Abstract
This study examined the role of β-adrenergic and opioid receptors in spinal reflex bladder activity and in the inhibition induced by pudendal nerve stimulation (PNS) or tibial nerve stimulation (TNS). Spinal reflex bladder contractions were induced by intravesical infusion of 0.25% acetic acid in α-chloralose-anesthetized cats after an acute spinal cord transection (SCT) at the thoracic T9/T10 level. PNS or TNS at 5 Hz was applied to inhibit these spinal reflex contractions at 2 and 4 times the threshold intensity (T) for inducing anal or toe twitch, respectively. During a cystrometrogram (CMG), PNS at 2T and 4T significantly (P < 0.05) increased bladder capacity from 58.0 ± 4.7% to 85.8 ± 10.3% and 96.5 ± 10.7%, respectively, of saline control capacity, while TNS failed to inhibit spinal reflex bladder contractions. After administering propranolol (3 mg/kg iv, a β1/β2-adrenergic receptor antagonist), the effects of 2T and 4T PNS on bladder capacity were significantly (P < 0.05) reduced to 64.5 ± 9.5% and 64.7 ± 7.3%, respectively, of the saline control capacity. However, the residual PNS inhibition (about 10% increase in capacity) was still statistically significant (P < 0.05). Propranolol treatment also significantly (P = 0.0019) increased the amplitude of bladder contractions but did not change the control bladder capacity. Naloxone (1 mg/kg iv, an opioid receptor antagonist) had no effect on either spinal reflex bladder contractions or PNS inhibition. At the end of experiments, hexamethonium (10 mg/kg iv, a ganglionic blocker) significantly (P < 0.05) reduced the amplitude of the reflex bladder contractions. This study indicates an important role of β1/β2-adrenergic receptors in pudendal inhibition and spinal reflex bladder activity.
Keywords: urinary bladder, neuromodulation, pudendal, tibial, cat
overactive bladder (oab) is a significant clinical problem that is estimated to affect more than 30 million adults in the United States (2). OAB is characterized by urinary urgency, frequency, and often incontinence, which can significantly impact the quality of life. Currently, antimuscarinic drugs are first-line therapy for OAB, but these drugs are associated with significant side effects and moderate efficacy causing a mediocre long-term compliance (1, 4). The U.S. Food and Drug Administration recently approved mirabegron (a β3-adrenergic receptor agonist) for the treatment of OAB with reduced side effects (3). In addition to the drug treatments, both pudendal and tibial neuromodulation have also been shown to be effective in OAB treatment (19, 20). However, the mechanisms underlying neuromodulation therapies for OAB are currently not fully understood.
Previous studies in cats have shown that both pudendal nerve stimulation (PNS) and tibial nerve stimulation (TNS) can inhibit bladder overactivity induced by bladder irritation with intravesical acetic acid (14, 15, 23). Opioid receptors play a major role in TNS inhibition of bladder overactivity but have no role in PNS inhibition (15, 23). However, PNS inhibition can involve multiple neurotransmitter mechanisms, including glutamate (14), GABA (25), and serotonin (22). These neurotransmitter mechanisms could occur at the spinal and/or supraspinal sites. Identification of the site of action of different neurotransmitter mechanisms involved in PNS or TNS inhibition of bladder overactivity in animals may provide insights into the mechanisms underlying the clinical use of pudendal or tibial neuromodulation for treatment of OAB.
The purpose of this study is to determine the role of β-adrenergic and opioid receptors in PNS or TNS inhibition of spinal reflex bladder activity in α-chloralose-anesthetized cats. A spinal cord transection (SCT) was performed at the thoracic T9/T10 spinal level to eliminate the nonnociceptive afferent Aδ-fiber-mediated supraspinal micturition reflex (7, 11). Acute SCT cats were studied instead of chronic SCT cats to investigate the effect of neuromodulation on the spinal bladder reflex that was not changed/reorganized by chronic SCT. Dilute (0.25%) acetic acid was then used to irritate the bladder, activate the nociceptive bladder afferent C-fibers (12), and induce the spinal reflex bladder contractions. PNS or TNS was applied to inhibit these spinal reflex bladder contractions. Propranolol (a β1/β2-adrenergic receptor antagonist with weaker affinity for β3-adrenergic receptors) or naloxone (an opioid receptor antagonist) was administered intravenously to examine the role of these receptors in nociceptive bladder afferent C-fiber-mediated spinal reflex and PNS/TNS inhibition of this spinal reflex.
METHODS
The protocol and animal use in this study were approved by Animal Care and Use Committee at the University of Pittsburgh.
Surgical procedure.
Ten cats (5 males and 5 females, 3.0–4.1 kg, Liberty Research, Waverly, NY) were anesthetized with isoflurane (2–3% in oxygen) during surgery and then with α-chloralose (65 mg/kg iv and supplemented as needed) during data collection. A tube was inserted via a tracheotomy to keep the airway patent. A catheter was placed into the right carotid artery to monitor blood pressure. Another catheter was placed into the right cephalic vein to allow for the intravenous injection of drugs or fluids. A pulse oximeter (9847V, NONIN Medical, Plymouth, MN) was placed on the tongue to monitor heart rate and oxygen saturation.
Through an abdominal incision, the ureters were isolated, tied, and cut for external drainage of urine. A double lumen catheter was inserted into the bladder through a small cut at the proximal urethra and secured by a ligature around the urethra. One lumen of the catheter was used to infuse (1–2 ml/min) either saline or dilute (0.25%) acetic acid (AA), while the other lumen was used to record intravesical pressure. The same infusion rate was used in the same animal throughout the entire experiment. A small incision on the medial side of the left ankle exposed the tibial nerve for implanting a bipolar cuff electrode (NC222pt, MicroProbe, Gaithersburg, MD). Another incision was made lateral to the tail in the right sciatic notch to expose the right pudendal nerve for implanting a tripolar cuff electrode (NC223pt, MicroProbe). The cuff electrodes were connected to a stimulator (S88; Grass Medical Instruments, Quincy, MA) via a constant voltage stimulus isolator (SIU5; Grass Medical Instruments). A switch was inserted between the electrodes and the stimulus isolator so that the pudendal or tibial nerve could be stimulated individually. A laminectomy was performed to expose the spinal cord at the thoracic (T9/T10) level for a complete SCT during the experiment. The spinal cord was covered with saline-soaked cotton and then all incisions were closed by sutures.
Experimental protocol.
On the basis of previous studies (14, 23), uniphasic rectangular pulses of 0.2-ms pulse width and 5-Hz frequency were used for PNS or TNS. The stimulation threshold (T) defined as the minimal intensity for inducing anal/toe twitch was determined at the beginning of the experiment. Initially, before transecting the spinal cord, cystometrograms (CMGs) were performed by slowly infusing the bladder with saline to determine the bladder capacity, defined as the bladder volume threshold required to induce a micturition contraction of large amplitude (>30 cmH2O) and long duration (>20 s). Because the urethra was ligated to prevent bladder emptying, the bladder was emptied manually after each CMG by withdrawing the saline through the catheter. Multiple CMGs were performed to ensure reproducibility of the saline control capacity. Then, the spinal cord was completely transected at the T9/T10 level. Thirty minutes following the SCT, a saline CMG was performed to confirm the disappearance of the large-amplitude micturition contraction. Then, bladder infusion was changed from saline to 0.25% AA to irritate the bladder, activate the nociceptive bladder afferent C-fibers, and induce spinal reflex bladder activity. During the AA CMG, bladder capacity was determined by the volume threshold required to induce a bladder contraction of amplitude greater than 10 cmH2O. To ensure reproducibility of the AA control capacity, multiple CMGs were performed by filling and manually emptying the bladder. Then, the animals were divided into two groups.
In the first group (n = 5 cats), four CMGs were performed with AA infusion: 1) control CMG without PNS, 2) CMG during 2T PNS, 3) CMG during 4T PNS, and 4) control CMG without PNS to determine any poststimulation effect. Then, the four CMGs were performed again with TNS instead of PNS. At the end of the last CMG, the bladder remained full, and isovolumetric bladder contractions were established. Then, propranolol (3 mg/kg iv, a β1/β2-adrenergic receptor antagonist; Sigma Aldrich, St. Louis, MO) was administered during the isovolumetric bladder contractions. Ten minutes after propranolol treatment, the bladder was emptied, and the same CMG protocol, as above, was performed again. In the second group (n = 5 cats), the same CMG protocol, as above, was used before and 5 min after administering naloxone (1 mg/kg iv, an opioid receptor antagonists, Sigma Aldrich). The dosage of propranolol or naloxone was chosen to maximally block β1/β2-adrenergic receptors (9) or opioid receptors (18, 21, 24) in the micturition reflex pathway. In both experimental groups, at the end of the experiment, when the bladder was filled with AA and maintained under isovolumetric conditions, hexamethonium (10 mg/kg iv, a ganglionic blocker; Sigma Aldrich) was administered to suppress the spinal reflex bladder activity.
Data analysis.
Bladder capacity measured during each CMG was normalized to initial saline control capacity prior to SCT in the same animal. The amplitude of maximal bladder contractions was measured before and after a drug treatment to indicate the effect on spinal reflex bladder activity. Measurements were averaged across the animals for the same conditions and reported with standard error of the mean. Statistical significance was determined by a paired t-test or ANOVA followed by Dunnett (one-way) or Bonferroni (two-way) post hoc tests.
RESULTS
Effects of propranolol and naloxone on spinal reflex bladder contractions.
During saline CMGs, micturition contractions of large amplitude (>30 cmH2O) and long duration (>20 s) could be induced after filling the bladder to a volume ranging from 9.1 to 18 ml, i.e., the bladder capacity (average 13.7 ± 0.7 ml, n = 10 cats, Fig. 1A). After a complete SCT at T9/T10 level, this large micturition reflex disappeared during saline CMGs, even after the baseline bladder pressure reached more than 40 cmH2O at a much larger bladder volume (157.8 ± 11.8% of the saline control capacity; Fig. 1A). Infusing AA into the bladder, which is known to activate nociceptive afferent C-fibers, induced small-amplitude (<30 cmH2O) and short-duration (<20 s) bladder contractions at a significantly (P < 0.0001) smaller bladder volume ranging from 5.3 to 11.3 ml (average 57.1 ± 5.1% of the saline control capacity, Fig. 1, A and B). These small bladder contractions were not detected during saline infusion after SCT (see the middle trace in Fig. 1A).
Fig. 1.

Spinal reflex bladder activity induced by bladder irritation after acute spinal cord transection (SCT) at T9/T10 level. A: cystrometrogram (CMG) during saline infusion demonstrates no reflex bladder contractions after acute SCT, but infusion of 0.25% acetic acid (AA) induces small-amplitude bladder contractions. B: bladder capacity during 0.25% AA infusion is smaller than the saline capacity measured before SCT (n = 10 cats). The downward arrow on the AA CMG trace indicates where the AA bladder capacity is measured. *Significant (P < 0.0001) difference (paired Student's t-test). Note: Bladder capacity (%) was normalized to initial saline control capacity prior to SCT in each cat.
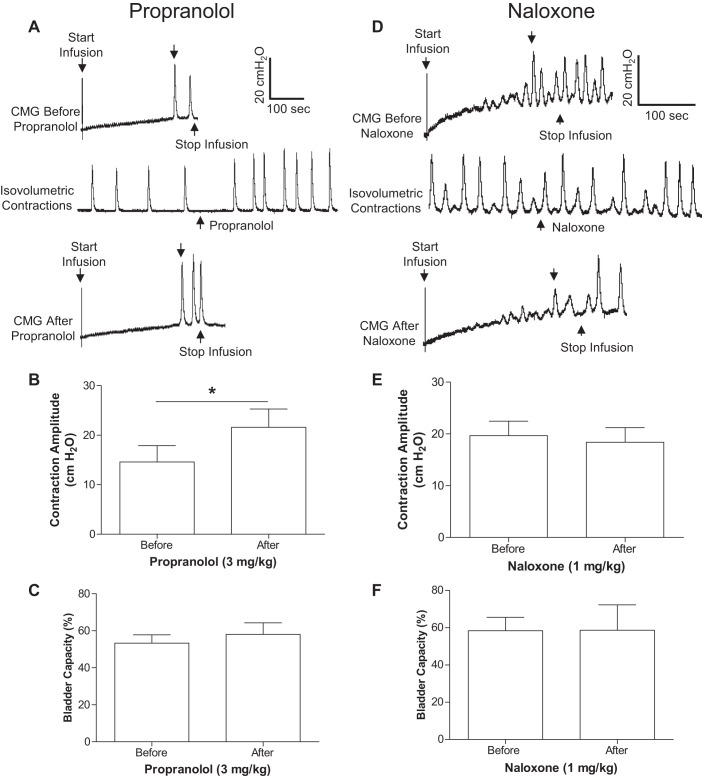
The small-amplitude bladder contractions were not only induced during AA infusion but also during isovolumetric conditions after stopping the AA infusion at bladder volumes about 20–30% above the AA bladder capacity (Fig. 2, A and D). Under these conditions, propranolol (3 mg/kg iv) significantly (P = 0.0019) increased the maximal amplitude of the bladder contractions from 14.9 ± 3.3 to 21.6 ± 3.7 cmH2O (Fig. 2B). However, it did not change the bladder capacity during CMGs (Fig. 2, A and C). Naloxone (1 mg/kg iv) did not alter either the maximal contraction amplitude or bladder capacity (Fig. 2, D–F). At the end of the experiment during AA-induced isovolumetric contractions, administration of a ganglionic blocking agent (hexamethonium, 10 mg/kg iv) significantly (P < 0.05) reduced the maximal contraction amplitude (>50%) in both propranolol and naloxone-pretreated cats (Fig. 3), showing that these bladder contractions were mediated by spinal reflex mechanisms since hexamethonium-sensitive peripheral ganglion reflexes have not been detected in the feline bladder.
Fig. 2.
Effects of propranolol or naloxone on spinal reflex bladder activity induced by acetic acid irritation. A–C: propranolol's effects on contraction amplitude and bladder capacity (n = 5 cats). D–F: naloxone's effects on contraction amplitude and bladder capacity (n = 5 cats). The downward arrow on CMG traces in A and D indicates where the bladder capacity is measured. *Significant (P = 0.0019) difference (paired Student's t-test). Note: Parts A–C and D–F were from different cats.
Fig. 3.
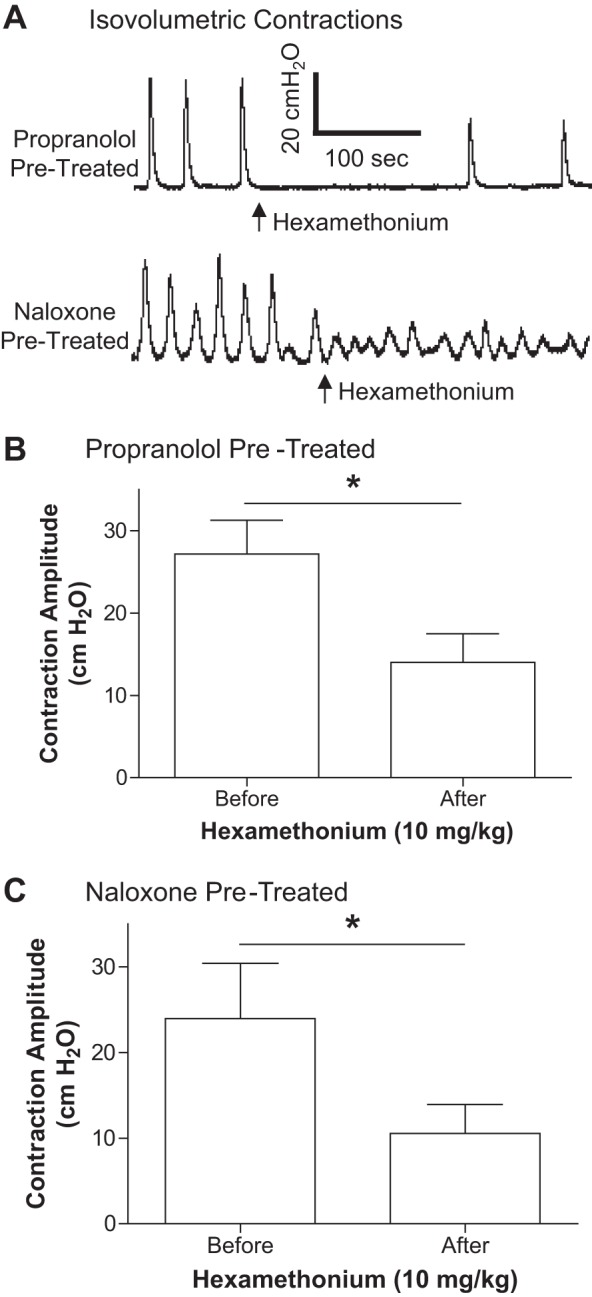
Hexamethonium reduced the amplitude of spinal reflex bladder contractions. Hexamethonium (10 mg/kg iv) was administered during isovolumetric contractions (A) and significantly reduced the bladder contraction amplitude in both propranolol (B) and naloxone (C)-treated cats. *Significant (P < 0.05) difference (paired t-test). n = 5 cats in both B and C.
Effects of propranolol and naloxone on PNS inhibition of spinal reflex bladder contractions.
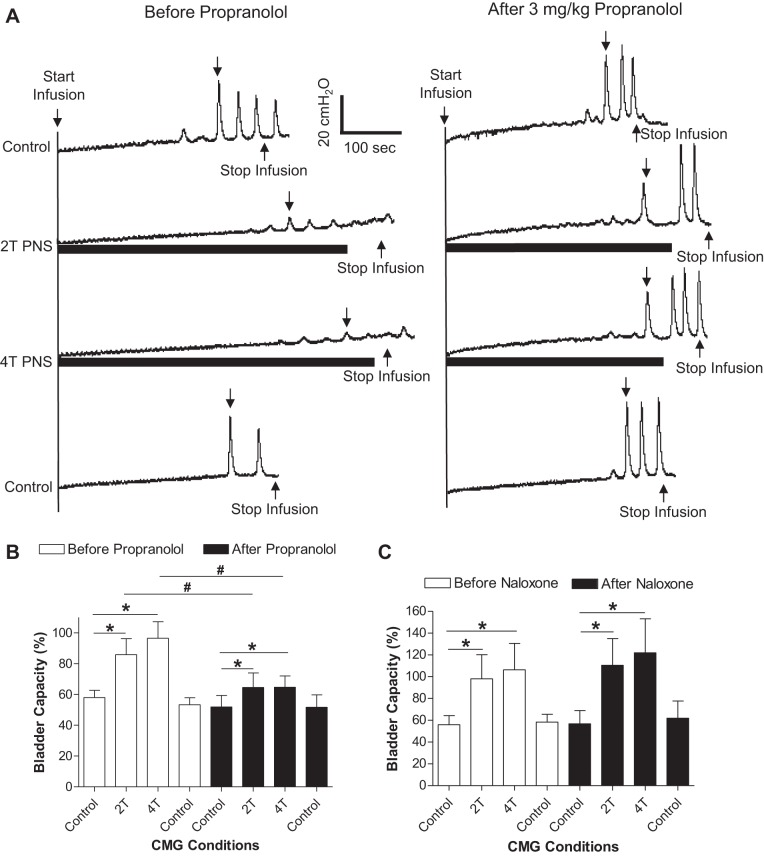
Before any drug treatment, PNS alone applied during AA CMGs significantly (P < 0.05) increased bladder capacity from 58.0 ± 4.7% to 85.8 ± 10.3% and 96.5 ± 10.7% at 2T and 4T intensity, respectively (Fig. 4 A and B). After administering propranolol (3 mg/kg i.v.), PNS inhibition was significantly (P < 0.05) reduced (Fig. 4, A and B). However, PNS still significantly (P < 0.05) increased the bladder capacity from 51.8 ± 7.6% of saline control to 64.5 ± 9.5% (2T) and 64.7 ± 7.3% (4T) after propranolol treatment (Fig. 4, A and B). In another group of cats, naloxone (1 mg/kg iv) did not alter the PNS inhibition (Fig. 4C).
Fig. 4.
Inhibition of spinal reflex bladder contractions by pudendal nerve stimulation (PNS) is reduced by propranolol, but not by naloxone. A: CMG tracings before and after propranolol administration. The downward arrow on the CMG tracing indicates where the bladder capacity is measured. B: propranolol (3 mg/kg) significantly reduced PNS inhibition (n = 5 cats). C: administration of naloxone (tracings not shown) had no effect on bladder capacity or PNS inhibition (n = 5 cats). *Significant (P < 0.05) difference from control (one-way ANOVA). #Significant (P < 0.05) difference before and after the drug treatment (two-way ANOVA).
TNS did not inhibit spinal reflex bladder contractions.
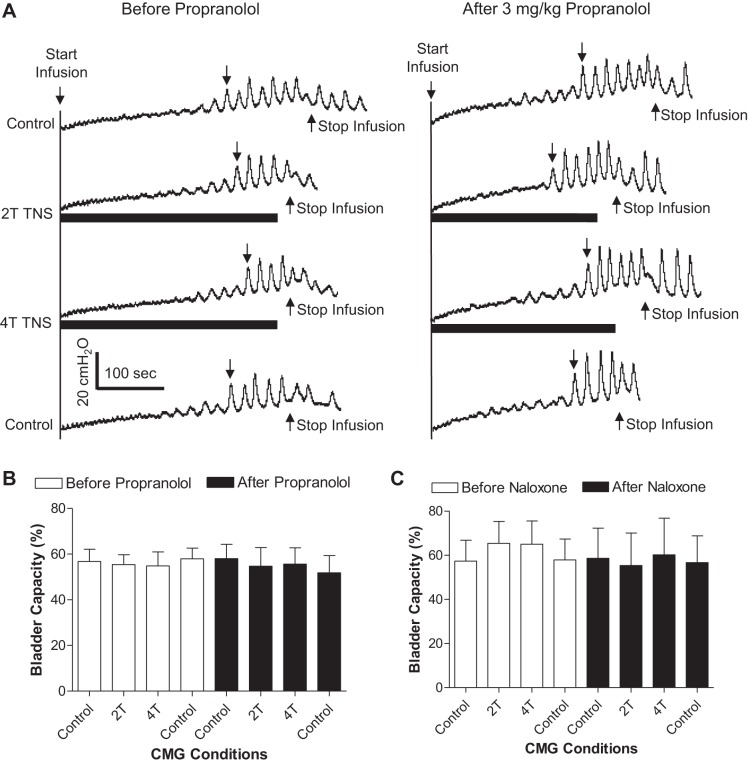
TNS at both 2T and 4T intensity did not significantly inhibit the spinal reflex bladder activity during AA CMGs (Fig. 5, A and B). Propranolol or naloxone treatment did not change the TNS effect on spinal reflex bladder activity (Fig. 5, B and C).
Fig. 5.
Tibial nerve stimulation (TNS) failed to inhibit spinal reflex bladder activity. A: CMG tracings before and after propranolol administration. The downward arrow on CMG tracing indicates where the bladder capacity is measured. B: TNS has no effect on bladder capacity before or after propranolol treatment (n = 5 cats). C: administration of naloxone (CMG tracings not shown) also had no effect on bladder capacity with/without TNS (n = 5 cats).
DISCUSSION
This study shows that in cats, spinal reflex bladder contractions can be induced after acute SCT by bladder irritation using 0.25% acetic acid (Figs. 1 and 3). PNS can inhibit these spinal reflex contractions (Fig. 4), but TNS has no effect (Fig. 5). Propranolol can significantly increase the amplitude of spinal reflex contractions (Fig. 2, A and B) and greatly reduce PNS inhibition (Fig. 4, A and B), but naloxone has no effect on either the spinal reflex contractions (Fig. 2, D–F) or PNS inhibition of these contractions (Fig. 4C).
It is known that bladder reflexes are initiated by two types of afferent nerves: Aδ-fibers and C-fibers (7, 11). During saline infusion, bladder distention activates the nonnociceptive afferent Aδ-fibers that trigger a spinobulbospinal micturition reflex (8), while the nociceptive afferent C-fibers are silent (12). Therefore, acute SCT at T9/T10 level eliminates the supraspinal reflex bladder contraction during saline distention (Fig. 1A). However, changing bladder infusion from saline to AA induces bladder contractions of small amplitude (<30 cmH2O) and short duration (<20 s) (Figs. 1A and 2, A and D). These small bladder contractions are mediated by spinal reflexes because they are sensitive to hexamethonium (a ganglionic blocking agent, Fig. 3) that blocks the efferent pathway from the spinal cord to the bladder. Although propranolol or naloxone was administered in this study before hexamethonium, our previous study showed the same sensitivity to hexamethonium in untreated cats (26). In addition, these small bladder contractions can be largely removed by lidocaine injection into the sacral spinal cord or transection of the sacral spinal roots and spinal cord (26). It is also known that the afferent C-fibers, which are inactive during saline distention, become activated during bladder irritation (12). Previous studies in cats have further demonstrated that the spinal C-fiber reflex can be activated after acute (5, 16) and chronic (7) SCT by electrical stimulation of bladder afferents and recording of efferent activity on the pelvic nerve. Therefore, it would be logical to conclude that in this study, AA irritation triggers a spinal bladder reflex by activating the nociceptive bladder afferent C-fibers.
Enkephalins and opioid receptors are known to play a role in the control of micturition in animals with an intact spinal cord (21). Naloxone can also enhance the bladder C-fiber reflex evoked by electrical stimulation of bladder afferents in the pelvic nerve (5, 17). However, this study shows that a large dose of naloxone (1 mg/kg iv) that is known to maximally block the opioid receptors in the spinobulbospinal micturition reflex pathway (18, 21) did not alter the bladder reflex activity in this study, indicating that the opioid receptors play no demonstrable role in the afferent C-fiber-mediated spinal bladder reflex induced by AA irritation (Fig. 2, D–F). Our previous studies in spinal intact cats (15, 23) have also shown that naloxone has no effect on bladder overactivity induced by AA irritation. These results indicate that while opioid receptors are involved in the regulation of the bladder C-fiber reflex elicited by electrical stimulation of bladder afferent nerves, their function is very limited in irritation-induced reflex bladder overactivity. In contrast, opioid receptors are very important in the supraspinal micturition reflex pathways mediated by nonnociceptive bladder afferent Aδ-fibers because naloxone intravenously, intracerebroventricularly, or intrathecally significantly enhances the micturition reflex in spinally intact cats (13, 18, 21). In addition, after chronic SCT, spinal reflex bladder activity can also be enhanced by naloxone (24), indicating a significantly different role of opioid receptors in bladder spinal reflexes after acute and chronic SCT.
On the other hand, β1/β2-adrenergic receptors appear to play a role in the modulation of the nociceptive afferent C-fiber-mediated spinal bladder reflex (Fig. 2, A–C). Propranolol increased the amplitude of spinal reflex bladder contractions (Fig. 2, A and B), indicating that tonic activation of β1/β2-adrenergic receptors presumably mediated by sympathetic efferent pathways in the hypogastic nerves or sympathetic chain can suppress the reflex detrusor contractions by acting at the sites in the bladder smooth muscle (6, 9, 10). Meanwhile, propranolol did not change bladder capacity (Fig. 2, A and C), which indicates that β1/β2-adrenergic receptors are not involved in controlling the afferent limb or the spinal pathways underlying the C-fiber-mediated spinal reflex.
Previous studies have shown that TNS can inhibit bladder overactivity induced by AA irritation in spinal intact cats (23). However, in this study, TNS failed to inhibit the AA-induced reflex bladder activity after acute SCT (Fig. 5). This result indicates that TNS inhibition of the bladder reflex requires intact supraspinal pathways. However, this does not necessarily mean that TNS inhibition only occurs in the brain because the afferent input to the brain activated by TNS could drive a descending spinal pathway to inhibit bladder reflexes in the spinal cord. SCT would eliminate the descending spinal pathway and the TNS inhibition. It is also possible that TNS inhibition only occurs on the supraspinal reflex activated by nonnociceptive Aδ bladder afferent. On the basis of this assumption, TNS inhibition of bladder overactivity induced by AA irritation in spinal intact cats (23) will be mainly due to inhibition of Aδ-fiber-mediated supraspinal reflex instead of the C-fiber-mediated spinal reflex. It seems clear that additional investigations are needed to determine the site of action for TNS inhibition. However, the result from this study supports that afferent input from the tibial nerve cannot directly inhibit the nociceptive afferent C-fiber-mediated spinal reflex bladder activity in the lumbosacral spinal cord. On the other hand, PNS is still able to inhibit the spinal reflex bladder activity after acute SCT (Fig. 4), indicating that significantly different mechanisms underlie PNS and TNS inhibition.
Propranolol significantly reduced PNS inhibition of the spinal reflex bladder contractions (Fig. 4), indicating that pudendal afferent input must drive the sympathetic efferent output in the hypogastric nerves to inhibit the bladder spinal reflex via β-adrenergic transmitter mechanisms. However, after propranolol treatment, PNS can still significantly inhibit spinal reflex bladder activity and increase bladder capacity (Fig. 4, A and B). This residual PNS inhibition could be due to sympathetic inhibition in bladder ganglia or muscle mediated by α2/β3-adrenergic receptors, or it might occur in the spinal cord where the pudendal afferent input can activate a group of inhibitory interneurons that have synaptic connections to the neurons in bladder spinal reflex pathway. These inhibitory spinal interneurons could be GABAergic (25) but not enkephalinergic since naloxone has no effect on PNS inhibition of the spinal reflex bladder activity (Fig. 4C). A previous study in spinally intact cats (15) also showed that opioid receptors play no role in PNS inhibition of bladder overactivity induced by AA irritation. Additional studies are needed to further determine the possible neurotransmitter mechanisms utilized by the spinal inhibitory interneurons during PNS inhibition.
Perspectives and Significance
This study in an acute SCT cat model revealed significantly different mechanisms between PNS and TNS inhibition of reflex bladder activity mediated by nociceptive afferent C-fibers. Sympathetic efferent activity and β1/β2-adrenergic receptors play an important role in modulating C-fiber-mediated spinal reflex bladder activity and in PNS inhibition of this spinal reflex activity, while opioid receptors play no role. Understanding neurotransmitter mechanisms underlying PNS or TNS inhibition is important for clinical application of pudendal or tibial neuromodulation in the treatment of bladder disorders.
GRANTS
This study is supported by the National Institutes of Diabetes and Digestive and Kidney Diseases under Grants DK-102427, DK-094905, DK-090006, and DK-091253.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.J.R., Z.X., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. conception and design of research; M.J.R., Z.X., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. performed experiments; M.J.R., Z.X., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. analyzed data; M.J.R., Z.X., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. interpreted results of experiments; M.J.R., Z.X., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. prepared figures; M.J.R., Z.X., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. drafted manuscript; M.J.R., Z.X., B.S., J.W., Z.S., J.R.R., W.C.d.G., and C.T. edited and revised manuscript; M.J.R., Z.X., B.S., J.W., Z.S., W.C.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1.Andersson KE, Pehrson R. CNS involvement in overactive bladder: pathophysiology and opportunities for pharmacological intervention. Drugs 63: 2595–2611, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology 77: 1081–1087, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Chapple CR, Kaplan SA, Mitcheson D, Klecka J, Cummings J, Drogendijk T, Dorrepaal C, Martin N. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β3-adrenoceptor agonist, in overactive bladder. Eur Urol 63: 296–305, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 54: 543–562, 2008. [DOI] [PubMed] [Google Scholar]
- 5.de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst 30 Suppl: S71–S77, 1990. [DOI] [PubMed] [Google Scholar]
- 6.de Groat WC, Lalley PM. Reflex firing in the lumbar sympathetic outflow to activation of vesical afferent fibres. J Physiol 226: 289–309, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst 3: 135–160, 1981. [DOI] [PubMed] [Google Scholar]
- 8.de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J Physiol 200: 87–108, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groat WC, Saum WR. Sympathetic inhibition of the urinary bladder and of pelvic ganglionic transmission in the cat. J Physiol 220: 297–314, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Groat WC, Theobald RJ. Reflex activation of sympathetic pathways to vesical smooth muscle and parasympathetic ganglia by electrical stimulation of vesical afferents. J Physiol 259: 223–237, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 425: 545–562, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo DC, Hisamitsu T, de Groat WC. A sympathetic projection from sacral paravertebral ganglia to the pelvic nerve and to postganglionic nerves on the surface of the urinary bladder and large intestine of the cat. J Comp Neurol 226: 76–86, 1984. [DOI] [PubMed] [Google Scholar]
- 14.Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Involvement of metabotropic glutamate receptor 5 in pudendal inhibition of nociceptive bladder activity in cats. J Physiol 589: 5833–5843, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mally AD, Matsuta Y, Zhang F, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Role of opioid and metabotropic glutamate 5 receptors in pudendal inhibition of bladder overactivity in cats. J Urol 189: 1574–1579, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazières L, Jiang CH, Lindström S. The C fibre reflex of the cat urinary bladder. J Physiol 513: 531–541, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazières L, Jiang CH, Lindström S. Recurrent inhibition of the bladder C fibre reflex in the cat and its response to naloxone. J Physiol 575: 603–615, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noto H, Roppolo JR, de Groat WC, Nishizawa O, Sugaya K, Tsuchida S. Opioid modulation of the micturition reflex at the level of the pontine micturition center. Urol Int 47 Suppl 1: 19–22, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn 24: 643–647, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, Siegel SW, Tate SB, Jarnagin BK, Rosenblatt PL, Feagins BA. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 182: 1055–1061, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Roppolo JR, Booth AM, de Groat WC. The effect of naloxone on the neural control of the urinary bladder of the cat. Brain Res 264: 355–358, 1983. [DOI] [PubMed] [Google Scholar]
- 22.Schwen Z, Matsuta Y, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Involvement of 5-HT3 receptors in pudendal inhibition of bladder overactivity in cats. Am J Physiol Renal Physiol 305: F663–F671, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 302: F1090–F1097, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thor KB, Roppolo JR, de Groat WC. Naloxone induced micturition in unanesthetized paraplegic cats. J Urol 129: 202–205, 1983. [DOI] [PubMed] [Google Scholar]
- 25.Xiao Z, Reese J, Schwen Z, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Role of spinal GABAA receptors in pudendal inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 306: F781–F789, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao Z, Rogers MJ, Shen B, Wang J, Schwen Z, Roppolo JR, de Groat WC, Tai C. Somatic modulation of spinal reflex bladder activity mediated by nociceptive bladder afferent nerve fibers in cats. Am J Physiol Renal Physiol 307: F673–F679, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]