Abstract
Background and Objectives
The present work was carried out to investigate the ability of Spirulina platensis to produce antimicrobial substance against bacteria and fungi.
Materials and Methods
The cells of the cyanobacterium were subjected to different extractions and the purified antagonistic compound proved to be effective against broad spectrum of bacteria and fungi. The antagonistic compound was purified using thin layer chromatography.
Results
The results indicated that the IR spectrum showed bands at 1269 cm-1, 1414 cm-1 (C-O-C), 1643 cm-1 (CO of amide),1563 cm-1 (C = C) and broad band 3441 cm-1 (of OH and NH)., 1HNMR showed δ 0.8 (-CH3), δ 1.2 (-CH2), δ 4.2(-OH), δ 7.2(-NH), δ 7.4 and δ 7.7 (aromatic CH)., Mass spectrum showed molecular ion beak at m/z = 341 (abundance (0.03%). Also, the elemental analysis gave molecular formula,C15H18NO8.
Conclusion
The purified antimicrobial compound produced by S. platensis was more active against Gram positive, Gram negative bacteria and unicellular fungi, C. albicans. The highest biological activity was recorded against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis and Aspergillus niger. The results of this investigation proved that cyanobacteria could be a good source for production of antimicrobial agents which could be effective when compared with contemporary antimicrobial compounds.
Keywords: Spirulina platensis, antimicrobial activities, MIC, MCC
INTRODUCTION
Most species of cyanobacteria are known to produce intracellular and extracellular metabolites with diverse biological activities such as antialgal, antibacterial, antifungal and antiviral activity (1- 3). The genus Spirulina has an international demand in healthy foods, feed, therapeutics and diagnostics industries (4). Pharmacological activities of Spirulina sp. have been previously reviewed by several publishers, where it exhibits antiviral (5), antibacterial (6), antiplatelet (7), anticardiotoxic (8), hypocholesterolemic (9), antinephratoxic (10) and anti-hepatoxic effect (11). It also has a variety of health benefits and therapeutic properties (12) and may play a role in reducing the cadmium toxicity (13).
Several solvents such as methanol, dichloromethane, petroleum ether, ethyl acetate have been used to extract the bioactive materials of Spirulina platensis that has an antimicrobial activity against bacteria in vitro (6). This work aimed to clarify the production, extraction, purification and identification of the active metabolic product(s) produced by Spirulina platensis isolated from Egypt and to test their antimicrobial activities.
MATERIALS AND METHODS
Organism
Spirulina platensis was isolated from freshwater channel of River Nile. The cyanobacterium was identified according to Prescott (14). The axenic culture was obtained using the method recommended by Bolch and Blackburn (15). The tested microorganisms were obtained from Hydrobiology Lab.; Microbiology, National institute of Oceanography and Fisheries, Cairo, Egypt.
Optimization of the medium constituents affecting S. platensis growth
The nitrogen source (NaNO3; 2.5 gl-1) in growth medium Aiba and Ogawa (16) replaced by Glycene (4.42 gl-1), urea (1.824 gl-1), ammonium sulphate (4.12 gl-1) and ammonium chloride (3.146 gl-1l), while phosphate (K2HPO4; 0.5 gl-1) in the standard medium was replaced by Na2HPO4 (0.734 gl-1), Na3PO4.12H2O (1.96 gl-1), KH2PO4 (0.9 gl-1) and NaH2PO4 (0.62 gl-1). Flasks were incubated at the same conditions then dry weight of organism was determined.
Growth conditions
Spirulina platensis was grown in modified Aiba and Ogawa, medium (16). Each flask was aerated using air pump (150 bubbles /min.) through a sterilized plastic tube (17), this step also provided agitation. Flasks were incubated at 30 ± 2°C and light intensity of 50 μ Em-2s-1 for 9 days at pH 9.0.
Antimicrobial activities of S. platensis
After 9 days growth, antimicrobial activity was tested using agarwell diffusion technique (18, 19). Gram +ve bacteria; Bacillus subtilis NCTC3610, Staphyllococcus aureus ATCC13565, Gram -ve bacteria; Pseudomonas aeruginosa ATCC6939, Eschrichia coli NCTC9132, yeast; Candida albicans ATCC10231, and fungi; Aspergillus flavous and Aspergillus niger were used as test organisms.
Four ml 0.15 M NaCl of fresh bacterial suspension (12 h. old) of each isolate with 106 CFU ml-1 for bacteria at OD 620 nm, and 104 cells ml-1 for yeast based on MacFerland’s scale (20), were mixed well with 100 ml melted warm (≈45°C) nutrient agar (NA). (1-140, Scharlau Chemie, S.A). The fungal suspension was prepared by six mm disk of the fungal growth (7 days old culture). in 0.15 M NaCl, 4 ml was mixed with 100ml potato dextrose agar (PDA) (1-483, Scharlau Chemie, S.A.) After the agar has set, 9 mm wells were cut in the agar with sterilized cork borer and filled with 100 μl of algal extract. Plates were kept for 6 hours in a refrigerator to allow the antimicrobial substance to diffuse through the inoculated medium, after 48-72 h incubation at 30 ± 2°C, the clearance zones were recorded in (mm) of three replicates.
Production and extraction of the antimicrobial material
Twenty liters flask with 10L of sterilized medium inoculated with 3% of nine days old culture of S. platensis, and aerated using air pump (150 bubbles min-1). through a sterilized plastic tube (Anaga & Abu, 1977) for 9 days at 30 ±2°C and light intensity of 50 μ Em-2s-1, the biomass harvested using 20 μ nylon mesh and extracted using diethyl ether (1,1 v/v) shake overnight at room temperature (27-30°C) at 100 rpm, collect the organic layer and recovery the solvent under reduced pressure using a rotate evaporator at 45°C (21).
The residues (Crude extract, with a known weight) were re-dissolved in 95% dimethyl sulphoxide (DMSO), 20 μl of the extract was loaded to sterilized 6 mm discs of Whatmann filter paper No.1 and allowed to dry at room temperature in laminar air flow bench (22).
Purification and identification of the antimicrobial material produced by S. platensis
Thin layer chromatography (TLC) plates were loaded by the crude extract and developed using chloroform, methanol 99:1 v/v solvent system. After migration (2 hours), the plate was removed and dried in a steam of warm air and detected by UV lamp (256 nm and 360 nm). Each spot was removed and re-eluted in diethyl ether, free from silica gel by washing several times by the same solvent on filter paper, then the antimicrobial activity of the filtrate was assayed by diffusion disc technique. Also column chromatography was used for the same purpose using silica gel column (1.0 × 25 cm). Five ml of the crude extract was loaded on the silica gel column and eluted using diethyl ether, chloroform, acetone and ethanol 95%. After 8 hours, eleven fractions were collected. Each fraction was free from solvent, re-dissolved in appropriate solvent and screened for its antimicrobial activity by disc diffusion method (22).Three trials for each were tested.
Structure determination of the antimicrobially active substance produced by S. platensis
In addition to classical color reactions that detect the presence of certain groups in the molecule as the aromatic nature, reducing group, sugar moiety, phenolic group, diketon bond, protein peptide bond, guandine and indocile groups, (23) the spectroscopic analysis UV (200 –400 nm) spectrum, infra-red (500-4000nm) spectrum, Nuclear magnetic resonance (1HNMR), C, N, H ratio and Mass spectrum were done at Cairo University Micro Analytical Center, Giza, Egypt.
Minimal inhibitory concentration (MICs) and Minimal cidal concentration (MCCs)
MIC and MCC of the purified bioactive compound using bacteria, yeast and filamentous fungi were determined by using a well microplate technique (24). A concentration cover the range between 0.0 and 1000 μg ml-1 of the product was made. MIC level was evaluated optically, which exhibited the lowest growth at optical density λ620nm compared to the control tube (25). MCC the concentration that showed no growth (99.9 % inhibition). of each inoculum using viable plate count method on agar plates for bacteria (26), and Zapex Dox (27) the effect was observed as no growth appear on the plates. The assay results were compared with those obtained when challenging the bacterial isolates with commercial antibiotic as streptomycin (10 μg) and polymyxin (300 IU).
RESULTS
The highest biomass concentration of Spirulina was achieved in the 9th day of growth and accounted for 111.70±1.13 mg dry wt/100 ml (Fig. 1). Also sodium nitrate and dipotassium phosphate (standard nitrogen and phosphorus sources) gave the highest value of biomass 132.267 ± 0.902 mg dry wt./100 ml, while a significant decrease in biomass production was observed when urea was used (95.6 ± 1.1012 mg/ 100 ml) and no significant differences in biomass was recorded with Na2HPO4 or Na3PO4 (110.133 ± 0.4163 and 109.667 ± 0.7571 mg dry wt./100 ml, respectively). However the lowest biomass concentration was observed when K2HPO4 was replaced by NaH2PO4 (96.067 ± 0.5033 mg dry wt/100 ml).
Fig. 1.
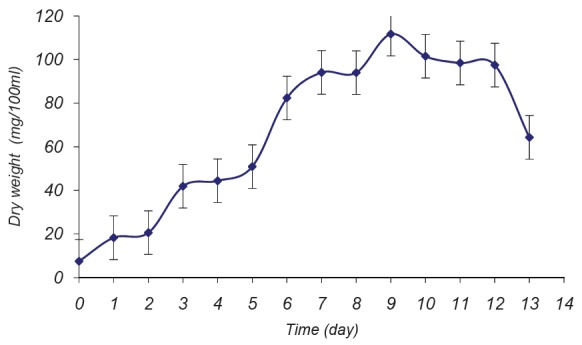
Growth curve of S. platensis measured as mg dry wt./100 ml.
It was found that the highest biological activity was recorded against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis and Aspergillus niger. The results revealed that diethyl ether and ethyl acetate exhibited antimicrobial activity against Gram +ve and Gram -ve bacteria, while petroleum ether exhibited antimicrobial activity against Gram -ve only and n-hexane had no activity against all test organisms. On the other hand, among the water-miscible solvents (acetone, methanol and ethanol) ethanol was the most effective solvent showed wide spectrum of antimicrobial activity against Gram +ve, Gram -ve bacteria and fungi as shown in Fig.2 and 3.
Fig. 2.
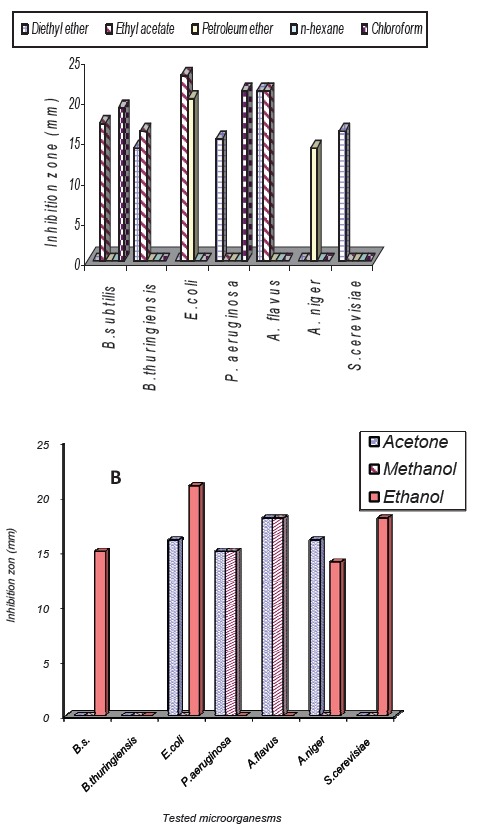
Antimicrobial activities of different solvents extracts of Spirulina platensis (A). water immiscible solvents (B). water miscible solvents
Fig. 3.
UV Spectrum of the antimicribially active compound produced by Spirulina platensis
The test microorganisms differed in relation to their susceptibility to purified antibiotic produced by S. platensis. The Gram positive bacterium Bacillus subtilis was the most susceptibile bacterial species, while the Gram negative bacterium P. aeruginosa was the least susceptible, the purified antibiotic produced by S. platensis was observed to be more active against Gram positive, Gram negative bacteria and unicellular fungi, C. albicans. On the other hand most resistance species were the multicellular fungi as recorded data C. albicans was the most susceptible test organism with minimal inhibition concentration of 30.0μg ml-1, while the Gram negative bacterium P. aeruginosa was the least susceptible one (MIC, 85μg ml-1), while the most resistance isolates were the multicellular fungi A. flavus and A. niger (Table 1).
Table 1.
MIC and MCC of purified antimicrobial substance produced by Spirulina platensis related to strptomycine and polymycin.
| Microorganism | MIC μg ml-1 | MCC μg ml-1 | Streptomycin μg ml-1 | Polymyxin (IU) |
|---|---|---|---|---|
| B. subtilis NCTC 3610 | 60.0 | 80.0 | 2.0 | ND |
| S. auerus ATCC 13565 | 65.0 | 90.0 | 2.5 | ND |
| E. coli NCTC 9132 | 80.0 | 110.0 | 3.0 | ND |
| P. aeruginosa ATCC 10145 | 85.0 | 120.0 | 3.50 | ND |
| C. albicans ATCC 10231 | 30.0 | 45.0 | 1.50 | 20* |
| Aspergillus flavus | > 120 | > 200 | ND | R |
| A. niger | > 120 | > 200 | ND | R |
ND, Not detected, R, Resistance, MIC, Minimum inhibition concentration.
MCC, Minimum cidal concentration.
300IU/300 μl
Characterization of the antimicrobial product produced by S. platensis
The purified compound was found to be yellowish green with no characteristic odor, soluble in methanol, diethyl ether, chloroform and dimethyl sulfoxide, but sparingly soluble in water and acetone with melting point 37-40°C (data not shown).
The spectroscopic analysis of the purified antimicrobial product indicated that the IR spectrum showed bands at 1269 cm-1, 1414 cm-1 (C-O-C), 1643 cm-1 (CO of amide),1563 cm-1 (C = C) and broad band 3441 cm-1 (of OH and NH), (Fig. 4). 1HNMR showed δ 0.8 (-CH3)., δ 1.2 (-CH2)., δ 4.2(-OH)., δ 7.2(-NH)., δ 7.4 and δ 7.7 (aromatic CH)., (Fig.5). Mass spectrum showed molecular ion beak at m/z = 341(abundance (0.03%) (Fig.6). Also, the elemental analysis gave molecular formula, C15H18NO8.
Fig. 4.
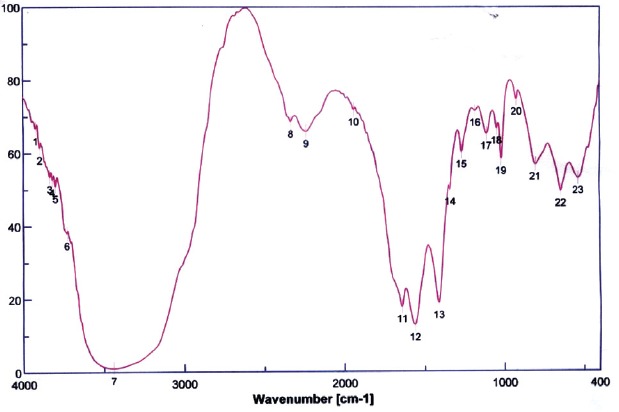
IR Spectrum of the antimicribially active compound produced by Spirulina platensis.
Fig. 5.
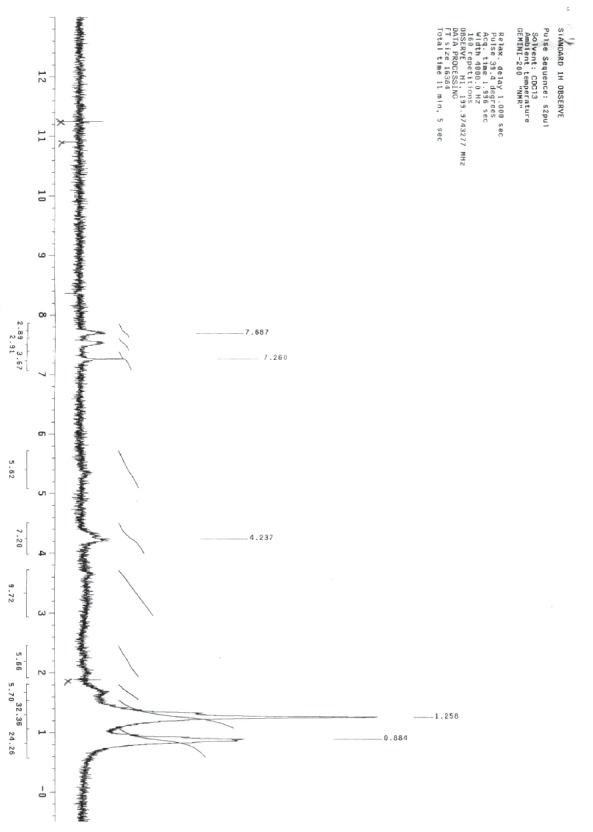
1HNMR Spectrum of the antimicribially active compound produced by Spirulina platensis.
Fig. 6.
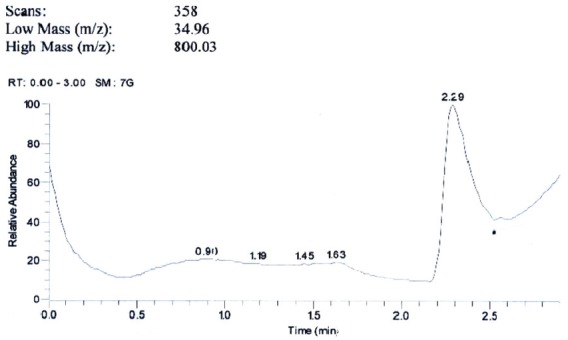
Mass Spectrum of the antimicribially active compound produced by Spirulina platensis.
DISCUSSION
Spirulina sp. represents one of the most important commercial microalga for the production of biomass as healthy food and animal feed (28). A large number of microalgal extracts and extracellular products have been found to have antibacterial activity. The algal extracts showed antibacterial and antifungal activities, both culture filtrate and whole culture (cells and exometabolites) have been proved to have wide spectrum antimicrobial activity, where Bacillus sublilis and Candida albicans were the most sensitive species. The antimicrobial activity of microalgae could be explained by the presence of cyclic peptides, alkaloids and lipopolysaccharides (29). This activity may be due to the toxins produced by its cells like a number of blue green algae that produce toxins which have potential pharmaceutical applications. The present results go in harmony with those obtained by Volk and Furkert (30) who found that some microalgae had high biological activity against Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa and Candida tropicalis.
Organic solvents always provides a higher efficiency in extracting compounds for antimicrobial activities compared to water-based methods (31). The results showed that ethane and ethyl acetate and diethyl ether were the best solvents (water miscible and immiscible solvents) for extraction of the active substances. Many of the tested solvents were effective against all microorganisms; however, ethanol extract indicated an antimicrobial activity against several microorganisms. This could be related to the presence of bioactive metabolites which are soluble in ethanol but not diethyl ether (32). The present results are in agreement with Abedin & Taha (33) who reported that acetone and diethyl ether extracts of Spirulina platensis gave the highest antimicrobial activity against Bacillus subtilis and Pseudomonas aeruginosa. Also, Santoyo et al., (34) reported that hexane and petroleum ether extracts were slightly active than ethanolic extracts.
The antimicrobial activities of S. platensis could be attributed to different compounds belonging to a diverse range of chemical classes (6). The antimicrobial activity found in S. platensis extracts could be due to contain γ-linolenic acid (35), active fatty acid (36), synergetic effect of lauric and palmitoleric acid (37). The test microorganisms differ significantly in relation to their susceptibility to S. platensis antimicrobial substances, Candida albicans was the most sensitive microorganism (MIC μ50 g ml-1) while Gram positive bacteria were more sensitive than the Gram negative bacteria. This may be attributed to the fact that cell wall in Gram positive bacteria consists of a single layer, whereas Gram negative bacterial cell wall is multilayered structure bounded by an outer cell membrane (38) and or due to the permeability barrier provided by the cell wall or to the membrane accumulation mechanism (39). On the other hand, some bacterial species did not respond to extracts of S. platensis where as the purified fractions showed broad-spectrum activity against different test organisms, this might be due to masking of antibacterial activity by the presence of some inhibitory compounds in the extract as observed by Sastry & Rao (40).
REFERENCES
- 1.Noaman NH, Khaleafa AM, Zaky SH. Factors affecting antimicrobial activity of Synechococcus leopoliensis. Microbiol Res. 2004;159:395–402. doi: 10.1016/j.micres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 2.El-Sheekh M, Osman ME, Dyab M, Amer MS. Production and characterization of antimicrobial active substance from the cyanobacterium Nostoc muscorum. Environ Toxicol & Pharmacol. 2006;21:42–50. doi: 10.1016/j.etap.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 3.El-Sheekh MM, Dawah AM, Abd El-Rahman AM, El-Adel HM, Abd El-Hay RA. Antimicrobial activity of the cyanobacteria Anabaena wisconsinense and Oscillatoria curviceps against pathogens of fish in aquaculture. Annals Microbiolh. 2008;58:527–534. [Google Scholar]
- 4.Becker EW. Microalgae, biotechnology and microbiology. Cambridge, Cambridge University Press; New York, USA: 1994. p. 291. [Google Scholar]
- 5.Hernandez-Corona A, Nieves I, Meckes M, Chamorro G, Barron BL. Antiviral activity of Spirulina maxima against herpes simplex virus type 2. Antiviral Res. 2002;56:279–285. doi: 10.1016/s0166-3542(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 6.Özdemir G, Karabay NU, Dalay MC, Pazarbasi B. Antibacterial activity of volatile component and various extracts of Spirulina platensis. Phytother Resh. 18:754–757. doi: 10.1002/ptr.1541. [DOI] [PubMed] [Google Scholar]
- 7.Hsiao G, Chou PH, Shen MY, Chou DS, Lin CH, Sheu JR. C- phycocyanin, a very potent and novel platelet aggregation inhibitor from Spirulina platensis. J Agric Food Chem. 2005;53:7734–7740. doi: 10.1021/jf051352y. [DOI] [PubMed] [Google Scholar]
- 8.Khan M, Shobha JC, Mohan IK, Naidu MUR, Sundaram C, Singh S, et al., editors. Protective effect of Spirulina against doxorubicin-induced cardiotoxicity. Phytother Res. 2005;19:1030–1037. doi: 10.1002/ptr.1783. [DOI] [PubMed] [Google Scholar]
- 9.Nagaoka S, Shimizu K, Kaneko H, Shibayama F, Morikawa K, Kanamaru Y, et al. A novel protein C-phcocyanin plays a crucial role in the Hypocholesterolemic action of Spirulina platensis concentrate in rats. J Nutr. 2005;135:2425–2430. doi: 10.1093/jn/135.10.2425. [DOI] [PubMed] [Google Scholar]
- 10.Khan M, Shobha JC, Mohan IK, Naidu MUR, Prayag A, Kutala VK. Spirulina attenuates cyclosporine-induced nephrotoxicity in rats. J Appl Toxico. 2006;26:444–451. doi: 10.1002/jat.1159. [DOI] [PubMed] [Google Scholar]
- 11.Mohan IK, Khan M, Shobha JC, Naidu MUR, Prayag A, Kuppusamy P, et al. Protection against cisplatin-induced nephrotoxicity by Spirulina in rats. Cancer Chemother Pharmacol. 2006;58:802–808. doi: 10.1007/s00280-006-0231-8. [DOI] [PubMed] [Google Scholar]
- 12.Dartsch PC. Antioxidant potential of selected Spirulina platensis preparations. Phytotherapy Research. 2008;22:627–633. doi: 10.1002/ptr.2310. [DOI] [PubMed] [Google Scholar]
- 13.Karadeniz A, Cemek M, Simsek N. The effects of Panax ginseng and Spirulina platensis on hepatotoxicity induced by cadmium in rats. Ecotoxicol Environ Saf. 2009;72:231–235. doi: 10.1016/j.ecoenv.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Prescott GW. How to Know Freshwater Algae. 3. Wm C Brown Company Publishers; Dubuque: 1978. [Google Scholar]
- 15.Bolch CJS, Blackburn SI. Isolation and purification of Australian isolates of the toxic cyanobacterium Microcystis aeruginosa. Kutz J Appl Phycol. 1996;8:5–13. [Google Scholar]
- 16.Aiba S, Ogawa T. Assessment of growth yield of a blue-green alga, Spirulina platensis, in axenic and continuous culture. J Gen Microbiol. 1977;102:179. [Google Scholar]
- 17.Anaga A, Abu G. A laboratory scale cultivation of Chlorella and Spirulina using waste effluent from a fertilizer company in Niger. Bioresource Technol. 1996;58:93–95. [Google Scholar]
- 18.Perez C, Pauli M, Bazerqu P. An antibiotic assay by agar-well diffusion method. Acta Biologiae et Medecine Experimentalis. 1990;15:113–115. [Google Scholar]
- 19.Traub WH, Geipel U, Leonhard B, Bauer D. Antibiotic Susceptibility Testing (Agar Disk Diffusion and Agar Dilution). of Clinical Isolates of Corynebacterium jeikeium. Chemotherapy. 1998;44:230–237. doi: 10.1159/000007119. [DOI] [PubMed] [Google Scholar]
- 20.Heritage J, Evan S, Killington RA. Intro- duction microbiology. Cambridge Univ Press. 1996:103–104. [Google Scholar]
- 21.Choudhury S, Sree A, Mukher Jee SC, Pathnaik P, Bapuji M. In vitro antibacterial activity of extracts of selected marine algae and mangroves against fish phatogens. Asian Fisheries Science. 2005;18:285–294. [Google Scholar]
- 22.Ratnam K V, Raju RRV. In vitro antimicrobial screening of the fruit extracts of two Syzygium species (Myrtaceae) Advan Biol Res. 2008;2:17–20. [Google Scholar]
- 23.Plummer DT. In: An introduction to practical bio-chemistry. Plummer David T., editor. Mc Grow-Hill Book Company (UK) Limited; 1978. [Google Scholar]
- 24.Kobayashi I, Muroaka H, Saika T, Nishida M, Fujioka T, Nasu M. Micro-broth dilution method with air-dried microplate for determining MICs of claritheramycin and amoxicillin for Helicobacter pylori isolates. J Med Microbiol. 2004;53:403–406. doi: 10.1099/jmm.0.05397-0. [DOI] [PubMed] [Google Scholar]
- 25.Lorian V, Atkinson B. Effect of subinhibitory concentra- tion of antibiotics on cross wall of cocci. Antimicrob Agent Chemother. 1976;9:1043–1055. doi: 10.1128/aac.9.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagayama K, Yoshitoshi I, Toshiyuki S, Izumi H, Takashi N. Bactericidial activity of phlorotanins from the brown alga Echonia kurome. Antimicrob Agent Chemother. 2002;50:889–893. doi: 10.1093/jac/dkf222. [DOI] [PubMed] [Google Scholar]
- 27.Thom C, Raper KB. An annual of the Aspergilli Williams and Williams Co. Baltimooore Lmd. 1945 [Google Scholar]
- 28.Vonshak A, Tomaselli L. Arthrospira (Spirulina), Systematics and ecophysiology. In: Whitton BA, Potts M, editors. The ecology of Cyanobacteria. published by Kluwer Academic Publishers; The Netherlands, USA: 2000. pp. 505–522. [Google Scholar]
- 29.Katircioglu H, Beyatli Y, Aslim B, Yüksekdag Z, Atici T. Screening for antimicrobial agent production of some microalgae in freshwater. Internet J Microbiol. 2006;2:2. www.ispub.com/ostia/index.php?xmlFilePath…/ijmb/…/algae. [Google Scholar]
- 30.Volk RB, Furkert FH. Antialgal, antibacterial and antifungal activity of two metabolites produced and extracted by cyanobacteria during growth. Microbiol Res. 2006;161:180–186. doi: 10.1016/j.micres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Lima-Filho JVM, Carvalho AFU, Freitas SM, Melo VMM. Antibacterial activity of extracts of six macroalgae from the northeastern Bazilian Coast. Braz J Microbiol. 2002;33:311–313. [Google Scholar]
- 32.Tüney I, Cadirci BH, Ünal D, Sukatar A. Antimicrobial activities of the extracts of marine algae from the coast of Urla (Izmir, Turkey) Turk J Biol. 2006;30:171–175. [Google Scholar]
- 33.Abedin RMA, Taha HM. Antibacterial and antifungal activity of cyanobacteria and green microalgae. Evaluation of medium components by Placket-Burman design for antimicrobial activity of Spirulina platensis. GJBBR. 2008;3:22–31. [Google Scholar]
- 34.Santoyo S, Herrero M, Señorans EJ, Cifuentes A, Ibáñez E, Jaime L. Functional characterization of pressurized liquid extracts of Spirulina platensis. Eur Food Res Technol. 2006;224:75–81. [Google Scholar]
- 35.Demule MCZ, Decaire GZ, Decano MS. Bioactive substances from Spirulina platensis (Cyanobacteria) International Journal of Experimental Botany. 1996;58:93–96. [Google Scholar]
- 36.Xue C, Hu Y, Saito H, Zhang Z, Li Z, Cai Y, et al. Molecular species composition of glycolipids from Spirulina platensis. Food Chemistry. 2002;77:9–13. [Google Scholar]
- 37.Mendiola JA, Jaime L, Santoyo S, Reglero G, Cifuentes A, Ibañez E, et al. Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chemistry. 2007;102:1357–1367. [Google Scholar]
- 38.Ergene A, Guler P, Tan S, Mirici S, Hamzaoglu E, Duran A. Antibacterial and antifungal activity of Heracleum sphondylium subsp. artvinense. African Journal of Biotechnology. 2006;5(11):1087–1089. [Google Scholar]
- 39.Abu-Shanab B, Adwan G, Abu Safiya D, Adwan K, Abu-Shanab M. Antibacterial activity of Rhus coriaria extracts growing in Palestine. Journal of Islamic University Gaza, Palestine (Natural Sciences series) 2005;13(2):147–153. [Google Scholar]
- 40.Sastry VMVS, Rao GRK. Antibacterial substances from marine algae, successive extraction using benzene, chloroform and methanol. Botanica Marina. 1994;37:357–360. [Google Scholar]


