Abstract
Psychological disorders are prevalent in patients with inflammatory bowel disease; the underlying mechanisms remain unknown. We tested the hypothesis that ulcerative colitis-like inflammation induced by dextran sodium sulfate (DSS) exacerbates the ongoing spontaneous activity in colon-projecting afferent neurons that induces abdominal discomfort and anxiety, and depressive-like behaviors in rats. In this study, we used the conditioned place preference and standard tests for anxiety- and depression-like behaviors. DSS rats developed anxiety- and depression-like behaviors 10 to 20 days after the start of inflammation. Single-fiber recordings showed an increase in the frequency of spontaneous activity in L6-S1 dorsal root ganglion (DRG) roots. Prolonged desensitization of transient receptor potential vanilloid 1 (TRPV1)-expressing colonic afferents by resiniferatoxin (RTX) suppressed the spontaneous activity, as well as the anxiety- and depressive-like behaviors. Reduction in spontaneous activity in colon afferents by intracolonic administration of lidocaine produced robust conditioned place preference (CPP) in DSS rats, but not in control rats. Patch-clamp studies demonstrated a significant decrease in the resting membrane potential, lower rheobase, and sensitization of colon-projecting L6-S1 DRG neurons to generate trains of action potentials in response to current injection in DSS rats. DSS inflammation upregulated the mRNA levels of transient receptor potential ankyrin 1 and TRPV1 channels and downregulated that of Kv1.1 and Kv1.4 channels. Ulcerative colitis-like inflammation in rats induces anxiety- and depression-like behaviors, as well as ongoing abdominal discomfort by exacerbating the spontaneous activity in the colon-projecting afferent neurons. Alterations in the expression of voltage- and ligand-gated channels are associated with the induction of mood disorders following colon inflammation.
Keywords: inflammatory bowel disease, spontaneous activity in visceral afferent neurons, anxiety, depression, visceral hypersensitivity
accumulating evidence shows that peripheral inflammation in chronic diseases, including Type 1 diabetes, cardiovascular disease, asthma, rheumatoid arthritis, chronic pulmonary disease, and chronic hepatitis C, associates with anxiety and depression in vulnerable patients (7). Inflammatory bowel disease (IBD) causes intense overt inflammation in the distal gut. The prevalence of psychological disorders is controversial in IBD patients (20). However, various reports show that as high as 80% of IBD patients suffer from anxiety and 60% from depression during relapse (1) and 29% to 35% during remission (3, 22). The cellular and neurological mechanisms by which peripheral inflammation induces anxiety and depression remain unknown.
Evoked pain induced by balloon distension in the colon in a laboratory setting has received wide attention because of its relevance to episodic abdominal pain (cramping) experienced by irritable bowel syndrome (IBS) (25) and IBD patients (21). In daily life, strong compression of the colon wall by giant migrating contractions serves as the mechanical stimulus for evoked pain in these patients (10). The pain lasts nearly as long as balloon distension or as long as a giant migrating contraction is present anywhere in the colon (26). However, most diseases that cause peripheral nerve lesions also induce a feeling of ongoing pain/discomfort (neuropathic pain), as well as other aversive sensations, such as burning, numbness, tingling, and pricking, suggesting that peripheral nerve lesions may induce multiple types of affective feelings in addition to evoked pain (28).
Experimental models of spinal cord injury show that peripheral nerve lesions generate ongoing spontaneous discharges of action potentials (spontaneous activity), as well as hyperalgesia and allodynia to mechanical, thermal, or chemical stimuli in the afferent and central neurons (5). Reflexive measures demonstrate hypersensitivity and allodynia evoked by balloon distension, but they do not assess the affective responses (aversive states), because they occur in the absence of balloon distension. The conditioned place preference (18) paradigm rewards the animals with analgesic-induced relief from aversive states, so that they prefer the chamber in which they received treatment (16, 18). Ulcerative colitis-like inflammation induces a robust inflammatory response and tissue damage in the affected colon (29). However, it remains unknown whether ulcerative colitis-like inflammation induces an aversive state/discomfort.
We hypothesized that ulcerative colitis-like inflammation aggravates the spontaneous activity, in the colon-projecting afferents that contributes to anxiety and depression, as well as abdominal discomfort. We found that 10–20 days after the induction of colonic inflammation by dextran sodium sulfate (DSS), the mean frequency of ongoing spontaneous activity significantly increased in the dorsal root filaments and the rats displayed anxiety- and depression-like behaviors. Intraluminal treatment of the distal colon with resiniferatoxin (RTX) suppressed the spontaneous activity, as well as anxiety- and depression-like behaviors. In addition, the DSS-treated rats developed a significant conditioned place preference (CPP) for the chamber paired with intracolonic lidocaine treatment, indicating relief from ongoing discomfort.
METHODS
Animals and induction of ulcerative colitis-like inflammation.
We used 6- to 10-wk-old male Sprague-Dawley rats (180–280 g; Harlan, Indianapolis, IN) housed at 22°C with a 12:12-h light-dark cycle starting at 7 AM. Inflammation was induced by oral consumption of 5% DSS in drinking water (29). Experiments were performed from day 10 to day 20 after the start of DSS treatment. The Institutional Animal Care and Use Committee of the University of Texas Medical Branch at Galveston, TX, approved all procedures.
CPP.
After habituation handling, the rats received a 15-min preconditioning preference test, allowing them to explore the entire CPP apparatus, followed by an 8-day conditioning period. During conditioning, 1 ml of 2% lidocaine hydrochloride jelly USP or vehicle was administered intraluminally to the DSS-treated and control rats under brief anesthesia with isoflurane. Ten minutes later, each rat was confined to one assigned choice compartment for 30 min. On days 1, 3, 5, and 7, the animals received vehicle, and on days 2, 4, and 6, they received lidocaine and were placed in the alternative compartment. Lidocaine-paired choice chamber assignment within each group was counterbalanced and unbiased. The effect of lidocaine conditioning on chamber preference was assessed on day 9 in the absence of drug or vehicle administration. The rats were allowed to explore freely the apparatus for 15 min. Chamber preference was expressed as the difference between the time spent in the lidocaine and vehicle-paired chambers. Change in preference was the difference between postconditioning and preconditioning chamber preferences.
Anxiety-like behavior.
We used three tests of anxiety-like behavior; the elevated plus-maze (EPM), cold stress defecation, and neophobia to a novel taste (sucrose) (6, 16). At the beginning of the EPM test, the rat was placed in the central area facing one of the open arms. The behavior during the 5-min test period and the number of entries into the open and closed arms were scored, and the duration of time spent in each type of arm was measured. All activities were scored using an automated video-tracking system (Any-Maze, San Diego Instruments, San Diego, CA). In the sucrose neophobia test, water was removed from the home cage at 1700. At 1900, a sucrose solution was introduced to the home cage. The amount of sucrose consumed was measured at 30 min and 24 h later.
Depression-like behavior.
We employed three tests to model distinct aspects of depression-like behavior: the sucrose preference test to model anhedonia, the social interaction test to model social withdrawal, and the forced swim test to model behavioral despair (16). In the sucrose preference test, rats were preexposed to 1% (wt./vol.) sucrose solution for 3 days; after 2 days of abstinence, they were tested for their preference for sucrose vs. water. In the social interaction test, rats were isolated for 24 h, after which interactions between pairs of familiar cagemates were observed. A forced swim test was performed in a clear plastic cylinder (diameter: 25 cm; height: 45 cm) filled with water at 24 ± 0.2°C. During the pretest exposure, rats were placed in the cylinders and forced to swim for 10 min. Behavior was assessed the next day during a 5-min swim test session. An experimenter blinded to the conditions scored swimming immobility episodes and time.
In vivo single fiber recording of L6-S1 dorsal root ganglion rootlets.
Isoflurane, 2.5%, followed by 50 mg/kg ip pentobarbital sodium induced general anesthesia; 25 mg·kg−1·h−1 intravenous infusion of Nembutal maintained anesthesia (5). Adequacy of anesthesia was confirmed by the absence of corneal and pupillary reflexes and stability of end-tidal CO2 level. A tracheotomy tube connected to a ventilator provided a mixture of room air and oxygen (3.0 ml; 54 breaths/min). Expired CO2 was maintained between 2.2 and 4.5%. Body temperature was maintained at 37°C by a servo-controlled heating blanket. A laminectomy from T12 to S2 exposed the spinal cord. The head was stabilized in a stereotaxic frame. The dura was gently opened, and a warm mineral oil pool, contained by skin flaps, covered the exposed spinal cord and roots. Multiunit afferent discharges were recorded from the distal ends of L6-S1 dorsal rootlets decentralized close to their entry into the spinal cord. A bundle of multiunit fibers was distinguished into 2–6 single units off-line using wave mark template matching in Spike 2 software that differentiates spikes by shape and amplitude. Colonic afferent fibers were identified by their response to colorectal distension (CRD).
The spontaneous activity of the dorsal root fiber was quantitated as the number of action potentials per second during the 60-s period prior to CRD. The response of a fiber to CRD was determined as the average increase in discharge rate during CRD over the average spontaneous activity. Excitatory responses to visceral stimuli were calculated by subtracting spontaneous activity from the activity during a stimulus. Fibers were considered responsive when changes in responses to CRD increased more than 30% from the baseline. Mechanosensitive single units were classified as high threshold (HT) (>20 mmHg) and low threshold (LT) (≤ 20 mmHg) on the basis of their response threshold and profile during graded or ramp colon distensions (9).
Whole cell patch-clamp recordings from dissociated dorsal root ganglion neurons.
The colon projecting dorsal root ganglion (DRG) neurons were labeled and dissociated, as described previously (33). The glass coverslip containing DRG neurons was transferred to the recording chamber, perfused at 1.5 ml/min with solution containing (in mM): 130 NaCl, 5 KCl, 2 KH2PO4, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH adjusted to 7.4 with NaOH (300 mosmol/kg H2O) at room temperature. Recording pipettes, pulled from borosilicate glass tubing, with resistance of 1–5 MΩ, were filled with solution containing (in mM): 100 KmeSO3, 40 KCl, and 10 HEPES, pH 7.25 adjusted with KOH (290 mosmol/kg H2O). DiI-labeled neurons were identified under fluorescent microscope. Whole cell current and voltage were recorded from DiI-labeled neurons using Dagan 3911 patch-clamp amplifier. Data were acquired and analyzed by pCLAMP 9.2 (Molecular Devices, Sunnyvale, CA). While still under voltage clamp, the Clampex Membrane Test program (Molecular Devices) was used to determine membrane capacity, Cm, and membrane resistance, Rm, during a 10-ms, 5-mV depolarizing pulse from a holding potential of −60 mV. The configuration was then switched to current clamp (0 pA) for determining other electrophysiological properties. After stabilizing for 2–3 min, resting membrane potential (RMP) was measured. The minimum acceptable RMP was −40 mV. The spike firing threshold and rheobase were detected under current clamp conditions. Action potentials were evoked and recorded by 2 to 3 times rheobase current stimulation (pulse width: 300 ms). Action potentials generated by application of a threshold level current over two 30-s periods separated by 60 s without stimulation were recorded and averaged (5).
Laser capture microscopic dissection.
We injected CTB-488 (Invitrogen, Carlsbad, CA), 4 mg/ml in PBS, into the colon wall (six injections of 2 μl each/rat); dorsal root ganglia were collected 6 days later and frozen in OTC on dry ice. We identified CTB-488-labeled neuronal profiles and captured them with a Pixel IIe laser capture microscope (Applied Biosytems, Foster City, CA). RNA was prepared with a Qiagen microRNA kit. SYBR Green RT-PCR was performed with Applied Biosystems reagents and Step One Plus real-time PCR apparatus. We used 18S rRNA as a normalizer and compared fold change to control by using the DDCt (fold change in threshold cycle compared to control) procedure. Primers were designed using Primer Express Software (Applied Biosystems) and were validated through control experiments: a single amplimer was observed by melting curve analysis; no amplimer was produced without reverse transcription or template; amplification efficiency was 100%.
Pharmacological agents.
DSS (ICN Biomedicals, Aurora, OH) was dissolved in the drinking water (5% wt/vol). The 2% lidocaine hydrochloride jelly USP was purchased from UTMB Pharmacy (Arorn, Lake Forest, IL). The vehicle jelly was prepared fresh each day in saline, and the pH was adjusted to 7. RTX was purchased from Sigma (St. Louis, MO).
The fluorescent retrograde tracer, 1,1′-dioleyl-3,3,3′,3′-tetramethylindocarbocyanine methanesulfonate (50 mg/ml in DMSO) was purchased from Invitrogen (Carlsbad, CA).
Statistics.
Data are expressed as means ± SE. P < 0.05 was considered statistically significant. Anxiety and depression tests comparing two groups were analyzed by t-test; RTX effects on anxiety- and depression-like behavior in DSS and control rats were analyzed by two-factor ANOVA. For stimulus response curves generated by graded CRD, the area under the curve was calculated for each fiber or rat, and comparison of group means was made by Student's t-test. CPP data were analyzed by two-way repeated-measures ANOVA. Preconditioning and postconditioning data were assessed for effects of DSS-induced colitis and lidocaine conditioning on chamber preference to confirm lack of chamber preference before conditioning and to detect significant changes in chamber preference after conditioning. Preconditioning and postconditioning data within each group were analyzed to detect conditioning-induced changes in chamber preference. Where significant CPP was detected, the conditioning-induced change in preference for the lidocaine-paired chamber was calculated for control and DSS rats and means compared by t-test. Cytokine data were compared using ANOVA.
RESULTS
Anxiety- and depression-like behavior in DSS-induced colitis.
In the EPM test, DSS-treated rats displayed significantly fewer entries into the open arm (2.1 ± 0.4 vs. 7.3 ± 1.3 entries in 5 min, P < 0.05; Fig. 1A) and spent significantly less time in the open arm than the control rats (5.6 ± 1 vs. 13.4 ± 4 s, P < 0.05; Fig. 1B). There was no significant difference between the control and DSS-treated rats in the average total distance traveled in EPM (11.7 ± 0.7 vs. 11.0 ± 0.6 m, P = 0.5; Fig. 1C). The DSS rats exhibited normal grooming behavior and muscle tone and had no substantial weight loss during this test, indicating that the results of behavior tests were not confounded by colitis-induced sickness behavior. Sucrose consumption in the sucrose neophobia test was not significantly different between DSS and control rats (data not shown). The EPM results suggested greater anxiety-like behavior in DSS vs. control rats.
Fig. 1.
Dextran sodium sulfate (DSS) rats exhibited greater anxiety- and depression-like behaviors compared with controls. DSS rats made significantly fewer entries (A) and spent significantly less time in the open arms of the elevated plus-maze (EPM) (B). C: there was no significant difference between Ctr. and DSS in the average total distance traveled in EPM. In the sucrose preference test, sucrose consumption as a percentage of total liquid consumed was lower in DSS vs. the control rats (D), DSS rats spent significantly more time immobile in the forced swim test (E), and engaged in significantly fewer social contacts compared with controls (F). *P < 0.05 DSS vs. control rats.
In the sucrose preference test, the DSS rats showed less preference for sucrose, consuming sucrose 48 ± 8.1% of total fluid intake vs. 82 ± 4.1% by control rats (n = 7 or 8; P < 0.05; Fig. 1D). Overall, the DSS rats consumed less sucrose than the control (21.1 ± 3.3 vs. 41.8 ± 4.7 g, P < 0.05). In the forced swim test, the DSS rats were immobile for a longer period than the control rats (74.8 ± 18.6 s vs. 23.1 ± 9.4 s, P < 0.05; Fig. 1E). The results of these two tests suggested greater depression-like behavior in DSS vs. control rats. In the social interaction test, however, DSS-treated rats displayed significantly reduced contact frequency vs. the control rats (1.1 ± 0.12 vs. 2.4 ± 0.3 contacts/min, P < 0.05; Fig. 1F), but significantly greater contact time (24.3 ± 0.9 vs. 20.1 ± 1.3 s/30 min, P < 0.05). The combination of decreased frequency and increased contact time likely represents a behavioral response to the ongoing discomfort/pain from the DSS treatment (see below).
Spontaneous activity and sensitization of afferent neurons by DSS-induced colitis.
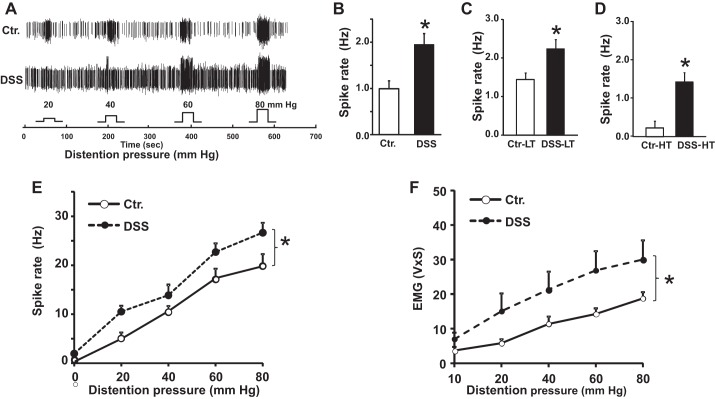
Single-fiber recordings showed that 86.5% (77 out of 89) of L6-S1 dorsal roots from DSS rats generated spontaneous activity vs. 68.5% (37 out of 54) in the control rats. The mean frequency of spontaneous activity in these roots was twofold greater in DSS than in control rats (Fig. 2, A and B; 2.0 ± 0.2 vs. 1.0 ± 0.2 Hz, P < 0.05). Both, low-threshold (LT) and high-threshold (HT) fibers from DSS rats showed a significant increase in firing frequency vs. the control rats (Fig. 2, C and D; 2.2 ± 0.2 vs. 0.9 ± 0.2 Hz, P < 0.05 for LT fibers, and 1.4 ± 0.3 vs. 0.2 ± 0.1 Hz, P < 0.05 for HT fibers).
Fig. 2.
Colonic spontaneous activity and sensitization to colorectal distension (CRD) increased in DSS rats. A: wave mark tracings show multiunit afferent fiber activity from S1 dorsal roots from a Ctr. and a DSS rat during graded CRD, on day 20. B, C, and D: mean frequencies of spontaneous activity in CRD-responsive single afferent fibers from control and DSS rats: all active fibers, Ctr. (B; n = 54), DSS (n = 89), low threshold (LT) fibers Ctr. (n = 34), DSS, (n = 58) (C), high-threshold (HT) fibers Ctr. (n = 21), DSS, (n = 32) (D). E: average distention-evoked fiber activity was significantly higher in fibers from DSS (n = 89) vs. those from control rats (n = 54). F: visceromotor response to graded CRD was significantly greater in DSS (n = 5 rats) vs. control rats (n = 6). *P < 0.05, DSS vs. rats. EMG, electromyographic activity.
Next, we investigated whether DSS colitis concurrently sensitizes the evoked responses to colorectal distension. The firing frequencies of neurons in response to CRD in DSS rats were significantly greater than in control rats (Fig. 2E, P < 0.05). The firing frequencies in evoked responses ranged from 10.5 ± 1.2 Hz to 26.5 ± 2.0 Hz in DSS and control rats at distension pressures of 20 mmHg to 80 mmHg. The frequencies of action potentials in all evoked responses were several-fold greater than the frequency of spontaneous activity. We also found a significant increase in visceromotor response (VMR) to graded CRD in DSS rats vs. control rats (Fig. 2F, P < 0.05). These findings indicated concurrent increase in the frequency of spontaneous activity and evoked response to CRD in DSS rats.
Ongoing abdominal discomfort/pain in DSS rats.
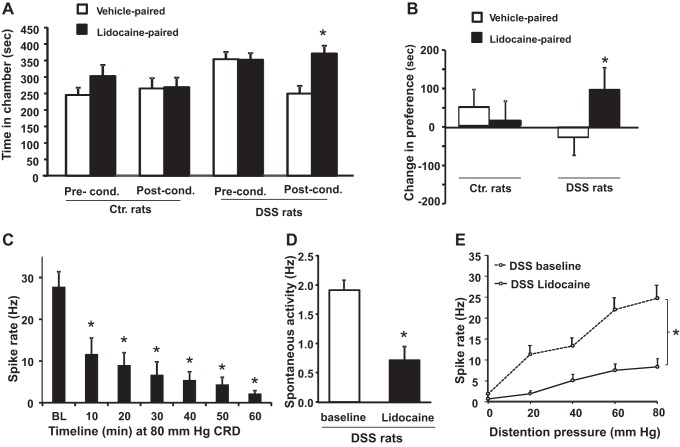
We used CPP test to investigate whether DSS treatment induced ongoing abdominal discomfort/aversive state. As previously shown in models of neuropathic or chronic inflammatory pain, relief of pain/discomfort by analgesic treatment is rewarding and reinforces behaviors associated with the treatment (11, 18). We infused the colon with 2% lidocaine jelly to impair afferent nerve activity, and we assessed the ability of control and DSS rats to associate analgesic treatment with a chamber providing relief. Prior to conditioning, neither DSS nor control rats exhibited a significant preference for either chamber (Fig. 3A). Following conditioning, lidocaine-treated DSS rats spent significantly greater time in the lidocaine-associated chamber compared with the vehicle-paired chamber (371 ± 24 s vs. 250 ± 23 s, P < 0.05; Fig. 3A); lidocaine-treated controls displayed no significant difference in time spent in lidocaine vs. vehicle paired chamber (303 ± 34 s vs. 245 ± 22 s, P = >0.05, Fig. 3A). When we calculated the change in preference for the lidocaine-paired chamber induced by conditioning, only the lidocaine-conditioned DSS rats displayed a significantly greater change in preference for the lidocaine-paired chamber compared with the control rats (94.3 ± 24.3 vs. −31.8 ± 58.9; P < 0.05). Change in preference exhibited by lidocaine-conditioned controls was not significantly different from that of unconditioned vehicle-treated controls (13.4 ± 67.5 vs. 48.9 ± 46.6) (Fig. 3B).
Fig. 3.
Reduction of colon afferent fiber activity by intracolonic application of lidocaine produces a conditioned place preference in DSS, but not in control rats. A: time spent in the lidocaine- or vehicle-paired chambers before conditioning and during the postconditioning test in DSS and control rats (n = 15 and 12, respectively). Lidocaine-treated DSS rats spent a significantly greater time in the lidocaine-paired chamber, whereas controls showed no significant difference. B: after conditioning, lidocaine-conditioned DSS rats exhibited a significantly greater increase in average change in preference for the lidocaine-paired chamber than the lidocaine-conditioned control rats. C: intracolonic lidocaine produced significant decrease in average single fiber activity recorded during a 20-s 80-mmHg colon distention delivered at 10-min intervals in DSS rats. D: lidocaine treatment significantly decreased spontaneous activity of CRD-responsive fibers in DSS rats. E: lidocaine significantly inhibited distention evoked single fiber activity in DSS rats at all distention pressures tested. *P < 0.05 DSS vs. control (Ctr.) rats.
We confirmed that lidocaine jelly significantly reduced colon afferent activity by measuring the ongoing spontaneous activity and evoked responses to CRD in anesthetized DSS rats before and after lidocaine treatment. The application of intraluminal lidocaine time dependently decreased action potential frequency in response to 80 mmHg distension of the colon (Fig. 3C). Within 10 min of its application, lidocaine treatment produced a significant decrease in ongoing spontaneous nerve discharge rate compared with pretreatment baseline; the decrease lasted for at least 1 h. (Fig. 3D). Lidocaine treatment also significantly decreased the CRD-evoked discharge frequencies at all distention pressures tested (Fig. 3E). These findings demonstrate that lidocaine treatment significantly suppresses both the ongoing spontaneous activity and afferent nerve hypersensitivity to CRD. Note that no CRD was applied during CPP conditioning. Therefore, the ongoing pain/discomfort likely originated from the increase of spontaneous activity generated by colon afferents in DSS rats. The lack of lidocaine induced place preference in control rats suggested that colonic lidocaine did not produce reward in these rats.
RTX reverses anxiety- and depression-like behavior in DSS rats.
We investigated whether increased spontaneous activity in DSS rats contributed to anxiety- and depression-like behavior by inducing prolonged desensitization of transient receptor potential vanilloid 1 (TRPV1) expressing colonic afferents. We infused RTX into the colons of DSS and control rats on days 6, 7, and 8 after the start of DSS treatment and performed the anxiety and depression tests between day 10 and day 20 after the start of DSS treatment. In a test of anxiety-like behavior, RTX treatment significantly increased the number of entries into the open arm of the EPM without altering behavior in control rats (Fig. 4A). In the forced swim test for depression-like behavior, RTX treatment significantly decreased the number of immobile episodes (Fig. 4C), and the immobile time in DSS, but not in control rats (Fig. 4D). Following behavior testing, we measured the ongoing spontaneous activity and CRD-evoked response activity. RTX treatment significantly reduced both spontaneous and CRD evoked activities in colon afferents (Fig. 4, D and E). These findings indicated that the spontaneous activity in colon afferents contributed to the generation of anxiety- and depression-like behaviors in DSS rats, as CRD was not applied during the above period, except for testing the efficacy of RTX in blocking colon afferent nerve activity at the end.
Fig. 4.
Resiniferatoxin (RTX) treatment reduced afferent nerve activity, as well as anxiety- and depression-like behaviors in DSS rats. A: number of open arm entries made by DSS+RTX rats in the EPM was not significantly different from Ctr. + Veh. However, DSS+Veh. rats made significantly fewer open arm entries vs. Ctr.+Veh. rats. The number of immobile episodes (B) and the time spent immobile (C) in the forced swim test by DSS+RTX rats were not significantly different from those in Ctr.+Veh. However, DSS+Veh. rats had significantly more immobile episodes and spent more time immobile vs. Ctr.+Veh rats. D: RTX treatment significantly reduced the frequency of spontaneous activity in DSS rats vs. DSS+Veh. rats. E: RTX treatment significantly reduced colon afferent firing frequencies in response to graded CRD in DSS rats. *P < 0.05, DSS vs. control (Ctr.) rats.
Sensitization of L6-S2 DRG neurons.
We identified the colon-projecting neurons by uptake of the retrograde label DiI, as described previously (33). In patch-clamp studies, 51.1% (47 out of 92, n = 7 rats) of colon-projecting afferent neurons isolated from DSS rats displayed sustained action potential firing for a mean duration of 20.3 ± 6.7 s following current injection for 30 s. By contrast, only 19.4% (7 out of 36, n = 7 rats; P < 0.05 vs. DSS rats) of neurons from control rats fired action potentials for a mean duration of 11.5 ± 4.2 s (P < 0.05 vs. DSS rats) (Fig. 5, A and B). The frequency of evoked action potentials was significantly greater in DSS (3.58 ± 0.39/s) vs. control rats (1.11 ± 0.23/s; n = 7, P < 0.05). The resting membrane potential in colon-projecting DRG neurons from the DSS rats (−45.1 ± 0.49 mV) was significantly reduced compared with that of neurons from the control rats (−53.0 ± 1.3 mV; n = 7, P < 0.05; Fig. 5C). The rheobase was significantly lower in DSS than in control rats (Fig. 5C). The number of action potentials induced by 2× and 3× rheobase was significantly greater in neurons from the DSS (1.96 ± 0.08 and 3.39 ± 0.16) vs. the control rats (1.29 ± 0.18 and 2.29 ± 0.29; Fig. 5, E and F). These findings suggested an increase in the sensitivity of colon-specific DRG neurons in generating a series of spontaneous activity-like action potentials (5).
Fig. 5.
Evaluation of excitability of acutely dissociated DiI-labeled colon projecting lumbar-sacral dorsal root ganglion (DRG) neurons in patch-clamp experiments. A: representative traces show action potentials evoked by 30-s constant current injection recorded from a DiI-labeled neuron from a Ctr. (top) or DSS (bottom) rat. B: bar graph shows the percent of DiI-labeled neurons from control and DSS rats that exhibited sustained action potential firing in response to current injection. Average resting membrane potential (C; RMP) was significantly depolarized and rheobase (D) was significantly less in neurons from DSS vs. control. The average number of action potentials evoked in neurons during a 400-ms current pulse from DSS rats was significantly greater than in Ctr. rats at 2× rheobase (E) and 3× rheobase (F). *P < 0.05 DSS vs. control (Ctr.) rats.
Effect of DSS-induced colitis on the expression of sodium, potassium, and TRP channels and brain-derived neurotrophic factor.
We found that DSS inflammation significantly enhanced brain-derived neurotrophic factor (BDNF) (Fig. 6A; n = 6, P < 0.05), transient receptor potential ankyrin 1 (TRPA1) and TRPV1 mRNA levels (Fig. 6B; n = 5 or 6, P < 0.05) in colon-projecting DRG neurons, while it decreased the mRNA expression of Kv1.4 and Kv1.1 vs. the control rats (Fig. 6B; n = 5 or 6; P < 0.05). The expression of Nav1.8 mRNA increased, but it did not reach statistical significance (Fig. 6B; n = 5 or 6, P = 0.09).
Fig. 6.

Bar graphs show quantitative RT-PCR. mRNA levels of brain-derived neurotrophic factor (BDNF) (A) and five cation channels, Kv1.1, Kv1.4, Nav1.8, transient receptor potential vanilloid 1 (TRPV1), and transient receptor potential ankyrin 1 (TRPA1) (B) in colon projecting S1 DRG neurons from DSS and Ctr. rats. *P < 0.05 DSS vs. control (Ctr.) rats.
Inflammation.
We measured the generation of select inflammatory mediators in the muscularis externa (ME), mucosa/submucosa (M/SM), plasma and cerebrospinal fluid to investigate whether they correlate with affective symptoms. DSS-treatment significantly increased myeloperoxidase (MPO) activity, IL-1β and IL-6 in the ME and MPO, IL-1β, and TNF-α in the M/SM by day 7 (Fig. 7, A, B, D). MPO activity decreased significantly by day 15 in both ME and M/SM but remained significantly upregulated in the ME vs. controls at this time (Fig. 7A). However, IL-1β, IL-6, and TNF-α remained upregulated in the ME, but not in M/SM on day 15. By contrast, none of the inflammatory mediators was elevated in the plasma or the CSF during DSS inflammation. (Fig. 7, B, C, and D). Together, these findings indicate that DSS-induced colitis did not provoke a systemic or central nervous system (CNS) inflammatory response.
Fig. 7.
Inflammation is restricted to the colon in DSS rats on day 7 and day 15. A: myeloperoxidase (MPO) in extracts from the colon muscularis externa (ME) and mucosa/submucosa (MSM) from DSS and Ctr. rats on day 7 and day 15. B–D: bar graphs comparing IL-1β concentrations in colon ME and MSM, cerebrospinal fluid (CSF), and plasma from control and DSS rats on day 7 and day 15. *P < 0.05 DSS vs. control (Ctr.) rats.
DISCUSSION
Inflammation induced by spinal cord injury induces intractable spontaneous pain in humans (30). Studies in animal models found that nerve lesions in these injuries sensitize the afferent and spinal cord neurons to increase the frequency of spontaneous activity and evoked responses (5, 12). CPP tests showed that the blockade of spontaneous activity with lidocaine or clonidine induced negative reinforcement, indicating relief of spontaneous neuropathic pain. In contrast to the spinal cord injury, inflammation in ulcerative colitis patients is not known to induce ongoing spontaneous pain; these patients experience intermittent abdominal pain, each episode of which is evoked by a strong compression of the colon by a giant migrating contraction (10), indicating mechanical hyperalgesia. Our findings show for the first time that ulcerative colitis-like inflammation may instead induce an aversive/discomfort state (28). The DSS rats showed preference for the lidocaine-paired chamber in the CPP test, suggesting ongoing discomfort. The same dose of lidocaine applied to the colon in anesthetized rats significantly suppressed the spontaneous activity, suggesting that the ongoing spontaneous activity may induce discomfort in ulcerative colitis-like inflammation.
The precise nature of discomfort remains unidentified in animals. However, abdominal discomfort is one of the prominent symptoms in IBS patients in the absence of overt inflammation (25). An animal model of IBS-like visceral hypersensitivity to colorectal distension induced by neonatal colon irritation found an increase of spontaneous activity in afferent neurons (19). It appears, therefore, that the increase of spontaneous activity in the colon-projecting afferents by inflammatory or noninflammatory mechanisms may induce abdominal discomfort.
We found for the first time that in addition to abdominal discomfort, ulcerative colitis-like inflammation also induces anxiety- and depression-like behaviors in rats. These mood disorders persisted after the inflammation had subsided in the mucosa/submucosa, suggesting persistent sensitization of the visceral afferents. These findings agree with clinical observations that anxiety and depression develop at the onset of IBD or shortly thereafter, and the severity of anxiety and depression correlates positively with the intensity of disease activity, and that the neurological symptoms continue during remission (14). However, controversy has persisted about whether the onset of IBD causes mood disorders or mood disorders cause the onset of IBD. Our findings provide evidence that colon inflammation can induce anxiety- and depression-like behaviors by sensitizing colon afferents to increase the frequency of spontaneous activity; the long-term blockade of spontaneous activity by RTX reversed anxiety- and depression-like behaviors.
Regarding depression-related behavior, the forced swim test and the sucrose preference test results offer good evidence that ulcerative colitis-like inflammation produces a depressive phenotype in rats. The results of the social interaction test were less clear, as rats spent an “increased” amount of time in contact rather than the predicted “decrease”. This particular social interaction test has been used successfully in rats (15) to model the social withdrawal seen as a major symptom of major depression. However, these prior experiments were employed for addiction studies, not for chronic discomfort. We interpret the increased contact time combined with decreased number of contacts as comfort-seeking rather than typical social behavior. It is noteworthy that intrarectal lidocaine treatment provides relief from intermittent evoked pain in IBS patients; however, lidocaine's effect on ongoing abdominal discomfort was not reported in this study (31).
The average total distance traveled in EPM by DSS rats was not significantly different from controls, suggesting no impairment of the ambulatory activity in DSS rats. This lack of effect on mobility makes it unlikely that reduced swimming time in FSS was due to sickness behavior. It is likely that acute DSS-induced colitis produces sickness behavior during the height of the inflammatory response (e.g., day 5). However, we performed the behavior tests during the period of day 10 to day 20, when colonic inflammation had significantly subsided. In addition, the DSS rats exhibited normal grooming behavior and muscle tone and no substantial weight loss during the behavior studies. These considerations make it unlikely that the results of the social interaction test, sucrose neophobia, or sucrose preference were due to sickness behavior.
It appears that peripheral inflammation/injury might induce multiple types of neuropathies, including sensitization of evoked pain, ongoing neuropathic pain, ongoing aversive state/discomfort, and anxiety and depression-like behaviors. The mechanisms by which peripheral inflammation/injury cause different types of neuropathies are multifactorial and remain largely unknown. However, the coding of afferent signals is the initiating factor for neuropathies induced by a peripheral insult. The frequency of afferent discharges is one of the factors that distinguish evoked pain from other neuropathies. The frequency of spontaneous activity in naïve rats ranges from ∼0.3 Hz to 1.0 Hz. (32, 36). Colon inflammation increased this frequency to ∼2 Hz in our study, while modified spinal nerve axotomy/nerve ligation increased it to about 4 Hz. (8, 12). The ongoing discharges within this range were associated with aversive state/discomfort and anxiety- and depression-like behaviors. By contrast, the discharge frequency of evoked action potentials is several-fold greater (10 to 30 Hz in colon afferents and 10 to 120 Hz in dorsal horn neurons at various distension pressures). It is likely that other parameters of spontaneous activity, such as the recruitment of different types of afferent fibers, (C and Aδ, Aβ fibers) (8, 17, 32, 35) and their differential projection sites in the brain structures also play a role in inducing specific neuropathies in clinical diseases, such as autoimmune diseases, metabolic disease, cardiovascular disease, and cancer. Each disease or type of injury (4) induces a unique profile of inflammatory mediators (cytokines, chemokines, free radicals, iNOS, and metabolites of arachidonic acid) and, therefore, the nerve lesion and the resulting modifications of afferent neurons to induce different neuropathies.
The intracolonic lidocaine treatment significantly suppressed the spontaneous activity, as well as the anxiety- and depression-like behavior. There is little systemic accumulation of lidocaine at this dose (37). The brain-gut axis consists of neurological, hormonal and immunological routes of communication. Our data support the hypothesis that the ongoing spontaneous activity in pelvic afferent neurons induces the anxiety- and depression-like behaviors in ulcerative colitis-like inflammation. We found no significant increases in plasma or cerebrospinal fluid levels of inflammatory cytokines in DSS rats, indicating that immunological inputs are unlikely to contribute to the above neuropathies. Some studies found that systemic administration of LPS or cytokines of the innate immunity at pharmacologic or therapeutic doses in animals and humans induced neurological disorders (24, 27). However, in most diseases, including IBD, the plasma concentrations of cytokines are low or not different from those in control subjects (13, 23), as seen in our study. Since intracolonic administration of lidocaine would also suppress vagal afferent activity, we cannot rule out a potential vagal contribution.
Voltage- and ligand-gated channels play critical roles in the generation and propagation of action potentials in excitable cells. We found that colon inflammation significantly increased the mRNA levels of TRPV1 and TRPA1, while it decreased those of Kv1.1 and Kv1.4 in colon-projecting neurons. The combined effect of these changes was to make the neurons more excitable by depolarizing the resting membrane potential and decreasing rheobase, resulting in an increase of spontaneous activity frequency (2). It is noteworthy that long-term desensitization of TRPV1-expressing afferents with the TRPV1 channel agonist, RTX, suppressed the spontaneous activity, as well as alleviated the anxiety- and depression-like behavior in DSS rats. Our findings and those of other investigators (5, 12, 34) show that peripheral inflammatory insult concurrently sensitizes both the evoked responses and spontaneous activity in both LT and HT fibers, which suggest that at least some of the molecular changes noted above contribute to the concurrent potentiation of both effects.
In conclusion, the novel findings of our study are 1) ulcerative colitis-like colon inflammation induces mood disorders and an aversive state, in addition to inducing hypersensitivity to colorectal distension; 2) increase in the frequency of spontaneous activity in colon afferents is one of the parameters that code the induction of ongoing discomfort and underlie anxiety- and depression-like behaviors; 3) the abdominal discomfort and mood disturbances persist after the inflammation has subsided in the colon mucosa and submucosa; 4) alterations in the expression of TRPV1, TRPA1, Nav1.8, Kv1.1, and Kv1.4 channels may contribute to the sensitization of colon afferents in ulcerative colitis-like inflammation. These channels may serve as potential targets of therapeutic intervention for mood disorders in IBD patients.
Perspectives and Significance
Chronic diseases are often associated with anxiety and depression. The two likely sources of these neuropathic disorders are 1) psychological stress induced by persistent morbidity of disease affecting the neuronal circuits regulating anxiety and depression and 2) aberrant afferent signals induced by peripheral inflammation arriving in the CNS to modulate central neural circuits regulating anxiety and depression. The induction of anxiety and depression may persist after the inflammatory response has subsided, suggesting that inflammation may induce long-lasting alterations in the expression of neurotrophins and ion channels in afferent neurons by epigenetic programming. These findings suggest that therapeutic agents targeting the afferent neurons may serve in managing anxiety and depression in diseases associated with peripheral inflammation. Future research directions include whether adverse early life experiences alter ongoing signals in afferent neurons to induce anxiety and depression in diseases that do not show peripheral inflammation, such as functional bowel disorders.
GRANTS
This article was supported, in part, by National Institute of Diabetes and Digestive and Kidney Diseases Grant 5R01DK088796.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.C., J.H.W., Y.F., J.G., and K.L.J. performed experiments; J.C., J.H.W., and Y.F. analyzed data; J.C., J.H.W., X.-Z.S., T.A.G., and S.K.S. interpreted results of experiments; J.C., J.H.W., and Y.F. prepared figures; J.C. and S.K.S. drafted manuscript; J.H.W., T.A.G., and S.K.S. edited and revised manuscript; S.K.S. conception and design of research; S.K.S. approved final version of manuscript.
REFERENCES
- 1.Addolorato G, Capristo E, Stefanini GF, Gasbarrini G. Inflammatory bowel disease: a study of the association between anxiety and depression, physical morbidity, and nutritional status. Scand J Gastroenterol 32: 1013–1021, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Amir R, Michaelis M, Devor M. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci 19: 8589–8596, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews H, Barczak P, Allan RN. Psychiatric illness in patients with inflammatory bowel disease. Gut 28: 1600–1604, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 133: 433–447, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedi SS, Yang Q, Crook RJ, Du J, Wu Z, Fishman HM, Grill RJ, Carlton SM, Walters ET. Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci 30: 14,870–14,882, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res 125: 141–149, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139: 2102–2112, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Chapman V, Suzuki R, Chamarette HL, Rygh LJ, Dickenson AH. Effects of systemic carbamazepine and gabapentin on spinal neuronal responses in spinal nerve ligated rats. Pain 75: 261–272, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Winston JH, Sarna SK. Neurological and cellular regulation of visceral hypersensitivity induced by chronic stress and colonic inflammation in rats. Neuroscience 248C: 469–478, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol 96: 1499–1506, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Davoody L, Quiton RL, Lucas JM, Ji Y, Keller A, Masri R. Conditioned place preference reveals tonic pain in an animal model of central pain. J Pain 12: 868–874, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci 26: 1281–1292, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiocchi C, Fukushima K, Strong SA, Ina K. Pitfalls in cytokine analysis in inflammatory bowel disease. Aliment Pharmacol Ther 10Suppl 2: 63–69; discussion 70–61, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Goodhand JR, Wahed M, Mawdsley JE, Farmer AD, Aziz Q, Rampton DS. Mood disorders in inflammatory bowel disease: relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm Bowel Dis 18: 2301–2309, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Green TA, Alibhai IN, Hommel JD, DiLeone RJ, Kumar A, Theobald DE, Neve RL, Nestler EJ. Induction of inducible cAMP early repressor expression in nucleus accumbens by stress or amphetamine increases behavioral responses to emotional stimuli. J Neurosci 26: 8235–8242, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, Bass CE, Terwilliger EF, Bardo MT, Nestler EJ. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry 67: 28–35, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kajander KC, Bennett GJ. Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in A beta and A delta primary afferent neurons. J Neurophysiol 68: 734–744, 1992. [DOI] [PubMed] [Google Scholar]
- 18.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci 12: 1364–1366, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res 971: 73–82, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Mikocka-Walus AA, Turnbull DA, Moulding NT, Wilson IG, Andrews JM, Holtmann GJ. Controversies surrounding the comorbidity of depression and anxiety in inflammatory bowel disease patients: a literature review. Inflamm Bowel Dis 13: 225–234, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Minderhoud IM, Oldenburg B, Wismeijer JA, van Berge Henegouwen GP, Smout AJ. IBS-like symptoms in patients with inflammatory bowel disease in remission; relationships with quality of life and coping behavior. Dig Dis Sci 49: 469–474, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Mittermaier C, Dejaco C, Waldhoer T, Oefferlbauer-Ernst A, Miehsler W, Beier M, Tillinger W, Gangl A, Moser G. Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow-up study. Psychosom Med 66: 79–84, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Pollmacher T, Haack M, Schuld A, Reichenberg A, Yirmiya R. Low levels of circulating inflammatory cytokines—do they affect human brain functions? Brain Behav Immun 16: 525–532, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55: 453–462, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sach J, Bolus R, Fitzgerald L, Naliboff BD, Chang L, Mayer EA. Is there a difference between abdominal pain and discomfort in moderate to severe IBS patients? Am J Gastroenterol 97: 3131–3138, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Sarna SK. Enteric descending and afferent neural signaling stimulated by giant migrating contractions: essential contributing factors to visceral pain. Am J Physiol Gastrointest Liver Physiol 292: G572–G581, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Schaefer M, Engelbrecht MA, Gut O, Fiebich BL, Bauer J, Schmidt F, Grunze H, Lieb K. Interferon alpha (IFNα) and psychiatric syndromes: a review. Prog Neuropsychopharmacol Biol Psych 26: 731–746, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Serra J. Overview of neuropathic pain syndromes. Acta Neurol Scand Suppl 173: 7–11; discussion 48–52, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Shi XZ, Winston JH, Sarna SK. Differential immune and genetic responses in rat models of Crohn's colitis and ulcerative colitis. Am J Physiol Gastrointest Liver Physiol 300: G41–G51, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103: 249–257, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Verne GN, Sen A, Price DD. Intrarectal lidocaine is an effective treatment for abdominal pain associated with diarrhea-predominant irritable bowel syndrome. J Pain 6: 493–496, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Weng X, Smith T, Sathish J, Djouhri L. Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current (Ih) in C- but not Adelta-nociceptors. Pain 153: 900–914, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology 138: 294–304e293, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, Weerahandi HM, Campbell JN, Griffin JW, Meyer RA. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J Neurosci 22: 7746–7753, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: Abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-l-carnitine. Pain 135: 262–270, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao WH, Bennett GJ. Persistent low-frequency spontaneous discharge in A-fiber and C-fiber primary afferent neurons during an inflammatory pain condition. Anesthesiology 107: 813–821, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Q, Price DD, Verne GN. Reversal of visceral and somatic hypersensitivity in a subset of hypersensitive rats by intracolonic lidocaine. Pain 139: 218–224, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]