Abstract
Background and Objectives
Bacillus species are attractive industrial organisms due to their rapid growth rates leading to a short fermentation cycle and for their capacity to secrete important enzymes and proteins such as xylanase into the extracellular medium. Considering the industrial importance of xylanase, in this current study, Bacillus spp. were isolated from different soils and were screened for their xylanase production.
Materials and Methods
Bacillus isolates used in this study were obtained from a national screening program carried out during 2006-2007 in which soil samples that covered areas throughout the interior of Syria were collected. The prepared inoculum from each of Bacillus isolates was aliquoted onto xylan agar plates, incubated at 30°C for 72 h and screened for xylanase synthesis.
Results
Xylanolytic isolates were selected depending on the clear zones of xylan hydrolysis. Fifteen isolates having the highest clearing zone were determined and grown in a solid state fermentation. Of the 15 isolates, three bacilli namely SY30A, SY185C and SY190E that showed maximum xylanase production, were identified using the 16S rDNA sequencing method. According to 16S rDNA gene sequence data, the closest phylogenetic neighbor for SY30A was Bacillus pumilus and for SY185C and SY190E isolates was Bacillus subtilis. Optimal pH and temperature for xylanase activity was 7.0 and 55ºC for SY30A and 6.0 and 60ºC for SY185C and SY190E, respectively. Under these conditions, the following activities were found to be around 1157 ± 58, 916 ± 46 and 794 ± 39 (U/g) for SY30A, SY185C and SY190E, respectivly.
Conclusions
Selected local Bacillus isolates were found to be a potential source of xylanase which was proven to be quite suitable for multiple biotechnological applications. These isolates might after extensive optimization steps be an alternative to commercially available strains.
Keywords: Bacillus sp, Xylanase, Solid state culture
INTRODUCTION
Xylans, major structural heteropolysaccharides in plants, are α-1,4-linked polymers of xylopyranosyl units with a degree of polymerization ranging from 70 to 200. Depending on their origin, xylans may also contain variable amounts of arabinosyl- and 4-O-methylglucuronic acid residues and acetyl groups (18, 21). Xylanolytic enzymes are a group of enzymes that hydrolyze xylan and arabinoxylan polymers. This group includes endo-α-1,4-xylanase, β-xylosidase, arabinofuranosidase and acetylxylan esterase (5). Endo-α-1,4-xylanase plays an important role in the animal feed as it increases the body weight gains (14). In pulp and paper industry, xylanases are employed in the prebleaching process to reduce the use of the toxic chlorine chemicals (27). In bread and bakery industry, xylanases are used to increase the dough viscosity, bread volume, and shelf life (19). Other potential applications include the conversion of xylan in wastes from agriculture and food industries into xylose, and the production of fuel and chemical feedstocks (26).
Many bacteria and fungi have been studied for xylanase production (4, 9, 10). However, Bacillus species have been the major workhorse industrial microorganisms for more than a thousand years. Bacillus species are attractive industrial organisms for several reasons, including their high growth rates leading to short fermentation cycle times and their capacity to secrete proteins into the extra cellular medium. It is estimated that Bacillus spp. enzymes make up about 50% of the total enzyme market (24).
For the production of any industrial enzyme, an inexpensive substrate and an efficient fermentation process are essential for commercial viability. It has been established that solid-state fermentation has several advantages over submerged fermentation, due to a smaller volume of solvent required for product recovery, resulting in higher productivity per unit volume, lower contamination and foaming problems and better exploitation of various agro-residues as substrates (2-7).
Considering the industrial importance of xylanase, in the present study, Bacillus spp. were isolated from different soils and screened for xylanase production in a solid state fermentation process. The investigation led to the identification of three high producing xylanase isolates, B. pumilus SY30A and B. subtilis SY185C and SY190E.
MATERIALS AND METHODS
Bacterial strains and growth conditions
Bacillus isolates analyzed in this study were obtained from a national screening programme carried out in 2006 and 2007 from soil samples distributed throughout the interior of Syria (1). Isolates were routinely cultured on nutrient agar plates (NA). Plates were incubated at 30°C until bacterial colonies developed, kept at 4°C and sub-cultured every fifteenth day. Subsequently, selected isolates were maintained in 20% glycerol at -80°C.
Inoculum preparation
Five milliliter of medium containing nutrient broth was transferred to a 50 ml tube and sterilized in an autoclave at 121°C for 20 min. After cooling, a loopful of bacterial culture was aseptically transferred and rotated overnight at 200 rpm and 30°C. 1% of this culture was used to inoculate 20 ml of the same medium in 100 ml flask and incubated in an orbital shaker at 30°C until the optical density at 600 nm (OD600) reached 0.15 (cell density about 2 × 108 colony-forming unit (CFU)/ml).
Screening of xylanase producing bacteria
Twenty five microliter of the prepared inoculum for each Bacillus isolate was aliquoted onto xylan agar plates, incubated at 30°C for 72 h and screened for xylanase synthesis. Positive xylanolytic isolates were decided according to the clear zones of hydrolysis on the xylan. Fifteen isolates having the highest clearing zone were selected and grown in solid state fermentation. The amount of xylanase produced by each isolate was determined from the extract culture filtrate. Subsequently, the selected isolates were grown on NA plates at 30°C and maintained at 4 ± 1°C. Isolates were routinely subcultured every two weeks on NA plates.
Solid state fermentation
Enzyme production was checked for selected Bacillus isolates in solid state fermentation using wheat bran procured from local market. Enzyme production was carried out in 100 ml Erlenmeyer flasks containing 5 g-of wheat bran and nutrients plus distilled water to adjust the moisture to 60%. The fermentation medium consisted of (g/L): K2HPO4, 1; NaCl,3; MgSO4.7H2O, 0.3; and yeast extract, 3 and peptone 5, as nitrogen source. 1 ml of the prepared inoculum for each Bacillus isolate was transferred into the solid medium and placed in an incubator. After 3 days of cultivation flasks were removed and the enzyme was extracted by the addition of 25 mL distilled water containing 0.1% Triton X 100. Flasks contents were stirred for 1.5 h on a magnetic stirrer and the clear supernatant was obtained by centrifugation at 9800 × g for 15 min and used as enzyme source.
Xylanase assay
Xylanase activity was determined as described by Bailey et al. (3) using 1% birch wood xylan as substrate. Xylan solution and the enzyme at an appropriate dilution were incubated at 55°C for 5 minutes and the reducing sugars were determined by the dinitrosalicylic acid (DNS) procedure with xylose as a standard (15). The released xylose was measured spectrophotometrically at 540 nm. One unit (U) of enzyme activity is defined as the amount of enzyme releasing 1 μmol xylose per ml per minute underthe described assay conditions.
Effect of temperature on xylanase activity and stability
Enzyme activity was evaluated by measuring the xylanase activity at different temperatures ranging from 40 to 75°C in 0.1 M sodium phosphate buffer (pH 7.0) and 1% soluble birchwoodxylan. To evaluate thermal stability, the remaining xylanase activity was determined after preincubation of the crude enzyme preparations at 30-90°C in buffers of optimal activity, without substrate, for 1 h.
Effect of pH on xylanase activity and stability
The optimal pH for enzyme activity was determined by changing the assay reaction mixture pH using the following buffers (0.1 M): sodium acetate (pH 5.0), sodium phosphate (pH 6.0–7.0), Tris–HCl (pH 8), glycine–NaOH buffer (pH 9–10) and 1% soluble birchwood as a substrate. To evaluate the stability of the enzyme at each pH, xylanase activity was measured after incubation the enzyme solution in pH buffers ranging from 4.0 to 10.0 at 25°C for 24 hours. Residual activity was determined under optimal assay conditions for each isolate.
Polymerase chain reaction (PCR) amplification and 16S rDNA sequencing
Two primers BacF (5’ -GTGCCTAATACATGCAAGTC-3’) and BcaR (5’-CTTTACGCCCAATAATTCC-3’) flanking a highly variable sequence region of 545 bp towards the 5’ end of the 16S rDNA region were used (16). Genomic DNA was extracted and purified using DNA extraction kit according to the manufacturer’s recommendations (BIOTOOLS, Cat. NO. 21.002). PCR mixtures were prepared using 10–20 ng of template DNA, 0.4 μM of each primer, 1U of Taq DNA polymerase (Promega), 0.2 mM each of dATP, dCTP, dGTP and dTTP (Promega), 2 mM MgSO4, and 3% dimethyl sulfoxide (DMSO). Amplification was done in a Bio-Rad T gradient thermocycler under the following conditions: a 5 min denaturation step at 95°C, followed by 30 amplification cycles (1 min at 95°C, 1 min at 56°C and 1 min at 72°C) and an extra extension step of 10 min at 72°C. PCR products were separated on a 1% agarose gel to which ethidium bromide was added and photographed under UV light. Amplification products were purified using QIAqick Gel Extraction kit (QIAGEN, Cat. No. 28704) and sequenced on both strands using an ABI 310 sequencer machine (Department of Molecular Biology and Biotechnology, AECS). The sequences were subjected to a BLAST search against the full EMBL/GenBank database available at NCBI public database (http://www.ncbi.nlm.-nih.gov).
RESULTS AND DISCUSSION
Screening of xylanase producing Bacilli
Five hundred and twenty-five bacterial isolates from 200 soil samples collected from different areas of Syria including cereal fields, olive fields, forests, desert and gardens, were evaluated for xylanase production. Fifteen of these isolates with a high xylanase production level were determined (Fig. 1) and thus three isolates SY30A, SY185C and SY190E were selected for further studies.
Fig. 1.
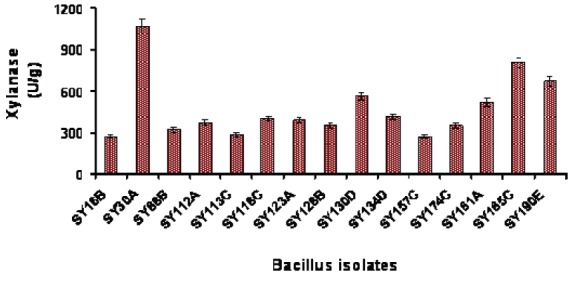
Extracellular xylanase production from Bacillus isolates.
Identification of the selected isolates
DNA sequence analysis methods are an objective, reproducible, and rapid means of species identification, therefore, they have been widely utilized (13). SY30A, SY185C and SY190E Identification was performed using the 16S DNA gene sequences. The nucleotide BLAST similarity search analysis, based on the 16S DNA gene sequence revealed that these isolates belong to the genus Bacillus. The closest phylogenetic neighbors according to the 16S DNA gene sequence data for SY30A was Bacillus pumilus and for the SY185C and SY190E isolates were Bacillus subtilis with a 100 % homology.
Effect of temperature on xylanase activity and stability
The effect of temperature on xylanase activity against birch wood xylan at pH 6.0 was examined in the temperature range of 40-75°C. Xylanases produced by SY30A exhibited maximum activity at 55°C compared with 60°C for the other two isolates, SY185C and SY190E (Fig. 2). Under these conditions, enzyme activity were 1013, 854 and 731 (U/g) for SY30A, SY185C and SY190E, respectively. Similar temperature optima have been reported by many other workers for xylanase production from varied sources. Sá-Pereira et al. (20) reported that optimal xylanase activity of a B. subtilis strain was at 60°C on phosphate buffer, at pH 6.0.
Fig. 2.
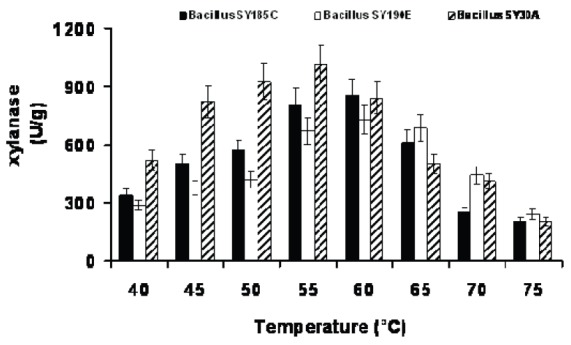
Effect of temperature on the xylanase activity of three Bacillus strains.
Additionally, xylanase from a thermo alkaliphilic bacterium showed optimum activity at 50°C (23). The highest activity of xylanase obtained from both B. circulans and B.amyloliquefaciens was at 50.0°C (11, 12). Sanghi et al. (22) found that xylanase produced from Bacillus subtilis ASH gave the best activity at 55°C
Enzyme stability is the most important factor in studying enzyme characteristics. Thus, thermal stability tests were carried out by pre-incubating xylanase for 60 min in a temperature range of 30 to 90°C (Fig. 3). Our results showed that there was no significant decrease in xylanase activity during 60 min incubation at 30 – 50°C, while at 50°C the residual xylanases activities were 93.4, 90.2 and 89.6% for SY30A, SY185C and SY190E, respectively. The enzyme was sensitive at 70°C, retaining 17.1% activity for SY30A, while retaining 58 and 70.8% activities for SY185C and SY190E, respectively. At higher temperature values xylanase stability gradually was found to decline. Thermal stability of xylanase is an important property due to its potential applications in several industrial processes. The industrial importance of an enzyme will be more when the effect of temperature input on its optimal activity is less. Strains isolated by us could be a good source for biotechnological applications.
Fig. 3.
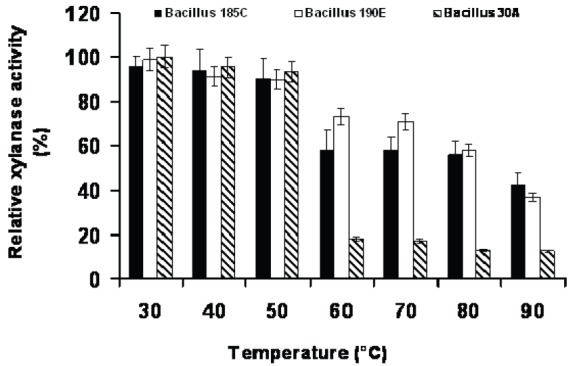
Effect of temperature on the xylanase stability of three Bacillus strains.
Effect of pH on xylanase activity and stability
Enzyme activity is markedly affected by pH because substrate binding and catalysis are often dependent on charge distribution on both, substrate and, in particular enzyme molecules (25). A pH range from 4 to 10 was used to study the effect of pH on xylanase activity and the results are given in (Fig. 4). Optimum pH was found to be 7 for SY30A and 6 for both SY185C and SY190E. Enzyme activity at pH 8 and 9 was 920 and 711 U/g for SY30A, 325 and 286 U/g for SY185C and 405 and 265 U/g for SY190E, respectively. Xylanase activity was shown to decrease at pH 10. These clearly indicates that SY30A produced enzyme is more suitable for any application in the pH range of 6.0-9.0 and 5-8 for the SY185C and SY190E enzymes.
Fig. 4.
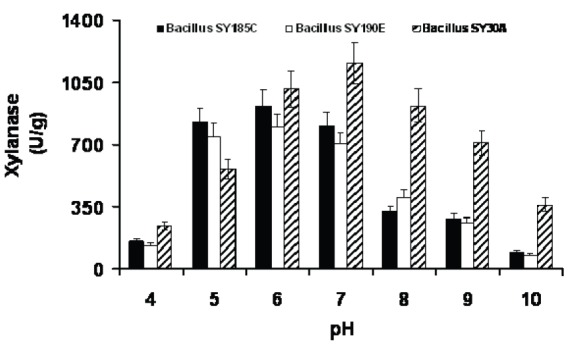
Effect of pH on the xylanase activity of three Bacillus strains.
Up to date, xylanases produced by most alkaliphiles are reported to have their optimum pH around neutrality. Nakamura et al. (17) reported the first alkaline xylanases produced by Bacillus sp. strain 41 M-1, which had an optimum pH at 9.0. Yang et al. (28) isolated an alkaliphilic Bacillus sp. VI-4 from a hard wood kraft pulp, which produced xylanase having an optimum pH of 6-8.5. Similarly, the optimum pH activity at 6.8-7.0 of xylanase reported from B. amyloliquefaciens (11). Thermostable alkaline xylanase from a Bacillus sp. showed three optimum peaks for pH 6.5, 8.5 and 10.5 (23). A wide range of pH activity from 5.0-8.0 was observed in B. circulans BL53 upon solid state cultivation (12). The activity persistence in a large range of pH is a desirable quality of an industrial enzyme. When pH stability was measured at values between 4 and 10, the xylanase stability was over a broad neutral to alkaline pH range (6-10) and retained more than 75% of its activity after 24 h of incubation at room temperature (Fig. 5). Stability at extreme pH values may be due to charged amino acid residues. The enzymes stable in alkaline conditions were characterized by a decreased number of acidic residues and an increased number of arginines (8).
Fig. 5.
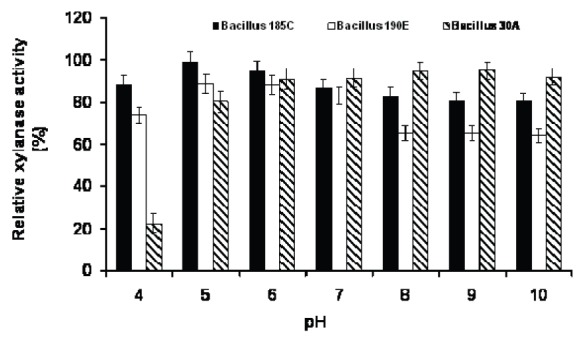
Effect of pH on the xylanase stability of three Bacillus strains.
CONCLUSION
Results obtained in the current study demonstrate that the local Bacillus isolates are a potential source of xylanase production. The temperature properties for enzyme activity and stability make the enzyme from some of the tested isolates a quite suitable for biotechnological applications. Therefore, these isolates could be alternative of the commercial strains. However, the process of xylanase production from the new local Bacillus isolates may be commercialized after further optimization for enhanced enzyme production.
Acknowledgments
The authors thank the Director General of AECS and the Head of the Molecular Biology and Biotechnology Department for their continuous support throughout this work.
REFERENCES
- 1.Ammouneh H, Hraba M, Idris E, Makee H. Isolation and characterization of native Bacillus thuringiensis isolates from Syrian soil and testing their insecticidal activities against some insect pests. Turk J Agric For. 2011;35:421–431. [Google Scholar]
- 2.Archana A, Satyanarayana T. Xylanase production by thermophilic Bacillus licheniformis A 99 in solid state fermentation. Enzyme and Microb Technol. 1997;21:12–17. [Google Scholar]
- 3.Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23:257–270. [Google Scholar]
- 4.Bakri Y, Jawhar M, Arabi MIE. Improvement of Xylanase Production by Cochliobolussativus in Solid State Fermentation. Brazi J Microbiol. 2008;39:238–240. doi: 10.1590/S1517-838220080003000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biely P. Biochemical aspects of the production of microbial hemicellulases. In: Coughlan MP, Hazlewood GP, editors. Hemicellulose and Hemicellulases. Portland Press; London: 1993. pp. 29–51. [Google Scholar]
- 6.Duarte MCT, Pellegrino ACA, Portugal EP, Ponezi AN, Franco TT. Characterization of alkaline xylanases from Bacillus pumilus. Brazi J Microbiol. 2000;31:90–94. [Google Scholar]
- 7.Gupta U, Kar R. Xylanase Production by a Thermo-tolerant Bacillus Species under Solid-state and Submerged Fermentation. Braz Arch Biol Techn. 2009;52:1363–1371. [Google Scholar]
- 8.Hakulinen N, Turunen O, Jänis J, Leisola M, Rouvinen J. Three-dimensional structures of thermophilic β-1,4-xylanases from Chaetomium thermophilum and Nonomuraea flexuosa comparison of twelve xylanases in relation to their thermal stability. Eur J Biochem. 2003;270:1399–1412. doi: 10.1046/j.1432-1033.2003.03496.x. [DOI] [PubMed] [Google Scholar]
- 9.Heck JX, Hertz PF, Ayab MAZ. Cellulase and xylanase production by isolated amazon Bacillus strains using soybean industrial residue based solid state cultivation. Brazi J Microbiol. 2002;33:213–218. [Google Scholar]
- 10.Kinegam S, Tanasupawat S, Akaracharanya A. Screening and identification of xylanase-producing bacteria from Thai soils. J Gen Appl Microbiol. 2007;53:57–65. doi: 10.2323/jgam.53.57. [DOI] [PubMed] [Google Scholar]
- 11.Javier DB, Faustino S, Mario DB, Guillermo RC, Rajni HK. Purification and characterization of a thermostable xylanase from Bacillus amylolique faciens. Enzyme Microb Technol. 1998;22:42–49. [Google Scholar]
- 12.Júlio XH, Luís HBS, Plinho FH, Marco AZA. Purification and properties of a xylanase produced by Bacillus circulansBL53 on solid-state cultivation. Biochem Eng J. 2006;32:179–184. [Google Scholar]
- 13.Li Y, Liu Z, Cui F, Xu Y, Zhao H. Production of xylanase from a newly isolated Penicillium sp. ZH-30. World J Microbiol Biotechnol. 2007;23:837–843. [Google Scholar]
- 14.Medel P, Baucells F, Gracia MI, De-Blas C, Mateos GG. Processing of barley and enzyme supplementation in diets for young pigs. Anim Feed Sci Tech. 2002;95:113–122. [Google Scholar]
- 15.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem. 1959;31:426–428. [Google Scholar]
- 16.Nair JR, Singh G, Sekar V. Isolation and characterization of a novel Bacillus strain from coffee phyllosphere showing antifungal activity. J f Appl Microbiol. 2002;93:772–780. doi: 10.1046/j.1365-2672.2002.01756.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura S, Wakabayashi K, Nakai R, Horikoshi K. Production of alkaline xylanase by a newly isolated alkaliphilic Bacillus sp. strain 41M-1. World J Microbiol Biotechnol. 1993;3:221–224. doi: 10.1007/BF00327842. [DOI] [PubMed] [Google Scholar]
- 18.Poutanen K, Rättö M, Puls J, Viikari L. Evaluation of different microbial xylanolytic systems. J Biotechnol. 1987;6:49–60. [Google Scholar]
- 19.Romanowska I, Polak J, Jonowska K, Bielecki S. The application of fungal endoxylanase in bread-making. Comm Agr Appl Biol Sci. 2003;68:317–320. [PubMed] [Google Scholar]
- 20.Sá-Pereira P, Costa-Ferreira M, Aires-Barros MR. Enzymatic properties of a neutral endo-1,3(4)-β-xylanaseXyl II from Bacillus subtilis. J Biotechnol. 2002;94:265–275. doi: 10.1016/s0168-1656(01)00436-9. [DOI] [PubMed] [Google Scholar]
- 21.Saha BC. Hemicellulose biocon version. J Ind Microbiol Biotechnol. 2003;30:279–291. doi: 10.1007/s10295-003-0049-x. [DOI] [PubMed] [Google Scholar]
- 22.Sanghi A, Garg N, Gupta VK, Mittal A, Kuhad RC. One step purification and characterization of cellulase free xylanase produced by alkalophilic Bacillus subtilis ASH. Brazi J Microbiol. 2010;41:467–476. doi: 10.1590/S1517-838220100002000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sapre M, Jha H, Patil M. Purification and characterization of a thermoalkalophilic xylanase from Bacillus sp. World J Microbiol Biotechnol. 2005;21:649–654. [Google Scholar]
- 24.Schallmey M, Singh A, Ward OP. Developments in the use of Bacillus species for industrial production. Can J Microbiol. 2004;50:1–17. doi: 10.1139/w03-076. [DOI] [PubMed] [Google Scholar]
- 25.Shah AR, Madamwar D. Xylanase production by a newly isolated Aspergillus foetidus strain and its characterization. Process Biochem. 2005;40:1763–1771. [Google Scholar]
- 26.Sunna A, Antranikian G. Xylanolytic enzymes from fungi and bacteria. Crit Rev Biotechnol. 1997;17:39–67. doi: 10.3109/07388559709146606. [DOI] [PubMed] [Google Scholar]
- 27.Wong KKY, James CS, Campion SH. Xylanase pre- and post-treatments of bleached pulps decrease absorption coefficient. J Pulp Pap Sci. 2000;26:377–383. [Google Scholar]
- 28.Yang VW, Zhuang Z, Elegir G, Jeffries TW. Alkaline active xylanase produced by an alkaliphilicBacillus spisolated from from kraft pulp. J Ind Microbiol. 1995;15:434–441. [Google Scholar]


