence of GHG-1: an 8% trypsinized rabbit erythrocyte suspension (200 μl) was prepared for normal, pre-operated and post-operated blood samples obtained from breast cancer patients, and treated in the presence and absence of GHG-1 (100 μg/ml). The degree of hemolysis was calculated by comparing with identical volume of erythrocytes mixed with distilled water which represented 100% lysis. Values shown are the mean ± S.E.M obtained from three observations.
Figure 4.
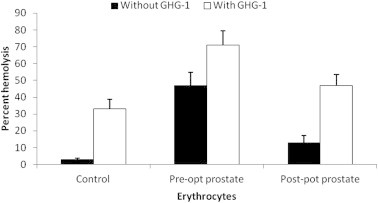
Hemolysis of human erythrocytes collected from normal, pre-operated and post-operated prostate cancer patients in the absence and presence of GHG-1: An 8% trypsinized rabbit erythrocyte suspension (200 μl) was prepared for normal, pre-operated and post-operated blood samples obtained from prostate cancer patients, and treated in the presence and absence of GHG-1 (100 μg/ml). The degree of hemolysis was calculated by comparing with identical volume of erythrocytes mixed with distilled water which represented 100% lysis. Values shown are the mean ± S.E.M obtained from three observations.
No significant change was observed in the hemolysis of lectin agglutinated erythrocytes collected from pre-operated breast cancer patients, whereas a significant increase was observed in normal healthy control and post-operated samples. This observation may be attributed to the decrease in the expression of β − 1 → 4 linked glycans on erythrocyte membrane surface in breast cancer patients and re-expression of β − 1 → 4 linked glycans after surgery. Owing to the pathological condition of cancer, an increase in the hemolysis of lectin untreated pre-operated erythrocytes was observed in comparison with the healthy control, and this finding was supported by an earlier study (Abou-Seif et al., 2000). Changes in binding qualities of blood group substances with lectins occur with the change in glyco-moieties of the glycoproteins (Hernández-Hernández et al., 2006). These changes in cell surface carbohydrates, during malignancy development involve blood antigens (Baxi et al., 1991). In contrast, an increase in hemolysis in lectin agglutinated erythrocytes was observed in prostate cancer patients, suggesting no change in glycosylation with respect to lectin specific sugars in erythrocytes of prostate cancer patients. These findings correlate galectin expression with the presence of erythrocytes in advanced stages of cancer and highlight potential role of galectins in antiangiogenic therapy (Laderach et al., 2013).
4. Conclusion
The effect of lectin induced agglutination on the rate of oxidative damage to the cell membrane revealed that lectin induced perturbations in cell membrane make it more vulnerable to oxidative attack. Leaks or pores formed in the lipid bilayer facilitate the superoxide ions to seep through the hydrophobic cellular membrane which was earlier impervious to these ions. No significant change was observed in the hemolysis of lectin agglutinated erythrocytes collected from pre-operated breast cancer patients, whereas a significant increase was observed in normal healthy control and post-operated samples. These findings suggested that lectins can be used as an excellent tool to detect the changes in the glycosylation pattern during malignancy which can be a better and handy method than analyzing biopsy samples.
Conflicts of interest
None declared.
Acknowledgements
The authors are grateful to the Aligarh Muslim University (Aligarh, India) for the facilities. Thanks are due to the Deanship of the Scientific Research (DSR) and King Fahd Medical Research Center (KFMRC), King Abdulaziz University (Jeddah, Saudi Arabia) for other facilities.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abou-Seif M.A., Rabia A., Nasr M. Antioxidant status, erythrocyte membrane lipid peroxidation and osmotic fragility in malignant lymphoma patients. Clin. Chem. Lab. Med. 2000;38:737–742. doi: 10.1515/CCLM.2000.104. [DOI] [PubMed] [Google Scholar]
- Ashraf G.M., Bilal N., Suhail N., Hasan S., Banu N. Glycosylation of purified buffalo heart galectin-1 plays crucial role in maintaining its structural and functional integrity. Biochemistry (Mosc.) 2010;75:1450–1457. doi: 10.1134/s0006297910120059. [DOI] [PubMed] [Google Scholar]
- Ashraf G.M., Rizvi S., Naqvi S., Suhail N., Bilal N., Hasan S., Tabish M., Banu N. Purification, characterization, structural analysis and protein chemistry of a buffalo heart galectin-1. Amino Acids. 2010;39:1321–1332. doi: 10.1007/s00726-010-0574-7. [DOI] [PubMed] [Google Scholar]
- Ashraf G.M., Banu N., Ahmad A., Singh L.P., Kumar R. Purification, characterization, sequencing and biological chemistry of galectin-1 purified from Capra hircus (goat) heart. Protein J. 2011;30:39–51. doi: 10.1007/s10930-010-9300-2. [DOI] [PubMed] [Google Scholar]
- Baxi B.R., Patel P.S., Adhvaryu S.G., Dayal P.K. Usefulness of serum glycoconjugates in precancerous and cancerous diseases of the oral cavity. Cancer. 1991;67:135–140. doi: 10.1002/1097-0142(19910101)67:1<135::aid-cncr2820670124>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Collard C.D., Montalto M.C., Reenstra W.R., Buras J.A., Stahl G.L. Endothelial oxidative stress activates the lectin complement pathway. Am. J. Pathol. 2001;159:1045–1054. doi: 10.1016/S0002-9440(10)61779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco C., Vona R., Cosimelli M., Matarrese P., Straface E., Scordati P., Giannarelli D., Casale V., Assisi D., Mottolese M., Moles A., Malorni W. Cell surface overexpression of galectin-3 and the presence of its ligand 90 k in the blood plasma as determinants in colon neoplastic lesions. Glycobiology. 2004;14:783–792. doi: 10.1093/glycob/cwh092. [DOI] [PubMed] [Google Scholar]
- Hajela K., Pande A.H., Sumati Carbohydrate induced modulation of cell membrane. VI. Binding of exogenous lectin induces susceptibility of erythrocytes to free radical damage: a spin label study. FEBS Lett. 1997;406:255–258. doi: 10.1016/s0014-5793(97)00272-x. [DOI] [PubMed] [Google Scholar]
- Hasan S.S., Ashraf G.M., Banu N. Galectins – potential targets for cancer therapy. Cancer Lett. 2007;253:25–33. doi: 10.1016/j.canlet.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Hernández-Hernández A., Rodríguez M.C., López-Revuelta A., Sánchez-Gallego J.I., Shnyrov V., Llanillo M., Sánchez-Yagüe J. Alterations in erythrocyte membrane protein composition in advanced non-small cell lung cancer. Blood Cells Mol. Dis. 2006;36:355–363. doi: 10.1016/j.bcmd.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Huang C.-S., Tang S.-J., Chung L.-Y., Yu C.-P., Ho J.-Y., Cha T.-L., Hsieh C.-C., Wang H.-H., Sun G.-H., Sun K.-H. Galectin-1 upregulates CXCR4 to promote tumor progression and poor outcome in kidney cancer. J. Am. Soc. Nephrol. 2014;25(7):1486–1495. doi: 10.1681/ASN.2013070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopitz J., André S., von Reitzenstein C., Versluis K., Kaltner H., Pieters R.J., Wasano K., Kuwabara I., Liu F.-T., Cantz M., Heck A.J.R., Gabius H.-J. Homodimeric galectin-7 (p53-induced gene 1) is a negative growth regulator for human neuroblastoma cells. Oncogene. 2003;22:6277–6288. doi: 10.1038/sj.onc.1206631. [DOI] [PubMed] [Google Scholar]
- Laderach D.J., Gentilini L.D., Giribaldi L., Delgado V.C., Nugnes L., Croci D.O., Nakouzi N.A., Sacca P., Casas G., Mazza O., Shipp M.A., Vazquez E., Chauchereau A., Kutok J.L., Rodig S.J., Elola M.T., Compagno D., Rabinovich G.A. A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease. Cancer Res. 2013;73:86–96. doi: 10.1158/0008-5472.CAN-12-1260. [DOI] [PubMed] [Google Scholar]
- Lahm H., André S., Hoeflich A., Kaltner H., Siebert H.-C., Sordat B., von der Lieth C.-W., Wolf E., Gabius H.-J. Tumor galectinology: insights into the complex network of a family of endogenous lectins. Glycoconjugate J. 2004;20:227–238. doi: 10.1023/B:GLYC.0000025817.24297.17. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Pugalendhi P., Manoharan S., Suresh K., Baskaran N. Genistein and daidzein, in combination, protect cellular integrity during 7,12-dimethylbenz[a]anthracene (DMBA) induced mammary carcinogenesis in Sprague-Dawley rats. Afr. J. Tradit. Complement. Altern. Med. 2010;8:91–97. doi: 10.4314/ajtcam.v8i2.63196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Lotan R. Lectin-like activities associated with human and murine neoplastic cells. Cancer Res. 1981;41:3642–3647. [PubMed] [Google Scholar]
- Robert W.L., Gusheng W., David B., Zi-Hua L., Hans-Joachim G. Galectins and Disease Implications for Targeted Therapeutics. American Chemical Society; 2012. Galectin-1 cross-linking of GM1 ganglioside in autoimmune suppression; pp. 107–121. (vol. 1115). [Google Scholar]
- Rorive S., Belot N., Decaestecker C., Lefranc F., Gordower L., Micik S., Maurage C.A., Kaltner H., Ruchoux M.M., Danguy A., Gabius H.J., Salmon I., Kiss R., Camby I. Galectin-1 is highly expressed in human gliomas with relevance for modulation of invasion of tumor astrocytes into the brain parenchyma. Glia. 2001;33:241–255. doi: 10.1002/1098-1136(200103)33:3<241::aid-glia1023>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Rotblat B., Niv H., André S., Kaltner H., Gabius H.-J., Kloog Y. Galectin-1(L11A) predicted from a computed galectin-1 farnesyl-binding pocket selectively inhibits Ras-GTP. Cancer Res. 2004;64:3112–3118. doi: 10.1158/0008-5472.can-04-0026. [DOI] [PubMed] [Google Scholar]
- Shetty R.K.S., Bhandary S.K., Kali A. Significance of serum l-fucose glycoprotein as cancer biomarker in head and neck malignancies without distant metastasis. J. Clin. Diagn. Res. 2013;7:2818–2820. doi: 10.7860/JCDR/2013/6681.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Tang J.-W., Owusu L., Sun M.-Z., Wu J., Zhang J. Galectin-3 in cancer. Clin Chim. Acta. 2014;431:185–191. doi: 10.1016/j.cca.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Toivanen A., Ihanus E., Mattila M., Lutz H.U., Gahmberg C.G. Importance of molecular studies on major blood groups – intercellular adhesion molecule-4, a blood group antigen involved in multiple cellular interactions. Biochim. Biophys. Acta. 2008;1780:456–466. doi: 10.1016/j.bbagen.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Vissers M.C.M., Winterbourn C.C. Oxidative damage to fibronectin: I. The effects of the neutrophil myeloperoxidase system and HOCl. Arch. Biochem. Biophys. 1991;285:53–59. doi: 10.1016/0003-9861(91)90327-f. [DOI] [PubMed] [Google Scholar]
- Vladoiu M.C., Labrie M., St-Pierre Y. Intracellular galectins in cancer cells: potential new targets for therapy (review) Int. J. Oncol. 2014;44:1001–1014. doi: 10.3892/ijo.2014.2267. [DOI] [PubMed] [Google Scholar]
- Winterbourn C.C. Free-radical production and oxidative reactions of hemoglobin. Environ. Health Perspect. 1985;64:321–330. doi: 10.1289/ehp.8564321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavodnik I.B., Lapshina E.A., Zavodnik L.B., Soszyński M., Bartosz G., Bryszewska M. Hypochlorous acid-induced oxidative damage of human red blood cells: effects of tert-butyl hydroperoxide and nitrite on the HOCl reaction with erythrocytes. Bioelectrochemistry. 2002;58:127–135. doi: 10.1016/s1567-5394(01)00126-8. [DOI] [PubMed] [Google Scholar]


