Abstract
Inflammation contributes significantly to cardiac dysfunction. Although the initial phase of inflammation is essential for repair and healing, excessive proinflammatory cytokines are detrimental to the heart. We found that adenine nucleotide translocator isoform-1 (ANT1) protein levels were significantly decreased in the inflamed heart of C57BL/6 mice following cecal ligation and puncture. To understand the molecular mechanisms involved, we performed small-interfering RNA-mediated knockdown of ANT1 and studied tumor necrosis factor-α (TNFα)-induced inflammatory responses in myocardium-derived H9c2 cells and cardiomyocytes. ANT1 knockdown significantly increased swollen mitochondria and mitochondrial reactive oxygen species, concomitant with increased TNFα-induced NF-κB reporter gene activity and interleukin-6 and TNFα expression. A mitochondrial-targeted antioxidant mito-TEMPO attenuated TNFα-induced mitochondrial reactive oxygen species, NF-κB reporter gene activity, and cytokine expression in ANT1 knockdown cells. Interestingly, TNFα or lipopolysaccharide (LPS) treatment significantly decreased ANT1 protein levels, suggesting a feed-forward regulation of proinflammatory cytokine expression activated by ANT1 downregulation. These data suggest that ANT1 downregulation contributes to cardiac inflammation post-cecal ligation and puncture. Preventing ANT1 downregulation could provide a novel molecular target to temper cardiac inflammation.
Keywords: adenine nucleotide translocator, cytokine, inflammation, sepsis
myocardial dysfunction is the predominant cause of death in severe septic patients. Approximately 60% of patients admitted to the intensive care units present cardiac dysfunction and, more importantly, with mortality rates up to 90% (6, 37, 51). Although the underlying mechanisms for sepsis-induced cardiac depression are complex and not well understood, recent studies demonstrate that proinflammatory cytokines play a crucial role (24, 29, 39). Inflammation is initially beneficial to coordinating defense and repair. However, excessive production of proinflammatory cytokines is detrimental to the heart (25).
In septic patients, TNFα is the prime mediator of the inflammatory response (47). It serves as an essential element of host defense. Moreover, it evokes the immune responses by inducing the production of a cascade of proinflammatory cytokines. Particularly, increased TNFα and IL-6 levels are associated with worse outcomes (17, 46, 50). Although originally TNFα and IL-6 were found to be secreted by activated neutrophils and macrophages, recent studies have demonstrated that cardiac cells produce these proinflammatory cytokines as well, suggesting that localized inflammatory cytokine plays a role in inflammation-induced cardiac dysfunction (20, 22).
We have recently demonstrated that ADP is protective to the opening of the mitochondrial permeability transition pore in the heart under pathological conditions, and adenine nucleotide translocator (ANT) is indispensable for its protection (45). ANT isoform 1 (ANT1) is the predominant ANT isoform in the heart. It drives the exchange of ADP/ATP between cytosol and mitochondria, using the electrochemical gradient (40, 41). Transgenic expression of ANT1 is protective to the heart (52–54). In contrast, ANT1 deficiency is associated with cardiac dysfunction (18, 27, 28, 32–34, 40, 48). Interestingly, recent studies have found that ANT1 is dysregulated in myocarditis and dilated cardiomyopathy (11–13). However, the mechanistic role of ANT1 in inflammation has not been elucidated.
In the present study, we investigated the role of ANT1 in TNFα-induced inflammatory response. Our data suggest that ANT1 downregulation is likely a key contributor to exacerbate cardiac inflammation at least partly through increasing inflammatory cytokine expression.
MATERIALS AND METHODS
Animals.
All animal experiments were conducted in accordance with experimental protocols approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University. Animals were maintained under pathogen-free condition (22 ± 0.5°C, 55% relative humidity, and 12-h:12-h light-dark cycle) at Thomas Jefferson University.
Antibodies.
Vascular cell adhesion molecule-1 was from Santa Cruz Biotechnology (Dallas, TX), voltage-dependent anion channel was from Cell Signaling (Dancers, MA); cyclophilin D (CypD) was from Abcam (Cambridge, MA), tubulin was from Sigma-Aldrich (St. Louis, MO), and ANT1 antibody was from Dr. Douglas C. Wallace of the University of Pennsylvania (18).
Reagents.
TNFα was from Roche Diagnostics (Indianapolis, MO), and ANT1-adenovirus was from Dr. Christopher Baines of the University of Missouri-Columbia (4). NF-κB luciferase construct and ANT1 small-interfering RNA (siRNA) were from Life Technologies (Grand Island, NY). Luciferase Reporter Assay System and β-galactosidase assay system were from Promega (Fitch, WI). mito-TEMPO (MTP) was from Santa Cruz Biotechnology.
Mouse cecal ligation and puncture model.
Male C57BL/6 mice of 8–12 wk old were used in the study. Mice were anesthetized with 1.5–2% isoflurane. The abdomens were shaved, and an ∼1-cm midline incision was made. The cecum, isolated and ligated below the ileocecal valve without causing bowel obstruction, was punctured with a 22-gauge needle and was then gently squeezed to ensure that the holes were patent. The cecum was placed back in the abdomen, and the incision was closed with sutures. The mice were then resuscitated with 1 ml of saline injected subcutaneously. Sham-operated controls were treated in an identical manner but without ligation and puncture. Survival was monitored every day for 72 h. Data were analyzed by log rank test using the statistical package R.
In vivo echocardiography.
Transthoracic two-dimensional echocardiography was performed by using echocardiographic imaging system (Vevo 2100, VisualSonic, Toronto, Canada) with a 40-MHz probe. Mice were anesthetized through the inhalation of isoflurane (∼1–2%). M-mode interrogation was performed in the parasternal short-axis view at the level of the greatest left ventricular end-diastolic dimension. Left ventricular wall thickness and internal dimensions were measured and used for calculation. Fraction shortening and ejection fraction values were exported from the echo program.
Cell culture, siRNA oligonucleotide treatment, and adenovirus infection.
Myocardial myoblast H9c2 cells were purchased from ATCC and cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum (Gibco, Grand Island, NY). Cells at passages 5–15 were used for experiments. Rat neonatal cardiomyocytes were isolated and cultured as previously described (7). To knock down ANT1, cells were treated with ANT1 siRNA. RNAiMAX (Life Technologies) was used according to the instructions of the manufacturer. Adenoviruses harboring mouse ANT1 were used to infect H9c2 cells as previously described (2).
Western blot analysis.
Western blots analysis (immunoblots) were performed as previously described (35, 36). The images were acquired and analyzed by Licor Odyssey system. Equal loading of protein was ensured by measuring tubulin expression.
Quantitative real-time PCR.
Qualitative real-time PCR was performed on iCycler iQ5 system using SYBR green (Bio-Rad). The primer sequences are as follows: TNFα, 5′-cccagaccctcacactcagat-3′ and 5′-ttgtcccttgaagagaacctg-3′; IL-6, 5′-tcaactccatctgcccttcag-3′ and 5′-aaggcaactggctggaagtct-3′; and 18S, 5′-tcaagaacgaaagtcggagg-3′ and 5′-ggacatctaagggcatcaca-3′.
Reporter gene assay.
Reporter gene and β-galactosidase activity assays were performed following the manufacturer's instructions. Briefly, cells cotransfected with reporter gene construct, together with β-galactosidase construct, were treated with TNFα (10 ng/ml) for 18 h and harvested 48 h after transfection. Luciferase activity was measured following the manufacturer's instructions. Data were normalized using β-galactosidase activity as the internal control.
Confocal and mitochondrial function assay.
Cells were loaded with MitoTracker Green, MitoSox Red, or tetramethylrhodamine ethyl ester following the manufacturer's instructions. Mitochondria images were acquired by Flouview (Olympus) using a ×60 oil objective and analyzed using National Institutes of Health ImageJ (v. 1.44). Oxygen consumption was measured using clark electrode as previously described (45). Respiration control ratio was determined by stages 3 and 4 oxygen consumption.
Statistical analysis.
Results represent at least three independent experiments if not mentioned specifically, and data are expressed as means ± SE. Where indicated, ANOVA was performed. One-way ANOVA was used for multiple group analysis, and paired Student's t-test was used for the comparison between two groups. P < 0.05 was considered significant in all experiments.
RESULTS
ANT1 is downregulated in the inflamed heart.
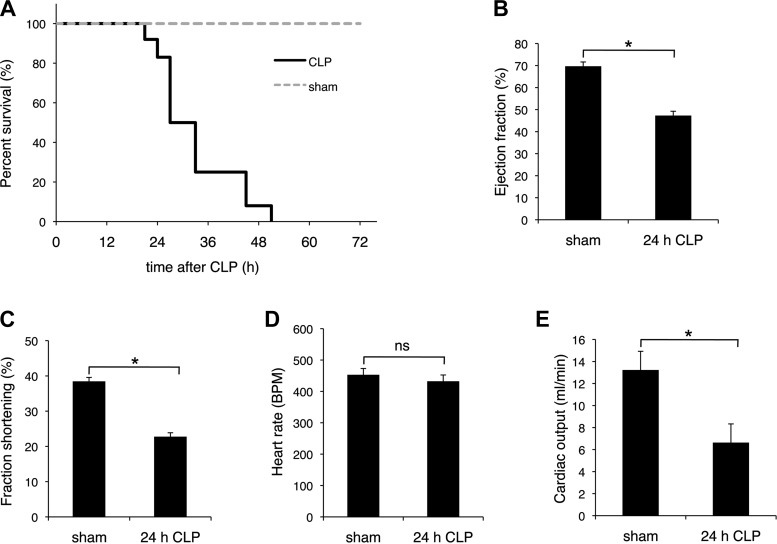
To understand the role for ANT1 in cardiac inflammation, we performed cecal ligation and puncture (CLP) in mice. Percent survival was significantly decreased after 24 h of CLP, likely because of CLP-induced organ failure (Fig. 1A). Therefore, we focused our studies on the initial 24-h post-CLP during which time inflammation was dominant. At 24 h, cardiac performance was significantly lower than that in sham-operated mice. The ejection fraction decreased from 66.1 ± 3.7 to 45.9 ± 18.5% (P < 0.05) and fraction shortening decreased from 35.6 ± 4.9% to 25.7 ± 16.9% (P < 0.05) as measured by echocardiography (Fig. 1, B and C). There was no significant change in heart rate at this 24 h time point (Fig. 1D). There was an ∼50% decrease in cardiac output (Fig. 1E).
Fig. 1.
Cecal ligation and puncture (CLP). C57BL/6 mice were subjected to CLP. A: survival curve. Mice were exposed to either CLP (solid line) or sham operation (dotted line) and monitored for 72 h postsurgery; n = 10 for each group. Ejection fraction (%; B), fractional shorting (%; C), heart rate [beats/min (bpm); D], and cardiac output (E) at 24 h postsurgery (24-h CLP) were analyzed by echocardiography. *P < 0.05 vs. sham; n = 4. NS, not statistically significant.
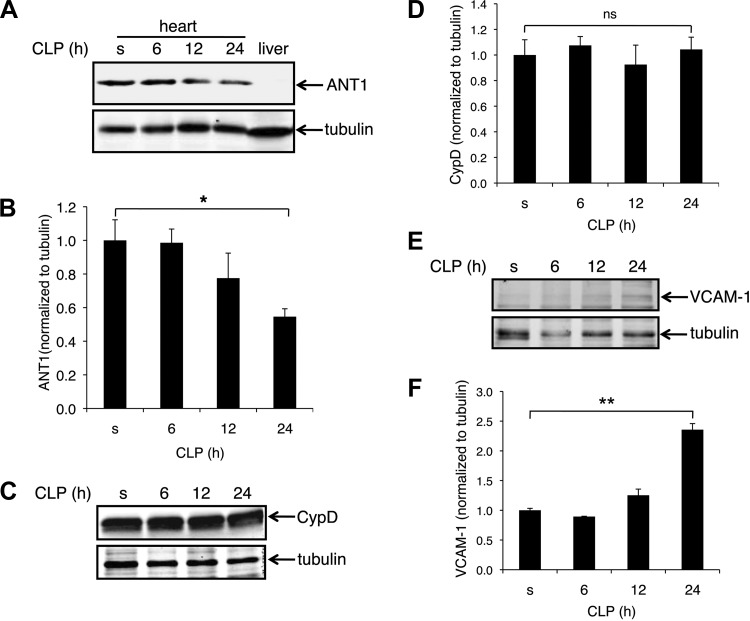
ANT1 protein levels were significantly decreased in the left ventricle of CLP mice at 24 h as assessed by Western blot (Fig. 2A). Protein extracts from liver were used as the control for antibody specificity (the ANT1 antibody detects only ANT1 in the heart, but not ANT2 in the liver) (Fig. 2A). In the heart, ANT1 decreased to 50% of sham at 24 h after CLP (Fig. 2B). In contrast, there was no significant difference in mitochondrial matrix protein CypD between sham and 24 h after CLP (Fig. 2, C and D). These data demonstrate that ANT1 is downregulated in the heart post CLP. Protein levels of vascular cell adhesion molecule-1, one of the indicators of inflammation, was increased ∼2.4-fold at 24 h post CLP (Fig. 2, E and F).
Fig. 2.
Adenine nucleotide translocator isoform 1 (ANT1) is downregulated in the left ventricle of C57BL/6 mice after CLP. Protein extracts from the left ventricles of sham (s) or CLP mice (6-, 12-, and 24-h post-CLP) were analyzed for ANT1, cyclophilin D (CypD), and VCAM-1 protein levels by Western blot analysis. A: representative Western blot showing ANT1 protein levels after CLP. Protein extract from liver was loaded as the control, showing the antibody was specific for ANT1 (liver expresses ANT2). B: quantified results of A, expressed as the ratio to tubulin; n = 4. *P < 0.05 vs. sham. C: representative Western blot showing no change in CypD protein levels after CLP. D: quantified results of C, expressed as the ratio to tubulin; n = 4. P = 0.75 vs. sham. E: representative Western blot showing VCAM-1 protein levels after CLP. F: quantified results of E, expressed as the ratio to tubulin; n = 3. **P < 0.01 vs. sham.
ANT1 knockdown results in a reduction of mitochondrial respiration ratio and an increase in mitochondrial membrane potential in myocardium-derived H9c2 cells.
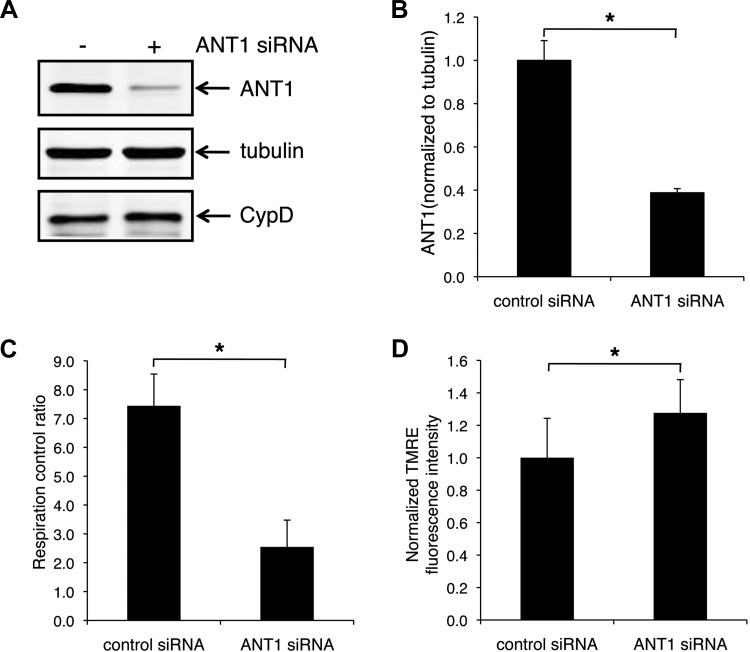
To understand the effect of ANT1 downregulation on mitochondrial function, we performed siRNA studies to knock down ANT1 in H9c2 cells. ANT1 siRNA decreased ANT1 protein levels to 40% of control cells (Fig. 3, A and B) without affecting other mitochondrial proteins such as CypD (Fig. 3A, bottom) and voltage-dependent anion channel (data not shown). Mitochondrial respiration control ratio was decreased by ANT1 knockdown (from 7.4 ± 14.9% to 2.6 ± 18%, P < 0.05) (Fig. 3C). In addition, mitochondrial membrane potential was ∼1.3-fold higher in ANT1 knockdown cells (Fig. 3D).
Fig. 3.
ANT1 knockdown results in a decrease in respiration control ratio and an increase in mitochondrial membrane potential in H9c2 cells. A: representative Western blot showing ANT1 knocking down by small-interfering RNA (siRNA). B: quantified results of A, expressed as the ratio to tubulin; n = 3. C: respiration control ratio in control and ANT1 knockdown cells; n = 3. D: mitochondrial membrane potential in control and ANT1 knockdown cells from 3 independent experiments. *P < 0.05 vs. control siRNA. TMRE, tetramethylrhodamine ethyl ester.
ANT1 knockdown increases swollen and donut/blob-shaped mitochondria in H9c2 cells.
During sepsis, there is a marked and rapid increase in TNFα followed by other proinflammatory cytokines (38, 47). Therefore, to understand the meaning of ANT1 downregulation in inflammation, we studied TNFα-induced immune responses in ANT1 knockdown H9c2 cells.
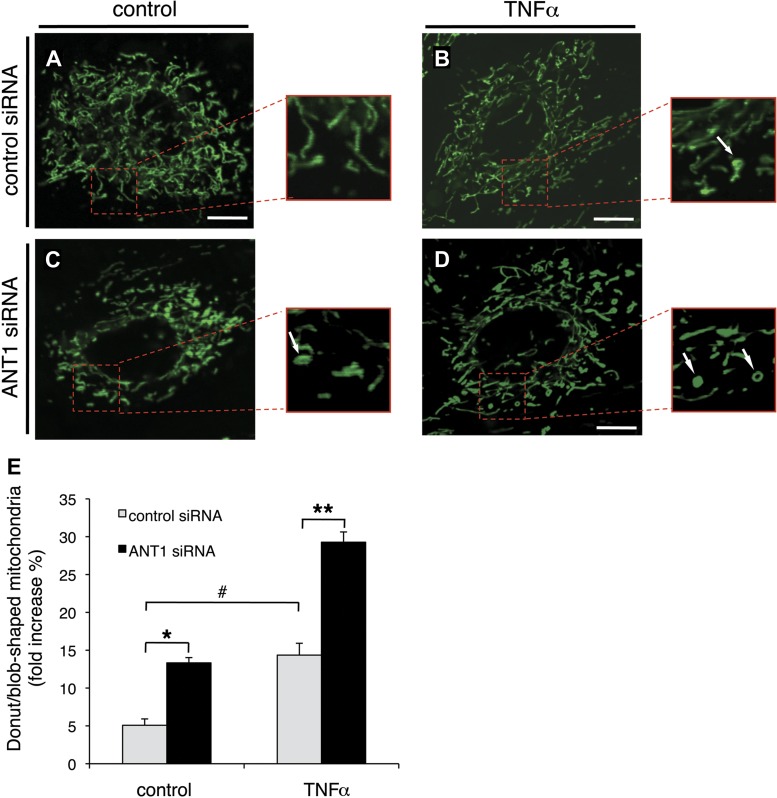
We first studied the effect of ANT1 knockdown on TNFα-induced mitochondrial morphological changes by live cell confocal imaging. To compare mitochondrial morphology objectively among different groups of cells, individual mitochondria were measured in high magnification. Mitochondria are tubular under normal conditions but change to a donut shape under mild stress and to a blob shape under high stress (1). Control siRNA-transfected cells primarily displayed tubular and long mitochondria. The mitochondria form networks and spread throughout the cells (Fig. 4A). In contrast, ANT1 knockdown cells displayed an increased proportion of swollen and donut/blob-shaped mitochondria (Fig. 4C).
Fig. 4.
ANT1 knockdown increases swollen mitochondria in H9c2 cells. Confocal microscopy analysis of mitochondrial morphology in control and ANT1 siRNA-transfected cells treated with either vehicle or TNFα (10 ng/ml) for 24 h is shown. Cells were loaded with MitoTracker Green. Representative images from cells transfected with control siRNA treated with vehicle (A), control siRNA treated with TNFα (B), ANT1 siRNA treated with vehicle (C), and ANT1 siRNA treated with TNFα (D) are shown. Arrows indicate donut/blob-shaped mitochondria. Bar = 20 μm. E: quantified results for donut/blob-shaped mitochondria from 3 independent experiments in control siRNA or ANT1 siRNA-transfected cells treated with either vehicle or TNFα. *P < 0.05 vs. control siRNA treated with vehicle; **P < 0.01 vs. control siRNA treated with TNFα; #P < 0.05 vs. vehicle.
Because TNFα treatment causes the formation of donut/blob-shaped mitochondria (1), we next quantified whether ANT1 knockdown would favor TNFα-induced donut/blob-shaped mitochondria formation. In control siRNA-transfected cells, TNFα treatment significantly increased the percentage of donut/blob-shaped mitochondria (from 5.1 ± 0.2 to 14.4 ± 0.1, P < 0.05) (Fig. 4, B and E). Interestingly, ANT1 knockdown further increased donut/blob-shaped mitochondria (Fig. 4, C–E). In ANT1 knockdown cells, the percentage of donut/blob-shaped mitochondria was significantly increased after TNFα treatment (to 29.3 ± 0.05, P < 0.01 vs. matched control siRNA group), indicating that ANT1 knockdown sensitizes mitochondria to stress/TNFα-induced morphological changes (Fig. 4, C–E).
ANT1 knockdown increases mitochondrial reactive oxygen species generation in H9c2 cells.
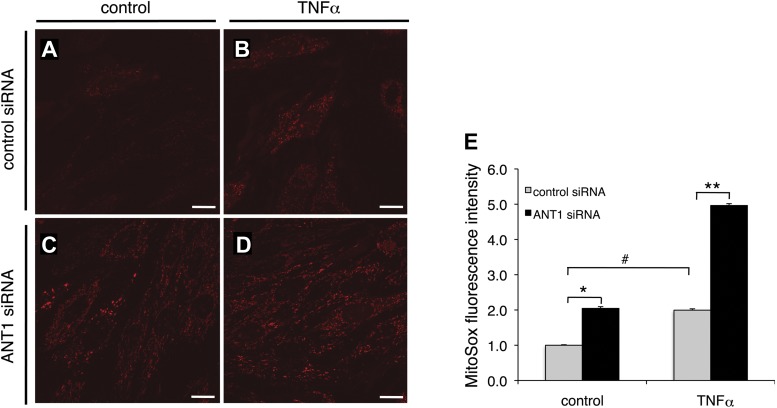
We next examined whether ANT1 knockdown increases mitochondrial reactive oxygen species (mtROS) because TNFα treatment induces mtROS generation (9). In control siRNA-transfected cells, TNFα treatment caused an ∼2.0-fold increase in mtROS as indicated by the increased MitoSox Red fluorescent intensity (Fig. 5, A, B, and E). ANT1 knockdown alone (vehicle treatment) increased mtROS by ∼2.0-fold (Fig. 5, C–E). Moreover, there was an ∼5.0-fold increase in mtROS in ANT1 knockdown cells treated with TNFα (Fig. 5, D and E). Our data indicate that ANT1 downregulation promotes mtROS generation in H9c2 cells.
Fig. 5.
ANT1 knockdown increases mitochondrial reactive oxygen species (mtROS) in H9c2 cells. Confocal microscopy analysis of mtROS in control and ANT1 siRNA-transfected cells treated with either vehicle or TNFα (10 ng/ml) for 24 h is shown. Cells were loaded with MitoSox Red. Representative images from cells transfected with control siRNA treated with vehicle (A), control siRNA treated with TNFα (B), ANT1 siRNA treated with vehicle (C), and ANT1 siRNA treated with TNFα (D) are shown. Bar = 50 μm. E: quantified results for mtROS from 3 independent experiments in control siRNA- or ANT1 siRNA-transfected cells treated with either vehicle or TNFα. *P < 0.01 vs. control siRNA treated with vehicle; **P < 0.01 vs. control siRNA treated with TNFα; #P < 0.05 vs. vehicle.
ANT1 knockdown increases TNFα-induced NF-κB reporter gene activity and IL-6 and TNFα expression in H9c2 cells.
In both human patients and rodent models of sepsis, inflammation is associated with significant increases in ROS generation, NF-κB activation, and inflammatory cytokine production (3, 17, 42, 46, 50). Therefore, we next determined whether ANT1 modulates NF-κB reporter gene activity in response to TNFα.
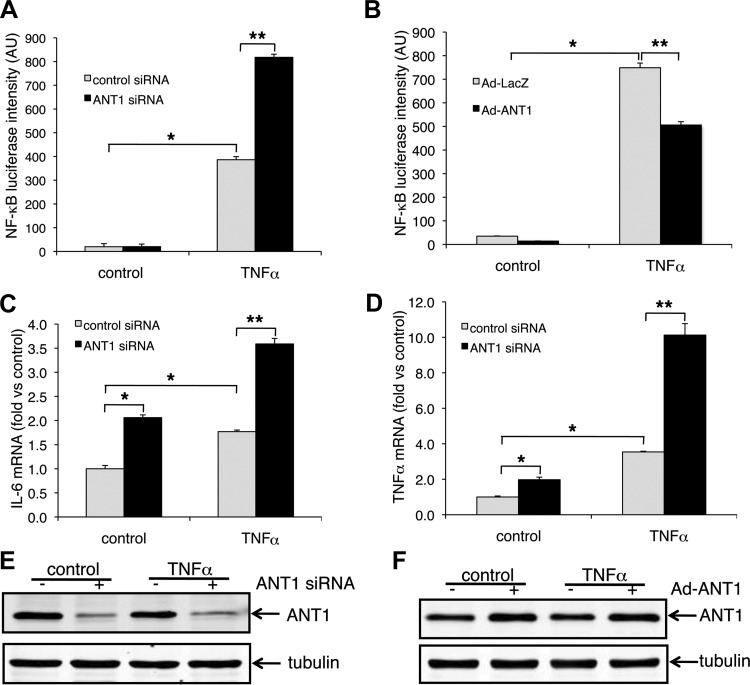
In control siRNA-transfected cells, TNFα significantly increased NF-κB luciferase activity (from 19.7 ± 1.3 to 386.3 ± 1.7, P < 0.01) (Fig. 6A). More importantly, ANT1 knockdown further increased TNFα-induced NF-κB reporter gene activity (to 817.9 ± 1.3, P < 0.01 vs. TNFα-treated control group), suggesting that ANT1 knockdown enhances TNFα-induced NF-κB activation (Fig. 6A).
Fig. 6.
ANT1 knockdown increases TNFα-induced NF-κB activation and IL-6 and TNFα expression in H9c2 cells. A: NF-κB luciferase reporter gene activity in ANT1 knockdown cells. ANT1 knockdown and control H9c2 cells were transfected with NF-κB luciferase reporter gene plasmid using transfected β-galactosidase plasmid as the internal control. Cells were treated with 10 ng/ml TNFα for 18 h before harvest for assays. Results were from 3 independent experiments normalized by β-galactosidase activity are shown. *P < 0.01 vs. control siRNA treated with vehicle; **P < 0.01 vs. control siRNA treated with TNFα. AU, arbitrary units. B: NF-κB luciferase reporter gene activity in ANT1 overexpressed cells. ANT1 was transiently expressed in H9c2 cells using ANT1 adenovirus (Ad-ANT1). Adenovirus expressing LacZ (Ad-LacZ) was used as the control. NF-κB luciferase activity was analyzed as in A. Data were from 3 independent experiments. *P < 0.01 vs. Ad-LacZ treated with vehicle; **P < 0.01 vs. Ad-LacZ treated with TNFα. Expression of IL-6 (C) and TNFα (D) in ANT1 knockdown cells is shown. ANT1 was knocked down by siRNA in H9c2 cells. Cells were treated with ether vehicle or TNFα (10 ng/ml) for 8 h before being harvested at 72 h after siRNA treatment. mRNA was extracted from the cells, and IL-6 and TNFα expression were examined by qualitative real-time PCR analysis. Data from 3 independent experiments were normalized to control siRNA treated with vehicle. *P < 0.01 vs. control siRNA plus vehicle treatment; **P < 0.05 vs. control siRNA plus TNFα. E: representative Western blots showing ANT1 was knocked down by the siRNA. F: representative Western blots showing overexpressed ANT1.
The role of ANT1 in NF-κB activation was further confirmed by infecting cells with either control adenovirus (Ad-LacZ) or mouse ANT1 adenovirus (Ad-ANT1) (Fig. 6B) (4). In control virus-transfected cells, TNFα significantly increased NF-κB luciferase activity (from 34.9 ± 3.1 to 748.9 ± 55.7, P < 0.01). More importantly, ANT1 overexpression significantly reduced TNFα-induced NF-κB luciferase activity (to 506.1 ± 39.7, P < 0.01 vs. TNFα-treated control group) (Fig. 6B), supporting a positive role for ANT1 downregulation in TNFα-induced NF-κB activation.
During inflammation, TNFα is the prime mediator of the inflammatory response and triggers the expression of other cytokines (47). Because TNFα and IL-6 are transcriptionally regulated by NF-κB, we next determined the effect of ANT1 knockdown on the expression of IL-6 and TNFα in H9c2 cells (Fig. 6, C and D). There was an ∼2.1-fold increase in endogenous IL-6 mRNA levels by ANT1 knockdown alone (plus vehicle treatment). Moreover, the combination of ANT1 knockdown and exogenous TNFα further caused an ∼3.6-fold increase in IL-6 mRNA (Fig. 6C). Similarly, ANT1 knockdown caused an ∼1.97-fold and an ∼10.1-fold increase in TNFα mRNA levels in vehicle- and recombinant TNFα-treated cells, respectively (Fig. 6D). Success in ANT1 knockdown or overexpression was confirmed by Western blot analysis (Fig. 6, E and F).
MTP attenuates mtROS generation, TNFα-induced NF-κB reporter gene activity, and IL-6 and TNFα expression in ANT1 knockdown cells.
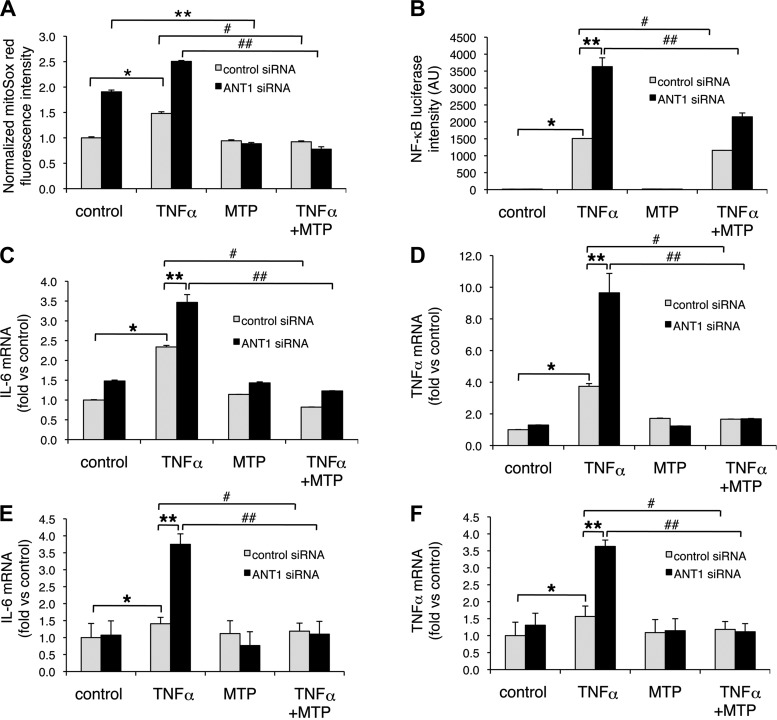
Because ANT1 knockdown increases mtROS generation (Fig. 5), we first determined whether mitochondrial-targeted antioxidant MTP would attenuate mtROS generation in control and ANT1 knockdown cells in response to vehicle or TNFα (Fig. 7A). In control siRNA group treated with MTP, no significant change was detected in vehicle-treated cells. However, MTP significantly decreased TNFα-induced mtROS (from 1.5 ± 0.03 to 0.9 ± 0.02, P < 0.05). In ANT1 knockdown group, MTP decreased mtROS in vehicle-treated cells (from 1.9 ± 0.03 to 0.9 ± 0.02, P < 0.05). More dramatic decrease of mtROS by MTP was found in TNFα-treated ANT1 knockdown cells (from 2.5 ± 0.02 to 0.8 ± 0.05, P < 0.05) (Fig. 7A).
Fig. 7.
mito-TEMPO (MTP) reduces mtROS generation, TNFα-induced NF-κB activation, and IL-6 and TNFα expression. A: mtROS generation. ANT1 knockdown, and control H9c2 cells were preincubated with 10 nM MTP for 1 h before being exposed to 10 ng/ml TNFα for 24 h. mtROS generation was measured by MitoSox Red fluorescence. Data were from 3 independent experiments. *P < 0.05 vs. control siRNA treated with vehicle; **P < 0.05 vs. ANT1 siRNA treated with vehicle; #P < 0.05 vs. control siRNA treated with TNFα; ##P < 0.05 vs. ANT1 siRNA treated with TNFα. B: NF-κB luciferase reporter gene activity. ANT1 knockdown and control H9c2 cells were transfected with NF-κB luciferase reporter gene plasmid and β-galactosidase plasmid as the internal control. Cells were preincubated with 10 nM MTP for 1 h before being exposed to 10 ng/ml TNFα for 18 h. Data were from 3 independent experiments normalized by β-galactosidase activity. *P < 0.05 vs. control siRNA treated with vehicle; **P < 0.05 vs. control siRNA treated with TNFα; #P < 0.05 vs. control siRNA treated with TNFα; ##P < 0.05 vs. ANT1 siRNA treated with TNFα. IL-6 (C) and TNFα (D) expression in H9c2 cells. Control and ANT1 knockdown cells were preincubated with 10 nM MTP for 1 h before TNFα treatment for 8 h (10 ng/ml). IL-6 and TNFα expression were examined by quantitative real-time PCR analysis. Data were from 3 independent experiments normalized to control siRNA with vehicle treatment. *P < 0.05 vs. control siRNA treated with vehicle; **P < 0.05 vs. control siRNA treated with TNFα; #P < 0.05 vs. control siRNA treated with TNFα; ##P < 0.05 vs. ANT1 siRNA treated with TNFα. IL-6 (E) and TNFα (F) expression in rat neonatal cardiomyocytes. Control and ANT1 knockdown cardiomyocytes were preincubated with 10 nM MTP for 1 h before TNFα treatment for 8 h (10 ng/ml). IL-6 and TNFα expression were examined by quantitative real-time PCR analysis. Data were from 3 independent experiments normalized to control siRNA with vehicle treatment are shown. *P < 0.05 vs. control siRNA treated with vehicle; **P < 0.05 vs. control siRNA treated with TNFα; #P < 0.05 vs. control siRNA treated with TNFα; ##P < 0.05 vs. ANT1 siRNA treated with TNFα.
We next determined whether mtROS plays a role in TNFα-induced NF-κB activation in control and ANT1 knockdown H9c2 cells (Fig. 7B). In control siRNA group treated with MTP, TNFα-induced NF-κB reporter gene activity was significantly decreased (from 1508.2 ± 18.9 to 1158.9 ± 42.2, P < 0.05). More dramatic changes of TNFα-induced NF-κB reporter gene activity were found in ANT1 knockdown groups treated with MTP (from 3630.4 ± 184.9 to 2149.7 ± 80.7, P < 0.05) (Fig. 7B).
We further determined whether MTP reduces TNFα-induced IL-6 and TNFα expression after ANT1 knockdown in H9c2 cells (Fig. 7, C and D). ANT1 knockdown increased TNFα-induced IL-6 and TNFα expression as we previously observed. MTP had little effect on the expression of IL-6 and TNFα in vehicle-treated control siRNA or ANT1 siRNA groups (MTP group vs. control group) (Fig. 7, C and D). However, in TNFα-treated cells, MTP decreased IL-6 expression in control siRNA groups (from ∼2.3 ± 0.07 to 0.8 ± 0.003, P < 0.05) (Fig. 7C). More importantly, MTP decreased IL-6 expression in ANT1 siRNA groups treated with TNFα (from 3.5 ± 0.14 to 1.2 ± 0.01, P < 0.05) (Fig. 7C). Similarly, MTP decreased TNFα expression in control siRNA groups treated with exogenous TNFα (from 3.7 ± 0.13 to 1.7 ± 0.01, P < 0.05) (Fig. 7D). Moreover, MTP dramatically decreased TNFα expression in ANT1 siRNA groups treated with exogenous TNFα (from 9.7 ± 0.88 to 1.7 ± 0.02, P < 0.05) (Fig. 7D).
The effect of MTP on IL-6 and TNFα expression was further confirmed using isolated cardiomyocytes (Fig. 7, E and F). ANT1 knockdown increased TNFα-induced IL-6 and TNFα expression (to 3.8 ± 0.4, P < 0.05, and 3.6 ± 0.3, P < 0.05, respectively) (Fig. E and F). MTP had little effect on the expression of IL-6 and TNFα in vehicle-treated control siRNA or ANT1 siRNA groups (MTP group vs. control group) (Fig. E and F). However, in TNFα-treated cells, MTP decreased IL-6 expression in control siRNA groups (from 1.4 ± 0.3 to 1.2 ± 0.3, P < 0.05) (Fig. 7E). More importantly, MTP significantly decreased IL-6 expression in ANT1 siRNA groups treated with TNFα (from 3.8 ± 0.4 to 1.1 ± 0.5, P < 0.05) (Fig. 7E). Similarly, MTP decreased TNFα expression in control siRNA groups treated with exogenous TNFα (from 1.6 ± 0.4 to 1.2 ± 0.3, P < 0.05) (Fig. 7F). Moreover, MTP dramatically decreased TNFα expression in ANT1 siRNA groups treated with exogenous TNFα (3.6 ± 0.3 to 1.1 ± 0.3, P < 0.05) (Fig. 7F). Taken together, our data support a role for mtROS in TNFα-induced NF-κB activation and proinflammatory cytokine expression after ANT1 knockdown.
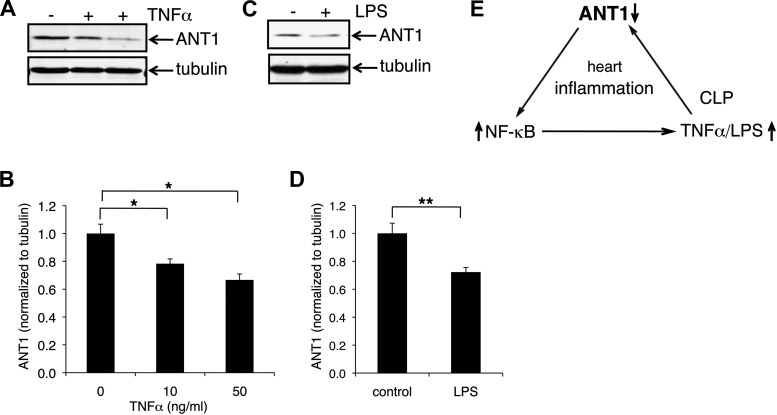
TNFα or LPS treatment decreases ANT1 protein levels in H9c2 cells.
We found that ANT1 is downregulated in the inflamed heart of CLP mice (Fig. 2). Because CLP significantly increases inflammatory cytokines, we investigated whether inflammatory cytokines per se could induce ANT1 downregulation. We assayed the effect of recombinant TNFα and LPS on ANT1 protein levels in H9c2 cells (Fig. 8). Indeed, TNFα decreased ANT1 protein levels in a dose-dependent manner (to 78% at 10 ng/ml and to 66.7% at 50 ng/ml) (Fig. 8, A and B). Similarly, ANT1 protein levels were significantly decreased by LPS treatment (to 72%) (Fig. 8, C and D).
Fig. 8.
ANT1 is downregulated in TNFα or LPS treated H9c2 cells. H9c2 cells were treated with TNFα at 0, 10, and 50 ng/ml (A and B) or LPS at 250 ng/ml (C and D) for 24 h. ANT1 protein levels were examined by Western blot analysis. Data were from 3 independent experiments. *P < 0.01 vs. control; **P < 0.05 vs. control. E: model for the role of ANT1 downregulation in cardiac inflammation.
DISCUSSION
In the present study, we provide a novel target for mitochondrial regulation of cardiac inflammation. We found that ANT1 protein levels were significantly downregulated in the inflamed heart in our murine model of systemic inflammation induced by CLP. Characterization of mitochondria in ANT1 knockdown cells indicated a decrease in mitochondrial respiration control ratio, an increase in mitochondrial membrane potential and mtROS generation. Further mechanistic studies support a role for mtROS in ANT1 downregulation-induced inflammatory response, because mitochondria-targeted antioxidant MTP reduced NF-κB reporter gene activity, as well as IL-6 and TNFα expression stimulated by recombinant TNFα. These data are consistent with previous observations that TNFα causes the formation of donut/blob-shaped mitochondria and are supported by the finding that ANT1 negatively regulates ROS generation (1, 14, 23). Interestingly, TNFα and LPS treatments that mimic the environment in inflamed cells in turn decreased ANT1 protein levels, implicating a feed-forward regulation of inflammatory cytokine production mediated by ANT1 downregulation.
Our data support a model in which increased production of TNFα during CLP triggers ANT1 downregulation in the heart. In turn, ANT1 downregulation exacerbates TNFα-induced NF-κB activation and TNFα and IL-6 expression, leading to excessive inflammation in the heart (Fig. 8E).
During inflammation, immune factors such as cytokines and chemokines are secreted to coordinate defense and repair. However, excessive proinflammatory cytokines cause cellular and mitochondrial damage. In septic patients, one of the most severe and frequent complications is inflammation-induced cardiac dysfunction. Its presence is associated with significantly increased mortality rates (6, 31, 37, 51). Although the molecular mechanisms for sepsis-induced cardiac dysfunction are complicated and not completely understood, a growing body of evidence indicates that increased inflammatory cytokine production contributes significantly to its pathogenesis (21, 30). Similarly, a sustained increase in TNFα or IL-6 has also been reported in several pathological processes, such as ischemic myocardial injury, cardiac hypertrophy, myocardial infarction, and chronic heart failure (5, 15, 19, 26, 49).
We demonstrate that ANT1 downregulation enhances TNFα-induced NF-κB reporter gene activity. In septic patients, NF-κB is a critical indicator for survival because NF-κB activity is significantly higher in nonsurvivors and correlates strongly with the severity of the illness (3). The pathological role of NF-κB activation is also supported by the finding that inhibiting NF-κB prevents CLP-induced organ dysfunction and reduces cardiac hypertrophy and heart failure (8, 19). Interestingly, our data support a role for ANT1 downregulation in mtROS-mediated NF-κB activation. Because sepsis-induced organ failure is associated with increased NF-κB activation and oxidative damage induced by mitochondrial dysfunction (10, 16), preventing ANT1 downregulation could be beneficial, diminishing mtROS generation and NF-κB activation.
Studies to date indicate that mitochondria participate in the detection of microorganisms and cellular damage to activate innate immune responses (43, 44). Here, we provide further evidence for ANT1 modulation of cardiac inflammation and assert the importance of cross talk between mitochondrial proteins and cytosolic pathways in the regulation of inflammation. Our data suggest that ANT1 downregulation, NF-κB activation, and subsequent inflammatory cytokine production form a vicious cycle that exacerbates inflammation. Although how much of an effect increasing myocardial ANT1 would have in CLP mice is still not clear at this point. ANT1 downregulation could be a novel molecular target in sepsis-induced cardiac inflammation, especially since mitochondrial mass and protein synthesis do not change during sepsis (16). Further studies on the regulatory mechanisms of ANT1 will shed light on its role in preventing excessive inflammation.
GRANTS
This work is supported by National Heart, Lung, and Blood Institute Grants HL110371 and HL093671 (to S. S Sheu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.P. conception and design of research; S.P., N.W., S.B., and B.Y. performed experiments; S.P. and N.W. analyzed data; S.P. and S.-S.S. interpreted results of experiments; S.P. and N.W. prepared figures; S.P. drafted manuscript; S.P., N.W., and S.-S.S. edited and revised manuscript; S.P., N.W., S.B., B.Y., and S.-S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The ANT1-encoding adenovirus was a generous gift form Dr. Christopher Baines of the University of Missouri-Columbia. The ANT1 antibody was a generous gift from Dr. Douglas C. Wallace of the University of Pennsylvania.
REFERENCES
- 1.Ahmad T, Aggarwal K, Pattnaik B, Mukherjee S, Sethi T, Tiwari BK, Kumar M, Micheal A, Mabalirajan U, Ghosh B, Sinha Roy S, Agrawal A. Computational classification of mitochondrial shapes reflects stress and redox state. Cell Death Dis 4: e461, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexis JD, Wang N, Che W, Lerner-Marmarosh N, Sahni A, Korshunov VA, Zou Y, Ding B, Yan C, Berk BC, Abe J. Bcr kinase activation by angiotensin II inhibits peroxisome-proliferator-activated receptor gamma transcriptional activity in vascular smooth muscle cells. Circ Res 104: 69–78, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnalich F, Garcia-Palomero E, Lopez J, Jimenez M, Madero R, Renart J, Vazquez JJ, Montiel C. Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infect Immun 68: 1942–1945, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baines CP, Molkentin JD. Adenine nucleotide translocase-1 induces cardiomyocyte death through upregulation of the pro-apoptotic protein Bax. J Mol Cell Cardiol 46: 969–977, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman MR, Kao RH, McCune SA, Holycross BJ. Myocardial tumor necrosis factor-alpha secretion in hypertensive and heart failure-prone rats. Am J Physiol Heart Circ Physiol 277: H543–H550, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Celes MR, Prado CM, Rossi MA. Sepsis: going to the heart of the matter. Pathobiology 80: 70–86, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Yi B, Sun J. Inhibition of cardiomyocyte hypertrophy by protein arginine methyltransferase 5. J Biol Chem 289: 24325–24335, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coldewey SM, Rogazzo M, Collino M, Patel NS, Thiemermann C. Inhibition of IkappaB kinase reduces the multiple organ dysfunction caused by sepsis in the mouse. Dis Model Mech 6: 1031–1042, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol 24: 762–768, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Crouser ED. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion 4: 729–741, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Dorner A, Giessen S, Gaub R, Grosse Siestrup H, Schwimmbeck PL, Hetzer R, Poller W, Schultheiss HP. An isoform shift in the cardiac adenine nucleotide translocase expression alters the kinetic properties of the carrier in dilated cardiomyopathy. Eur J Heart Fail 8: 81–89, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Dorner A, Pauschinger M, Schwimmbeck PL, Kuhl U, Schultheiss HP. The shift in the myocardial adenine nucleotide translocator isoform expression pattern is associated with an enteroviral infection in the absence of an active T-cell dependent immune response in human inflammatory heart disease. J Am Coll Cardiol 35: 1778–1784, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Dorner A, Schulze K, Rauch U, Schultheiss HP. Adenine nucleotide translocator in dilated cardiomyopathy: pathophysiological alterations in expression and function. Mol Cell Biochem 174: 261–269, 1997. [PubMed] [Google Scholar]
- 14.Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci USA 96: 4820–4825, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem 13: 1877–1893, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Garrabou G, Moren C, Lopez S, Tobias E, Cardellach F, Miro O, Casademont J. The effects of sepsis on mitochondria. J Infect Dis 205: 392–400, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Gordon AC, Lagan AL, Aganna E, Cheung L, Peters CJ, McDermott MF, Millo JL, Welsh KI, Holloway P, Hitman GA, Piper RD, Garrard CS, Hinds CJ. TNF and TNFR polymorphisms in severe sepsis and septic shock: a prospective multicentre study. Genes Immun 5: 631–640, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Graham BH, Waymire KG, Cottrell B, Trounce IA, MacGregor GR, Wallace DC. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat Genet 16: 226–234, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Young D, Maitra RK, Gupta A, Popovic ZB, Yong SL, Mahajan A, Wang Q, Sen S. Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB. J Mol Biol 375: 637–649, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gwechenberger M, Mendoza LH, Youker KA, Frangogiannis NG, Smith CW, Michael LH, Entman ML. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation 99: 546–551, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Heard SO, Perkins MW, Fink MP. Tumor necrosis factor-alpha causes myocardial depression in guinea pigs. Crit Care Med 20: 523–527, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Horton JW, Maass D, White J, Sanders B. Nitric oxide modulation of TNF-alpha-induced cardiac contractile dysfunction is concentration dependent. Am J Physiol Heart Circ Physiol 278: H1955–H1965, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Kim EH, Koh EH, Park JY, Lee KU. Adenine nucleotide translocator as a regulator of mitochondrial function: implication in the pathogenesis of metabolic syndrome. Korean Diabetes J 34: 146–153, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koller-Strametz J, Pacher R, Frey B, Kos T, Woloszczuk W, Stanek B. Circulating tumor necrosis factor-alpha levels in chronic heart failure: relation to its soluble receptor II, interleukin-6, and neurohumoral variables. J Heart Lung Transplant 17: 356–362, 1998. [PubMed] [Google Scholar]
- 25.Kremer JP, Jarrar D, Steckholzer U, Ertel W. Interleukin-1, -6 and tumor necrosis factor-alpha release is down-regulated in whole blood from septic patients. Acta Haematol 95: 268–273, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res 81: 627–635, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Levy SE, Chen YS, Graham BH, Wallace DC. Expression and sequence analysis of the mouse adenine nucleotide translocase 1 and 2 genes. Gene 254: 57–66, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Li K, Warner CK, Hodge JA, Minoshima S, Kudoh J, Fukuyama R, Maekawa M, Shimizu Y, Shimizu N, Wallace DC. A human muscle adenine nucleotide translocator gene has four exons, is located on chromosome 4, and is differentially expressed. J Biol Chem 264: 13998–14004, 1989. [PubMed] [Google Scholar]
- 29.MacGowan GA, Mann DL, Kormos RL, Feldman AM, Murali S. Circulating interleukin-6 in severe heart failure. Am J Cardiol 79: 1128–1131, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Meng X, Ao L, Meldrum DR, Cain BS, Shames BD, Selzman CH, Banerjee A, Harken AH. TNF-alpha and myocardial depression in endotoxemic rats: temporal discordance of an obligatory relationship. Am J Physiol Regul Integr Comp Physiol 275: R502–R508, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Merx MW, Weber C. Sepsis and the heart. Circulation 116: 793–802, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Narula N, Zaragoza MV, Sengupta PP, Li P, Haider N, Verjans J, Waymire K, Vannan M, Wallace DC. Adenine nucleotide translocase 1 deficiency results in dilated cardiomyopathy with defects in myocardial mechanics, histopathological alterations, and activation of apoptosis. JACC Cardiovasc Imaging 4: 1–10, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ning XH, Zhang J, Liu J, Ye Y, Chen SH, From AH, Bache RJ, Portman MA. Signaling and expression for mitochondrial membrane proteins during left ventricular remodeling and contractile failure after myocardial infarction. J Am Coll Cardiol 36: 282–287, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Palmieri L, Alberio S, Pisano I, Lodi T, Meznaric-Petrusa M, Zidar J, Santoro A, Scarcia P, Fontanesi F, Lamantea E, Ferrero I, Zeviani M. Complete loss-of-function of the heart/muscle-specific adenine nucleotide translocator is associated with mitochondrial myopathy and cardiomyopathy. Hum Mol Genet 14: 3079–3088, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Pan S, Berk BC. Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circ Res 100: 213–219, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Pan S, World CJ, Kovacs CJ, Berk BC. Glucose 6-phosphate dehydrogenase is regulated through c-Src-mediated tyrosine phosphorylation in endothelial cells. Arterioscler Thromb Vasc Biol 29: 895–901, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med 113: 227–242, 1990. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen BK. The diseasome of physical inactivity—and the role of myokines in muscle—fat cross talk. J Physiol 587: 5559–5568, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petretta M, Condorelli GL, Spinelli L, Scopacasa F, de Caterina M, Leosco D, Vicario ML, Bonaduce D. Circulating levels of cytokines and their site of production in patients with mild to severe chronic heart failure. Am Heart J 140: E28, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Portman MA. Adenine nucleotide translocator in heart. Mol Genet Metab 71: 445–450, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Portman MA. The adenine nucleotide translocator: regulation and function during myocardial development and hypertrophy. Clin Exp Pharmacol Physiol 29: 334–338, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Rocha M, Herance R, Rovira S, Hernandez-Mijares A, Victor VM. Mitochondrial dysfunction and antioxidant therapy in sepsis. Infect Disord Drug Targets 12: 161–178, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Saitoh T, Akira S. Regulation of innate immune responses by autophagy-related proteins. J Cell Biol 189: 925–935, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulze K, Witzenbichler B, Christmann C, Schultheiss HP. Disturbance of myocardial energy metabolism in experimental virus myocarditis by antibodies against the adenine nucleotide translocator. Cardiovasc Res 44: 91–100, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Sokolova N, Pan S, Provazza S, Beutner G, Vendelin M, Birkedal R, Sheu SS. ADP protects cardiac mitochondria under severe oxidative stress. PLoS One 8: e83214, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song M, Kellum JA. Interleukin-6. Crit Care Med 33: S463–465, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Spooner CE, Markowitz NP, Saravolatz LD. The role of tumor necrosis factor in sepsis. Clin Immunol Immunopathol 62: S11–S17, 1992. [DOI] [PubMed] [Google Scholar]
- 48.Stepien G, Torroni A, Chung AB, Hodge JA, Wallace DC. Differential expression of adenine nucleotide translocator isoforms in mammalian tissues and during muscle cell differentiation. J Biol Chem 267: 14592–14597, 1992. [PubMed] [Google Scholar]
- 49.Stetson SJ, Perez-Verdia A, Mazur W, Farmer JA, Koerner MM, Weilbaecher DG, Entman ML, Quinones MA, Noon GP, Torre-Amione G. Cardiac hypertrophy after transplantation is associated with persistent expression of tumor necrosis factor-alpha. Circulation 104: 676–681, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Turnbull IR, Javadi P, Buchman TG, Hotchkiss RS, Karl IE, Coopersmith CM. Antibiotics improve survival in sepsis independent of injury severity but do not change mortality in mice with markedly elevated interleukin 6 levels. Shock 21: 121–125, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 36: 1701–1706, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Vogelpohl I, Vetter R, Heger J, Ebermann L, Euler G, Schultheiss HP, Dorner A. Transgenic overexpression of heart-specific adenine nucleotide translocase 1 positively affects contractile function in cardiomyocytes. Cell Physiol Biochem 27: 121–128, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Walther T, Tschope C, Sterner-Kock A, Westermann D, Heringer-Walther S, Riad A, Nikolic A, Wang Y, Ebermann L, Siems WE, Bader M, Shakibaei M, Schultheiss HP, Dorner A. Accelerated mitochondrial adenosine diphosphate/adenosine triphosphate transport improves hypertension-induced heart disease. Circulation 115: 333–344, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Ebermann L, Sterner-Kock A, Wika S, Schultheiss HP, Dorner A, Walther T. Myocardial overexpression of adenine nucleotide translocase 1 ameliorates diabetic cardiomyopathy in mice. Exp Physiol 94: 220–227, 2009. [DOI] [PubMed] [Google Scholar]