Abstract
Objective
Some previous studies have reported that ADHD is often comorbid with anxiety disorders. The aim of the present study was to evaluate the effectiveness of reboxetine in treating outpatient children and adolescents with ADHD and comorbid anxiety disorders.
Method
In this open-label study, 25 outpatient children and adolescents, aged 6-16 years were selected by convenient sampling and underwent treatment with 4mg reboxetine for four weeks. Data were collected at baseline, two weeks and four weeks after the start of the medication using Conners’ Parent Questionnaire, Hamilton’s Rating Scale for Anxiety, Clinical Global Assessment Scale, Clinical Global Impression-Severity Scale and Side Effects Form. Data were analyzed using repeated measure, analyses of variance (ANOVA), Tukey post hoc test and paired t-test.
Results
There were significant reduction in the total score of ADHD (F = 31.441; P <0.001) at the end of the treatment compared to baseline (Table 1). The differences between T0 and T2 in the subscales of attention deficit, hyperactivity and confrontation (F = 20.691; P <0.001, F = 28.810; P < 0.001, and F = 17.463; P <0.001, respectively) were also significant. Findings also indicated significant differences between T0 and T1 (P<0.01) and T1 and T2 (P<0.01) in all of the subscales except for confrontation. A significant improvement was observed in the severity of ADHD and anxiety disorders during different courses of the treatment (p<0.001). No significant changes were observed in systolic and diastolic blood pressure and pulse and weight of the patients during the study. The most common complications were headache and anorexia.
Conclusions
The short-term treatment with reboxetine was effective in improving ADHD with comorbid anxiety disorders. Therefore, reboxetine could be used as a treatment option for ADHD in those children who experience comorbid anxiety disorders or in those who are non-responsive or intolerant to methylphenidate.
Keywords: Attention deficit-hyperactivity disorder (ADHD), Reboxetine, Conners’ Parent questionnaire, Hamilton’s Anxiety Rating Scale
Attention deficit-hyperactivity disorder (ADHD) is one of the most common neurobehavioral and psychiatric disorders in children and adolescents. About 3-7% of school age children and adolescents have been reported to be affected by ADHD.1 This disorder often causes impairment in academic performance and interpersonal relationships; and researches indicate concurrent symptoms of mood, anxiety and substance abuse disorders in patients with ADHD; 2 thus, its treatment is of prime importance.
Dopaminergic and noradrenergic agonists or stimulants are first-line medications in treatment of patients with ADHD; however, due to some limitations (e.g., non- responsiveness of some patients, worsening of anxiety in patients with comorbid anxiety symptoms and potential risk for abuse),several clinical trials have considered non-stimulants drugs to treat ADHD. 3,4
Although approximately all antidepressants can be used to treat ADHD, the clinical use of tricyclic antidepressants such as imipramine and desipramine is limited due to risk of adverse effects like cardiovascular complications and sudden death.5, 6,7 Atomoxetineis often suggested rather than tricyclic antidepressant in cases of non-responsiveness to stimulants or in presence of depression or anxiety symptoms. Atomoxetine is a selective norepinephrine reuptake inhibitor, with little effects on other receptors or neurotransmitters.8 some studies showed that atomoxetine is an effective and economic alternative for the treatment of ADHD.9 Reboxetine is also a selective norepinephrine reuptake with similar effects, but a longer half-life of approximately 12 hours.10, 11
Several recent studies have revealed the efficacy and tolerability of reboxetine in treatment of ADHD; 12, 10,13, 14, 15, 16, 17, 18 but to our knowledge, there is none considering the effectiveness of reboxetine in treatment of patients with ADHD and concurrent anxiety disorders. The aim of this study was to evaluate the effect of reboxetine on ADHD with comorbid anxiety disorders in children and adolescents.
Material and Methods
In this open-label study, 25 outpatient children and adolescents (men age: 8.7 and range: 6-16 years) diagnosed with ADHD (combined subtype) and comorbid anxiety disorders (including General Anxiety Disorder, Social Phobia Disorder or Specific Phobia Disorder)were selected from the outpatient clinic of child and adolescent psychiatry of Golestan educational hospital in Ahvaz/Iran using convenient sampling.
Parents Complaints were ADHD. After explaining the procedure and the purpose of the study, informed consent was obtained from the parents.
Diagnosis of the disorders according to DSM-IV-TR criteria and physical examination including blood pressure, peripheral pulse and weight were made by two child and adolescent psychiatrists, and Raven IQ test was performed by a clinical psychologist.
Exclusion criteria included the presence of heart problem, high blood pressure, kidney and liver dysfunction, epilepsy, drug abuse during the last month, intolerance to reboxetine, mental retardation (IQ≤70) and any other psychiatric disorders.
All subjects received treatment with reboxetine for four weeks. To minimize the side effects, reboxetine was started at a single dose of 1mg in the morning of the first day and continued to two divided doses in the morning and afternoon by the end of first week. Then, the dose increased to 4mg daily (2mg in the morning and 2mg in the afternoon) from the second to forth weeks. The average dose was 3.8 mg/day.
One of the participants could not tolerate the drug and developed butterfly sensation after taking the first dose (1mg) and gave up the study.
The participants were evaluated at baseline, two weeks and four weeks after the start of the medication. Assessments included completing the side effects form and filling out the Canner’s Parent questionnaire (by children’s parents), Hamilton Rating Scale for Anxiety (HRSA), Clinical Global Assessment Scale (CGAS) and Clinical Global Impression-Severity scale (CGIS-S)(by a child and adolescent psychiatrist)
Questionnaire
Conner’s Parent Rating Scale–Revised Short Version [(CPRS –RS]):
Conner’s Parent Rating Scale–Revised Short Version is an instrument for assessing ADHD symptoms according to the fourth edition of diagnostic and classification of mental disorders (DSM-IV); its subscales include attention deficit, hyperactivity and confrontation.19
Shahaeian A, Shahim S, Bashash L, usofi F. (2006) used factor analysis toassess the validity of the instrument. They obtained 4 factors of conduct problems, social problems, anxiety- shyness and psychosomatic problems. Shahaeian et al. evaluated the reliability of the instrument by calculating Cronbach’s alpha as a measure of internal consistency reliability. They found the measure to be reliable. Coefficient consistency was 0.73 for the total score and 0.57 to 0.86 for the subscales.20
Child Functional Assessment Scale (CGAS)
The Child performance assessment (CGAS) runs from zero to 100 (highest performance in all areas, including social, occupational and psychological). 21 The joint reliability of the scale has been reported quite high ranging from 0.83 to 0.91 in research settings. However, in typical clinical settings, only moderate agreement has been demonstrated (0.53–0.66). In evaluation of validity in a community study in Puerto Rico, the CGAS score differentiated between children identified by diagnostic research interviews as patients and as non-patients.22 The validity and reliability of this test was in agreement with the Diagnostic and Statistical Manual diagnosis of mental disorders (DSM). The face validity of the Persian version of the scale was checked by 5psychiatry faculty members at the Psychiatric Department of Ahvaz Jundishapur University of Medical Sciences.
Clinical Global Impression-severity Scale (CGI-S)
The Clinical Global Impression-Severity Scale is used for clinical assessment of the severity of impairment, with scores from zero (normal condition) to seven (maximum severity). The scale has been previously used in the Iranian population.18
Hamilton Anxiety Rating Scale (HARS)
This instrument runs from zero (absence of symptom) to 4 (very severe and disabling); 23 it must be completed by the therapist based on the patient and parent interviews at the same time 24. HARS is a suitable instrument to measure general symptoms of anxiety in adolescents.25 In the present study, we used this instrument to assess the severity of comorbid anxiety disorders although the diagnoses of anxiety disorders were made by two child and adolescent psychiatrists.
Convergent validity correlation of this instrument has been reported 0.63 with Covi anxiety scale, and some studies have reported inter-rater reliability of the instrument 0.82, 0.92,0.92. 26
Statistical Tests
Analyses of variance with repeated measure were used to compare the symptoms of ADHD and at all the three time points. Three measurements during the study were considered as the within-subjects factor in the analysis.
Tukey post hoc test for pair comparisons was used. Paired t-test was used to assess the changes in the severity of ADHD and anxiety before and after the treatment. Statistical analyses were carried out using SPSS version 17.
Result
Of the total of 25 patients who participated in the study, 24 cases (20(84%) boys and 4(16%) girls) tolerated the reboxetine and completed the treatment.
The result of repeated measure was statistically significant (p<0.001). We found significant reduction in total score of ADHD (F = 31.441; P <0.001) at the end of the treatment compared to baseline. The differences between T0 and T2 in the subscales of attention deficit, hyperactivity and confrontation (F = 20.691; P <0.001, F = 28.810; P <0.001, and F = 17.463; P <0.001, respectively) were also statistically significant. Significant differences also reported between T0 and T1 (P<0.01) and T1 and T2 (P<0.01) in all of the subscales excluding confrontation. In other words, although the reduction of scores of confrontation at baseline and two weeks after the start of medication was significant, these changes were not significant between the second and fourth weeks (Table 1).
Table 1.
Comparison of the drop of subscale scores in attention deficit, hyperactivity, confrontation during different study time courses
| Scoring index | T0 | T1 | T2 | P-value a | P-value b |
|---|---|---|---|---|---|
| Attention deficit, Mean ± SD | 18.80 ± 4.63 | 13.36 ± 4.13 | 10.13 ± 3.13 | 0.000 | 0.001 |
| hyperactivity, Mean ± SD | 15.72 ± 4.2 | 11.58 ± 3.98 | 8.3 ± 3.0 | 0.001 | 0.037 |
| Confrontation, Mean ± SD | 8.72 ± 2.6 | 6.33 ± 2.18 | 4.88 ± 2.0 | 0.002 | 0.032 |
| Total score, Mean ± SD | 50.36 ± 10.64 | 37.21 ± 10.68 | 27.92 ± 8.36 | 0.000 | 0.005 |
Beginning of the study (T0), after 2-week (T1) and after 4-week (T2)
p-values between T0 and T1
p-values between T1 and T2
As shown in Table 2, the overall reduction in the severity of attention deficit-hyperactivity disorder was significant at the end of the treatment.
Table 2.
Comparison of the severity of ADHD and anxiety during different study time courses
| severity | T0 | T2 | T | P-value |
|---|---|---|---|---|
| severity of ADHD, Mean ± SD | 5.60 ± 0.5 | 3.25 ± 0.44 | 17.39 | 0.001 |
| severity of anxiety, Mean ± SD | 4.80 ± 0.645 | 2.33 ± 0.87 | 11.31 | 0.001 |
Beginning of the study (T0), and after 4-week (T2)
There were also significant differences between T0 and T1 (P<0.01) and between T1 and T2 (P<0.01) in the Hamilton’s test scores, which indicated that the scores of anxiety reduced during the treatment (Figure 1). The overall reduction in the severity of the anxiety disorders was significant at the end of the treatment compared to baseline ((F = 83.879; P <0.001) (Table 2). According to the results, anxiety reduced during the treatment, and this intervention had been effective in reduction of anxiety.
Fig 1.
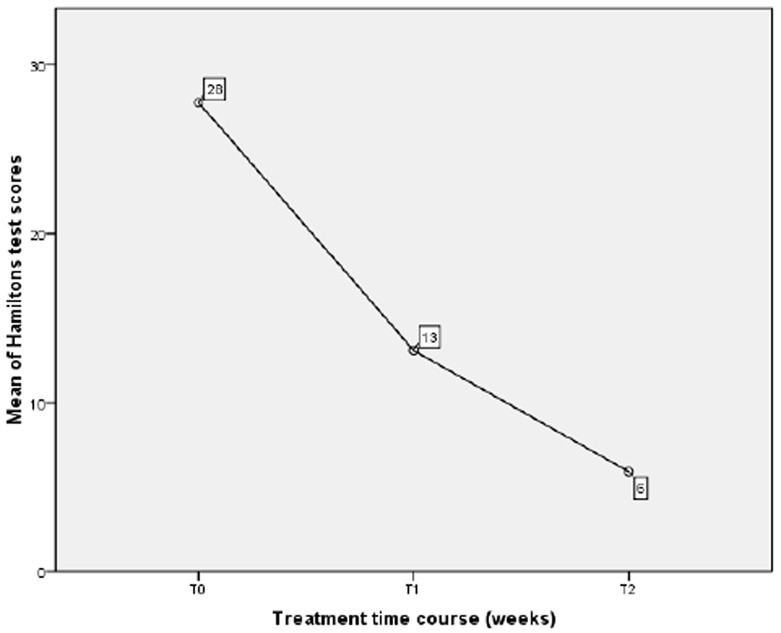
Comparison of the Hamilton’s test scores between different study time courses
Performance scores of participants were 41-50 (lowest level of performance) at the beginning of the treatment. Thirteen cases (52%) reached to 71 -80 score, 10 participants (40%) to 61-70 score, and one participant (4%) to 50-61 score at the end of the study (Figure 2).
Fig 2.
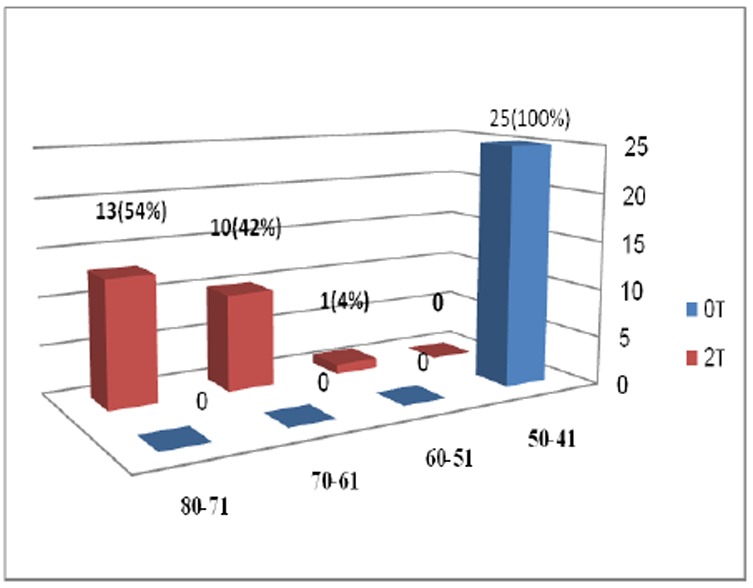
Changes in performance level of 24 patients who completed the study
The results of paired t-test revealed no dramatic changes in systolic and diastolic blood pressure, pulse and weight of the patients during the study (Table 3).
Table 3.
change in systolic and diastolic blood pressure, pulse rate and body weight of the patient during the study
| Scoring index | T0 | T1 | T2 | P-valuea | P-valueb |
|---|---|---|---|---|---|
| SBP, Mean ± SD | 99.56 ± 9.90 | 100.20 ± 9.38 | 100.40 ± 11.4 | 0.954 | 0.991 |
| DBP, Mean ± SD | 65.80 ± 7.99 | 64.17 ± 9.51 | 65.58 ± 8.36 | 0.775 | 0.857 |
| PR, Mean ± SD | 84.92 ± 8.36 | 86.25 ± 7.62 | 87.54 ± 6.77 | 0.488 | 0.852 |
| BW, Mean ± SD | 38.24 ± 17.95 | 38.75 ± 18.03 | 39.08 ± 18.32 | 0.987 | 0.798 |
Beginning of the study (T0), after 2-week (T1) and after 4-week (T2); SBP, Systolic blood pressure; DBP, Diastolic blood pressure; PR, Pulse rate; BW, Body weight
p-values between T0 and T1
p-values between T1 and T2
Some side effects were observed in the study including headache, low appetite, somnolence, irritability, butterfly sensation and bruxism. The most common complications were headache and anorexia (Table 4). Thirteen participants (52%) did not experience any side effects and two experienced headache and low appetite simultaneously.
Table 4.
Adverse Effects on Reboxetine Treatment (N=25)
| Side effects | Frequency (%) |
|---|---|
| Headache | 4(16%) |
| Low appetite | 2(8%) |
| Somnolence | 2(8%) |
| Irritability | 2(8%) |
| Butterfly sensation | 1(4%) |
| Bruxism | 1(4%) |
Beginning of the study (T0), after 2-week (T1) and after 4-week (T2)
significant p-value (<0.001) between T0 and T1
significant p-value (<0.001) between T1 and T2
Discussion
The therapeutic effects of noradrenergic drugs on core symptoms of ADHD have been suggested in some studies.5, 27 The role of norepinephrine dysfunction in anxiety disorders 28, 29 also has been proved in some studies.
In the present study, it was assumed that reboxetine which is a selective noradrenaline reuptake inhibitor can be effective in the treatment of ADHD with comorbid anxiety disorders. In previous studies, the patients with ADHD did not have any comorbid disorder10, 12, or ADHD was concurrent with mood disorders.30
In this open-label trial, the maximum dose of 4 mg daily of reboxetine was effective in the reduction of all the three ADHD subscales including hyperactivity, attention deficit and confrontation as well as anxiety disorders. These findings were in line with the previous studies which reported the positive effect of reboxetine in reducing symptoms of ADHD 10, 12, 13, 14 and its effects on anxiety disorders as well.31, 32
Inconsistent with previous studies, we found a significant reduction in ADHD symptoms after two weeks of treatment (12,30).
Approximately, 44% reduction in ADHD symptoms was observed after four weeks of treatment with reboxetine. This result was fairly close to previous reports of 46% 12 and 42% 10.
In our study, the reduction of anxiety symptoms using Hamilton’s anxiety tool after four weeks was 78%, which was higher than 69.63 % in depressed children and 24% in ADHD children in previous research.24 These findings suggest that reboxetine is significantly effective in reduction of symptoms of ADHD and anxiety symptoms in children who met the criteria for both of these disorders
To explain the efficacy of reboxetine in treatment of ADHD, it can be stated that the noradrenergic system is involved in the modulation of higher cortical functions including attention, alertness, vigilance and executive functions12. Low concentration of norepinephrine in the prefrontal cortex could cause lack of self-control, inattention and hyperactivity 27. Therefore, increasing norepinephrine level in this area of the brain by a selective norepinephrine reuptake inhibitor improves ADHD symptoms12. Reboxetine increases dopamine in the prefrontal cortex and can have beneficial effects on ADHD symptoms. 33
We assessed the cardiovascular system in terms of systolic and diastolic blood pressure, pulse and also weight of the patients and this was the strength of this study compared to some previous studies; however, it should be noted that no dramatic changes were observed10. This clears the superiority of reboxetine over TCA with regards to the cardiac side effects.
In the present study, the side effects were tolerable and diminished with continuation of the treatment .
In previous studies, drowsiness and loss of appetite have been reported as the most common side effects10, 12, but in our study, headache was the most common side effect. These differences may be due to differences in the target groups.
Inconsistent with some studies, we did not have side effects of drowsiness, dry mouth, stomachache or nausea.10
In this study, the participants with OCD and PTSD were excluded because of their different neurobiology, which is one of the strengths of this research as well as the blindness of the patients and their families about the medication in use.
Conclusion
The short-term treatment (4-week) with reboxetine was effective in reduction of symptoms of AHDH with comorbid anxiety disorders in children and adolescents. Considering the role of the noradrenergic system in the path physiology of ADHD and anxiety disorders, limitations of using stimulants34, 35, limitations of using tricyclic antidepressants36 and also by considering the tolerability of reboxetine10, 12,13,15 and its little side effects (because of low tendency towards receptors of serotonin, histamine and cholinergic)28, 37, and its shorter plasma half-life compared to atomoxetine1 , it seems that reboxetine is a safe medicine for patients suffering from ADHD with concurrent anxiety. Reboxetine is a non-stimulant drug without a risk of abuse that can be prescribed for adolescents. It may also be beneficial to patients with ADHD with comorbid Tic.
Limitations
The limitations of the present study include the relatively small sample size, no control group and short duration of the study. Double-blind controlled trials, with larger sample size and longer- term evaluation are suggested for the future researches.
Acknowledgments
This study was approved by the Ethical Committee of Jundishapur University of medical sciences (No: P.8.20.8158) and has been submitted under IRCT138905033979N3 in IRCT.
We appreciate the support of the staff at the RCC (Research Consulting Center) of Research Deputy at the Jundishapur University of Medical Sciences.
Footnotes
Conflict of interest:
The authors declare no conflict of interests
References
- 1.Wilens TE, Spencer TJ. Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgrad Med. 2010;122:97–109. doi: 10.3810/pgm.2010.09.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ek U, Westerlund J, Holmberg K, Fernell E. Academic performance of adolescents with ADHD and other behavioural and learning problems -a population-based longitudinal study. Acta Paediatr. 2010;100:402–406. doi: 10.1111/j.1651-2227.2010.02048.x. [DOI] [PubMed] [Google Scholar]
- 3.Banaschewski T, Roessner V, Dittmann RW, Santosh PJ, Rothenberger A. Non-Stimulant Medications in the Treatment of Adhd. Eur Child Adolesc Psychiatry. 2004;13(Suppl 1):I102–116. doi: 10.1007/s00787-004-1010-x. [DOI] [PubMed] [Google Scholar]
- 4.Pliszka SR. Non-stimulant treatment of attention-deficit/hyperactivity disorder. CNS Spectr. 2003;8:253–8. doi: 10.1017/s1092852900018460. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J, Faraone SV, Spencer T, et al. Patterns Of Psychiatric Co morbidity, Cognition, And Psycho Social functioning in Adults with Attention Deficit Hyperactivity Disorder. Am J psychiatry. 1993;150:1792–1798. doi: 10.1176/ajp.150.12.1792. [DOI] [PubMed] [Google Scholar]
- 6.Biederman J, Faraone S, Milberger S, Guite J, Mick E, Chen L, et al. A Prospective 4-Year Follow-up Study of Attention-Deficit Hyperactivity and Related Disorders. Arch Gen Psychiatry. 1996;53:437–446. doi: 10.1001/archpsyc.1996.01830050073012. [DOI] [PubMed] [Google Scholar]
- 7.Prasad S, Furr AJ, Zhang S, Ball S, Allen AJ. Baseline values from the electrocardiograms of children and adolescents with ADHD. Child Adolesc Psychiatry Ment Health. 2007;1:11. doi: 10.1186/1753-2000-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11:203–226. doi: 10.2165/00148581-200911030-00005. [DOI] [PubMed] [Google Scholar]
- 9.Hong J, Dilla T, Arellano J. A Modelled Economic Evaluation Comparing Atomoxetine with Methylphenidate in the Treatment of Children with Attention-Deficit/Hyperactivity Disorder in Spain. BMC Psychiatry. 2009;9:15. doi: 10.1186/1471-244X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arabgol F, Panaghi L, Hebrani P. Reboxetine versus methylphenidate in treatment of children and adolescents with attention deficit-hyperactivity disorder. Eur Child Adolesc Psychiatry. 2009;18:53–59. doi: 10.1007/s00787-008-0705-9. [DOI] [PubMed] [Google Scholar]
- 11.Cohen-Yavin I, Yoran-Hegesh R, Strous RD, Kotler M, Weizman, Spivak B. Efficacy of Reboxetine in the Treatment of Attention-Deficit/Hyperactivity Disorder in Boys with Intolerance to Methylphenidate: An Open-Label, 8-Week, Methylphenidate-Controlled Trial. Clin Neuropharmacol. 2009;32:179–182. doi: 10.1097/WNF.0b013e318183796d. [DOI] [PubMed] [Google Scholar]
- 12.Tehrani-Doost M, Moallemi S, Shahrivar Z. An open-label trial of reboxetine in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2008;18:179–184. doi: 10.1089/cap.2006.0034. [DOI] [PubMed] [Google Scholar]
- 13.Toren P, Ratner S, Weizman A, Lask M, Ben-Amitay G, Laor N. Reboxetine Maintenance Treatment in Children with Attention-Deficit/Hyperactivity Disorder: A Long-Term Follow-up Study. Journal of child and adolescent psychopharmacology. 2007;17:803–812. doi: 10.1089/cap.2006.0145. [DOI] [PubMed] [Google Scholar]
- 14.Cak HT, Cetin FC. Reboxetine use in the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2006;16:803–804. doi: 10.1089/cap.2006.16.803. [DOI] [PubMed] [Google Scholar]
- 15.Ratner S, Laor N, Bronstein Y, Weizman A, Toren P. Six-week open-label reboxetine treatment in children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:428–433. doi: 10.1097/01.chi.0000155327.30017.8c. [DOI] [PubMed] [Google Scholar]
- 16.Mozes T, Meiri G, Ben Amity G, Sabbagh M, Weizman A. Reboxetine as an optional treatment for hyperkinetic conduct disorder: a prospective open-label trial. J Child Adolesc Psychopharmacol. 2005;15:259–269. doi: 10.1089/cap.2005.15.259. [DOI] [PubMed] [Google Scholar]
- 17.Otka JE, Mercadante MT, Scahill L, Leckman JF. Reboxetine as a Potentially Effective Treatment for Attention Deficit Hyperactivity Disorder. J Child Adolesc Psychopharmacol. 2001;11:203–204. doi: 10.1089/104454601750284135. [DOI] [PubMed] [Google Scholar]
- 18.Riahi F, Tehrani-Doost M, Shahrivar Z, Alaghband-Rad J. Efficacy of reboxetine in adults with attention-deficit/hyperactivity disorder: A randomized, placebo-controlled clinical trial. Hum Psychopharmacol. 2010;25:570–576. doi: 10.1002/hup.1158. [DOI] [PubMed] [Google Scholar]
- 19.Conners KC. Conners Rating Scales Manual. New York: Multi-Health; 1997. [Google Scholar]
- 20.Shahaeian A, Shahim S, Bashash L, usofi F. Standardization, factor analysisandreliability of the short form of Conner’s parent rating scalefor6 to 11years old childreninShiraz. Educational Psychological studies. 2007;3:97–120. [Google Scholar]
- 21.Dyrborg J, Larsen FW, Nielsen S, Byman J, Nielsen BB, Gautre-Delay F. The Children’s Global Assessment Scale (Cgas) and Global Assessment of Psychosocial Disability (Gapd) in Clinical Practice--Substance and Reliability as Judged by Intraclass Correlations. Eur Child Adolesc Psychiatry. 2000;9:195–201. doi: 10.1007/s007870070043. [DOI] [PubMed] [Google Scholar]
- 22.Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A Children’s Global Assessment Scale (Cgas) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 24.Andreescu C, Belnap BH, Rollman BL, Houck P, Ciliberti C, Mazumdar S, et al. Generalized anxiety disorder severity scale validation in older adults. Am J Geriatr Psychiatry. 2008;16:813–818. doi: 10.1097/JGP.0b013e31817c6aab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman JB, Gareia AM, Leonard HL. Anexity disorders. In: Lewis M, editor. Child and adolescent psychiatry: a comprehensive text book new haven. New York: Williams and Wilkins; 2002. [Google Scholar]
- 26.McDowell I. Measurement Health, A Guide to Rating Scales and Questionnaires. 3. New York: Oxford University Press; 2006. [Google Scholar]
- 27.Biederman J, Spencer T, Wilens T. Evidence –based pharmacotherapy for attention –deficit hyper activity disorder. Int J Neuropsychopharmacol. 2004;7:77–97. doi: 10.1017/S1461145703003973. [DOI] [PubMed] [Google Scholar]
- 28.Schuler P, Seibel K, Chevts V, Schaffler K. [Analgesic Effect of the Selective Noradrenaline Reuptake Inhibitor Reboxetine] Nervenarzt. 2002;73:149–154. doi: 10.1007/s00115-001-1226-7. [DOI] [PubMed] [Google Scholar]
- 29.Versiani M, Cassano G, Perugi G, Benedetti A, Mastalli L, Nardi A, et al. Reboxetine, a Selective Norepinephrine Reuptake Inhibitor, Is an Effective and Well-Tolerated Treatment for Panic Disorder. J Clin Psychiatry. 2002;63:31–37. doi: 10.4088/jcp.v63n0107. [DOI] [PubMed] [Google Scholar]
- 30.Tashakori A, Arablgol F, Panahy L. Effect of Reboxetine on Reduction of Anxiety Symptoms in Depressed Children and Adolescents. Scientific Medical Journal. 2006;6:210–218. [Google Scholar]
- 31.Kasper S, EI Giamal N, Hilger E. Reboxetine: The first selective noradrenalin reuptake inhibitor. Expert Opin Pharmacother. 2000;1:771–782. doi: 10.1517/14656566.1.4.771. [DOI] [PubMed] [Google Scholar]
- 32.Taner E, Demir EY, Cosar B. Comparison of the effectiveness of reboxetine versus fluoxetine in patients with atypical depression: a single-blind, randomized clinical trial. AdvTher. 2006;23:974–987. doi: 10.1007/BF02850218. [DOI] [PubMed] [Google Scholar]
- 33.Valentini V, Frau R, Di Chiara G. Noradernalin Transporter Blockers raise Extracellular dopamine In Medial Prefrontal but not Parietal and Occipital Context: Differences With mianserin and Clozapin. J Neurochem. 2004;88:917–927. doi: 10.1046/j.1471-4159.2003.02238.x. [DOI] [PubMed] [Google Scholar]
- 34.Spencer T, Biederman J, Wilens T, et al. Pharmacotherapy of attention deficit hyperactivity disorder across the Life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35:409–432. doi: 10.1097/00004583-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Taylor E, Dopfner M, Sergeant J, Asherson P, Banaschewski T, Buitelaar J, et al. European Clinical Guidelines for Hyperkinetic Disorder -- First Upgrade. Eur Child Adolesc Psychiatry. 2004;13(Suppl 1):I7–30. doi: 10.1007/s00787-004-1002-x. [DOI] [PubMed] [Google Scholar]
- 36.Labellarte MJ, Crosson JE, Riddle MA. The relevance of prolonged QTc measurement to pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry. 2003;42:642–450. doi: 10.1097/01.CHI.0000046860.56865.25. [DOI] [PubMed] [Google Scholar]
- 37.Taner E, Demir EY, Cosar B. Comparison of the effectiveness of reboxetine versus fluoxetine in patients with atypical depression: a single-blind, randomized clinical trial. Adv Ther. 2006;23:974–987. doi: 10.1007/BF02850218. [DOI] [PubMed] [Google Scholar]


