Abstract
Investigations of human myogenic responses typically use maneuvers that evoke robust changes in transmural pressure. Although this strategy has demonstrated peripheral myogenic responsiveness in the limbs, particularly in glabrous skin of the hand or foot, it has not considered the potential influence of the myogenic mechanism in beat-to-beat blood flow (BF) control during unprovoked rest. In the present study, we examined the interactions of spontaneous beat-to-beat mean arterial pressure (MAP; Finapres) with BF (Doppler ultrasound) supplying the forearm (brachial artery), lower leg (popliteal artery), and hand (ulnar artery) during 10 min of supine rest in healthy young men. Cross-correlation analyses revealed a negative association between MAP and BF, which was more prominent in the forearm than lower leg. The strongest correlation resulted when a −2-heart beat offset of MAP was applied (R = −0.53 ± 0.04 in the forearm and −0.23 ± 0.05 in the leg, P < 0.05), suggesting an ∼2-s delay from instances of high/low MAP to low/high BF. Negatively associated episodes (high MAP/low BF and low MAP/high BF) outnumbered positively associated data (P < 0.05). BF during low MAP values was greater than the steady-state average BF and vice versa. Wrist and ankle occlusion blunted the strength of correlations, homogenized the incidence of MAP and BF pairings, and reduced the magnitude of deviation from steady-state values. In contrast, these relationships were matched or accentuated for hand BF. Overall, these results suggest that myogenic responses are present and occur rapidly in human limbs during rest, overwhelm perfusion pressure gradient influences, and are primarily mediated by the distal limb circulation.
Keywords: brachial artery, popliteal artery, blood flow, vascular regulation
according to Poiseuille's law, a fundamental hydraulic theory for cardiovascular physiology, blood pressure (BP) is positively related with blood flow (BF). However, this relationship may not fully explain the complexity of in vivo physiology. Indeed, variations in compliance throughout the arterial tree, along with vasodilation and vasoconstriction elicited from sympathetic nerve activity, hormones, and/or local autoregulatory reactions, can also influence any association between BP and BF. Furthermore, vascular beds such as the brain and kidney exhibit near perfect autoregulation of BF over a wide range of arterial BP from 50–150 mmHg, i.e., little direct correlation between BP and BF (13, 17, 19). Thus, numerous factors can influence the relationship between BP and BF in vivo.
To our knowledge, the physiological relationship between BP and BF in human limbs during normal rest has never been systematically tested. This deficit primarily stems from limitations in acquiring and analyzing duplex ultrasonography on a beat-by-beat basis in human studies. We recently developed a data-acquisition system and custom software to allow such studies to be performed. To our initial surprise, examination of continuous beat-by-beat data collected at rest in young healthy men actually revealed a negative relationship between mean arterial pressure (MAP) and forearm BF, wherein transient MAP elevations were followed by a decrease rather than an increase in BF. This seemed to oppose the concepts of Poiseuille's law and our basic presumptions of hemodynamics in vivo. Furthermore, it suggested that a myogenic mechanism might be prominent and acting dynamically on a beat-by-beat basis during rest in humans. Thus, we designed a series of experiments to further explore this relationship and to determine contributing factors to the presence of a myogenic mechanism in resting limb BF control.
To better understand the extent to which the myogenic mechanism may be engaged and to examine potential limb dependency in BF control, we determined the relationship between resting BP and BF supplying the forearm (brachial artery) and lower leg (popliteal artery) of young men during periods of supine rest. The brachial and popliteal arteries were selected for between-limb comparisons because these two arteries are of similar size and exhibit relatively similar resting blood flows. Given that recent evidence suggests glabrous skin of the palm (or foot) exhibits accentuated myogenic responses compared with nonglabrous skin (3, 18), BF was measured during wrist and ankle occlusion to exclude glabrous skin. Additionally, to directly examine BF supplying glabrous skin, measurements were obtained at the ulnar artery, just proximal to the wrist. The data from these protocols were then thoroughly interrogated to reveal important aspects of the association between beat-by-beat arterial BP and limb BF, and the magnitude and prevalence of the negative relationships were calculated. Collectively, we used these methods to test the hypothesis that a myogenic mechanism is present in human limbs on a beat-to-beat basis and that it contributes to the control of resting BF.
METHODS
Eighteen healthy young men with a mean age of 26 ± 1 yr, height of 176 ± 1 cm, and weight of 78 ± 2 kg (means ± SE) were recruited for voluntary participation in this study. Exclusion criteria included hypertension (resting BP > 140/90 mmHg), obesity (body mass index > 30 kg/m2), smoking, and any known neurological, pulmonary, metabolic, or cardiovascular disease. Subjects received verbal and written explanation of the study objectives, measurement procedures, and risks and benefits associated with the investigation before they provided written informed consent for their participation. The University of Missouri Health Sciences Institutional Review Board approved all procedures, which were performed in accordance with the Declaration of Helsinki. On the experimental day, subjects reported to the laboratory ≥3 h after a meal and after ≥12 h of abstaining from alcohol, caffeine, and strenuous physical activity.
Experimental measurements.
Heart rate was monitored using a lead II electrocardiogram (Quinton Q710, Bothell, WA). Arterial BP was measured noninvasively by a servo-controlled finger photoplethysmograph (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands) placed on the middle finger of the left hand. The Finometer software corrects finger artery pressure to the brachial artery. Briefly, the Finometer uses a “return-to-flow” procedure that calibrates finger pressure to brachial artery pressure. Systolic pressure is determined twice by the upper arm cuff, and the finger pulse pressure is then corrected with the average of the two systolic pressure measures (6). The corrected finger pulse pressure is acquired for data analysis. As described in detail below, Finometer arterial pressure waveforms were directly acquired into LabView (while correcting for the 1-s processing delay) with simultaneous Doppler diameter and velocity. We then calculate MAP using the arithmetic mean (between R waves) of the Finometer BP waveform. Automated brachial artery sphygmomanometry (Welch Allyn, Skaneatles Falls, NY) was also used to verify absolute Finometer values. We acknowledge that our estimation of BP from the Finometer to other regional circulations (i.e., popliteal) without having local pressure measurements has limitations; however, our questions are mainly focused on changes in BP. Respiratory movements were monitored using a strain-gauge pneumograph placed in a stable position over the abdomen (Pneumotrace, UFI, Morro Bay, CA).
BF was measured from the brachial artery (supply to the forearm), popliteal artery (supply to the lower leg), and ulnar artery (supply to the hand) using high-resolution duplex Doppler ultrasonography (Logiq 7 or P5, GE Medical Systems, Milwaukee, WI). Standardized locations were used: the brachial artery was imaged ∼5 cm proximal to the antecubital fossa, the ulnar artery was imaged 2–3 cm proximal to the wrist, and the popliteal artery was imaged within or just distal to the popliteal fossa. Diameter and velocity were simultaneously and continuously measured in pulsed-wave mode using an insonation angle of 60°, and the depth, Doppler, and ultrasound frequencies were adjusted to optimize signal quality for each artery. The sample volume of each measurement contained the entire vessel lumen and did not extend beyond it. Consistent and stable imaging of the brachial and ulnar arteries was accomplished by securing the upper extremity at heart level using a vacuum pillow and fixing the transducer in place using a custom-designed clamp. Popliteal artery imaging was aided by stabilizing the transducer between the leg and the padding of the medical exam table. Skin markings were used to verify that transducer placement remained constant.
Experimental protocol.
Subjects were positioned comfortably supine in a quiet, dimly lit, climate-controlled room (23 ± 1°C). Each subject was instrumented for the simultaneous measurements of heart rate, BP, BF, and respiration and prepared for one of three protocols. Protocol 1 (n = 12) examined forearm BF during rest, from studies previously performed in our laboratory. Protocol 2 (n = 7, 5 subjects also performed protocol 1) compared forearm and lower leg BF with and without wrist or ankle cuff occlusion to exclude glabrous skin of the palm and foot, respectively. The order between subjects was randomized. Cuff occlusion was induced by rapid inflation (220 Torr) of an air bladder secured just proximal to the wrist or ankle. Cuff occlusion induces its own brief alteration to BF; thus, a 1-min delay (8) was incorporated before recording. Protocol 3 (n = 8, 3 subjects also performed protocol 1 and 1 subject also performed protocol 2) simultaneously compared BF of the whole forearm with the hand (greater proportion of glabrous skin). These protocols involved repeat recordings in some subjects (e.g., resting forearm BF). This was performed to allow simultaneous collection of data for all protocols with the exception of cuff occlusion protocols, where data were acquired with and without cuff inflation in the same limb. Repeated data in a subject were reproducible, although only within-session data were used for analysis and primary comparisons in each protocol. For all protocols, experimental variables were recorded for 10 min while the subject lay resting quietly and awake.
Data and statistical analyses.
As previously described in detail (4, 5, 14, 16), our laboratory has developed a custom LabView acquisition program to acquire Doppler ultrasound images and velocity signals (as 30-Hz video output) simultaneously with ECG, Finometer-derived BP waveforms, and up to two additional variables of interest. Importantly, data acquisition in this manner permits continuous measures of arterial diameter and mean blood velocity to be collected simultaneously with other variables of interest (e.g., BP). Thus, we can confidently investigate beat-to-beat changes of all measured variables in relationship to calculated beat-to-beat BF measures without realignment concerns when signals are acquired in two different acquisition systems. This system of acquiring data allowed for the systematic examination of the relationships between MAP and limb BF on a beat-to-beat basis in the present study. All group data are presented as means ± SEs.
Linear regressions.
Beat-to-beat MAP and BF were associated using cross correlations (Pearson correlations as a function of time) for each subject, similar to the methods of Lossius et al. (10). The full 10-min period of beat-to-beat BF values were related to the corresponding MAP values (614 ± 36 data pairs/subject). This was first performed in real time (i.e., within the same heart beat) and then as a function of a time lag in which BF values were related to MAP values offset by −10 to +10 heart beats. In other words, MAP was systematically shifted backward and forward in relation to its real time-associated BF value. As such, these analyses generated a total of 21 correlation coefficients for each subject. The TDIST Excel function (α = 0.05) determined the t-value for individual coefficients and was considered significant when exceeding its critical value. The full array of correlation coefficients was compared between experimental conditions and among MAP offsets using two-way ANOVAs and a Student-Newman-Keuls post hoc test to test the level of significance (P < 0.05) and interaction.
Bivariate histograms.
The prevalence of negatively related data was examined as a bivariate histogram. For each subject, all MAP and BF values were independently assigned a percent rank and binned into 10% classes. The relative number of occurrences that each MAP bin coincided with each BF bin was calculated for each subject and averaged over the group. This analysis was first performed for protocol 1, using a range of MAP offsets to determine which alignment produced the greatest trend in the bivariate histogram (which presumably identifies a regulated physiological effect). In agreement with linear regression analyses, the −2-heart beat MAP offset resulted in the greatest trend, whereas the other offsets produced flatter, more homogenous bivariate histograms. Thus, the −2-heart beat MAP offset was chosen to examine all experimental conditions. ANOVAs compared the mean occurrences among negatively (≥70% MAP with ≤30% BF or ≤30% MAP with ≥70% BF), intermediate (30–70% MAP with 30–70% BF), and positively related (≥70% MAP with ≥70% BF or ≤30% MAP with ≤30% BF) data occurrences.
Probability distributions.
The magnitude that instances of negatively related data deviated away from its averaged steady-state value was determined by comparing the distribution of BF associated with four quartiles of MAP. All MAP values were ranked and divided equally into four groups for quartile assignments. For each subject, BF values were expressed as percentages of their 10-min mean BF and segregated by their associated MAP quartile (using the established −2-heart beat MAP offset). To compare BF distribution across multiple subjects with wide-ranging BF variability, BF values were transformed into normalized probabilities (the likelihood of BF to occur at any percentage of the steady-state average BF, where 0% = no flow, 100% = steady-state mean BF, and 200% = 2 × mean BF). Normalized probabilities were grouped into 5% BF bins and plotted together, comparing BF distributions between the four quartiles of MAP. To summarize these probability distributions, a regression was constructed of the mean value of MAP and BF from each of the four quartiles. These regressions were expressed both as absolute changes and percent changes relative to each subject's steady-state mean values. ANOVAs were used to examine this summary data.
RESULTS
Pearson correlations.
The inherent variability in MAP and forearm BF (brachial artery) at rest from one subject is shown in Fig. 1A. Interestingly; the variability appeared to interact systematically. Time-aligned data for the resting 10-min period demonstrated a relatively weak, negative Pearson correlation that was markedly strengthened after all MAP values were offset by −2 heart beats (Fig. 1B). The full cross-correlation analysis for this subject (Fig. 1C) as well as the summary data for the group demonstrated that MAP-BF correlations were stronger and more negative when MAP was negatively offset (Fig. 1D). Moreover, group cross-correlation results demonstrated significant negative MAP-BF correlations using MAP offsets ranging from −10 to +4 heart beats (Fig. 2A), although it was the strongest at −2 heart beats. Wrist occlusion significantly attenuated the negative correlations, limiting the range for significant MAP offsets to −2 through 0 heart beats. The lower leg (popliteal artery) exhibited weaker correlations then the forearm (Fig. 2B) but demonstrated similar patterns of time lag and responses to occlusion. Hand BF (ulnar artery) results were equivalent to the simultaneously acquired forearm BF (Fig. 2C).
Fig. 1.
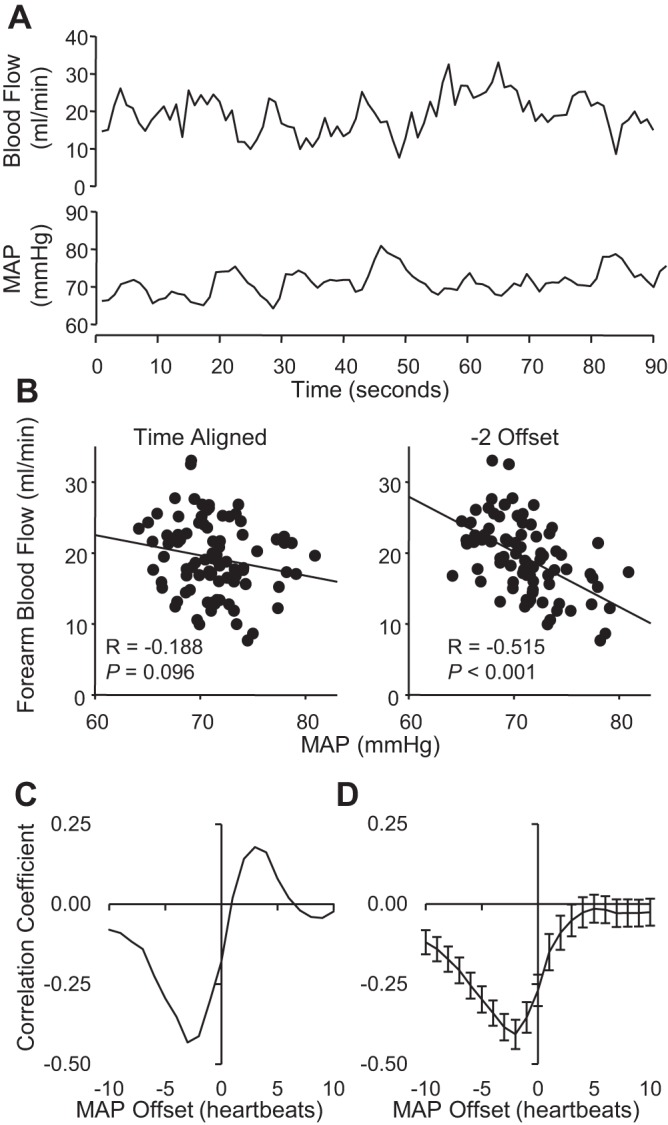
A: original beat-by-beat recording of forearm blood flow (BF) and mean arterial pressure (MAP) during 90 s of rest. B: MAP-BF correlations using time-aligned data (0 offset) and data incorporating a −2-heart beat offset. C: cross correlation of MAP and BF from one subject with MAP offsets ranging from −10 to +10 heart beats. D: summary data of cross correlations between MAP and forearm BF (n = 12, means ± SE).
Fig. 2.
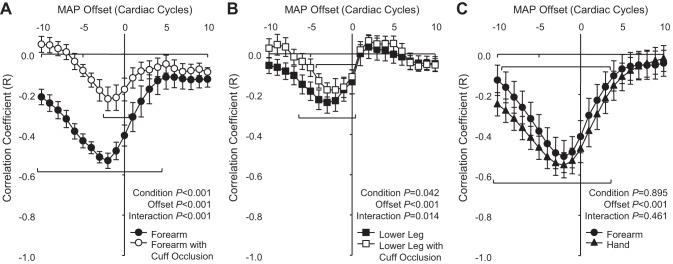
Summary data of cross correlations between MAP and BF comparing between limbs and between proximal and distal limb circulations. Protocol 2 (n = 7, means ± SE) compared cuff occlusion (open symbols) with control conditions (closed symbols) among the forearm (brachial artery; A) and lower leg (popliteal artery; B), whereas protocol 3 (n = 8, means ± SE) directly compared forearm with hand BF (ulnar artery; C).
Bivariate histograms.
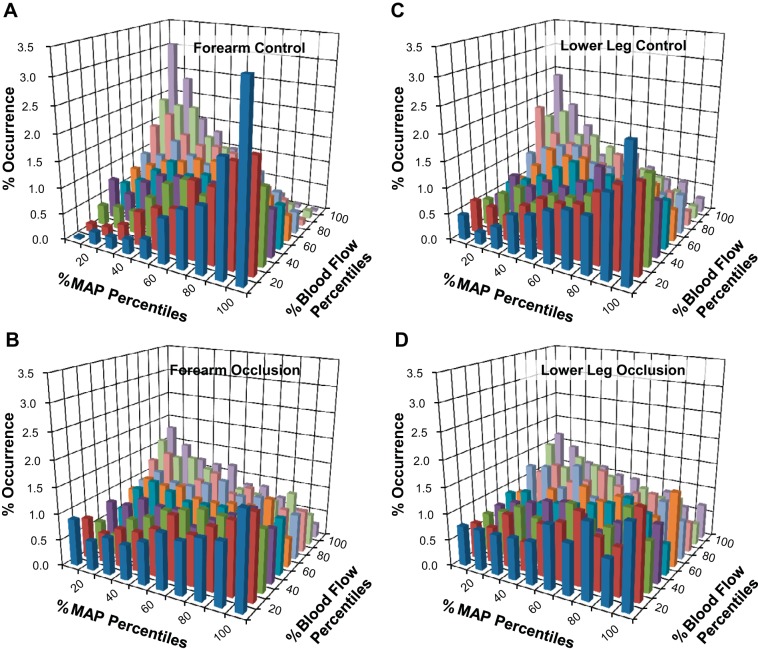
The percent occurrence for specific MAP-BF pairings during each experimental condition was demonstrated using bivariate histograms. The distribution of occurrences for the forearm depended on the MAP offset (time lag) used (Fig. 3A). As with cross correlations, the greatest trends resulted from a −2-heart beat MAP offset (Fig. 3B). When we examined resting forearm BF from protocol 1, negatively related data combinations (≥70% MAP with ≤30% BF or ≤30% MAP with ≥70% BF) occurred significantly more frequently than intermediate (30–70% MAP with 30–70% BF) or positively related data combinations (≥70% MAP with ≥70% BF or ≤30% MAP with ≤30% BF). Thus, to examine data relating to myogenic responses, all subsequent histograms used a −2-heart beat offset.
Fig. 3.
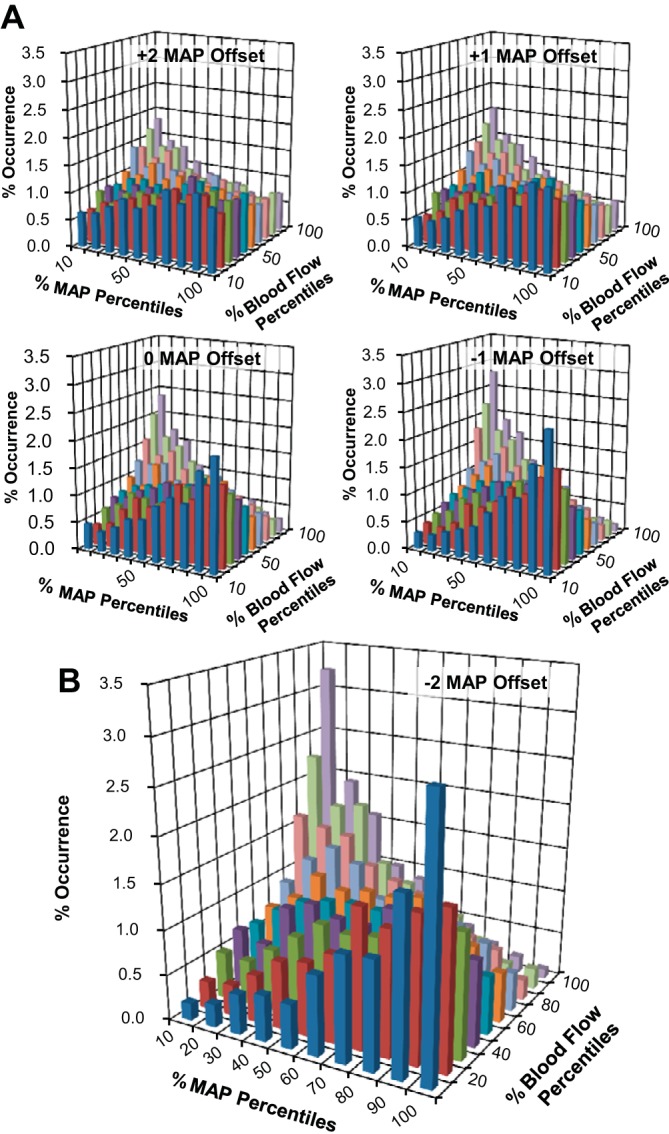
Bivariate histograms relating the relative occurrences of coincident data pairings between percentiles of MAP and forearm BF (protocol 1, n = 12). MAP offsets ranging from +2 to −1 heart beats are shown in A along with the −2 offset, which resulted in the greatest emerging trend (B).
Figure 4 shows bivariate histogram data for the forearm and lower leg during control conditions (Fig. 4, A and C) and with cuff occlusion (Fig. 4, B and D). Elimination of BF supplying the hand or foot significantly attenuated the trend for negatively related data pairings, producing a more uniform distribution compared with control conditions (Fig. 4, B and D). Also, similar to cross-correlation analyses, histogram results from direct measures of hand BF (ulnar artery) did not differ from the forearm (brachial artery) as a whole (Fig. 5).
Fig. 4.
Bivariate histograms from protocol 2 (n = 7) comparing the relative occurrences of coincident MAP and BF data supplying the forearm (A and B) and lower leg (C and D) during control conditions (A and C) and during cuff occlusion (B and D).
Fig. 5.
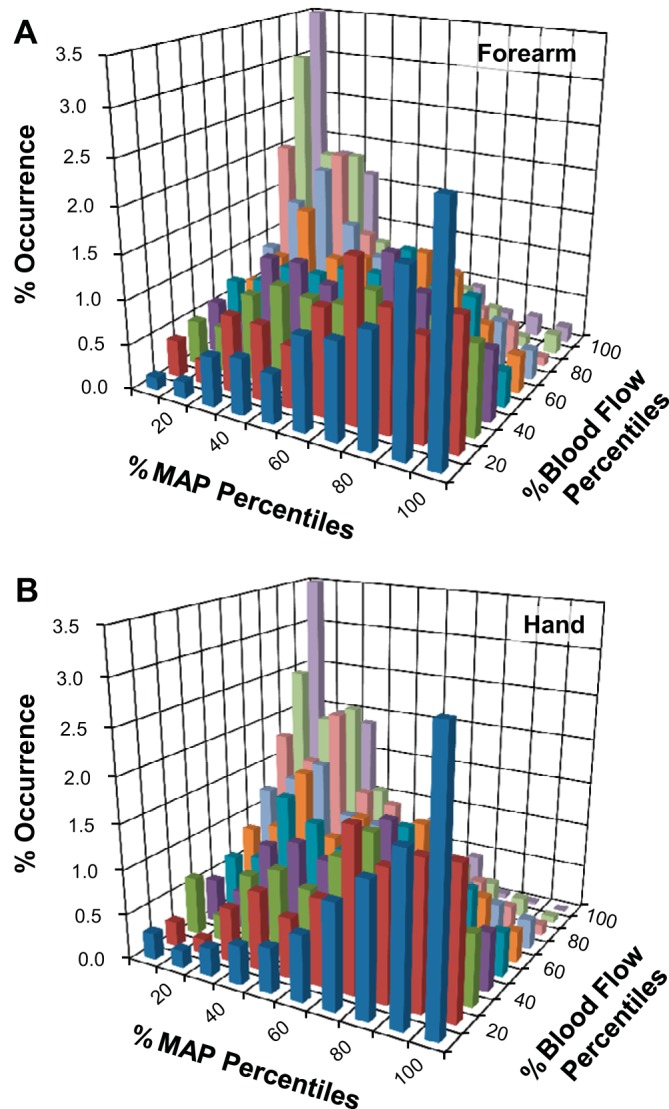
Bivariate histograms from protocol 3 (n = 8) comparing the relative occurrences of coincident MAP and BF data supplying the forearm (A) and hand (B).
Probability distributions.
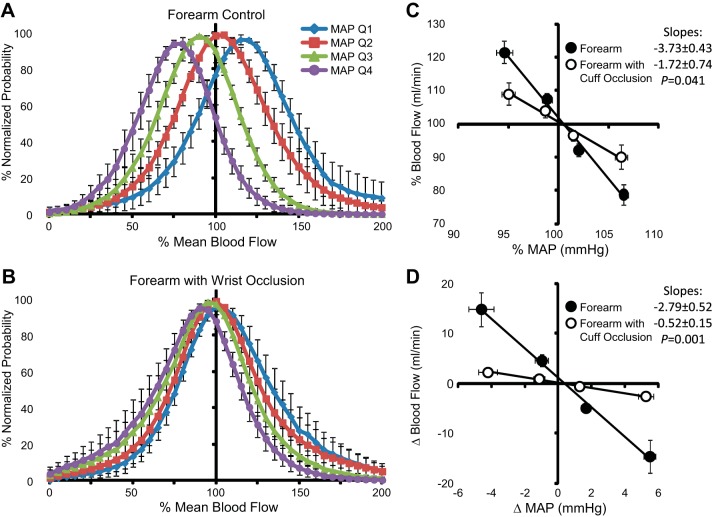
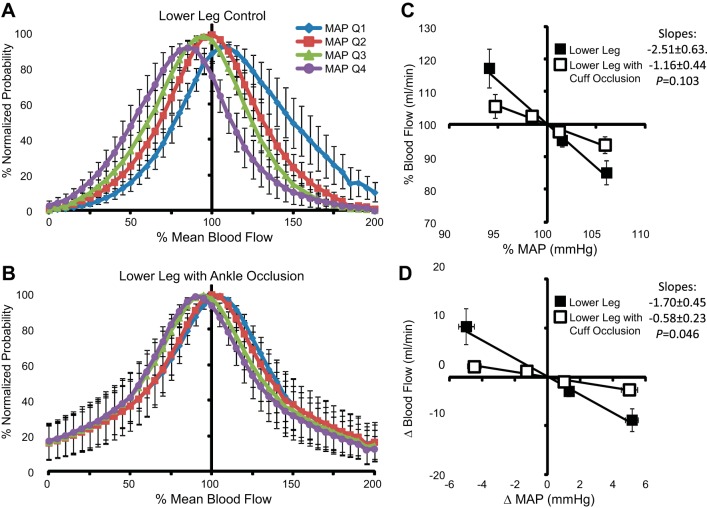
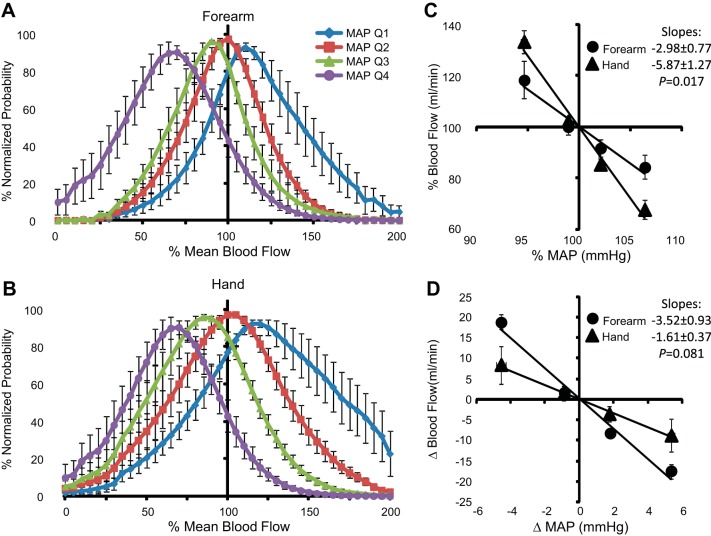
Forearm BF values associated with the lowest 25% of MAP (quartile 1) values were distributed above the overall mean (>100% mean blood flow), whereas BF values associated with the highest 25% of MAP (quartile 4) values were situated below the overall mean (Fig. 6A). In comparison, forearm BF during wrist occlusion exhibited minimal separation between the various MAP quartiles (Fig. 6B) and generally represented a normal distribution. Mean values of each quartile are summarized as deviations from the overall mean values of MAP and BF during each experimental condition (Fig. 6, C and D). The lowest MAP quartile decreased below mean MAP by −4.6 ± 0.7 mmHg (−5.4 ± 0.8%) and was paired with an increase in forearm BF of +14.8 ± 3.4 ml/min (+21.5 ± 3.3%). Reciprocal, similar magnitude changes resulted from spontaneous elevations in MAP. During wrist occlusion, changes in MAP were maintained, but the BF deviation was significantly attenuated relative to measures without occlusion, increasing only +2.3 ± 0.7 ml/min (+9.1 ± 3.4%) above the mean. Comparable results were observed for the lower leg before and during ankle occlusion (Fig. 7). The distributions of hand BF (ulnar artery) were directionally similar to the forearm (brachial artery) measures (Fig. 8). However, because of the large difference in mean values between forearm and hand BF, larger percent change deviations were observed among hand BF, whereas forearm BF exhibited larger absolute changes. To compare the three experimental conditions with the upper limbs, mean percent changes in BF to mean deviations in MAP were plotted together (Fig. 9). In response to a decrease in MAP, BF to the hand exhibited the greatest relative increase (+33.3 ± 7.4%) followed by BF to the forearm (+18.5 ± 4.3%). The smallest BF increase occurred when cuff occlusion was applied to the wrist to exclude glabrous skin of the palm from the forearm BF measures (+9.1 ± 3.4%).
Fig. 6.
A and B: normalized probability distributions of forearm BF among four MAP quartiles [quartiles 1–4 (Q1–Q4)] during control conditions (A) and wrist occlusion (B). C and D: mean values of MAP and BF among each MAP quartile relative to the overall mean expressed as percentages (C) and absolute changes (D) ± SE.
Fig. 7.
A and B: normalized probability distributions of lower leg BF among four MAP quartiles during control conditions (A) and ankle occlusion (B). C and D: mean values of MAP and BF among each MAP quartile relative to the overall mean expressed as percentages (C) and absolute changes (D) ± SE.
Fig. 8.
A and B: normalized probability distributions of forearm (A) and hand BF (B) among four MAP quartiles. C and D: mean values of MAP and BF among each MAP quartile relative to the overall mean expressed as percentages (C) and absolute changes (D) ± SE.
Fig. 9.
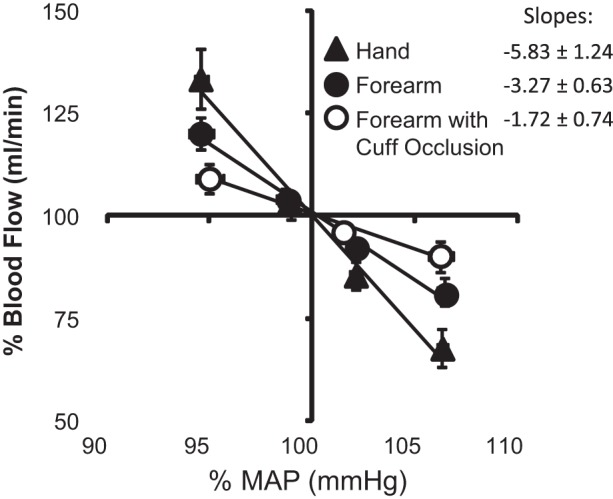
Summary data (means ± SE) comparing the hand, forearm, and forearm during wrist occlusion among each MAP quartile as a percentage of the overall mean MAP and BF value.
DISCUSSION
Hemodynamics based on Poiseuille's law predicts that regional BF is positively related with MAP due to the propulsive force of the perfusion pressure gradient. However, in an intact cardiovascular system, regulatory mechanisms can counteract these pressure-dependent effects and minimize the subsequent change in BF. The present examination was the first to assess the beat-to-beat BP and limb BF relationship in humans under resting unprovoked conditions, and it revealed novel and unexpected information about in vivo hemodynamics. Cross-correlation analyses revealed significantly negative correlations between MAP and BF supplying the forearm, hand, and lower leg. The strongest negative relationships for all regions occurred when MAP values were shifted 2 heart beats back in relationship to BF, suggestive of a ∼2-s delay from instances of high/low MAP to low/high BF. Bivariate histograms showed that instances of negatively associated data were the most abundant of any data combination. Probability distributions revealed the importance of these negative relationships by demonstrating the magnitude of deviation away from the overall steady-state values. Together, these observations strongly suggest that a myogenic mechanism is active in human limbs during rest, that it exhibits a rapid response time (most consistently ∼2 s), and that myogenic responses appear to dominate over passive pressure-dependent influences in establishing the overall relationship between resting MAP and BF.
The directional offset resulting in the greatest negative relationship between MAP and limb BF has important implications toward data interpretation. The strongest correlations appeared when a change in MAP preceded a change in BF, not vice versa. Many signals can induce a local vasoconstriction, which would then increase regional vascular resistance, decrease BF, and eventually contribute to an elevation in MAP. Importantly, these events would produce negative relationships wherein MAP changes follow BF. However, the application of multiple analyses revealed that MAP temporally leads BF, demonstrating that BP is the instigating variable of the interaction, which points to a myogenic mechanism.
Although some combination of myogenic, metabolic, and shear stress-mediated effects can contribute to BF autoregulation during rest, our results are consistent with a prevailing myogenic mechanism. Since metabolic demand is low during supine rest, the range of metabolic changes are presumably very small and its influence on blood flow is likely also small. Furthermore, potential metabolic-induced changes in BF are unlikely to be time bound to the spontaneous variations in BP. In regards to shear stress, flow-mediated responses in the microcirculation exhibit a time delay of tens of seconds (9, 15) serving to oppose vasoconstriction and thus, if anything, might contribute to a positive MAP-BF relationship. So, while we cannot definitively discount the contribution of other local autoregulatory mechanisms, we conclude that the myogenic response is the primary plausible explanatory mechanism for our observed relationships. Moreover, there is precedent for the concept that the myogenic response can be quite rapid in vivo, with renal afferent arterioles being the fastest responders (<0.5-s time constant) (2, 12). Although some studies have suggested skeletal muscle arterioles and skin may not be as fast, rapid myogenic responses (∼2 s) have been demonstrated in human limbs in response to ±50-Torr changes in forearm transmural pressure (11). The current data are the first documentation of rapid myogenic responses resulting from normal fluctuations in BP and BF in the unprovoked, resting state.
A potential source of these myogenic responses was investigated by eliminating glabrous skin BF using wrist/ankle occlusion or by directly measuring ulnar artery BF. Glabrous skin contains a high density of arteriovenous anastomoses, which are specialized vascular structures capable of selectively diverting blood from arterial beds directly to venous venules (7, 18). These structures provide an opportunity to bypass or incorporate additional capillaries during variations in MAP, buffering pressure-dependent flow outcomes. Recently, lower body negative pressure was used to reveal enhanced dynamic autoregulation in glabrous skin compared with nonglabrous skin (18). Taking advantage of these established regional variations in dynamic autoregulation and myogenic mechanisms, we compared the BF responses of the whole limb with the proximal limb and distal limb. Compared with the whole limb, elimination of hand and foot BF via cuff occlusion significantly attenuated the strength of negative correlations, the occurrence of negatively related data pairs, and the magnitude of deviations from the overall mean MAP and BF values. Myogenic patterns did remain apparent, although weakened suggesting a primary contribution of the distal limb circulation. In agreement, hand BF showed enhanced negative relationships or outcomes similar to whole limb responses (calculated as relative or absolute changes, respectively). Although obtained for different scientific purposes, Lossius and colleagues (10) showed similar findings from measurements in the distal limb. These results suggest that myogenic responses occur throughout the limb circulations of the arms and legs but that the fully realized response requires participation of the distal limb.
Perspectives
It is puzzling why the resting limb would frequently and robustly oppose pressure-dependent BF changes during rest. Without obvious threats to metabolism or mechanical strain, why should it be necessary to resist pressure-dependent flow? One prospective utility for a strong resting myogenic response would be to increase BF variability during rest. Indeed, for the same mean BF, increased flow variability associated with arteriolar vasomotion enhances tissue dialysis, interstitial fluid dynamics, and nutrient exchange across the capillary wall (1). Alternatively, for situations when MAP increases, an ability to offset Poiseuille-dependent BF may serve as an additional mechanism to regulate limb flow. Additional studies will be necessary to test these proposed purposes of a persistent myogenic response at rest as identified in the present set of experiments. Furthermore, examination of these relationships during sustained changes in BP will be important. Although our conclusions appear clear for the beat-to-beat regulation of blood flow that encompasses the inherent fluctuations in BP under resting conditions, this may not be the case when BP is elevated or lowered for a sustained period of time.
In conclusion, a potential role for arteriovenous anastomoses within the distal limb circulation is suggested to mediate a major proportion of beat-by-beat dynamic myogenic responses. These results are the first to demonstrate an active and dynamic role for myogenic responses in the regulation of beat-by-beat BF in human limbs during rest.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-093167 (to P. J. Fadel) and P01-HL-095486 (to M. J. Davis) and by American Heart Association Grant 14SDG20320006 (to J. Padilla).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.T.F., J.P., L.C.V., and P.J.F. conception and design of research; S.T.F., J.P., and S.W.H. performed experiments; S.T.F. analyzed data; S.T.F., J.P., L.C.V., M.J.D., and P.J.F. interpreted results of experiments; S.T.F. prepared figures; S.T.F. drafted manuscript; S.T.F., J.P., S.W.H., M.J.D., and P.J.F. edited and revised manuscript; S.T.F., J.P., L.C.V., S.W.H., M.J.D., and P.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
The time and effort expended by all volunteer subjects is greatly appreciated.
REFERENCES
- 1.Aalkjaer C, Boedtkjer D, Matchkov V. Vasomotion–what is currently thought? Acta Physiol (Oxf) 202: 253–269, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension 54: 393–398, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durand S, Zhang R, Cui J, Wilson TE, Crandall CG. Evidence of a myogenic response in vasomotor control of forearm and palm cutaneous microcirculations. J Appl Physiol 97: 535–539, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Fairfax ST, Holwerda SW, Credeur DP, Zuidema MY, Medley JH, Dyke PC 2nd, Wray DW, Davis MJ, Fadel PJ. The role of α-adrenergic receptors in mediating beat-by-beat sympathetic vascular transduction in the forearm of resting man. J Physiol 591: 3637–3649, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fairfax ST, Padilla J, Vianna LC, Holwerda SH, Davis MJ, Fadel PJ. Influence of spontaneously occurring bursts of muscle sympathetic nerve activity on conduit artery diameter. Am J Physiol Heart Circ Physiol 305: H867–H874, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gizdulich P, Imholz BP, van den Meiracker AH, Parati G, Wesseling KH. Finapres tracking of systolic pressure and baroreflex sensitivity improved by waveform filtering. J Hypertens 14: 243–250, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Hurley HJ Jr, Mescon H, Moretti G. The anatomy and histochemistry of the arteriovenous anastomosis in human digital skin. J Invest Dermatol 27: 133–145, 1956. [DOI] [PubMed] [Google Scholar]
- 8.Kerslake DM. The effect of the application of an arterial occlusion cuff to the wrist on the blood flow in the human forearm. J Physiol 108: 451–457, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol 259: H1063–H1070, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Lossius K, Eriksen M, Walloe L. Fluctuations in blood flow to acral skin in humans: connection with heart rate and blood pressure variability. J Physiol 460: 641–655, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lott ME, Herr MD, Sinoway LI. Effects of transmural pressure on brachial artery mean blood velocity dynamics in humans. J Appl Physiol 93: 2137–2146, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ Res 90: 1316–1324, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Navar LG. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol Renal Fluid Electrolyte Physiol 234: F357–F370, 1978. [DOI] [PubMed] [Google Scholar]
- 14.Padilla J, Simmons GH, Vianna LC, Davis MJ, Laughlin MH, Fadel PJ. Brachial artery vasodilatation during prolonged lower limb exercise: role of shear rate. Exp Physiol 96: 1019–1027, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pohl U, Herlan K, Huang A, Bassenge E. EDRF-mediated shear-induced dilation opposes myogenic vasoconstriction in small rabbit arteries. Am J Physiol Heart Circ Physiol 261: H2016–H2023, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH, Fadel PJ. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: role of thermoregulatory vasodilation. J Appl Physiol 110: 389–397, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke 26: 1014–1019, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Wilson TE, Zhang R, Levine BD, Crandall CG. Dynamic autoregulation of cutaneous circulation: differential control in glabrous versus nonglabrous skin. Am J Physiol Heart Circ Physiol 289: H385–H391, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 274: H233–H241, 1998. [DOI] [PubMed] [Google Scholar]