Abstract
Sodium/hydrogen exchanger (NHE) 8 is expressed at the apical membrane of the epithelial cells and plays important roles in neutral sodium absorption in the gastrointestinal tract and the kidney. It also has an important role in epithelial mucosal protection in the gastric gland and the intestine. Although NHE8 has broad tissue distribution, the precise location and the physiological role of NHE8 in the eye remain unknown. In the present study, we successfully detected the expression of NHE8 in the ocular surface by PCR and Western blot in human and mouse eyes. Immunohistochemistry staining located NHE8 protein at the plasma membrane of the epithelial cells in the conjunctiva, the cornea, and the lacrimal gland both in human and mouse. We also detected the expression of downregulated-in-adenoma (DRA, a Cl−/HCO3− transporter) in the ocular surface epithelial cells. Using NHE8−/− mouse model, we found that loss of NHE8 function resulted in reduced tear production and increased corneal staining. These NHE8−/− mice also showed increased expression of TNF-α and matrix metalloproteinase 9 (MMP9) genes. The expression of epithelial keratinization marker genes, small proline-rich protein 2h (Sprr2h) and transglutaminase 1 (Tgm1), were also increased in NHE8−/− eyes. Furthermore, DRA expression in NHE8−/− mice was reduced in the conjunctiva, the cornea, and the lacrimal glands in association with a reduction in conjunctival mucosal pH. Altered ocular surface function and reduced epithelial DRA expression in NHE8−/− mice suggest that the role of NHE8 in ocular surface tissue involve in tear production and ocular epithelial protection. This study reveals a potential novel mechanism of dry eye condition involving abnormal NHE8 function.
Keywords: NHE8, conjunctiva, cornea, lacrimal gland, epithelia
sodium/hydrogen exchangers (NHEs) are a group of membrane proteins that exchange extracellular Na+ for intracellular H+, and they display broad tissue distribution. NHEs have various physiological functions including intracellular pH homeostasis, cell volume regulation, acid-base regulation, and electroneutral NaCl transport (22, 53). The mammalian NHE family has ten members, and each of them has its own cellular localization and tissue distribution. Among them, five NHEs are detected at the plasma membrane in the epithelial cells in the intestine and the kidney. NHE1 is detected in almost all tissues and is located at the basolateral membrane in the epithelial cells (39, 42). NHE2 and NHE3 are mainly expressed at the apical membrane in the intestinal and the renal epithelial cells (9, 14, 39). NHE4 is also detected in the gastric epithelial cells and renal epithelial cells (39). NHE8, the latest characterized NHE isoform, is expressed at the apical membrane in the intestinal and renal epithelial cells (25, 49). Functional loss of these NHEs resulted in various phenotypes. Loss of NHE1 expression in mice displays growth retardation (7). Interruption of NHE2 and NHE4 expression in mice shows defects in gastric function (21, 43). Mice lacking NHE3 protein have altered sodium absorption (44). In the absence of NHE8 expression, reduced mucin production, elevated TNF-α expression, and decreased expression of downregulated-in-adenoma (DRA), a Cl−/HCO3− transporter, were detected in the gastrointestinal tract in mice (50, 51).
Tear film covers the ocular surface and functions as a protective barrier against microorganism invasion from the external environment (13, 23, 38). Tear film contains secretions from the cornea, the conjunctiva, and the lacrimal gland (24, 52). Compromise of any component of this system could lead to tear film disorders, most commonly dry eye (30). Similar to the epithelia of the lungs, gastrointestinal tract, and kidneys, the ocular epithelia regulate water transport through active electrolyte transport (3, 4, 45, 48). Studies have demonstrated that the NHE family plays an important role in sodium and water absorption in the epithelial cells in the intestine and the kidney. Interestingly, among the ten NHE family members, only NHE1 was detected in the ocular surface epithelial cells, and its function involves cell volume regulation (47). Since NHE8 is expressed in broad tissue types throughout the body, and NHE8 plays important roles in electrolyte absorption and mucosal protection in the intestinal epithelia (34, 49–51), we wonder if NHE8 is also expressed in the ocular tissues and plays a similar role in the ocular epithelia. Here, we conducted the present study to determine the expression of NHE8 in the ocular tissues and to explore the role of NHE8 in the ocular tissues using a NHE8−/− mouse model. Our data showed for the first time that NHE8 is highly expressed at the plasma membrane in epithelial cells of the conjunctiva, the corneas, and the lacrimal glands in both human and mouse eyes. Loss of NHE8 function in mice leads to morphological and functional alterations at the ocular surface.
MATERIALS AND METHODS
Animals.
The creation of the NHE8−/− mouse model was described in details previously (51). Ten- to 12-wk-old mice (males and females) were used in this study. All animal studies were approved by the University of Arizona Institutional Animal Care and Use Committee and conducted in compliance with the Tenets of the Declaration of Helsinki and ARVO statement for the use of animals in Ophthalmic and Visual Research.
Tissue collection.
Human eye tissues were obtained in accordance with good clinical practice, Institutional Review Board approval, and written informed consent regulations of the University of Arizona, and the tenets of the Declaration of Helsinki. Normal human palpebral conjunctival epithelia were harvested from freshly excised eyelid tissues during an ectropion or entropion repair. After a rinse with PBS, tissues were collected for RNA/protein extraction and histological processing. Mouse eyeballs were enucleated with attached conjunctiva and processed in the same fashion as fresh human tissue. Mouse extraorbital lacrimal glands and corneas were also collected.
RNA isolation and PCR assay.
Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) from human and mouse eye samples. RNA (500 ng) was reverse-transcribed using M-MLV Reverse Transcriptase (Promega, Madison, WI) following manufacturer's instruction. Real-time PCR was performed using TaqMan technology on an iCycler PCR thermal cycler (Bio-Rad, Hercules, CA). TBP gene was used as endogenous reference to normalize expression levels. TaqMan probes used in this study were purchased from Applied Biosystems (Foster City, CA). Data were analyzed using the comparative cycle threshold (Ct) method. The target gene cycle thresholds are adjusted relative to a calibrator (normalized Ct value obtained from control groups) and expressed as 2−ΔΔCt (Applied Biosystems User Bulletin No. 2: Rev B “Relative Quantitation of Gene Expression”).
Protein preparation and Western blotting.
Total protein was prepared in RIPA buffer following methods described previously (14). Briefly, tissues were homogenized in a small volume of RIPA buffer on ice. The resulting tissue lysates were then centrifuged for 10 min at 15,000 rpm at 4°C. The supernatants were collected and used for Western blot. The antibodies used in this study are NHE8 antibody (1:2,000 dilution) (49), DRA antibody (1:1,000 dilution; Sigma-Aldrich, St Louis, MO), GAPDH antiserum (1:100,000 dilution; Millipore, Billerica, MA), and β-actin antiserum (1:20,000 dilution; Sigma-Aldrich). Western detection was performed using the BM chemiluminescence Western blotting kit (Roche Diagnostics, Indianapolis, IN), and reviewed under a gel doc system (G:Box, Syngene; Frederick, MD). A ratio of target protein intensity over GAPDH or β-actin protein intensity was used for quantitation.
Tissue histological observation and immunohistochemistry staining.
Tissues fixed in 10% formalin were embedded in paraffin and sectioned. Eight-micrometer-thick sections were cut and stained with hematoxylin and eosin (H and E) or periodic-acid Schiff's reagent (PAS). All sections and staining works were done at the pathology services laboratory (University Animal Care, Tucson, AZ). Tissue sections with H and E staining or PAS staining were reviewed under a Zeiss Axioplan microscope. Immunohistochemical labeling and detection on NHE8 and DRA were performed as previously described (49). Briefly, NHE8 or DRA antiserum was incubated with tissue sections overnight at a 1:200 dilution for NHE8 or 1:100 dilution for DRA. The tissue slides were subsequently incubated with secondary antiserum (Alexa Fluor 647 goat anti-rabbit IgG; Molecular Probes, Eugene, OR) at 1:400 dilution, and then visualized under MRC-1024ES laser scanning confocal microscope (Bio-Rad).
Ocular surface evaluation.
Age- and sex-matched wild-type and NHE8−/− mice were used in this study. The ocular surface evaluation was conducted in an examiner-masked fashion. To measure the surface pH of the conjunctiva, narrow pH-range paper (pH 6.0 to 8.0; Micro Essential Laboratory; Brooklyn, NY) was used. Briefly, a pH paper strip (1 mm wide) was inserted into the lateral canthus of the conjunctival fornix for 30 s. The pH value was recorded using the color standards provided by the manufacturer. Tear volume was determined using ZoneQuick phenol red thread (Showa Yakuhin Kako; Tokyo). The lower eyelid was slightly pulled to place a 1-mm portion of the thread into the lower fornix at approximately 1/3 of the distance from the lateral canthus. Each eye was tested with the eye open for 15 s, and the wet portion of the thread was measured under a microscope. To determine the corneal surface damage, corneal fluorescein staining was used. A 2.5% sodium fluorescein solution (Sigma-Aldrich, St. Louis, MO) was diluted in sterile PBS to 0.25% before use. Mouse eye was applied with 1 μl of the diluted fluorescein. After 1 min, the excess fluorescein was blotted with cotton tips. Fluorescein solution treated corneas were digitally photographed individually using the Motic SMZ-168 microscope equipped with cobalt blue light. The images were scored using a standard National Eye Institute grading system (33). Briefly, the cornea is divided into five areas: central, superior, nasal, inferior, and temporal; punctate fluorescein staining in each area was graded on a scale of 0 to 3, and the total score (0 to 15) was the sum of scores from all five areas.
Statistical analysis.
One-way ANOVA and Chi-square test were used to compare data between groups with significance level set at P ≤ 0.05.
RESULTS
Expression and localization of NHE8 in the ocular surface in human and mouse eyes.
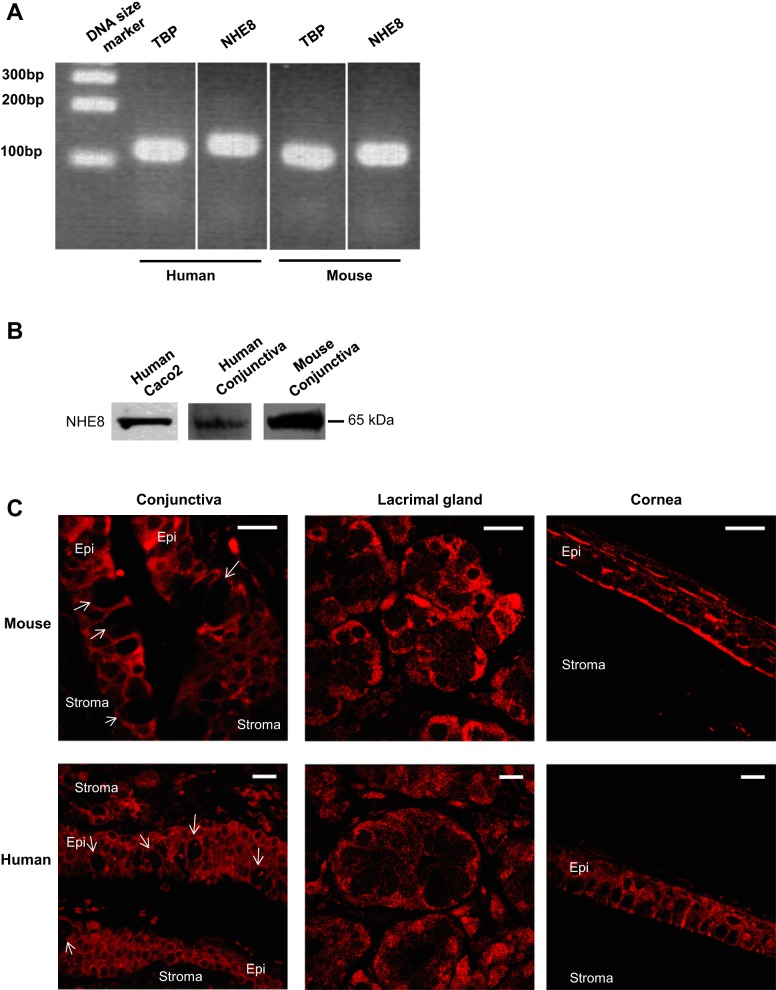
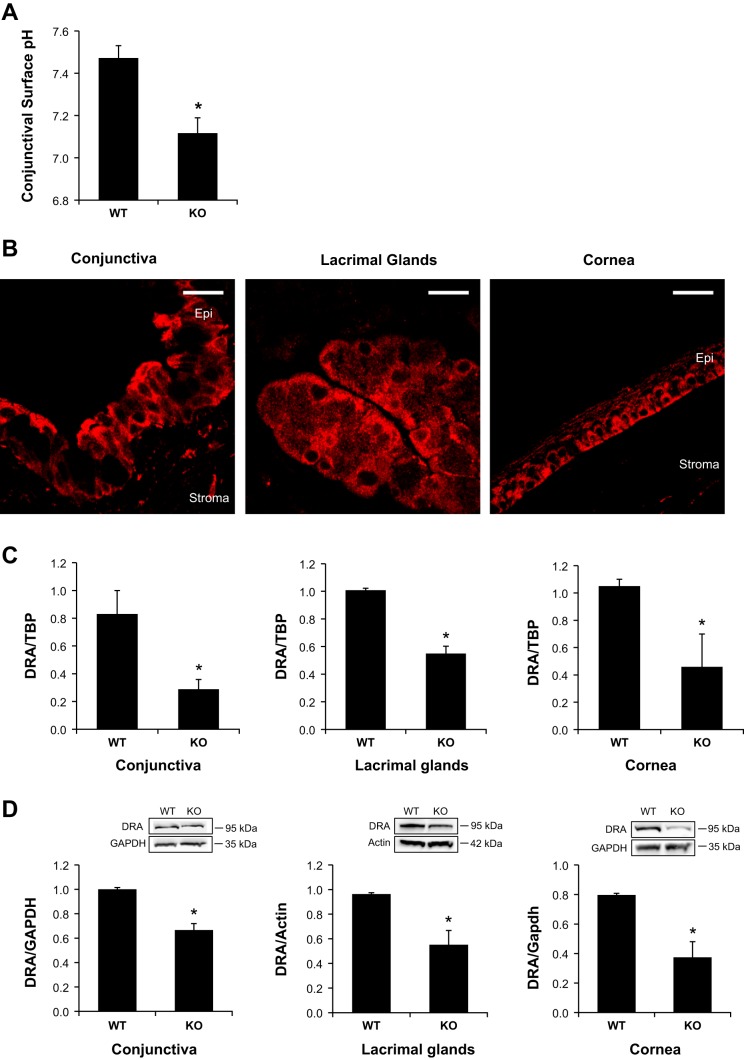
Although NHE8 displays a broad tissue distribution, it was unknown if NHE8 is expressed in the ocular surface. Here, we detected NHE8 expression by PCR and Western blot, and located NHE8 protein by immunohistochemistry technique. As shown in Fig. 1A, NHE8 mRNA was amplified from human and mouse conjunctival tissue using human- and mouse-specific NHE8 probes (Fig. 1A). Western blot also confirmed the expression of NHE8 protein in the conjunctival tissue lysates from human and mouse samples (Fig. 1B). Confocal microscope detected the plasma membrane localization of NHE8 in both stratified epithelial cells and goblet cells in human and mouse conjunctival tissues. In the cornea and the lacrimal glands, NHE8 was also detected at the plasma membrane in the epithelial cells in human and mouse (Fig. 1C).
Fig. 1.
Sodium/hydrogen exchanger (NHE) 8 expression in conjunctiva of human and mouse. Conjunctival tissue was collected from human subjects and mice. Total RNA and tissue lysate were prepared and were used for PCR and Western blotting. RT-PCR products were separated on 2% agarose gel and observed under UV light. Tissue lysates were reacted with NHE8 antibody and exposed on Syngene G:Box. Tissue sections were reacted to NHE8 antibody and observed under MRC-1024ES laser scanning confocal microscope. A: PCR detection of conjunctival NHE8 expression in human and mouse eyes. B: Western detection on NHE8 protein (65 kDa) in human intestinal epithelial cells (Caco2), human conjunctiva, and mouse conjunctiva. C: NHE8 localization in human and mouse eyes. Arrow in the conjunctival section indicates goblet cells. Epi, epithelium. Scale bar, 20 μm.
Morphological changes of the conjunctiva, cornea, and lacrimal gland in NHE8−/− mice.
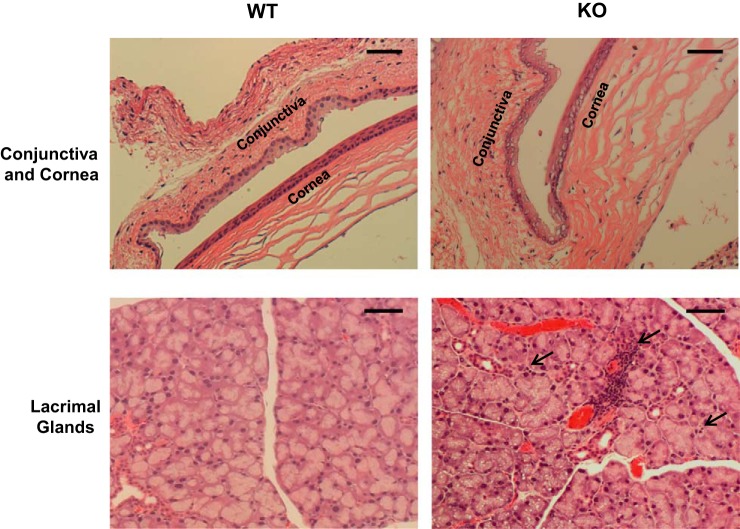
Since NHE8 is expressed in the conjunctiva, the cornea, and the lacrimal gland, we wanted to know whether loss of NHE8 function results in any abnormalities in the eye. Indeed, significant abnormalities were observed in NHE8−/− mouse eye tissues. Intracellular vacuoles were seen in the conjunctival and the corneal epithelia with more detected in the basal epithelial layer in NHE8−/− mice (Fig. 2, top). Clusters of an early stage of inflammation started around the blood vessels, and inflammatory cells were seen around the lacrimal glands in the NHE8−/− mouse (5 of 5 mice), but not in the wild-type mice (0 of 4 mice) (Fig. 2, bottom).
Fig. 2.
Morphological assessment of conjunctiva, cornea, and lacrimal gland in NHE8−/− mice. The eyes and the lacrimal glands were collected from mice and fixed in 4% paraformaldehyde at 4°C overnight, dehydrated, and embedded in paraffin. Sections were stained with hematoxylin and eosin and observed under microscope. WT, wild-type mice. KO, NHE8−/− mice. Dashed arrow in the lacrimal gland section indicates inflammatory infiltrates. Scale bar, 50 μm.
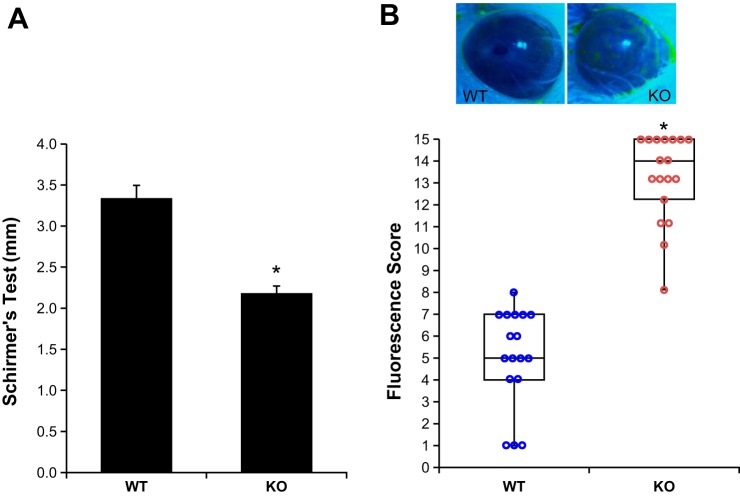
Decreased tear volume and increased corneal fluorescein staining in NHE8−/− mice.
Phenyl red thread test showed that the tear volume was significantly reduced in NHE8−/− mice compared with their wild-type littermates. The tear volume was reduced from 3.3 ± 0.2 mm in wild-type mice to 2.2 ± 0.1 mm in NHE8−/− mice (n = 35 eyes, P = 0.000003) (Fig. 3A). To assess the health of corneal surface, fluorescein staining was conducted to show the disruption of the integrity of corneal epithelium. As indicated in Fig. 3B, the median score of fluorescein staining differs dramatically between the wild-type mice and the NHE8−/− mice. The median score of fluorescein staining was 5 and 14 for wild-type mice and NHE8−/− mice, respectively (n = 35 eyes, P < 0.001).
Fig. 3.
Ocular function evaluation. A: Schirmer's test was used to measure tear volume in age- and sex-matched WT and NHE8−/− mice. Data are means ± SE from 35 eyes (one eye/mouse). *P < 0.001 for WT vs. NHE8−/− mice (KO). B: typical fluorescein staining to evaluate corneal surface health in WT and NHE8−/− mice. Data are means ± SE from 35 eyes (one eye/mouse). *P < 0.001 for WT vs. NHE8−/− mice (KO).
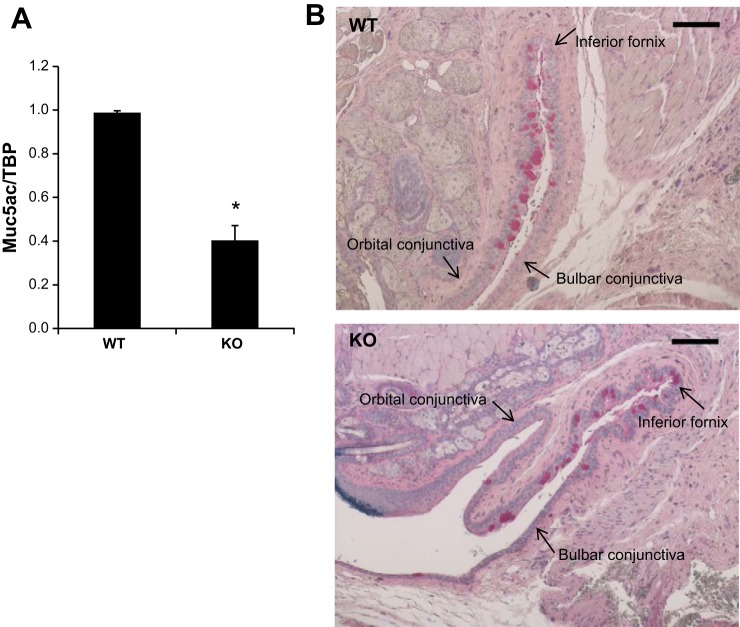
Reduced Muc5ac production in the conjunctiva of NHE8−/− mice.
Muc5ac is a major secreted mucin produced by conjunctival goblet cells. To determine if Muc5ac expression is impaired in NHE8−/− mice, we analyzed Muc5ac gene expression by RT-PCR. As indicated in Fig. 4, Muc5ac mRNA was significantly reduced in NHE8−/− conjunctiva (0.99 ± 0.02 in wild-type mice vs. 0.40 ± 0.07 in NHE8−/− mice; n = 6 groups, P = 0.00003) (Fig. 4A). PAS staining in the fornical region of mouse conjunctiva showed less stained cells in NHE8−/− mice than that in wild-type mice (Fig. 4B).
Fig. 4.
Mau5ac gene expression in the conjunctiva in NHE8−/− mice. A: RNA was isolated from the conjunctiva and used for PCR analysis. Data are means ± SE from 6 groups of samples (6 eyes from 6 mice in each group). *P ≤ 0.001 for WT vs. NHE8−/− mice (KO). B: PAS staining of the conjunctival mucin content in WT and NHE8−/− mice (KO). Scale bar, 50 μm.
Elevated TNF-α gene expression in the conjunctiva, the lacrimal gland, and the cornea in NHE8−/− mice.
To evaluate TNF-α expression in the ocular surface tissues in NHE8−/− mice, we compared ocular TNF-α expression levels between wild-type mice and NHE8−/− mice. As shown in Fig. 5, TNF-α mRNA expression was significantly increased in the ocular tissues in NHE8−/− mice. Compared with wild-type mice, the expression of TNF-α in NHE8−/− mice was increased from 1.02 ± 0.01 in wild-type conjunctiva to 1.98 ± 0.17 in NHE8−/− conjunctiva (n = 6 groups, P < 0.0001), from 1.02 ± 0.04 in wild-type lacrimal glands to 1.91 ± 0.12 in NHE8−/− lacrimal glands (n = 3 groups, P = 0.001), and from 0.95 ± 0.05 in wild-type cornea to 2.04 ± 0.55 in NHE8−/− cornea (n = 4 groups, P = 0.047).
Fig. 5.
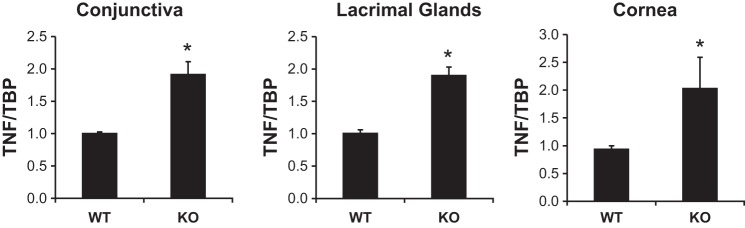
TNF-α expression in the ocular tissues in NHE8−/− mice. RNA was isolated from conjunctiva, lacrimal glands, and cornea and used for RT-PCR. Data are means ± SE from total 3–6 groups of samples (each group contains 6 eyes from 6 mice). *P < 0.05 for WT vs. NHE8−/− mice (KO).
Increased MMP9, Sprr2h, and Tgm1 gene expression in the conjunctiva and cornea of NHE8−/− mice.
To assess the epithelial integrity of the ocular surface in NHE8−/− mice, we evaluated the expression of wound repair marker MMP9, and keratinization markers Sprr2h and Tgm1 in the conjunctiva and the cornea in NHE8−/− mice. As shown in Fig. 6, the expression of these genes was significantly increased in NHE8−/− ocular tissues. The expression of MMP9 was increased from 0.98 ± 0.10 in wild-type conjunctiva to 2.25 ± 0.62 in NHE8−/− conjunctiva (n = 4 groups, P = 0.04), and from 1.00 ± 0.01 in wild-type cornea to 2.36 ± 0.26 in NHE8−/− cornea (n = 2 groups, P = 0.02) (Fig. 6A). The expression of Sprr2h was increased from 1.00 ± 0.03 in wild-type conjunctiva to 1.87 ± 0.44 in NHE8−/− conjunctiva (n = 5 groups, P = 0.04), but not in NHE8−/− cornea (Fig. 6B). The expression of Tgm1 was also elevated from 1.00 ± 0.03 in wild-type conjunctiva to 1.51 ± 0.22 in NHE8−/− conjunctiva (n = 5 groups, P = 0.02), and from 1.00 ± 0.01 in wild-type cornea to 1.35 ± 0.05 in NHE8−/− cornea (n = 2 groups, P = 0.01) (Fig. 6C).
Fig. 6.
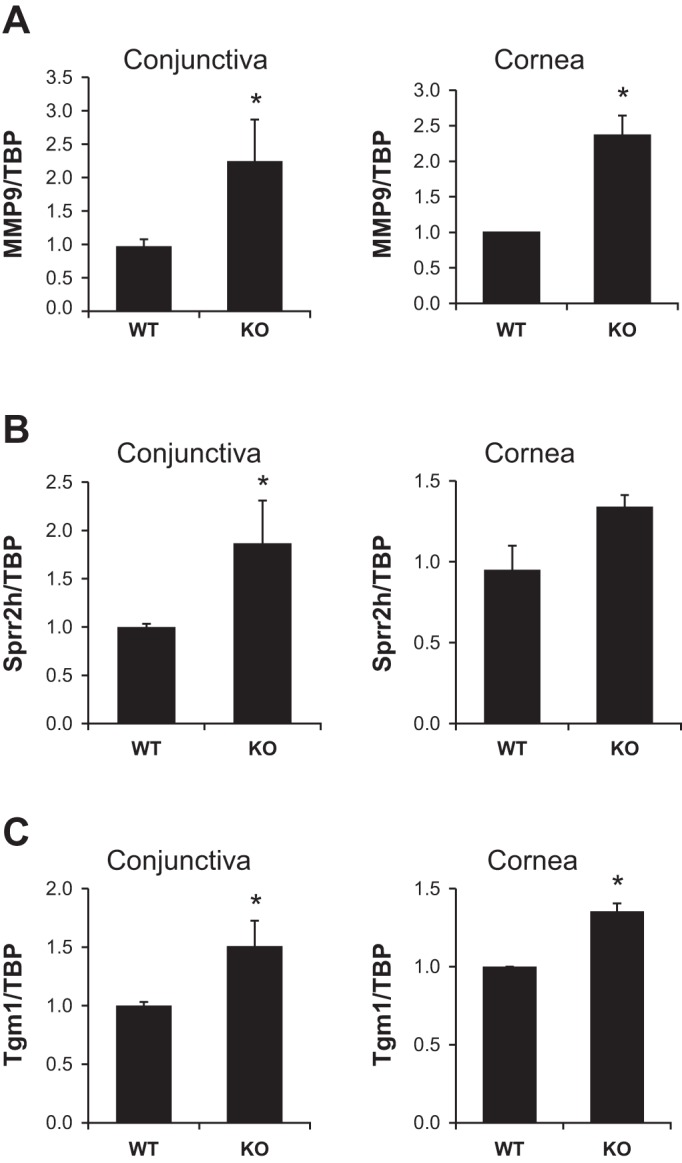
MMP9, Sprr2h and Tgm1 gene expression in the ocular tissues in NHE8−/− mice. RNA was isolated from conjunctival and corneal tissues and used for RT-PCR analysis. Data are means ± SE from total 2–5 groups of samples (each group contains 6 eyes from 6 mice). *P < 0.05 for WT vs. NHE8−/− mice (KO). A: MMP9 gene expression. B: Sprr2h gene expression. C: Tgm1 gene expression.
Significant reduction of ocular surface pH and DRA expression in NHE8−/− mice.
We have reported that the mucosal surface pH is reduced in the absence of NHE8 expression in the gastrointestinal tract (50, 51); here we compared the conjunctival epithelial surface pH in wild-type and NHE8−/− mice. As indicated in Fig. 7A, the average pH in the conjunctiva of wild-type mice is 7.5 ± 0.1, but this value was significantly decreased to 7.1 ± 0.1 in NHE8−/− mice (n = 36 eyes, P = 0.0006). We previously reported that DRA plays a role in maintaining mucosal surface pH in the gastrointestinal tract and loss of NHE8 resulted in decreased DRA expression in the intestine (50, 51). To further explore if this association also applies to the conjunctival surface pH, we assessed the expression of DRA in the ocular surface tissues in NHE8−/− mice. As shown in Fig. 7, DRA was indeed expressed in the conjunctiva, the cornea, and the lacrimal glands in mice. Confocal imaging showed the plasma membrane localization of DRA in the epithelial cells in the conjunctiva, the cornea, and the lacrimal glands in mice (Fig. 7B). Interestingly, the expression of DRA mRNA was significantly reduced in NHE8−/− eye tissues. In the conjunctiva, DRA mRNA was reduced from 0.83 ± 0.17 in wild-type mice to 0.29 ± 0.07 in NHE8−/− mice (n = 6 groups, P = 0.009). In the lacrimal glands, DRA mRNA expression was reduced from 1.03 ± 0.02 in wild-type mice to 0.55 ± 0.05 in NHE8−/− mice (n = 4 groups, P = 0.0001). In the cornea, DRA mRNA expression was reduced from 1.05 ± 0.06 in wild-type mice to 0.46 ± 0.28 in NHE8−/− mice (n = 4 groups, P = 0.02) (Fig. 7C). Similar to the RNA expression, DRA protein expression was also significantly reduced in NHE8−/− eye tissues. In the conjunctiva, DRA protein was reduced from 1.00 ± 0.02 in wild-type mice to 0.67 ± 0.07 in NHE8−/− mice (n = 4 groups, P = 0.002). In the lacrimal glands, DRA protein expression was reduced from 0.96 ± 0.01 in wild-type mice to 0.55 ± 0.12 in NHE8−/− mice (n = 3 groups, P = 0.012). In the cornea, DRA protein expression was reduced from 0.80 ± 0.01 in wild-type mice to 0.37 ± 0.11 in NHE8−/− mice (n = 2 groups, P = 0.029) (Fig. 7D).
Fig. 7.
Conjunctival epithelial surface pH and DRA expression in NHE8−/− mice. A: ocular surface pH measurement. A pH-paper strip was inserted into the lateral canthus of conjunctival fornix for 30 s. The pH value was recorded immediately. Data are means ± SE from 36 eyes (one eye/mouse). *P ≤ 0.0006 for WT vs. NHE8−/− mice (KO). B: DRA localization in the ocular tissues in the eye. Tissue sections were reacted to DRA antibody and observed under MRC-1024ES laser scanning confocal microscope. Epi, epithelium. Scale bar, 20 μm. C: DRA mRNA expression in NHE8−/− mouse eyes. Data are means ± SE from 4–6 group samples (each group contains 6 eyes from 6 mice). *P < 0.05 for WT vs. NHE8−/− mice (KO). D: DRA protein expression in NHE8−/− mouse eyes. Bar chart shows the DRA expression indicated as means ± SE in the sum of a total of 2–4 group samples (each group contains 6 eyes from 6 mice). *P < 0.05 for WT vs. NHE8−/− mice (KO). Inset: the corresponding Western blot image.
DISCUSSION
NHE8, the latest characterized NHE isoform in the NHE family, has been shown to participate in transmembrane electrolyte exchange and mucosal protection in the intestine and the kidney (25, 49–51). Although NHE8 is expressed in the epithelial cells in the intestine and the kidney, whether NHE8 is expressed in the ocular surface epithelia remains unknown (25, 49). In the present study, we showed that NHE8 mRNA is expressed in human and mouse eyes. Similar to NHE8 protein localization in the intestinal epithelial cells (49, 50), NHE8 is also located at the plasma membrane of the epithelial cells in the conjunctiva, the cornea, and the lacrimal glands in human and mouse eye tissues. The presence of NHE8 in the ocular surface epithelia suggests a possible role of this protein in ocular function.
Tear film plays a very important role in ocular protection. It is believed that the majority of tears is produced by lacrimal glands, but the ocular epithelial cells, especially conjunctiva, could also contribute to tear production. Studies have suggested that conjunctival epithelium could be the source of tears under unstimulated, normal conditions (12, 16, 19). To understand the physiological role of NHE8 in the ocular surface, we used a NHE8−/− mouse model in this study. We found that NHE8−/− mice have significantly reduced tear volume (∼34%) compared with that of wild-type littermates. The reduced tear volume in NHE8−/− mice might be due to the altered lacrimal gland function. In fact, the presence of focal inflammatory infiltrates and foamy vacuoles within acinar cells in the lacrimal gland, and the elevated TNF-α expression (∼191% increase) in the lacrimal glands in NHE8−/− mice suggested a compromised lacrimal gland function in NHE8−/− eyes. Moreover, the abnormal morphology of the conjunctival epithelial layer and the elevated TNF-α expression (∼194% increase) in the conjunctiva may also contribute to the reduced tear volume in NHE8−/− mice since conjunctiva participate in tear volume regulation (12, 16, 19). Decreased tear volume, increased corneal stain, and elevated inflammatory cytokines in ocular surface are the hallmarks of dry eye disease (1, 6). The ocular surface alterations observed in NHE8−/− mice are similar to human dry eye condition. Unlike other dry eye animal models, such as aging-related dry eye (36) or goblet-deficiency induced dry eye (35), NHE8−/− mice display reduced tear production while the others showed increased tear volume. Therefore, NHE8 indeed plays an important role in maintaining ocular surface moisture.
Muc5ac, a major secretory mucin in the ocular surface, is produced by conjunctival goblet cells in the eye (24, 28). A decrease in Muc5ac production has been linked to ocular surface inflammation (5, 11, 15, 18, 32). We have previously reported that loss of NHE8 expression in mice resulted in reduced Muc2 expression in the intestine (51). Here we observed a similar pattern in the NHE8−/− eyes. The expression of Muc5ac was reduced by 60% in the conjunctiva in NHE8−/− mice. These observations suggested that NHE8 may also play a role in goblet cell function in the conjunctiva.
Since ocular damage displays elevated expression of MMP9, Sprr2h, and Tgm1 (2, 17, 35), we wondered if these markers were also changed in NHE8−/− mice. MMP9 belongs to the matrix metalloproteinases (MMPs) family. MMP9 function has been shown to be involved in ocular surface injury (2, 17), and wound repair (10, 37). In NHE8−/− mice, MMP9 expression was increased by 2.3-fold in the conjunctiva and 2.4-fold in the cornea, indicating ocular epithelial damage in the absence of NHE8 function. Sprr2h, a member of the small proline rich (Sprr) protein family, is required to create an epidermal cornified cell envelope in terminal differentiated keratinocytes to prevent the loss of water and ions and to protect from environmental hazards (40). Tgm1, a protein encoding for the TGase-1 enzyme, is also involved in the formation of the cornified cell envelope (8, 31, 41, 46). Both Sprr2h and Tgm1 genes have been shown to increase in the conjunctival epithelial cells in a dry eye model induced by Spdef deficiency (35). Spdef is involved in the induction of goblet cell differentiation; lack of Spdef expression resulted in impaired goblet cell maturation (26). In NHE8−/− mice, the expression of Sprr2h was increased by 190% in the conjunctiva, and the expression of Tgm1 was increased by 150% in the conjunctiva and 140% in the cornea. These observations further indicated the important function of NHE8 in maintaining epithelial layer integrity.
Bicarbonate is an important player in protecting the mucosal layer that lines all epithelia (20). NHE8−/− mice displayed conjunctival mucosal acidification, suggesting a possible defect in bicarbonate secretion. DRA is a transmembrane glycoprotein originally discovered in the intestine, and it functions to secrete bicarbonate in exchange for extracellular Cl− (27, 29). We have previously showed that NHE8 and DRA are closely coupled in absorbing electrolytes and excreting bicarbonate and proton in the stomach and the colon (50, 51). In this study, we detected the expression of DRA in the ocular tissues by PCR and Western blot. We also localized DRA protein at the plasma membrane in the epithelial cells in the conjunctiva, the cornea, and the lacrimal gland. Interestingly, the expression of DRA in NHE8−/− mouse eyes displayed a similar pattern as observed in the stomach and in the intestine. Loss of NHE8 in the ocular surface resulted in decreased conjunctival surface pH and reduced conjunctival DRA expression (65% reduction at mRNA level and 33% reduction at protein level). A similar reduction was also seen in the lacrimal glands and the cornea (50–60% reduction at both mRNA and protein levels in both tissues). These observations together suggested that NHE8 is important for normal DRA expression and bicarbonate secretion in the ocular surface.
In summary, our study showed that NHE8 is highly expressed in the ocular tissues in human and mouse. Ablation of NHE8 function in mice resulted in reduced tear volume, increased corneal staining, and elevated TNF-α expression in the ocular surface, and hence possibly led to dry eye phenotype. These results suggested that NHE8 plays important roles in ocular surface protection by participating in tear production and bicarbonate secretion through coupling with DRA function. Therefore, abnormal NHE8 function might be a potential novel mechanism of dye eye conditions.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-073638 (F. K. Ghishan and H. Xu), Research to Prevent Blindness Foundation (M. Wang), and University of Arizona Faculty Research Seed Grant (M. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.X., M.W., and F.K.G. conception and design of research; H.X., Y.Z., J.L., and M.W. performed experiments; H.X., Y.Z., J.L., F.L., M.G., and F.K.G. analyzed data; H.X., Y.Z., J.L., M.W., F.L., and M.G. interpreted results of experiments; H.X., Y.Z., and J.L. prepared figures; H.X. drafted manuscript; H.X., M.W., and F.K.G. edited and revised manuscript; H.X. and F.K.G. approved final version of manuscript.
REFERENCES
- 1.Anonymous. Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocular Surf 5: 179–193, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Acera A, Vecino E, Duran JA. Tear MMP-9 levels as a marker of ocular surface inflammation in conjunctivochalasis. Invest Ophthalmol Vis Sci 54: 8285–8291, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez LJ, Candia OA, Turner HC, Zamudio AC. Phorbol ester modulation of active ion transport across the rabbit conjunctival epithelium. Exp Eye Res 69: 33–44, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez LJ, Zamudio AC, Candia OA. Cl− secretory effects of EBIO in the rabbit conjunctival epithelium. Am J Physiol Cell Physiol 289: C138–C147, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci 43: 1004–1011, 2002. [PubMed] [Google Scholar]
- 6.Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Progr Retin Eye Res 31: 271–285, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol Cell Physiol 276: C788–C795, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Boeshans KM, Mueser TC, Ahvazi B. A three-dimensional model of the human transglutaminase 1: insights into the understanding of lamellar ichthyosis. J Mol Modeling 13: 233–246, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Brant SR, Yun CH, Donowitz M, Tse CM. Cloning, tissue distribution, and functional analysis of the human Na+/H+ exchanger isoform, NHE3. Am J Physiol Cell Physiol 269: C198–C206, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Buisson AC, Zahm JM, Polette M, Pierrot D, Bellon G, Puchelle E, Birembaut P, Tournier JM. Gelatinase B is involved in the in vitro wound repair of human respiratory epithelium. J Cell Physiol 166: 413–426, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Cai RR, Wang Y, Xu JJ, Zhang CR. [The effects of hyperosmotic stress on rabbit ocular surface and mucin 5AC expression]. [Zhonghua yan ke za zhi] Chin J Ophthalmol 47: 252–259, 2011. [PubMed] [Google Scholar]
- 12.Candia OA, Alvarez LJ. Fluid transport phenomena in ocular epithelia. Progr Retin Eye Res 27: 197–212, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandler JW, Gillette TE. Immunologic defense mechanisms of the ocular surface. Ophthalmology 90: 585–591, 1983. [DOI] [PubMed] [Google Scholar]
- 14.Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol Cell Physiol 273: C1937–C1946, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Corrales RM, Narayanan S, Fernandez I, Mayo A, Galarreta DJ, Fuentes-Paez G, Chaves FJ, Herreras JM, Calonge M. Ocular mucin gene expression levels as biomarkers for the diagnosis of dry eye syndrome. Invest Ophthalmol Vis Sci 52: 8363–8369, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Dartt DA. Regulation of mucin and fluid secretion by conjunctival epithelial cells. Progr Retin Eye Res 21: 555–576, 2002. [DOI] [PubMed] [Google Scholar]
- 17.De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD 3rd, Fang B, Zheng X, Ma P, Farley WJ, Siemasko KF, Niederkorn JY, Stern ME, Li DQ, Pflugfelder SC. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol 2: 243–253, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dogru M, Matsumoto Y, Okada N, Igarashi A, Fukagawa K, Shimazaki J, Tsubota K, Fujishima H. Alterations of the ocular surface epithelial MUC16 and goblet cell MUC5AC in patients with atopic keratoconjunctivitis. Allergy 63: 1324–1334, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Fischbarg J. Water channels and their roles in some ocular tissues. Mol Aspects Med 33: 638–641, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Flemstrom G, Isenberg JI. Gastroduodenal mucosal alkaline secretion and mucosal protection. News Physiol Sci 16: 23–28, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Gawenis LR, Greeb JM, Prasad V, Grisham C, Sanford LP, Doetschman T, Andringa A, Miller ML, Shull GE. Impaired gastric acid secretion in mice with a targeted disruption of the NHE4 Na+/H+ exchanger. J Biol Chem 280: 12781–12789, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Ghishan FK, Kiela PR. Small intestinal ion transport. Curr Opin Gastroenterol 28: 130–134, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gipson IK. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci 48: 4390; 4391–4398, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gipson IK, Inatomi T. Cellular origin of mucins of the ocular surface tear film. Adv Exp Med Biol 438: 221–227, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Goyal S, Mentone S, Aronson PS. Immunolocalization of NHE8 in rat kidney. Am J Physiol Renal Physiol 288: F530–F538, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, Peters PJ, Clevers H. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology 137: 1333–1345, e1331–e1333, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Haila S, Saarialho-Kere U, Karjalainen-Lindsberg ML, Lohi H, Airola K, Holmberg C, Hastbacka J, Kere J, Hoglund P. The congenital chloride diarrhea gene is expressed in seminal vesicle, sweat gland, inflammatory colon epithelium, and in some dysplastic colon cells. Histochem Cell Biol 113: 279–286, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Inatomi T, Tisdale AS, Zhan Q, Spurr-Michaud S, Gipson IK. Cloning of rat Muc5AC mucin gene: comparison of its structure and tissue distribution to that of human and mouse homologues. Biochem Biophys Res Commun 236: 789–797, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology 122: 709–724, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Johnson ME, Murphy PJ. Changes in the tear film and ocular surface from dry eye syndrome. Progr Retin Eye Res 23: 449–474, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Kim IG, McBride OW, Wang M, Kim SY, Idler WW, Steinert PM. Structure and organization of the human transglutaminase 1 gene. J Biol Chem 267: 7710–7717, 1992. [PubMed] [Google Scholar]
- 32.Kunert KS, Keane-Myers AM, Spurr-Michaud S, Tisdale AS, Gipson IK. Alteration in goblet cell numbers and mucin gene expression in a mouse model of allergic conjunctivitis. Invest Ophthalmol Vis Sci 42: 2483–2489, 2001. [PubMed] [Google Scholar]
- 33.Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J 21: 221–232, 1995. [PubMed] [Google Scholar]
- 34.Liu C, Xu H, Zhang B, Johansson ME, Li J, Hansson GC, Ghishan FK. NHE8 plays an important role in mucosal protection via its effect on bacterial adhesion. Am J Physiol Cell Physiol 305: C121–C128, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marko CK, Menon BB, Chen G, Whitsett JA, Clevers H, Gipson IK. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol 183: 35–48, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClellan AJ, Volpe EA, Zhang X, Darlington GJ, Li DQ, Pflugfelder SC, de Paiva CS. Ocular surface disease and dacryoadenitis in aging C57BL/6 mice. Am J Pathol 184: 631–643, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, Lee Y, McCarthy BE, Wollenberg KR, Jester JV, Wang M, Welgus HG, Shipley JM, Senior RM, Fini ME. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem 277: 2065–2072, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Montes-Mico R, Cervino A, Ferrer-Blasco T, Garcia-Lazaro S, Madrid-Costa D. The tear film and the optical quality of the eye. Ocular Surf 8: 185–192, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Orlowski J, Kandasamy RA, Shull GE. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem 267: 9331–9339, 1992. [PubMed] [Google Scholar]
- 40.Patel S, Kartasova T, Segre JA. Mouse Sprr locus: a tandem array of coordinately regulated genes. Mamm Genome 14: 140–148, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Robinson NA, Lapic S, Welter JF, Eckert RL. S100A11, S100A10, annexin I, desmosomal proteins, small proline-rich proteins, plasminogen activator inhibitor-2, and involucrin are components of the cornified envelope of cultured human epidermal keratinocytes. J Biol Chem 272: 12035–12046, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Sardet C, Franchi A, Pouyssegur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell 56: 271–280, 1989. [DOI] [PubMed] [Google Scholar]
- 43.Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest 101: 1243–1253, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Selvam S, Thomas PB, Gukasyan HJ, Yu AS, Stevenson D, Trousdale MD, Mircheff AK, Schechter JE, Smith RE, Yiu SC. Transepithelial bioelectrical properties of rabbit acinar cell monolayers on polyester membrane scaffolds. Am J Physiol Cell Physiol 293: C1412–C1419, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem 270: 17702–17711, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Turner HC, Alvarez LJ, Candia OA. Identification and localization of acid-base transporters in the conjunctival epithelium. Exp Eye Res 72: 519–531, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Walcott B, Birzgalis A, Moore LC, Brink PR. Fluid secretion and the Na+-K+-2Cl− cotransporter in mouse exorbital lacrimal gland. Am J Physiol Cell Physiol 289: C860–C867, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol 289: G36–G41, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Xu H, Li J, Chen H, Wang C, Ghishan FK. NHE8 plays important roles in gastric mucosal protection. Am J Physiol Gastrointest Liver Physiol 304: G257–G261, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu H, Zhang B, Li J, Wang C, Chen H, Ghishan FK. Impaired mucin synthesis and bicarbonate secretion in the colon of NHE8 knockout mice. Am J Physiol Gastrointest Liver Physiol 303: G335–G343, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu DF, Chen Y, Han JM, Zhang H, Chen XP, Zou WJ, Liang LY, Xu CC, Liu ZG. MUC19 expression in human ocular surface and lacrimal gland and its alteration in Sjogren syndrome patients. Exp Eye Res 86: 403–411, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005. [DOI] [PubMed] [Google Scholar]