Abstract
The synthesis of new bone in response to a novel applied mechanical load requires a complex series of cellular signaling events in osteoblasts and osteocytes. The activation of the purinergic receptor P2X7R is central to this mechanotransduction signaling cascade. Recently, P2X7R have been found to be associated with caveolae, a subset of lipid microdomains found in several cell types. Deletion of caveolin-1 (CAV1), the primary protein constituent of caveolae in osteoblasts, results in increased bone mass, leading us to hypothesize that the P2X7R is scaffolded to caveolae in osteoblasts. Thus, upon activation of the P2X7R, we postulate that caveolae are endocytosed, thereby modulating the downstream signal. Sucrose gradient fractionation of MC3T3-E1 preosteoblasts showed that CAV1 was translocated to the denser cytosolic fractions upon stimulation with ATP. Both ATP and the more specific P2X7R agonist 2′(3′)-O-(4-benzoylbenzoyl)ATP (BzATP) induced endocytosis of CAV1, which was inhibited when MC3T3-E1 cells were pretreated with the specific P2X7R antagonist A-839977. The P2X7R cofractionated with CAV1, but, using superresolution structured illumination microscopy, we found only a subpopulation of P2X7R in these lipid microdomains on the membrane of MC3T3-E1 cells. Suppression of CAV1 enhanced the intracellular Ca2+ response to BzATP, suggesting that caveolae regulate P2X7R signaling. This proposed mechanism is supported by increased mineralization in CAV1 knockdown MC3T3-E1 cells treated with BzATP. These data suggest that caveolae regulate P2X7R signaling upon activation by undergoing endocytosis and potentially carrying with it other signaling proteins, hence controlling the spatiotemporal signaling of P2X7R in osteoblasts.
Keywords: caveolin-1, purinergic signaling, P2X7R, endocytosis, osteoblasts
purinergic signaling plays a critical role in regulation of skeletal mechanotransduction, an intricate process through which a physical stimulus is converted into a biochemical response (7, 23). We and others have shown that ATP and other nucleotides are released in response to fluid shear stress in vitro and activate purinergic (P2) receptors on the membrane of the osteoblasts (13, 20, 29, 38). Although many subtypes of P2 receptors are present in osteoblasts (4), knockout of the purinergic receptor P2X7R, a ligand-gated ion channel, resulted in an osteopenic phenotype (22), suggesting that this receptor is important in skeletal maintenance. Further in vivo loading experiments show that load-induced bone formation is significantly abrogated when the P2X7R is genetically deleted, demonstrating the importance of this receptor in mechanotransduction (23).
Several characteristics distinguish the P2X7R from the other P2X receptors. The P2X7R is 10–30 times more specific for 2′(3′)-O-(4-benzoylbenzoyl)ATP (BzATP) than ATP (28), and P2X7R activation by BzATP induces dynamic membrane blebbing (39). P2X7R activation has been associated with opening of a large pore on the plasma membrane that allows entry of up to 900-Da molecules (28, 46), although a recent report suggests that this pore is actually a pannexin (19). Although prolonged activation of this receptor has been known to trigger apoptosis in certain cell types, we have observed that there is no caspase-3 activation in osteoblasts stimulated with BzATP (23). P2X7R stimulation with BzATP in cells of the osteoblastic lineage results in expression of osteogenic markers such as osterix and osteocalcin (31) and release of PGE2 (23), all of which can eventually lead to osteogenesis. Signaling via the P2X7R is also known to activate PLD and PLA2, leading to synthesis of lysophosphatidic acid, which has been attributed to membrane blebbing (31). P2X7R activation also induces ERK phosphorylation in a PKC-dependent manner in osteoblasts (26). The receptor has a significant role in inflammatory responses; P2X7R activation has been shown to induce the release of inflammatory cytokines from macrophages and osteoclasts (42). Overall, this ligand-gated ion channel has a major role in several intracellular processes in a cell type-specific manner.
Recent reports suggest that a subpopulation of P2X7R exists in caveolae, 60- to 100-nm invaginations of the plasma membrane (30, 49), of cells of the submandibular glands, lung alveolae, and macrophages (3, 12, 16). These plasma membrane structures are widely expressed in adipocytes, fibroblasts, endothelial cells, and osteoblasts (34, 43, 44). Caveolae are formed from a subset of components of lipid rafts, such as sphingolipids and cholesterol, and are stabilized by 22-kDa integral proteins called caveolins (34). There are three caveolin isoforms: CAV1, CAV2, and CAV3. CAV1 and CAV2 are found in nonmuscle cells, whereas CAV3 is primarily found in skeletal muscle cells and some smooth muscle cells (47). Removal of CAV1 and CAV3 prevents formation of caveolae in these cells, but there were no apparent changes when CAV2 was suppressed (5, 11). Numerous studies have demonstrated the importance of caveolae in cell signaling and scaffolding of signaling molecules in several cell types (24, 27, 35). Besides anchoring second messengers, caveolae can also attenuate signaling by endocytosis and therefore, regulate the presence of the receptors, along with other signaling proteins, on the plasma membrane (17, 36).
The importance of CAV1 and caveolae in regulation of the skeleton was emphasized in a study in which knockout of CAV1 in mice increased trabecular and cortical bone, which appears to result from increased osteoblast function (40). Because P2X7R and CAV1 are important in skeletal maintenance and the response of bone to mechanical loading, we hypothesized that the P2X7R is present in caveolae of osteoblasts and, further, that disruption of caveolae will alter P2X7R-mediated signaling.
MATERIALS AND METHODS
Cell culture.
MC3T3-E1 cells, a murine preosteoblast-like cell line (American Type Culture Collection), were grown in α-modified Eagle's medium that was supplemented with 10% FBS (Gemini), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained in a humidified incubator in 5% CO2-95% air and subcultured every 72 h. MC3T3-E1 cells were serum-starved for 12–16 h prior to all experiments. All cell culture media and antibiotics were purchased from Sigma-Aldrich (St. Louis, MO).
Reagents and antibodies.
Adenosine 5'-triphosphate disodium salt (ATP), BzATP triethylammonium salt (BzATP) and puromycin dihydrochloride (puromycin) were purchased from Sigma-Aldrich. ATP (250 μM; from a stock of 5 mM in PBS) and BzATP (150 μM; from a stock of 6 mM in distilled water) were used for activation of P2X7R. A-839977 (500 nM; from a stock of 500 μM in DMSO), a potent P2X7R antagonist, was purchased from Tocris Bioscience. Rabbit polyclonal anti-CAV1 and mouse monoclonal anti-CAV1 were obtained from BD Biosciences (San Jose, CA). Rabbit polyclonal anti-P2X7R and FITC-conjugated rabbit anti-P2X7R were purchased from Alomone Labs (Jerusalem, Israel). Anti-GAPDH antibody (Abcam, Cambridge, MA) was used as loading control in Western blots. The caveolar marker Alexa Fluor 555-cholera toxin B (CTX-B) and Alexa Fluor 488-donkey anti-rabbit IgG were purchased from Life Technologies (Carlsbad, CA). siRNA against CAV1 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and the SureSilencing plasmid vectors coding shRNA against CAV1 with puromycin resistance (KM03639P) were purchased from SABiosciences (Frederick, MD).
Detergent-free isolation of caveolin-enriched membrane fractions.
Sucrose gradient fractionation was used to isolate caveolin-enriched membrane fractions, as described by Song et al. (45) with some modifications. Briefly, MC3T3-E1 cells were grown to 80–90% confluency in 150-mm petri dishes and washed with cold PBS. In the experiments with agonist treatment, the cells were grown in reduced (0.2%) serum for 14–16 h prior to treatment. The cells were scraped into 2 ml of ice-cold 500 mM sodium carbonate containing protease inhibitors (Calbiochem, San Diego, CA), the cell lysate was homogenized by sonication and centrifuged at 9,000 g for 10 min, and the supernatant was collected. Protein concentration was measured using the bicinchoninic acid assay, and equal concentrations were subjected to discontinuous sucrose gradient fractionation.
A 45% sucrose-lysate solution was made by addition of 90% sucrose in MBS (25 mM MES and 150 mM NaCl, pH 6.5) to the lysate. This mixture was placed at the bottom of an ultracentrifuge tube, and a discontinuous gradient was prepared on top of this mixture by layering with 4 ml of 35% and 5% sucrose in MBS containing 250 mM sodium carbonate. The gradient was subjected to ultracentrifugation for 18 h at 39,000 rpm at 4°C with a SW41-TI rotor (Beckman Coulter). A light-scattering band was typically observed at the 5%-35% interface, where most of the caveolae were enriched. Twelve 1-ml fractions were collected from the top of the gradient, and the proteins in each fraction were precipitated using trichloroacetic acid-acetone. The precipitates were resuspended in sample buffer, and equal volumes of the fractions were subjected to Western blotting for the respective proteins.
Immunocytochemistry.
Cells were seeded at a density of 2.5 × 103/cm2 onto glass coverslips coated with rat tail type I collagen (100 μg/ml in 0.02 N acetic acid; Becton Dickinson, Franklin Lakes, NJ) and grown to 80–90% confluency. Experimentally, cells were treated with 250 μM ATP or 150 μM BzATP. One group of cells were pretreated with 500 nM A-839977 for 30 min prior to addition of 150 μM BzATP. After 10 min of stimulation, the cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) containing 0.1% Triton X-100. Fixed cells were washed with PBS and blocked with blocking buffer containing 3% BSA and 10% donkey serum for 2 h. Rabbit anti-CAV1 antibody (0.5 μg) was then added, and the samples were incubated overnight at 4°C. After incubation, the samples were washed with blocking buffer, and the secondary antibody Alexa Fluor 488-donkey anti-rabbit IgG was added. The cells were mounted on microscopy slides with Prolong Gold (Life Technologies) and visualized using the Zeiss LSM 780 confocal microscope with a EC Plan-Neofluar ×40/1.30 oil objective lens. The number of cells with a strong membrane staining pattern for CAV1 within the field of view was counted manually. A minimum of 30 cells were counted for each treatment, and the percentage of cells with plasma membrane CAV1 was calculated. Each experiment was repeated three times with different cell passages. The line profile of CAV1 staining illustrating the intensity of labeling across the cell was created using ImageJ software.
Triton X-100 extraction.
MC3T3-E1 cells grown on 100-mm petri dishes were washed three times with ice-cold PBS and lysed in 500 μl of cold Triton X-100 lysis buffer (1% Triton X-100, 50 mM Tris, and 150 mM NaCl, pH 6.5) containing protease inhibitors. The cells were scraped off the plate and proteins were extracted for 30 min at 4°C. The lysate was centrifuged at 9,000 g for 30 min, and supernatant was collected as the soluble fraction. The pellet containing the detergent-resistant caveolae was resuspended in 500 μl of RIPA buffer (50 mM Tris, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, and protease inhibitors) and sonicated to obtain the insoluble fraction. Equal volumes of both fractions were subjected to Western blotting and probed for CAV1 and the P2X7R.
siRNA strategy for transient knockdown of CAV1.
siRNA was used to transiently knock down CAV1 in MC3T3-E1 cells according to the manufacturer's instructions. Briefly, MC3T3-E1 cells were seeded at a density of 2.5 × 103 cells/cm2 onto rat tail type I collagen-coated (100 μg/ml; Becton Dickinson) glass-bottom dishes (MatTek, Ashland, MA) and grown to 50–60% confluency prior to transfection. For transfection, the culture medium was removed, the cells were washed with PBS, and 800 μl of Opti-MEM (Life Technologies) were added. siRNA against CAV1 (0.5 μM; sc-29942) and 6 μl of Oligofectamine (Life Technologies) were diluted in 100 μl of Opti-MEM. The reagents were gently mixed and incubated for 20 min at room temperature and then added to each dish. After 5–7 h of incubation with the siRNA, fresh culture medium containing 10% FBS was added to the cells. Experiments were conducted 72 h posttransfection, since Western blotting demonstrated maximum suppression after 72 h. Scrambled siRNA (sc-37007) and PBS were used as controls.
Generation of stable CAV1 knockdown cells.
To generate a clonal line of MC3T3-E1 cells with stable knockdown of CAV1 (CAV1 KD cells), SureSilencing shRNA plasmid with puromycin resistance was used. Five sets of plasmids were provided: four were specifically targeted against CAV1 mRNA, and one was a nonspecific scrambled plasmid that served as a control. Following the manufacturers protocol, each of the plasmids was amplified after transformation into the competent DH5α cells. The plasmids were isolated using Qiagen Miniprep and concentration was measured.
Prior to transfection with the shRNA encoding plasmids, MC3T3-E1 cells (2.5 × 103/cm2) were grown on a 12-well plate for 24 h. Six microliters of the transfection reagent Lipofectin (Life Technologies) were diluted in 100 μl of Opti-MEM and mixed with 100 μl of Opti-MEM containing 1.2 μg of the plasmid and incubated for 20 min at room temperature. Meanwhile, the growth medium on the cells was replaced with 800 μl of fresh Opti-MEM. After incubation, the transfection mixture was added to the MC3T3-E1 cells and incubated for 5–7 h at 37°C. The transfection reagents were then removed, and fresh culture medium containing 10% FBS was added. At 72 h posttransfection, the culture medium was supplemented with puromycin at 5 μg/ml, the minimum inhibitory concentration based on the dose response for MC3T3-E1 cells. Cells that did not take up the plasmid failed to develop a resistance to the antibiotic and died. Within 1 wk, colonies of MC3T3-E1 cells with puromycin resistance were beginning to develop. These colonies were isolated for ∼8 wk and continuously screened for antibiotic resistance. A control group of untransfected MC3T3-E1 cells were also screened to ensure that the antibiotic concentration remained effective to completely kill the nontransfected cells. Four different clones, including the scrambled control, were isolated and the extent of CAV1 knockdown was measured using immunoblotting. GAPDH was used as the loading control.
Western blotting.
To determine the protein levels of CAV1 following knockdown or the siRNA or shRNA treatment, MC3T3-E1 cells were lysed using RIPA buffer, and equal amounts of protein were subjected to SDS-PAGE. To detect CAV1, P2X7R, and GAPDH in the sucrose fractionation studies and in the Triton X-100 extraction experiments, equal volumes of the samples were loaded onto 4%-12% gradient NuPAGE gels (Life Technologies). After electrophoresis, the proteins were transferred to a nitrocellulose membrane and blocked overnight with 5% milk in Tris-buffered saline containing 0.1% Tween 20. The blots were probed with antibodies against CAV1, P2X7R, and GAPDH using the respective antibodies.
Ca2+ imaging.
Intracellular Ca2+ concentration ([Ca2+]i) was measured in MC3T3-E1 cells using the fluorescent Ca2+ chelator fluo 4 and imaged using a Zeiss 510 confocal microscope with a Zeiss ×10 C-Apochromat water-immersion lens (0.45 numerical aperture). As previously described, MC3T3-E1 cells were grown on rat tail type I collagen-coated glass-bottom dishes until cells reached 70–80% confluency. Cells were then loaded with the Ca2+ indicator fluo 4 (10 μM; Life Technologies) for 20 min at 37°C, washed with Hanks' balanced saline solution to remove excess dye, and allowed to recover for 15 min before observation. A 488-nm excitation laser was used to visualize [Ca2+]i, and changes in intensity were measured as a time lapse. Images were collected over a 1-min period prior to addition of BzATP to establish the baseline and continued for 4 min after addition of BzATP. The data were analyzed using the Zeiss ZEN Physiology software module (version 2011) and the change in intensity over baseline was calculated to determine the increase in [Ca2+]i over baseline. The Ca2+ response to BzATP was measured in control and CAV1-suppressed MC3T3-E1 cells. The experiment was repeated three times from different cell passages, with ≥15 cells for each condition.
Colocalization of P2X7R and CTX-B.
Alexa Flour 555-CTX-B (5 μg/ml) was added to MC3T3-E1 cells grown on coverslips, which were incubated on ice for 20 min. Subsequently, the cells were washed with PBS and fixed in 4% paraformaldehyde for 20 min at 4°C. FITC-conjugated rabbit anti-P2X7R antibody was used to target the extracellular domain of the receptor after the fixed cells were blocked with 3% BSA. The immunostained cells were mounted on a slide using Prolong Gold and imaged using the Zeiss ELYRA PS.1 superresolution microscope with the Plan-Apochromat ×63 oil-immersion objective (1.4 numerical aperture). Structural illumination data were acquired with five rotations and three phases to obtain improved resolution and quantification. The excitation lasers were 488 and 561 nm. To minimize artifacts, channels were aligned with an affine channel alignment from a multicolor bead calibration, auto noise-filtered, and displayed using a baseline cut for accurate representation for colocalization analysis. Mander's correlation coefficient was measured by setting an appropriate threshold for each channel using the Zen 2011 software. The values describe the total amount of P2X7R colocalized with the CTX-B over the entire CTX-B-bound region (8). Mander's correlation coefficient was measured for 12 randomly selected individual cells over 2 different experiments and averaged.
Mineralization of CAV1 KD cells with BzATP treatment.
CAV1 KD cells were seeded in a 12-well plate at 2.5 × 103 cells/cm2 and grown to confluency; then the cell culture medium was supplemented with 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate. The cells were treated with the P2X7R agonist BzATP (150 μM), and after 24 h fresh medium was added. The same process was carried out for 21 days, with BzATP addition on alternative days. The control cells with the scrambled shRNA were subjected to the same treatment. The extent of mineralization was measured using von Kossa staining or Alizarin red staining.
von Kossa staining.
After 21 days in culture, the cells were washed with cold PBS and fixed in 4% paraformaldehyde for 20 min at 4°C. The cells were rinsed with distilled water, and 5% aqueous silver nitrate was added. The samples were exposed to UV light for 30 min, and the excess silver nitrate was washed off with water. The regions of mineral deposits were seen as dark nodules on the plate. Digital images of the wells were taken, and the extent of mineralization was quantified using ImageJ software. The intensity of the control untreated cells was set as the threshold, and comparisons were made with the suppression of CAV1 and with BzATP treatment in CAV1 KD cells. Data were normalized to the extent of mineralization in the control untreated cells and expressed as fold change.
Alizarin red staining.
After 21 days in culture, the cells were washed gently with PBS, fixed in 100% ethanol for 20 min, and air-dried. Alizarin red stain (Lifeline Cell Technology, Fredrick, MD) was added to the samples and washed away with running water after 15 min. Images encompassing different regions of the well were taken randomly using the Nikon SMZ 1500 light microscope and quantified using ImageJ.
Statistical analysis.
Densitometric analysis was carried out to quantify the extent of CAV1 translocation from the buoyant fractions to the denser fractions using ImageJ software. The samples were normalized to the protein concentration of the lysate. Mean peak [Ca2+]i response was expressed as percent increase in intensity over baseline intensity levels. Experiments were repeated at least three times with cells from three different cell passages, and values are means ± SE. Significance of all experiments was considered by one-way analysis of variance, and significance of multiple comparisons was determined using the Tukey-Kramer post hoc test.
RESULTS
P2X7R activation induces CAV1 endocytosis.
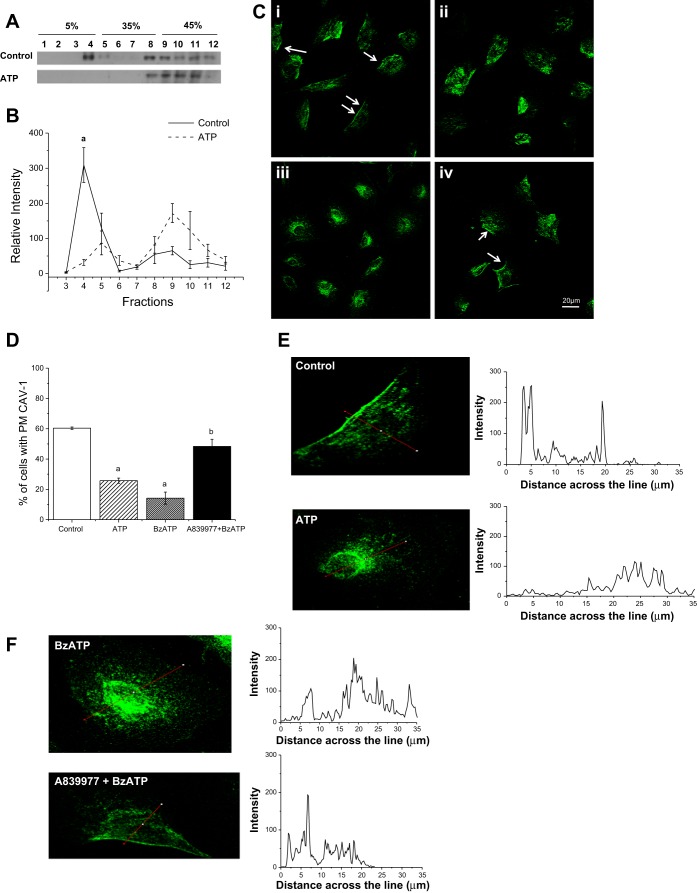
To determine if purinergic signaling can stimulate caveolar endocytosis, MC3T3-E1 cells were treated with 250 μM ATP and subjected to sucrose gradient fractionation. Because of the high concentration of cholesterol and sphingolipids in the caveolae, CAV1 is found in the less-dense fractions of the gradient when the sample is subjected to ultracentrifugation. However, when CAV1 translocates from the membrane microdomains, it loses the buoyant characteristic associated with the lipid microdomains and is found in the denser fractions with other cytosolic components (21). Figure 1A shows that, in control conditions, CAV1 was mostly found in fractions 3–6 of the gradient, equivalent to the 5%-35% gradient interface. However, when the cells were stimulated with 250 μM ATP for 10 min at 37°C, most of the CAV1 was found in the denser fractions (fractions 9–12). Normalized densitometric analysis of three separate experiments shows a significant reduction in CAV1 in lipid microdomain fractions (Fig. 1B).
Fig. 1.
Activation of the purinergic receptor P2X7R induces CAV1 endocytosis. A: MC3T3-E1 cells were subjected to sucrose gradient centrifugation, and 1-ml fractions were collected from the top of the gradient and immunoblotted for caveolin 1 (CAV1). Addition of 250 μM ATP caused a translocation of CAV1 from the lipid fractions (fractions 3–6) to the denser cytosolic fractions (fractions 9–12), indicating loss of CAV1 from the plasma membrane microdomains. B: quantification of CAV1 translocation with ATP treatment shows a significant loss of CAV1 from the buoyant lipid microdomain fractions (fractions 3–6). aP < 0.01. C: CAV1 is present on the plasma membrane of MC3T3-E1 cells under control conditions (i, arrows), but the staining pattern was strongly reduced when the cells were stimulated for 10 min with 250 μM ATP (ii) or 150 μM 2′(3′)-O-(4-benzoylbenzoyl)ATP (BzATP, iii). CAV1 remained on the plasma membrane when the cells were pretreated with 500 nM A-839977 before addition of 150 μM BzATP (iv). D: percentage of control and ATP-, BzATP-, and A-839977 + BzATP-treated cells in which CAV1 is present on the plasma membrane (PM). There was a significant reduction in the number of cells with CAV1 on the plasma membrane within 10 min of ATP or BzATP treatment and a significant difference between BzATP-treated cells and cells pretreated with A-839977. aP < 0.05 vs. control; bP < 0.05 vs. BzATP (by Tukey-Kramer test). E and F: line profile showing intensity of CAV1 staining across the cell, as indicated by the red line across the cell. In control cells, intensity peaks on the membrane region and tapers down toward the inside of the cell. When the cell is treated with ATP or BzATP, the intensity profile is distributed across the cytoplasm of the cell, and the profile of A-839977 + BzATP is similar to the control.
To test our hypothesis that CAV1 is endocytosed due to P2X7R activation, MC3T3-E1 cells were treated with ATP, BzATP, or BzATP after pretreatment with the specific P2X7R inhibitor A-839977 for 10 min. Changes in CAV1 localization were determined using immunocytochemistry. Under control conditions, CAV1 was localized to the plasma membrane (arrows in Fig. 1C, i); however, when the cells were treated with 250 μM ATP (Fig. 1C, ii) or 150 μM BzATP (Fig. 1C, iii), the characteristic membrane CAV1 staining was significantly reduced. Treatment with P2X7R agonists resulted in a diffuse and cytosolic staining pattern. Finally, this change in CAV1 localization in response to purinergic stimulation was significantly attenuated when the cells were pretreated with 500 nM A-839977 (Fig. 1C, iv), which blocks BzATP-induced Ca2+ influx (18). Quantification of the images under control conditions shows characteristic CAV1 staining on the plasma membrane in ∼60% of cells that was significantly reduced with ATP or BzATP (P < 0.05). However, BzATP addition after pretreatment with A-839977 had no significant effect on the percentage of cells with plasma membrane CAV1 compared with control cells (Fig. 1D). The intensity profile of CAV1 immunostaining created using ImageJ software highlights the intensity of the stain on the plasma membrane in control and A-83997 + BzATP samples, whereas the ATP- and BzATP-treated samples show cytoplasmic staining (Fig. 1, E and F).
A subpopulation of P2X7R is present in caveolae of osteoblasts.
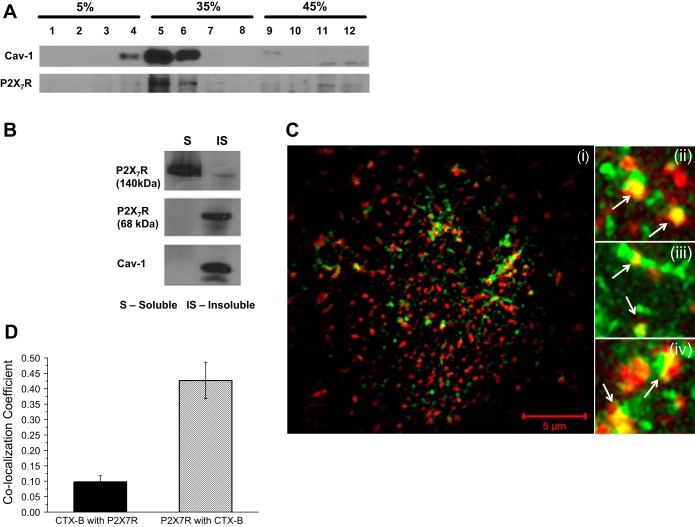
We hypothesize that P2X7R are scaffolded to caveolae to control downstream signaling. If this is true, then P2X7R and CAV1 should be observed in the same fractions in the sucrose gradient. Figure 2A shows a Western blot of sucrose gradient fractions in control MC3T3-E1 cells. As shown in Fig. 1A, CAV1 is found in the less-dense fractions (fractions 3–6) of the gradient. Stripping and reprobing the blot for P2X7R show that the P2X7R and CAV1 are found in similar fractions, suggesting that at least a subset of these purinergic receptors are present in the caveolae.
Fig. 2.
A subpopulation of P2X7R is present in caveolae of osteoblasts. A: P2X7R cofractionated with CAV1 in cells subjected to sucrose gradient centrifugation. One-milliliter fractions were collected from the top of the gradient and an equal volume of each fraction was immunoblotted for P2X7R and CAV1. B: representative Western blot showing P2X7R, along with CAV1, in the Triton X-100-insoluble (IS) fractions. C: superresolution structured illumination microscopy photomicrograph (i) illustrates colocalization of P2X7R (green) with cholera toxin B (CTX-B, red), seen as yellow regions. Magnified images (ii–iv) show subpopulation of P2X7R in caveolae (arrows). D: colocalization coefficients from the superresolution structured illumination microscopy images after appropriate threshold values are set show Mander's correlation coefficient of 0.45 for the P2X7R with CTX-B. This indicates that up to 45% of P2X7R in a cell is present in the CTX-B-enriched caveolae of MC3T3-E1 cells.
To support the fractionation data, we used Triton X-100 extraction to show that the P2X7R is present in the caveolar microdomains of osteoblasts. Based on the observation that caveolae are resistant to solubilization by detergents due to the elevated cholesterol and sphingolipid concentrations (25), we used 1% Triton X-100 to isolate Triton-insoluble and -soluble fractions from the lysate of MC3T3-E1 cells. Figure 2B shows that CAV1 and P2X7R were present in the insoluble fraction. However, the P2X7R was also found in the soluble fractions, suggesting that there could be two different pools of P2X7R with different functions associated with these different fractions, as reported previously (12).
The ganglioside GM1 has been well studied as a cell surface receptor for cholera toxin and has been shown to be concentrated in caveolae (32). To ascertain if the P2X7R colocalized with caveolae, we used CTX-B binding to GM1, combined with staining for P2X7R, to determine the colocalization of caveolae and P2X7R with superresolution structured illumination microscopy. MC3T3-E1 cells were treated with Alexa Fluor 555-CTX-B (red). P2X7R were found to be colocalized with the CTX-B and are seen as yellow spots (arrows in Fig. 2C, ii–iv). We determined the Mander's correlation coefficients and found that ∼45% of the total P2X7R colocalized with the CTX-B-stained caveolae in MC3T3-E1 cells (Fig. 2D). These data suggest that a subpopulation of P2X7R is present in the caveolae of osteoblasts.
Suppression of CAV1 enhanced P2X7R-mediated [Ca2+]i signaling.
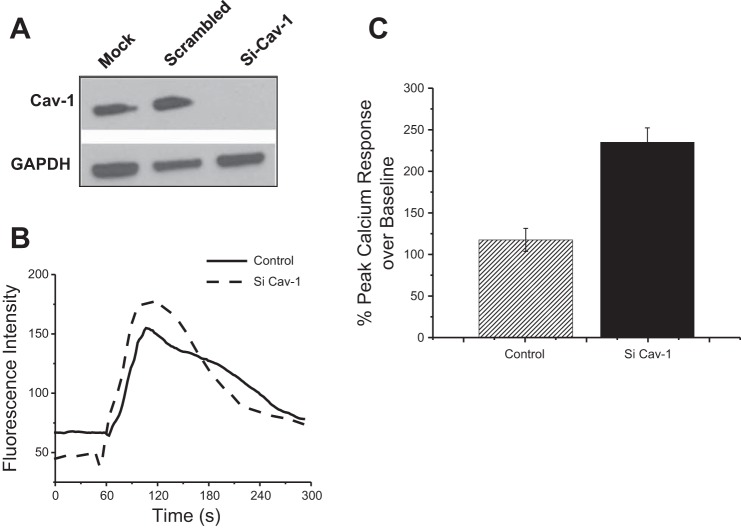
Because caveolae and P2X7R colocalized and CAV1 was internalized with activation of the P2X7R, we used siRNA strategy to determine the effects of CAV1 on P2X7R signaling. Western blotting demonstrates a 90% reduction in CAV1 protein expression in cells treated with siRNA compared with the GAPDH control (Fig. 3A). Treatment with scrambled siRNA sequence or the transfection reagent alone showed no change in CAV1 protein levels.
Fig. 3.
Suppression of CAV1 enhanced P2X7R-mediated intracellular Ca2+ ([Ca2+]i) signaling. A: Western blot showing suppression of CAV1 in MC3T3-E1 cells treated with siRNA (siCAV1). B: representative [Ca2+]i trace showing change in fluorescence intensity with 150 μM BzATP treatment over time. Fluo 4 was used to measure the change in [Ca2+]i. C: mean fold change in peak Ca2+ response to BzATP in CAV1 knockdown (siCAV1) cells showed a significant increase compared with control (P < 0.05).
The P2X7R is a ligand-gated ion channel, and activation of the receptor by BzATP leads to an influx of Ca2+ from the extracellular milieu. Addition of 150 μM BzATP produced a significantly (P < 0.05) higher Ca2+ response in MC3T3-E1 cells without CAV1 compared with the control, suggesting that CAV1 regulates P2X7R signaling (Fig. 3, B and C).
Mineralization due to P2X7R activation is amplified in CAV1 KD cells.
To determine if the CAV1 knockdown that led to increased [Ca2+]i signaling translated into a functional response in osteoblasts, we examined changes in mineralization of cultured MC3T3-E1 cells in response to BzATP with and without suppression of CAV1. Stable CAV1 KD cells were generated using shRNA. Clones selected by treatment with 5 μg/ml puromycin displayed a 70% knockdown in CAV1 protein expression (Fig. 4A).
Fig. 4.
Mineralization due to P2X7R activation is amplified in CAV1 KD cells. A: immunoblot showing extent of CAV1 suppression in stable MC3T3-E1 CAV1 KD cells generated using shRNA. B: mineralization nodules observed after von Kossa staining on MC3T3-E1 cells after 21 days. Addition of 150 μM BzATP clearly increased mineralization in CAV1 KD cells, as seen by the nodules. C: extent of mineralization shows a significant increase CAV1 KD cells with BzATP treatment. *P < 0.001. D: Alizarin red staining shows a strong increase in mineralization in CAV1 KD cells treated with BzATP compared with untreated (vehicle) cells. E: extent of mineralization was quantified using ImageJ. aP < 0.05 vs. control.
With von Kossa staining as a measure of mineralization, control and CAV1 KD MC3T3-E1 cells were differentiated with ascorbic acid and β-glycerophosphate for 21 days. Addition of BzATP (150 μM) every other day significantly increased mineralization of the CAV1 KD osteoblasts compared with control cells treated with BzATP (Fig. 4B). The extent of mineralization was quantified by ImageJ software. Differentiated, but untreated, MC3T3-E1 cells were used as control. Mineralization was significantly increased in CAV1 KD cells compared with all other groups (Fig. 4C; P < 0.001). Although there was a slight increase in mineralization with addition of BzATP in control cells, these changes were not significant.
To confirm the mineralization observed by von Kossa staining, Alizarin red staining was also carried out. These results complemented the results from von Kossa staining (Fig. 4D). Upon quantification, it was determined that mineral deposits significantly increased when CAV1 KD cells were treated with BzATP (Fig. 4E).
DISCUSSION
Lipid microdomains are highly ordered, 50- to 300-nm structures on the plasma membrane that are created by close association of cholesterol and sphingolipids (41). These domains, commonly called lipid rafts, have emerged as important regulators of receptor signaling and membrane trafficking (2, 17). Caveolae, a subset of these lipid microdomains, are characterized by plasma membrane invaginations that are stabilized by the binding of caveolins to cholesterol (37). Recently, CAV1 knockout mice have been shown to exhibit a significant increase in bone mass and strength, suggesting that these domains play a regulatory role in skeletal maintenance (40). Because caveolae have been linked to regulation of cell signaling and endocytosis of receptors such as the epidermal growth factor receptor, platelet-derived growth factor receptor, insulin receptor, glutamate receptor, and secondary messengers (9, 10, 36), we postulated that caveolae may be important in regulating the accessibility of membrane proteins involved in bone formation. We previously showed that several proteins, such as the mechanosensitive cation channel, voltage-gated Ca2+ channel, and purinergic receptors, are critical for mechanotransduction and regulation of bone formation by osteoblasts (6, 23). Specifically, the P2X7R has a significant effect on skeletal phenotype (22) and load-induced bone formation and has been shown to associate with caveolae in some cell types (3, 16). Mechanotransduction requires a high degree of spatiotemporal synchronization of signals in response to mechanical loads, and caveolae would serve as ideal candidates to sequester several of the proteins required for this signaling cascade.
We show that CAV1 is internalized within 5–10 min following application of exogenous ATP or BzATP to MC3T3-E1 osteoblasts in vitro. ATP is an important autocrine/paracrine signaling molecule released by osteoblasts and osteocytes in response to mechanical stimulation in vitro (15). Several studies have reported that activation of the purinergic receptors by ATP is required for NF-κB translocation, ERK1/2 activation, Cox-2 expression, and PGE2 release, all major anabolic factors for bone formation (13, 14). Although translocation and redistribution of CAV1 have been reported with mechanical stretch in type II epithelial cells (48), our novel findings indicate that CAV1 is redistributed from the lipid microdomain fractions into the denser cytosolic fractions when MC3T3-E1 cells were stimulated with ATP. We postulate that this translocation is due to activation of a purinergic receptor, and our immunostaining data indicate a loss of CAV1 from the plasma membrane with P2X7R activation, suggesting that caveolae are endocytosed. Treatment with A-839977, a P2X7R antagonist, significantly reduced the BzATP-induced CAV1 endocytosis. These data suggest that endocytosis of the caveolae could remove the important signaling proteins from the plasma membrane required for downstream signaling upon P2X7R activation.
Since CAV1 is endocytosed upon purinergic stimulation in MC3T3-E1 cells, we went on to determine if caveolae scaffolds the P2X7R. Here we show that, upon isolation of the caveolae-enriched microdomains from the osteoblasts using a detergent-free fractionation technique, P2X7R and CAV1 were found in the same fractions. However, we found two distinct pools of P2X7R, as observed by the presence of the receptor in the Triton X-100-soluble and -insoluble fractions, similar to previous reports in other cell types (12). These data were also supported by the partial colocalization of P2X7R with Alexa Fluor 555-CTX-B, which specifically binds to GM1 ganglioside enriched in caveolae. Using superresolution microscopy with quantification of the correlation coefficients, we found that ∼45% of the total P2X7R was associated with the lipid microdomains in osteoblasts. Proteins can be differentially segregated into the lipid microdomains on the basis of the lipid modification attached to it, and the P2X7R has been reported to be palmitoylated, which would enable its association with caveolae (16). This is a crucial finding with respect to P2X7R signaling in osteoblasts, since prolonged activation of this receptor triggers apoptosis in most cells. However, previous studies have shown no caspase-3 activation in osteoblasts where the P2X7R was activated (23). One possible explanation for the presence of P2X7R in different domains of the cell could be that P2X7R activation in the caveolae could trigger different downstream effectors to enhance gene expression and eventual bone formation.
Although these receptors were found to be associated with caveolae, coimmunoprecipitation showed no evidence of direct physical interaction between CAV1 and P2X7R (data not shown). These results were not entirely surprising, because proteins can be present in the caveolae without direct interaction with CAV1. It should be noted that caveolae are 60- to 100-nm-wide signaling platforms that encompass numerous membrane receptors and downstream effectors. Many components of the actin cytoskeleton and microtubules are also known to interact with caveolae and regulate endocytosis (33). Thus these may act as intermediaries between the receptor and CAV1. Another interesting possibility is the interaction of P2X7R with noncaveolar CAV1. To investigate this scenario, the caveolar CAV1 has to be isolated using additional caveolar markers such as cavin-1 [also known as polymerase 1 and transcript release factor (PTRF)] (33). However, cofractionation of P2X7R with CAV1 in the sucrose gradient centrifugation indicates that the receptor is associated with the lipid microdomains.
Our findings suggest that CAV1 attenuates P2X7R-mediated signaling in osteoblasts and acts as a regulatory mechanism to prevent continual signaling. This idea is supported by the increased peak [Ca2+]i response to BzATP in the CAV1-suppressed cells. Our data would suggest that caveolae act as a critical modulator of P2X7R signaling. The entry of [Ca2+]i via the P2X7R can activate the downstream signaling proteins and induce translocation of NF-κB, which is required for Cox-2 gene expression (14). Using stable CAV1 KD clones, we determined that suppression of CAV1 resulted in an increase in the mineralization capacity of osteoblasts in response to BzATP. This approach was used because of the transient nature of siRNA-induced protein suppression. Although a role for P2X7R and CAV1 in mineralization by osteoblasts separately has been established (31, 40), our results suggest a combined effect of CAV1 suppression- and P2X7R activation-induced mineralization. Data obtained by von Kossa staining provide strong evidence that the absence of CAV1 and, consequently, caveolae enhanced mineralization with BzATP up to threefold compared with untreated control.
The increase in signaling and the eventual functional response to P2X7R support a earlier report of higher bone mass and strength in CAV1−/− mice (40). The role of caveolae in cell signaling is extremely diverse, and a recent review describes two possible mechanisms for the signaling changes due to the loss of CAV1 in cells: 1) a direct loss in the interaction between membrane signaling protein and CAV1 or 2) indirect effects due to loss of compartmentalization of downstream signaling molecules (33). Since we did not find a direct interaction between P2X7R and CAV1, we postulate that the enhanced P2X7R signaling in the absence of CAV1 could be due to difference in localization of P2X7R on the plasma membrane. Agarwal et al. (1) observed similar changes in the functional response of a cell based on the localization of receptors in caveolae in adult cardiac myocytes. They found that the differences in the distribution of the β1-adrenegic receptor (β1-AR) and the E-type prostaglandin receptors on the plasma membrane led to different functional responses, although activation of both of these receptors led to an increase in cAMP production. They also found a differential response due to the distribution of the β1-AR in caveolar and noncaveolar fractions, whereas E-type prostaglandin receptors were found in the noncaveolar fractions.
Our data suggest that the presence of the P2X7R in caveolae is crucial for regulation of downstream signaling in osteoblasts. P2X7R activation triggers caveolar endocytosis and could be the potential mechanism of P2X7R signaling regulation. Endocytosis of caveolae can alter the availability of the proteins required for a signaling pathway and change the cellular response upon stimulation of P2X7R. We predict that the loss of caveolae from the plasma membrane alters the availability of the various proteins associated with the P2X7R signaling pathway on the plasma membrane. Numerous proteins, such as integrins, fibronectin, albumin, epidermal growth factor receptor, transforming growth factor-β receptor, insulin receptor, metabotropic glutamate receptor, occludin, and dopamine receptor, have been reported to be endocytosed via the caveolae (36). Our study describes a new mechanism of regulation of the P2X7R in osteoblasts, although further work is needed to determine the other proteins that associate with caveolae and P2X7R in osteoblasts.
In summary, this study demonstrates that P2X7R exist in a subpopulation of caveolae and that activation of this receptor induces CAV1 endocytosis in osteoblasts. Our data also indicate that CAV1 attenuates the P2X7R-mediated signaling following activation of this receptor, suggesting that caveolae have a role in osteoblast function and bone mineralization. Given the significance of P2X7R in the response of osteoblasts and bone to mechanical load and the role of caveolae in mechanosensation in other cell types, we predict that this interaction could alter the response of osteoblasts to extended loading.
GRANTS
This work was funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants R01 AR-043222 and R01 AR-051901 (R. L. Duncan) and by the Delaware IDeA Network of Biomedical Research Excellence, with National Institute of General Medical Sciences Grant 8 P20 GM-103446-13.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
V.G., A.N., J.C., K.C., and R.L.D. conception and design of research; V.G. performed experiments; V.G. analyzed data; V.G. and R.L.D. interpreted results of experiments; V.G. and R.L.D. prepared figures; V.G. and R.L.D. drafted manuscript; V.G., A.N., J.C., K.C., and R.L.D. edited and revised manuscript; V.G., A.N., J.C., K.C., and R.L.D. approved final version of manuscript.
REFERENCES
- 1.Agarwal SR, MacDougall DA, Tyser R, Pugh SD, Calaghan SC, Harvey RD. Effects of cholesterol depletion on compartmentalized cAMP responses in adult cardiac myocytes. J Mol Cell Cardiol 50: 500–509, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso MA, Millan J. The role of lipid rafts in signalling and membrane trafficking in T lymphocytes. J Cell Sci 114: 3957–3965, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Barth K, Weinhold K, Guenther A, Young MT, Schnittler H, Kasper M. Caveolin-1 influences P2X7 receptor expression and localization in mouse lung alveolar epithelial cells. FEBS J 274: 3021–3033, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bowler WB, Buckley KA, Gartland A, Hipskind RA, Bilbe G, Gallagher JA. Extracellular nucleotide signaling: a mechanism for integrating local and systemic responses in the activation of bone remodeling. Bone 28: 507–512, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293: 2449–2452, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Duncan R, Misler S. Voltage-activated and stretch-activated Ba2+ conducting channels in an osteoblast-like cell line (UMR 106). FEBS Lett 251: 17–21, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 57: 344–358, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol 300: C723–C742, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagerholm S, Ortegren U, Karlsson M, Ruishalme I, Stralfors P. Rapid insulin-dependent endocytosis of the insulin receptor by caveolae in primary adipocytes. PLos One 4: e5985, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francesconi A, Kumari R, Zukin RS. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci 29: 3590–3602, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H Jr, Kneitz B, Edelmann W, Lisanti MP. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem 276: 21425–21433, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Marcos M, Perez-Andres E, Tandel S, Fontanils U, Kumps A, Kabre E, Gomez-Munoz A, Marino A, Dehaye JP, Pochet S. Coupling of two pools of P2X7 receptors to distinct intracellular signaling pathways in rat submandibular gland. J Lipid Res 47: 705–714, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res 20: 41–49, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genetos DC, Karin NJ, Geist DJ, Donahue HJ, Duncan RL. Purinergic signaling is required for fluid shear stress-induced NF-κB translocation in osteoblasts. Exp Cell Res 317: 737–744, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol 212: 207–214, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonnord P, Delarasse C, Auger R, Benihoud K, Prigent M, Cuif MH, Lamaze C, Kanellopoulos JM. Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J 23: 795–805, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Gumbleton M, Abulrob AG, Campbell L. Caveolae: an alternative membrane transport compartment. Pharm Res 17: 1035–1048, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Honore P, Donnelly-Roberts D, Namovic M, Zhong C, Wade C, Chandran P, Zhu C, Carroll W, Perez-Medrano A, Iwakura Y, Jarvis MF. The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1αβ knockout mice. Behav Brain Res 204: 77–81, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol 295: C752–C760, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorgensen NR, Geist ST, Civitelli R, Steinberg TH. ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J Cell Biol 139: 497–506, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kandror KV, Stephens JM, Pilch PF. Expression and compartmentalization of caveolin in adipose cells: coordinate regulation with and structural segregation from GLUT4. J Cell Biol 129: 999–1006, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, Gabel CA, Jee WS, Dixon SJ, Sims SM, Thompson DD. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol 17: 1356–1367, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem 280: 42952–42959, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Lisanti MP, Tang Z, Scherer PE, Kubler E, Koleske AJ, Sargiacomo M. Caveolae, transmembrane signalling and cellular transformation. Mol Membr Biol 12: 121–124, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Lisanti MP, Tang ZL, Sargiacomo M. Caveolin forms a hetero-oligomeric protein complex that interacts with an apical GPI-linked protein: implications for the biogenesis of caveolae. J Cell Biol 123: 595–604, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu D, Genetos DC, Shao Y, Geist DJ, Li J, Ke HZ, Turner CH, Duncan RL. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca2+- and ATP-dependent in MC3T3-E1 osteoblasts. Bone 42: 644–652, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol 161: 673–677, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Orriss IR, Burnstock G, Arnett TR. Purinergic signalling and bone remodelling. Curr Opin Pharmacol 10: 322–330, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Palade GE. An electron microscope study of the mitochondrial structure. J Histochem Cytochem 1: 188–211, 1953. [DOI] [PubMed] [Google Scholar]
- 31.Panupinthu N, Rogers JT, Zhao L, Solano-Flores LP, Possmayer F, Sims SM, Dixon SJ. P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J Cell Biol 181: 859–871, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parton RG. Ultrastructural localization of gangliosides: GM1 is concentrated in caveolae. J Histochem Cytochem 42: 155–166, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol 14: 98–112, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol 8: 185–194, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol 48: 359–391, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic 3: 311–320, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res 44: 655–667, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Reimer WJ, Dixon SJ. Extracellular nucleotides elevate [Ca2+]i in rat osteoblastic cells by interaction with two receptor subtypes. Am J Physiol Cell Physiol 263: C1040–C1048, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Roger S, Pelegrin P, Surprenant A. Facilitation of P2X7 receptor currents and membrane blebbing via constitutive and dynamic calmodulin binding. J Neurosci 28: 6393–6401, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubin J, Schwartz Z, Boyan BD, Fan X, Case N, Sen B, Drab M, Smith D, Aleman M, Wong KL, Yao H, Jo H, Gross TS. Caveolin-1 knockout mice have increased bone size and stiffness. J Bone Miner Res 22: 1408–1418, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Simons K, Ikonen E. Functional rafts in cell membranes. Nature 387: 569–572, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem 276: 125–132, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Solomon KR, Adolphson LD, Wank DA, McHugh KP, Hauschka PV. Caveolae in human and murine osteoblasts. J Bone Miner Res 15: 2391–2401, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Solomon KR, Danciu TE, Adolphson LD, Hecht LE, Hauschka PV. Caveolin-enriched membrane signaling complexes in human and murine osteoblasts. J Bone Miner Res 15: 2380–2390, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem 271: 9690–9697, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272: 735–738, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem 271: 2255–2261, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Maciejewski BS, Drouillard D, Santos M, Hokenson MA, Hawwa RL, Huang Z, Sanchez-Esteban J. A role for caveolin-1 in mechanotransduction of fetal type II epithelial cells. Am J Physiol Lung Cell Mol Physiol 298: L775–L783, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol 1: 445–458, 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]