Abstract
Hindbrain injection of a melanocortin-3/4 receptor agonist, MTII, reduces food intake primarily by reducing meal size. Our previously reported results indicate that N-methyl-d-aspartate-type glutamate receptors (NMDAR) in the nucleus of the solitary tract (NTS) play an important role in the control of meal size and food intake. Therefore, we hypothesized that activation of NTS NMDARs contribute to reduction of food intake in response to fourth ventricle or NTS injection of MTII. We found that coinjection of a competitive NMDAR antagonist (d-CPP-ene) with MTII into the fourth ventricle or directly into the NTS of adult male rats attenuated MTII-induced reduction of food intake. Hindbrain NMDAR antagonism also attenuated MTII-induced ERK1/2 phosphorylation in NTS neurons and prevented synapsin I phosphorylation in central vagal afferent endings, both of which are cellular mechanisms previously shown to participate in hindbrain melanocortinergic reduction of food intake. Together, our results indicate that NMDAR activation significantly contributes to reduction of food intake following hindbrain melanocortin receptor activation, and it participates in melanocortinergic signaling in NTS neural circuits that mediate reduction of food intake.
Keywords: satiation, nucleus of the solitary tract, proopiomelanocortin, vagus
gastrointestinal signals activate vagal afferent fibers that synapse in the dorsal vagal complex (DVC) of the caudal hindbrain, where they participate in the process of satiation and termination of food intake. Whereas gastrointestinal satiation signals convey information related to an ongoing meal (45), other nongastrointestinal signals reflect energy utilization or storage, and control food intake, in part, by modulating vagally mediated satiation signals over multiple meals (25). For example, a subpopulation of hypothalamic melanocortinergic neurons project to the nucleus of the solitary tract (NTS) and dorsal motor nucleus of the vagus (DMV) (41, 64, 65), where melanocortin-4 receptors (MC4R) are expressed (36, 57). In addition, the NTS itself harbors a small population of POMC neurons that also may innervate neurons in the NTS and DMV (41). Therefore, melanocortinergic endings are appropriately positioned to influence neural circuits involved in satiation.
Consistent with melanocortinergic modulation of satiation signals in the NTS, hindbrain injection of an MC3/4R agonist reduces 24-h food intake by reducing meal size (26, 60, 64). Conversely, hindbrain injection of an MC3/4R antagonist increases food intake by increasing meal size (26, 52, 64). Hindbrain MC4R activation also has been shown to participate in reduction of food intake by CCK, the archetypal gut satiation peptide (22, 52). Finally, reduction of meal size and 48-h food intake following leptin-induced activation of melanocortinergic neurons in the hypothalamic arcuate nucleus is significantly attenuated by NTS injection of an MC4R antagonist (65). These observations suggest that hindbrain melanocortin signaling plays an important role in modulating the process of satiation.
Reduction of food intake by hindbrain MTII injection is associated with distinct signaling mechanisms in vagal afferent endings and in NTS neurons. Fourth ventricle injection of MTII increases NTS ERK1/2 phosphorylation, and inhibition of ERK1/2 phosphorylation attenuates MTII-induced reduction of food intake (7, 52). Moreover, hindbrain MTII injection increases PKA-mediated synapsin I phosphorylation in vagal afferent endings (11). Phosphorylation of synapsin I is known to increase synaptic vesicle trafficking to the readily releasable pool, thereby increasing synaptic strength (for review, see Ref. 15). Consistent with these observations, we have found that inhibition of PKA activity in the NTS attenuates reduction of food intake by MTII and prevents MTII-induced synapsin phosphorylation in vagal afferent endings (11). Our results, and those of others, suggest that NTS neuronal ERK1/2 phosphorylation and PKA-mediated synapsin phosphorylation in vagal afferent endings are downstream of MC4R activation and participate in MTII-induced reduction of food intake.
The primary excitatory neurotransmitter in the DVC is glutamate, which is released by vagal afferent endings (2), NTS neurons (20), glial cells (29), and potentially by afferent inputs from other brain areas (43, 48, 65). Consistent with glutamate's role in vagal afferent transmission and control of meal size, antagonists of N-methyl-d-aspartate-type glutamate receptors (NMDAR) administered into the hindbrain increase food intake by delaying satiation (32, 55) and prevent reduction of food intake by CCK (14, 27, 61). Therefore, we hypothesized that reduction of food intake by hindbrain MC3/4R agonist injection requires NMDAR activation. Because ERK1/2 phosphorylation and synapsin I phosphorylation are coupled to calcium channel activation (15, 54), including NMDAR channel activation (8, 16, 33), we further postulated that NMDAR antagonism would attenuate MTII-induced ERK1/2 and synapsin 1 phosphorylation in the NTS.
MATERIALS AND METHODS
Animals and housing.
Male adult Sprague-Dawley rats (280–300 g; Simonsen Laboratories, Gilroy, CA) were individually housed in suspended wire mesh cages in a vivarium with a 12:12-h light-dark cycle and controlled temperature and humidity. Rats had ad libitum access to water and pelleted rodent diet (Teklad, Kent, WA), except during overnight fasts and food intake experiments, as described below. All animal housing and experiments reported here were conducted in compliance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals under a protocol approved by the Washington State University Institutional Animal Care and Use Committee.
Surgical procedures.
Rats were fasted overnight and anesthetized with a ketamine (50 mg/kg), xylazine (25 mg/kg), and acepromazine (2 mg/kg) mixture for all surgical procedures. For cannula implantations, rats were placed in a stereotaxic instrument and implanted with 26-gauge stainless-steel (McMaster-Carr, Santa Fe Springs, CA) guide cannulas aimed for the fourth ventricle (2.0 mm anterior to occipital suture, on midline, 6.6 mm ventral from dura) or NTS (0.1 mm anterior to occipital suture, −0.8 mm lateral to midline, and 7.8 mm ventral from skull).
For unilateral nodose ganglion removal, the cervical vagus nerve was exposed on one side via a midline cervical incision and blunt dissection. The vagus was severed just distal to the nodose, allowing retraction of the ganglion to visualize and section the vagus proximal to the nodose, removing the entire ganglion. After suturing the cervical incision, rats were implanted with a cannula aimed for the fourth ventricle, as described above. All rats were allowed at least 4 wk of recovery time and exceeded their presurgery weights before commencement of experiments.
Immunohistochemistry.
For all immunohistochemical experiments, rats were rapidly anesthetized with isoflurane anesthetic (Vedco, St. Joseph, MO) and then perfused intracardially with 0.1 M PBS followed by 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in 0.1 M phosphate buffer (pH 7.4). Immediately after perfusion, brains were collected, postfixed in the same fixative for 2 h, and cryoprotected in 0.1 M phosphate buffer containing 25% sucrose overnight at 4°C. Thirty-micrometer coronal cryostat sections of the hindbrain were collected for immunostaining. For detection of phosphorylated ERK1/2, hindbrain sections were incubated for 36 h at room temperature in mouse anti-pERK1/2 antisera (1:300, Thr-202/Thr-204 phospho-ERK1/2; Cell Signaling Technology, Beverly, MA). Then the sections were washed and incubated in Alexa Fluor 488-conjugated goat anti-mouse (1:600; Invitrogen, Carlsbad, CA). For detection of phosphorylated synapsin I, sections were incubated in rabbit anti-phospho-synapsin I (1:500, phospho-Ser-9; Abcam, Cambridge, MA) with subsequent incubation in Alexa Fluor 555-conjugated goat anti-rabbit (1:600, Invitrogen). Stained sections were mounted on slides and cover-slipped with ProLong Gold (Invitrogen) prior to microscope examination.
Quantification procedures.
For each animal, the number of pERK1/2-immunoreactive cell bodies was counted manually in four hindbrain sections corresponding to 14.1 mm, 13.8 mm, 13.6 mm, and 13.3 mm caudal to bregma, according to the stereotaxic atlas of Paxinos and Watson (42). Phosphorylated-ERK1/2-immunoreactive cell bodies in the NTS and DMV were counted on both sides of the hindbrain in each section, and the numbers of pERK1/2-immunoreactive cells are presented as averages for each brain area across all four rostrocaudal levels. Phosphorylated synapsin I (pSyn) immunoreactivity was quantified according to our previously published protocol (12). Briefly, mean fluorescence intensity was sampled from the NTS and DMV from both sides of the hindbrain in each of the four rostrocaudal hindbrain levels that were used to quantify pERK1/2 immunoreactivity. These values were normalized to the background fluorescence intensity, which was sampled from the gracile nucleus. The data are presented as the average fluorescence intensity for each brain area across the four rostrocaudal levels listed above. The fluorescence intensity was calculated by applying the following formula: pSyn Fluorescence Intensity = (100 × pSyn Intensity/Background Intensity) − 100.
Effect of hindbrain NMDAR antagonist administration on MTII-induced reduction of food intake and body weight.
To examine the effect of hindbrain NMDAR antagonism on MTII-induced reduction of food intake, a total of 12 rats were cannulated with fourth ventricle cannulas as described above. All rats significantly reduced food intake following MTII injection and, therefore, all rats were considered to have patent fourth ventricle cannulas and were included in behavioral analysis. In a crossover, counterbalanced experimental design, overnight-fasted rats (16 h) received a fourth ventricle injection of saline (0.9% NaCl), MTII (50 pmol; Phoenix Pharmaceuticals, Burlingame, CA), d-4-[(2E)-3-phosphono-2-propenyl]-2-piperazinecarboxylic acid (d-CPP-ene; 40 ng; Tocris, Ellisville, MO), or a cocktail containing d-CPP-ene and MTII in the above amounts. The selection of d-CPP-ene was based on our prior experience with the antagonist and its effectiveness in being able to block CCK-induced reduction of food intake when injected into the fourth ventricle or NTS (14, 61). In addition, functional electrophysiological experiments indicate that d-CPP-ene effectively blocks NMDAR-dependent contributions in vagus-to-NTS neurotransmission (63). Injection volumes of 3 μl were injected over a 2-min period using a Hamilton syringe (Reno, NV). Immediately after injection, rats were given access to a preweighed amount of pelleted rodent diet, and intake less spillage was recorded at 30 min, 60 min, 120 min, 240 min, 4 h, and 24 h after injection. At least 5 days elapsed between experiments, and every rat received each treatment condition twice. Data from the two repetitions of each treatment were averaged for presentation and analysis. Hence, each animal received a total of eight fourth ventricle injections.
Sixteen rats implanted with 26-gauge guide cannulas aimed for the medial NTS were used to assess the participation of NTS NMDAR activation in MTII-induced reduction of food intake. In a crossover, counterbalanced experimental design similar to the one described above, overnight-fasted rats (16 h) received a 200-nl NTS injection of saline, MTII (50 pmol), d-CPP-ene (20 ng), or a solution containing d-CPP-ene and MTII. Immediately after injection, the rats were given access to a preweighed pelleted rodent diet, and intake less spillage was recorded at 30 min, 60 min, 120 min, 240 min, 4 h, and 24 h after injection. Body weights were measured before injections and at the end of the experiment, 24 h after injection. As above, experiments were conducted at least 5 days apart. Each treatment condition was conducted once, a total of four injections per animal. After all behavioral experiments were completed, rats were euthanized, and hindbrain tissue was collected for histological verification of cannula placement. Three rats were excluded from behavioral and body weight analyses because their cannula tips were located outside the dorsal vagal complex. MTII injection did not reduce food intake or body weight in these rats, and injection of d-CPPene alone also was without effect (data not shown).
MTII-induced ERK1/2 phosphorylation time course.
A total of 28 rats with fourth ventricle cannulas were used to establish a time course for MTII-induced ERK1/2 phosphorylation in the hindbrain. Prior to the start of experimentation, all rats with fourth ventricle cannulas were screened for cannula placement and patency, as previously described (46). Briefly, we injected 90 μg/3 μl of 5-thio-d-glucose into the fourth ventricle. Blood was collected for measurement of plasma glucose prior to injection and 60 min postinjection. Rats that exhibited an increase of blood glucose of at least 50 mg·100 ml−1·60 min−1 postinjection were considered to have properly placed, patent 4th ventricle cannulas. Overnight-fasted rats received a fourth ventricle injection (3 μl) of saline or MTII (50 pmol). Saline-injected rats (n = 4) were perfused 15 min after injection. The remaining rats received a fourth ventricle injection of MTII and were perfused 5 min (n = 4), 15 min (n = 4), 30 min (n = 4), 45 min (n = 4), 60 min (n = 4), and 90 min (n = 4) after injection. Hindbrains were harvested for immunohistochemical analysis of pERK1/2 immunoreactivity.
Effect of hindbrain NMDAR antagonist injection on MTII-induced ERK1/2 phosphorylation.
After an overnight fast, a total of 16 rats received one of the following 3-μl fourth ventricle injections: 0.9% NaCl (n = 4), MTII (50 pmol; n = 4), d-CPP-ene (40 ng; n = 4), or a solution containing d-CPP-ene and MTII in the above amounts (n = 4). Rats were euthanized and perfused 30 min after injection, and the brains were collected for quantification of pERK1/2-immunoreactive cell bodies in the NTS and DMV.
Effect of NMDAR antagonist on MTII-induced synapsin I phosphorylation.
A group of rats (total n = 16) with fourth ventricle cannulas were used to assess the effect of d-CPP-ene on MTII-induced synapsin I phosphorylation. Overnight-fasted rats received one of the following fourth ventricle injections: saline (n = 4), MTII (50 pmol; n = 4), d-CPP-ene (40 ng; n = 4), or a cocktail containing d-CPP-ene and MTII (n = 4). Thirty minutes after fourth ventricle injection, rats were euthanized, and hindbrains were collected for analysis of pSyn immunoreactivity.
Effect of unilateral nodose ganglion removal on MTII-induced ERK1/2 phosphorylation.
A group of rats (total n = 8) with unilateral nodose ganglion removal and fourth ventricle cannulas were used to assess MTII-induced ERK1/2 phosphorylation. Overnight-fasted rats received a fourth ventricle injection of saline (n = 4) or MTII (50 pmol; n = 4) and were perfused 30 min after injection. Subsequently, brains were removed and hindbrain sections were prepared for comparison of MTII-induced pERK1/2 immunoreactivity in the NTS and DMV contralateral or ipsilateral to nodose ganglion removal. Sections were also stained for isolectin B4, which binds to central vagal afferent C-type fibers (35). Destruction of central vagal afferent endings was verified by the lack of isolectin B4 binding in the hindbrain ipsilateral to nodose ganglion removal.
Statistical analyses.
Appropriate one- and two-way repeated-measures ANOVAs were used to analyze data, followed by Holm-Sidac post hoc analysis. For analysis of behavioral data, the repeated factor was the treatment condition. The confidence limit for statistical significance was set at P < 0.05. However, wherever actual confidence limits were substantially less than 0.05, those P values are provided. Results are presented as means ± SE.
RESULTS
Attenuation of MTII-induced reduction of food intake by hindbrain NMDAR antagonism.
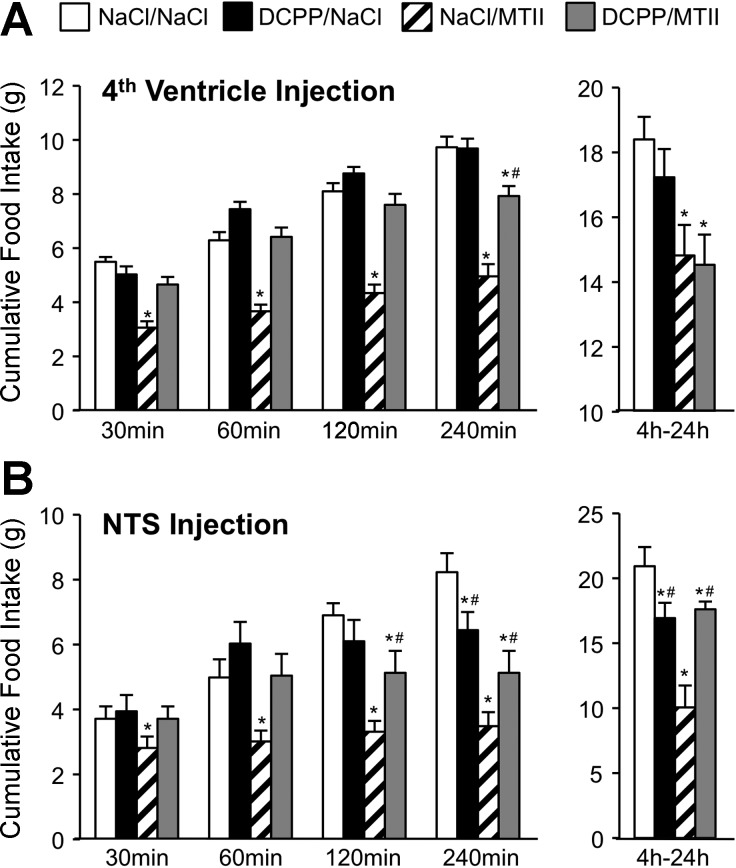
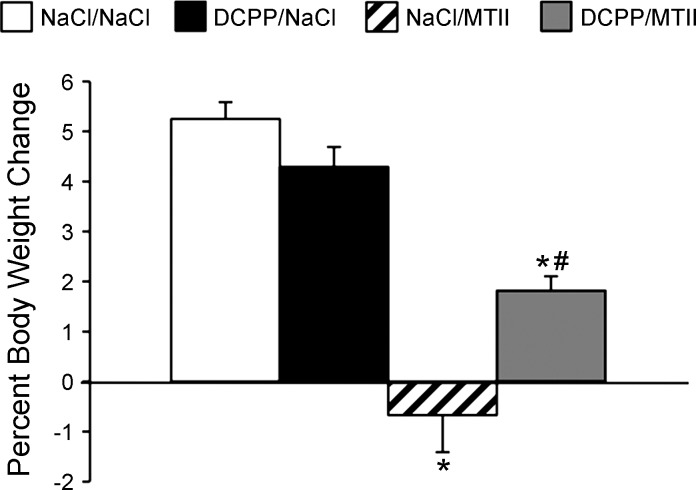
Consistent with prior reports (11, 26, 52, 60), injection of MTII into the fourth ventricle (50 pmol/3 μl) significantly reduced food intake relative to saline control injection, beginning 30 min postinjection, with reduced intake being sustained for 24 h post-MTII. Coinjection of d-CPP-ene (40 ng/3 μl) with MTII significantly attenuated MTII-induced reduction of food intake 30 min (P < 0.001), 60 min (P < 0.001), 120 min (P < 0.001), and 240 min (P < 0.01) after injection (Fig. 1A). However, fourth ventricle d-CPP-ene coinjection did not significantly attenuate reduction of food intake between 4 h and 24 h after MTII injection. As observed with fourth ventricular injection, MTII injection directly into the NTS (50 pmol/200 nl) significantly reduced cumulative food intake at 30 (P < 0.05), 60 (P < 0.01), 120 (P < 0.05), 240 min (P < 0.01), and 24 h post-MTII (P < 0.001). Furthermore, coinjection of d-CPP-ene (20 ng/200 nl) with MTII directly into the NTS significantly attenuated MTII-induced reduction of food intake at all time points, including 24 h after injection (Fig. 1B). NTS injection of MTII also significantly reduced body weight gain over 24 h after refeeding (P < 0.01), an effect that was significantly attenuated when MTII was coinjected with d-CPP-ene (P < 0.05) (Fig. 2).
Fig. 1.
Attenuation of a melanocortin-3/4 receptor agonist (MTII)-induced reduction of food intake following hindbrain administration of a competitive N-methyl-d-aspartate-type glutamate receptor (NMDAR) antagonist, d-CPP-ene. A: average cumulative food intake during refeeding by overnight-fasted rats (n = 12), following fourth ventricle injection of saline, MTII (50 pmol), d-CPP-ene (40 ng), or MTII with d-CPP-ene. B: food intake during refeeding by overnight-fasted rats (n = 13) following administration of saline, MTII (50 pmol), d-CPP-ene (20 ng), or MTII with d-CPP-ene injected directly into the NTS. Bars represent average cumulative food intake at 30 min, 60 min, 120 min, 240 min postinjection, and between 4 and 24 h after injection. Data are expressed as means ± SE. *Significantly different (P ≤ 0.05) from saline control treatment. #Significantly different (P ≤ 0.05) from MTII treatment.
Fig. 2.
Twenty-four-hour body weight change in overnight-fasted rats (n = 13) following administration of saline, MTII (50 pmol), d-CPP-ene (20 ng), or MTII with d-CPP-ene directly into the nucleus of the solitary tract (NTS). Data are expressed as means ± SE. *Significantly different (P ≤ 0.05) from saline control treatment. #Significantly different (P ≤ 0.05) from MTII treatment.
Attenuation of MTII-induced hindbrain ERK1/2 phosphorylation by NMDAR antagonist.
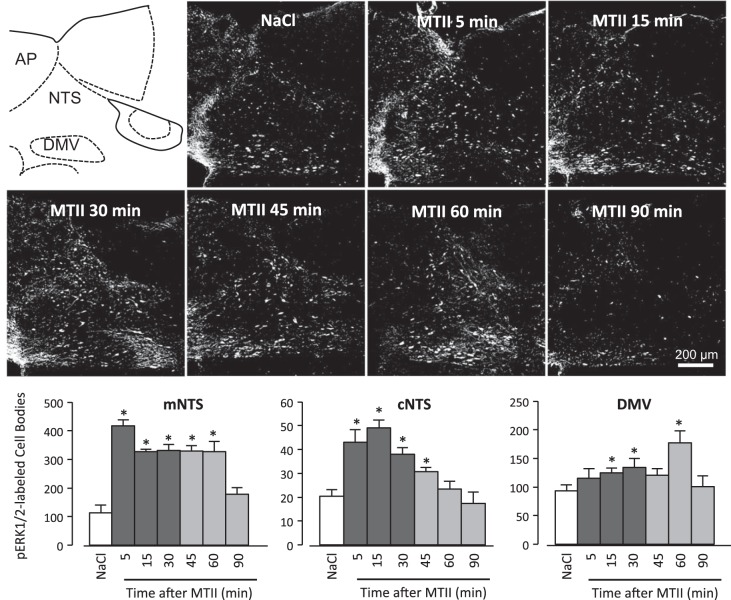
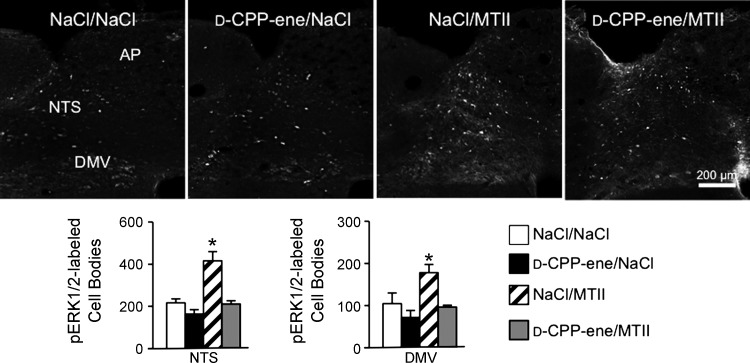
Compared with saline injection, fourth ventricle injection of MTII (50 pmol) significantly increased ERK1/2 phosphorylation in the NTS as early as 5 min (P < 0.01) after injection and by 30 min postinjection in the DMV (P < 0.05) (Fig. 3). Phosphorylated ERK1/2 (pERK1/2) remained significantly elevated (P < 0.01) for 60 min following MTII injection but was not different from saline-injected controls by 90 min after injection. Unlike ERK1/2 phosphorylation following systemic CCK administration (14), MTII-induced ERK1/2 phosphorylation was confined to NTS and DMV neurons and was not detectable in the neuropil. Consistent with NMDAR antagonist's attenuation of MTII-induced reduction of food intake, coinjection of d-CPP-ene with MTII abolished MTII-induced ERK1/2 phosphorylation in the NTS and DMV (Fig. 4).
Fig. 3.
Time course of MTII-induced ERK1/2 phosphorylation in hindbrain following fourth ventricle MTII injection. Schematic depicts the level of the hindbrain at which representative immunofluorescent images were captured. Immunofluorescent images represent the time course for the appearance of pERK1/2 immunoreactivity in the NTS and dorsal motor nucleus of the vagus (DMV) following fourth ventricle injection of MTII (50 pmol) in overnight-fasted rats. Graphs in the lower panel are quantification of pERK1/2-immunoreactive neurons in the NTS (commissural and medial) and DMV represented as averages for each time period, counted from coronal sections at four rostrocaudal levels of the hindbrain. AP, area postrema; DMV, dorsal motor nucleus of the vagus; NTS, nucleus of the solitary tract. *Significantly different (P ≤ 0.05) from saline control treatment.
Fig. 4.
Fourth ventricle injection of NMDAR antagonist attenuates MTII-induced pERK1/2 immunoreactivity in the hindbrain. Top: representative images of hindbrain sections stained to reveal pERK1/2 immunoreactivity following fourth ventricle injection of saline, d-CPP-ene (40 ng), MTII (50 pmol), or coinjection of d-CPP-ene with MTII. Bar graphs illustrate quantification of pERK1/2-immunoreactive neurons in the NTS and DMV for the aforementioned treatment conditions. Data are expressed as means ± SE. *Significantly different (P ≤ 0.05) from saline control treatment.
MTII-induced synapsin I phosphorylation requires hindbrain NMDAR activation.
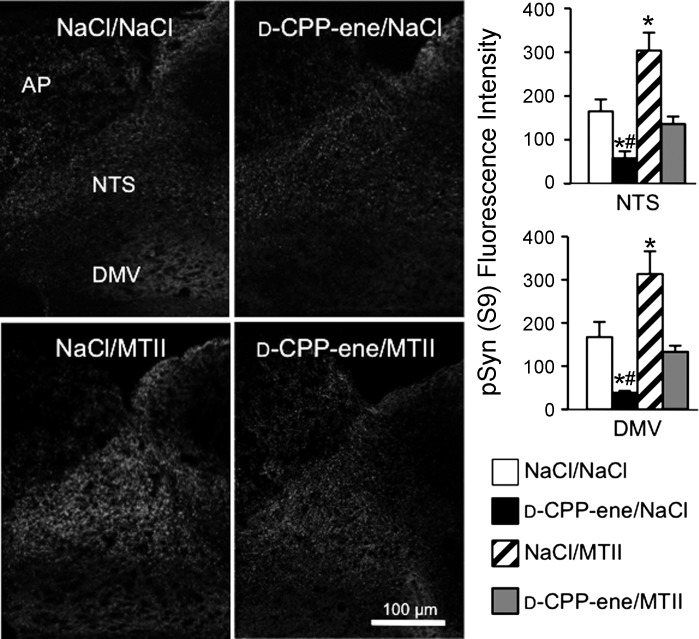
We previously demonstrated that MTII-induced, PKA-mediated synapsin phosphorylation is localized to vagal afferent endings, peaks by 30 min post-MTII, and persists for 6 h after MTII injection (11). Fourth ventricle injection of MTII significantly increased synapsin phosphorylation at the PKA-phosphorylated Ser-9 residue in the hindbrain (Fig. 5), and a significant treatment effect on hindbrain synapsin I phosphorylation [F(3,12) = 16.6, P < 0.001] was observed when d-CPP-ene or saline was injected with MTII. As predicted, fourth ventricle injection of the NMDAR antagonist, d-CPP-ene (40 ng/3 μl), prevented MTII-induced synapsin I phosphorylation in the NTS and DMV and also significantly reduced the level of immunoreactivity for phosphorylated synapsin in NaCl-injected control rats.
Fig. 5.
Fourth ventricle injection of NMDAR antagonist attenuates MTII-induced synapsin I phosphorylation (Ser-9) in the NTS and DMV. Immunofluorescent images show pSyn immunoreactivity following fourth ventricle injection of saline, MTII (50 pmol), d-CPP-ene (40 ng), or coinjection of d-CPP-ene with MTII. Hindbrain sections were stained using an antibody specific for phosphorylation of synapsin I at the PKA-specific site, Ser-9. Right: quantification of pSyn immunoreactivity expressed as the average fluorescence intensity of pSyn immunoreactivity sampled from the NTS and DMV across four rostrocaudal levels of the hindbrain and normalized to background intensity. *Significant difference (P ≤ 0.05) from saline control treatment. #Significant difference (P ≤ 0.05) from d-CPP-ene/MTII treatment.
Effects of destruction of central vagal afferent endings on MTII-induced ERK1/2 phosphorylation in the NTS.
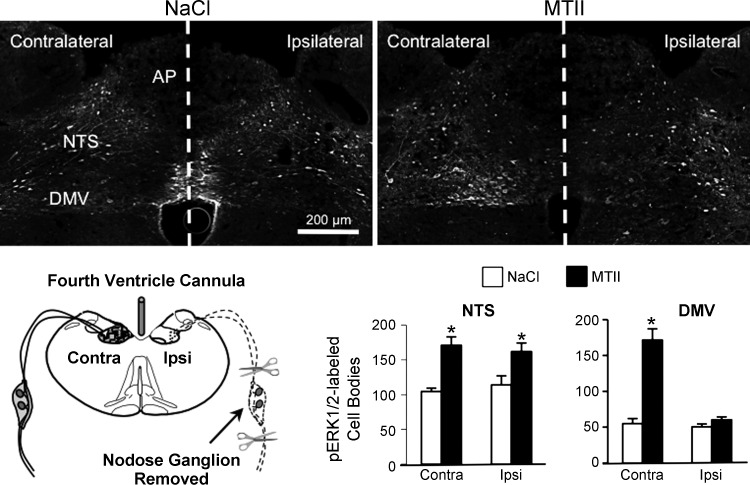
MTII-induced reduction of food intake and NTS synapsin phosphorylation is significantly attenuated by destruction of vagal afferent endings (11). Therefore, we postulated that MTII-induced activation of vagal afferent endings, with associated synapsin phosphorylation, might contribute to postsynaptic hindbrain ERK1/2 phosphorylation. Compared with saline injection, we found that MTII significantly increased ERK1/2 phosphorylation in the NTS (P < 0.01) and DMV (P < 0.01) contralateral to nodose ganglion removal (Fig. 6). Fourth ventricle MTII injection also significantly increased ERK1/2 phosphorylation in the ipsilateral NTS (P < 0.05), where vagal afferent endings were destroyed. However, we found that unilateral nodose ganglionectomy completely abolished MTII-induced ERK1/2 phosphorylation in the DMV ipsilateral to nodose ganglion removal, suggesting that MTII-induced activation of vagal afferent endings is necessary for induction of DMV ERK1/2 phosphorylation.
Fig. 6.
MTII-induced ERK1/2 phosphorylation in the NTS and DMV following unilateral nodose ganglion removal and destruction of vagal afferent endings in the ipsilateral hindbrain. Top: representative immunofluorescent images of pERK1/2 immunoreactivity 30 min after fourth ventricle injection of saline (left) or MTII (50 pmol, right) in rats that had undergone unilateral nodose ganglion removal. Schematic in bottom panel illustrates surgical removal of a nodose ganglion and degeneration of central vagal afferent endings in the ipsilateral but not contralateral hindbrain of fourth ventricle-cannulated rats. Bar graph illustrates numbers of pERK1/2-immunoreactive neurons in the NTS and DMV ipsilateral and contralateral to nodose ganglion removal. *Significantly different (P ≤ 0.05) from saline control treatment.
DISCUSSION
We found that fourth ventricle or NTS injection of d-CPPene, a competitive NMDAR antagonist significantly attenuated reduction of food intake by hindbrain MTII injection, indicating that NMDAR activation contributes to reduction of food intake by hindbrain MC4R activation. Interestingly, although both fourth ventricle and NTS NMDAR antagonist injections attenuated MTII-induced reduction of food intake during the first 4 h postinjection, only NTS injection attenuated reduction of food intake beyond 4 h. An explanation for this difference between fourth ventricle and NTS antagonist effects, currently, is not clear. However, because MC4R transcript is expressed in brain areas outside of the DVC (34, 36, 40), it is conceivable that our 3-μl fourth ventricle injections of MTII reached sites that participate in reduction of food intake independent of NMDAR activation. Nevertheless, our results are consistent with the hypothesis that DVC NMDAR activation plays an important role in reduction of food intake in response to DVC MC4R activation.
The cellular location of NMDAR that participate in the reduction of food intake by MTII remains uncertain. NMDAR subunit immunoreactivity and mRNA are expressed by NTS and DMV neurons (5, 10, 31, 59). In addition, NMDAR subunit immunoreactivity and mRNA also occur in nodose ganglion neurons (18, 47), and NMDAR subunit immunoreactivity has been localized ultrastructurally to vagal afferent endings in the NTS (1). Electrophysiological results also indicate that activation of presynaptic NMDAR increases glutamate release from vagal afferent endings in the DVC (3), while a recent report reveals that postsynaptic NMDAR on NTS neurons are critical for fidelity of transmission from vagal afferents to NTS neurons (63). Therefore, it is conceivable that NMDARs, expressed both by vagal afferents and NTS neurons, participate in the reduction of food intake by MTII.
NTS injection of MTII also was associated with reduced 24-h weight gain, and this effect was attenuated by NMDAR antagonism. We think this effect on body weight is largely accounted for by d-CPP-ene's attenuation of MTII-induced reduction of food intake. Nevertheless, we cannot exclude participation of NMDAR in reduction of body weight due to an MTII-induced increase in energy expenditure. MTII injection directly into the NTS is reported to increase energy expenditure (50), which may contribute to MTII-induced reduction of body weight. Interestingly, lipid infusion into the duodenum increases brown adipose tissue temperature, a process that requires peripheral CCKA receptor activation and NTS NMDAR activation (9). Given that NTS MC4R activation contributes to CCK-induced reduction of food intake (22, 52), it is conceivable that MTII activates a similar population of NTS neurons that may require NMDAR activation to increase energy expenditure.
Similar to NMDAR expression in the DVC, MC4R mRNA, but not MC3R mRNA, is expressed by some NTS and DMV neurons (34, 36), and is also expressed by ∼30% of vagal afferents in the nodose ganglia (24, 57). In fact, patch-clamp recordings from NTS neurons indicate that increased glutamate release from vagal afferent endings accounts for most MTII-evoked excitation of NTS neurons, suggesting a presynaptic mechanism of the MC3/4R agonist (57). Additionally, our recently reported results indicate that MTII-induced reduction of food intake requires the presence of central vagal afferent endings (11). While MTII may, indeed, have direct effects on NTS and DMV neurons, observations from our laboratory and others support the hypothesis that MTII-induced reduction of food intake is predominantly mediated via presynaptic MC4R activation of vagal afferent terminals. Consistent with a presynaptic MTII action, we recently reported that hindbrain MTII injection increases PKA-catalyzed phosphorylation of the Ser-9 residue of synapsin I in vagal afferent endings (11).
The MC4R is a Gαs receptor linked to the cAMP/PKA signaling cascade (23), and we have shown that reduction of food intake by hindbrain MTII is attenuated in the presence of a PKA inhibitor (11). Moreover, phosphorylation of the PKA-specific site (Ser-9) on synapsin I is a major mechanism by which presynaptic PKA activation increases synaptic vesicle trafficking and neurotransmitter release (39). Thus, phosphorylation of synapsin I and increased glutamate release from vagal afferent endings is a putative mechanism for MTII-induced reduction of food intake. Because NMDAR blockade attenuates MTII-induced reduction of food intake, and MTII reduces food intake via presynaptic MC4R activation, we hypothesized that MTII-induced NTS synapsin phosphorylation requires NMDAR activation. Indeed, we found that hindbrain administration of an NMDAR antagonist blocks MTII-induced synapsin phosphorylation. Interestingly, in our previous report, we also observed that synapsin phosphorylation was decreased following overnight food deprivation (11), but increased with refeeding. In the current study, NMDAR antagonism reduced the immunoreactivity for phosphorylated synapsin in NaCl control rats, as well as reversing the increase in synapsin phosphorylation following MTII. This result may indicate that NMDARs participate in maintaining a basal level of PKA-mediated synapsin phosphorylation, which is enhanced by MC4R activation. Our results suggest that NMDAR-dependent synapsin phosphorylation in vagal afferent endings is a mechanism by which NMDAR activation participates in MTII-induced reduction of food intake and that NMDAR may participate in setting a basal level of synapsin phosphorylation in response to endogenous signals as well.
Activation of the ERK1/2 signaling cascade in the NTS has been proposed as a mechanism by which the hindbrain integrates melanocortin signaling and gastrointestinal satiation signals (7). This hypothesis is based on the observations that both CCK and MTII increase NTS ERK1/2 phosphorylation, and inhibition of hindbrain ERK1/2 phosphorylation attenuates CCK and MTII-induced reduction of food intake (52, 53). However, the mechanisms by which hindbrain ERK1/2 phosphorylation contributes to reduction of food intake may differ for CCK and MTII. We observed that CCK increased ERK1/2 phosphorylation in vagal afferent endings in the NTS (14). Moreover, we found that pERK1/2-catalyzed phosphorylation of synapsin 1 at Ser-62 and Ser-67 and that the level of pERK1/2-catalyzed synapsin phosphorylation was correlated with CCK-induced reduction of food intake (12). Unlike CCK, we found MTII did not increase ERK1/2 phosphorylation in vagal afferent endings. Rather, our immunohistochemical results indicate that fourth ventricle MTII rapidly increases ERK1/2 phosphorylation in postsynaptic NTS and DMV neurons. Moreover, we found that NMDAR antagonism attenuated MTII-induced ERK1/2 phosphorylation in both NTS and DMV neurons, indicating that NMDAR activation is upstream of MTII-induced ERK1/2 phosphorylation. We conclude that NMDAR activation is necessary for MTII-induced ERK1/2 phosphorylation and that participation of ERK1/2 phosphorylation in MTII-induced reduction of food intake is limited to postsynaptic mechanisms.
In addition to increasing ERK1/2 phosphorylation in the NTS, we found that fourth ventricle MTII injection increased ERK1/2 phosphorylation in the DMV. This effect was surprising because the direct effect of MTII on DMV neurons reportedly is inhibitory (44, 51). We found that MTII-induced ERK1/2 phosphorylation in the DMV was attenuated by an NMDAR antagonist, indicating that DMV ERK1/2 phosphorylation requires NMDAR activation. Thus, the effect of MTII on DMV ERK1/2 phosphorylation may be due to indirect activation of DMV neurons following MTII-induced glutamate release from presynaptic endings on DMV neurons. Consistent with this hypothesis, we found that unilateral nodose ganglion removal, and destruction of vagal afferent endings in the ipsilateral hindbrain, abolished MTII-induced ERK1/2 phosphorylation in the DMV ipsilateral, but not contralateral, to nodose ganglion removal. Therefore, our results suggest that MTII-induced increase of pERK1/2 in DMV neurons depends on vagal afferent glutamate release. From these data, one might be tempted to postulate a role for vagal efferents in the reduction of food intake by MTII. While NTS injection of MC4R agonists has been shown to modulate gastric activity (44), vagal efferents are not likely to play a role in MTII-induced reduction of food intake. Williams et al. (60) clearly demonstrated that vagotomy, which results in degeneration of peripheral endings of vagal efferents, does not attenuate reduction of food intake by fourth ventricle MTII. In contrast to the DMV, unilateral nodose ganglion removal did not eliminate MTII-induced ERK1/2 phosphorylation in the ipsilateral NTS. This result suggests that at least part of MTII's effect on NTS neurons is independent of presynaptic vagal afferent activation (57).
The fact that NMDAR activation is necessary for MTII-induced reduction of food intake, as well as NTS neuronal ERK1/2 phosphorylation and synapsin phosphorylation in vagal afferent endings, suggests that glutamate from a currently unknown source is necessary for reduction of food intake by MTII. Of note, a subpopulation of POMC neurons releases glutamate (21), which may contribute to the reduction of food intake in the presence of endogenous or exogenous MC4R agonists. Glutamate released by astroglia (37) might also be sufficient to activate NTS neuronal NMDAR. Rogers and colleagues (29, 38) have demonstrated that NTS glia are activated by a variety of stimuli, including vagal afferent activation. Further investigation is needed to identify the sources of glutamate needed for MTII to increase NTS ERK1/2 phosphorylation and synapsin phosphorylation.
The cellular mechanisms by which MC4R activation and NMDAR activation interact in the reduction of food intake remains to be elucidated. Although modulation of NMDAR function by G protein-coupled receptors is well described (for review, see Ref. 62), interactions between MC4R and NMDAR have not yet been investigated. Nevertheless, NMDAR interaction with other Gαs receptors has been described. For example, activation of D1 dopamine receptors, VIP and PACAP receptors, can enhance calcium flux through NMDAR in a PKA-dependent manner (56, 58), and this interaction could contribute to ERK1/2 phosphorylation. Presynaptically, several reports indicate that phosphorylation of synapsin I at Ser-9 not only depends on PKA activation, but also requires participation of calcium-activated kinases (17, 39). Our results suggest that NMDAR activation may contribute to calcium influx needed for MC4R activation to increase synapsin I phosphorylation in vagal afferent terminals.
Experiments in this study were carried out using d-CPP-ene, a competitive NMDAR antagonist with high affinity for NR2A/B subunits (30). Our use of d-CPP-ene was based on our prior experience with the antagonist and its effectiveness in blocking CCK-induced reduction of food intake when injected into the fourth ventricle or NTS (13, 61). In addition, functional electrophysiological experiments indicate that d-CPP-ene effectively blocks NMDAR participation in vagus-to-NTS synaptic transmission (63). Although NTS and vagal afferent neurons have been found to express all known NR2 subunits (4, 18, 28), NR2B immunoreactivity is present in nearly all vagal afferent neurons that innervate the gut (19). Furthermore, Berthoud et al. (6) reported that a majority of NTS neurons that are activated by gastrointestinal stimuli are also NR2A/B-immunoreactive. Of note, NR2A/B subunits significantly contribute to PKA-mediated increase in NMDAR calcium permeability and LTP induction (49). Given that NTS MC4R activation reduces food intake via PKA-dependent pathways (11, 52) and that d-CPP-ene significantly attenuates MTII-induced reduction of food intake, our results are consistent with the hypothesis that NTS MC4R activation reduces food intake via PKA-dependent interaction with NR2A/B-containing NMDARs. Nevertheless, at the antagonist doses that we used, it is not possible to attribute these actions to selective antagonism of glutamate binding at a specific NR2 subunit.
Perspectives and Significance
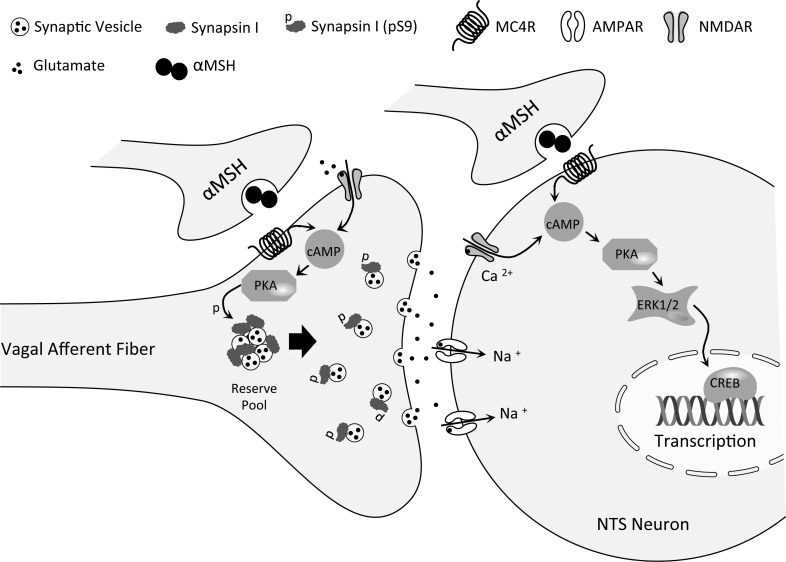
Our findings are consistent with NMDAR participation in control of food intake by modulation of vagal afferent neurotransmission, as outlined in Fig. 7. NTS NMDAR activation plays a critical role in reduction of food intake following hindbrain MC4R activation. NTS NMDAR activation modulates both MC4R-mediated NTS neuronal ERK1/2 phosphorylation and increased Ser-9 synapsin I phosphorylation in vagal afferent endings. Both ERK1/2 phosphorylation and synapsin phosphorylation comprise cellular mechanisms through which NMDAR signaling may contribute to vagal afferent/NTS synaptic function and, thereby, participate in the control of food intake. Hence, our current results reinforce the pivotal role played by hindbrain NMDAR in control of food intake by signals arising from within the brain, as well as by signals arising from the gastrointestinal tract.
Fig. 7.
Postulated interaction of NTS presynaptic and postsynaptic NMDA receptors in intracellular signaling and control of food intake by hindbrain MC4 receptors. PKA-catalyzed phosphorylation of synapsin I in vagal afferent endings is downstream of both NMDAR and MC4R activation. Synapsin 1 phosphorylation increases the readily releasable pool of glutamatergic synaptic vesicles and is expected to result in increased vagal afferent synaptic strength and reduction of food intake. Activation of postsynaptic NMDAR and MC4R on NTS neurons is upstream of phosphorylation of ERK1/2 via PKA. ERK phosphorylation may result in additional protein phosphorylations and transcriptional changes in NTS neurons. Note that although activation of central vagal afferent endings contributes to reduction of food intake by MC4R activation (11), results reported here indicate that activation of vagal afferent endings is not required for activation of the ERK signaling cascade in NTS neurons in response to MC4R agonist application. In contrast, ERK phosphorylation in DMV neurons, following MC4R agonist application, depends upon the presence of intact vagal afferent endings.
GRANTS
This work was supported by National Institutes of Health Grants DK-52849 and NS-20561 to R. C. Ritter.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C. Campos and R. Ritter designed the experiments; C. Campos conducted the experimental procedures; and C. Campos and R. Ritter analyzed the results and wrote the manuscript.
ACKNOWLEDGMENTS
The technical help of T. Duffy and N. Huston is gratefully acknowledged.
REFERENCES
- 1.Aicher SA, Sharma S, Pickel VM. N-methyl-d-aspartate receptors are present in vagal afferents and their dendritic targets in the nucleus tractus solitarius. Neuroscience 91: 119–132, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Allchin RE, Batten TF, McWilliam PN, Vaughan PF. Electrical stimulation of the vagus increases extracellular glutamate recovered from the nucleus tractus solitarii of the cat by in vivo microdialysis. Exp Physiol 79: 265–268, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Bach EC, Smith BN. Presynaptic NMDA receptor-mediated modulation of excitatory neurotransmission in the mouse dorsal motor nucleus of the vagus. J Neurophysiol 108: 1484–1491, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baude A, Strube C, Tell F, Kessler JP. Glutamatergic neurotransmission in the nucleus tractus solitarii: structural and functional characteristics. J Chem Neuroanat 38: 145–153, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Benke D, Wenzel A, Scheuer L, Fritschy JM, Mohler H. Immunobiochemical characterization of the NMDA-receptor subunit NR1 in the developing and adult rat brain. J Recept Signal Transduct Res 15: 393–411, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Berthoud HR, Earle T, Zheng H, Patterson LM, Phifer C. Food-related gastrointestinal signals activate caudal brainstem neurons expressing both NMDA and AMPA receptors. Brain Res 915: 143–154, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Berthoud HR, Sutton GM, Townsend RL, Patterson LM, Zheng H. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol Behav 89: 517–524, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Bertotto ME, Maldonado NM, Bignante EA, Gorosito SV, Cambiasso MJ, Molina VA, Martijena ID. Erk activation in the amygdala and hippocampus induced by fear conditioning in ethanol withdrawn rats: Modulation by mk-801. Eur Neuropsychopharmacol 21: 892–904, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Blouet C, Schwartz GJ. Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats. PLoS One 7: e51898, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broussard DL, Wiedner EB, Li X, Altschuler SM. NMDAR1 mRNA expression in the brainstem circuit controlling esophageal peristalsis. Brain Res Mol Brain Res 27: 329–332, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Campos CA, Shiina H, Ritter RC. Central vagal afferent endings mediate reduction of food intake by melanocortin-3/4 receptor agonist. J Neurosci 34: 12,636–12,645, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos CA, Shiina H, Silvas M, Page S, Ritter RC. Vagal afferent NMDA receptors modulate CCK-induced reduction of food intake through synapsin I phosphorylation in adult male rats. Endocrinology 154: 2613–2625, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campos CA, Wright JS, Czaja K, Ritter RC. CCK-induced reduction of food intake and hindbrain MAPK signaling are mediated by NMDA receptor activation. Endocrinology 153: 2633–2646, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campos CA, Wright JS, Czaja K, Ritter RC. CCK-induced reduction of food intake and hindbrain MAPK signaling are mediated by NMDA receptor activation. Endocrinology 153: 2633–2646, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol 91: 313–348, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Chandler LJ, Sutton G, Dorairaj NR, Norwood D. N-methyl d-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. J Biol Chem 276: 2627–2636, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Conti AC, Maas JW Jr, Moulder KL, Jiang X, Dave BA, Mennerick S, Muglia LJ. Adenylyl cyclases 1 and 8 initiate a presynaptic homeostatic response to ethanol treatment. PLoS One 4: e5697, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czaja K, Ritter RC, Burns GA. N-methyl-d-aspartate receptor subunit phenotypes of vagal afferent neurons in nodose ganglia of the rat. J Comp Neurol 496: 877–885, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czaja K, Ritter RC, Burns GA. Vagal afferent neurons projecting to the stomach and small intestine exhibit multiple N-methyl-d-aspartate receptor subunit phenotypes. Brain Res 1119: 86–93, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Davis SF, Derbenev AV, Williams KW, Glatzer NR, Smith BN. Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res 1017: 208–217, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dicken MS, Tooker RE, Hentges ST. Regulation of GABA and glutamate release from proopiomelanocortin neuron terminals in intact hypothalamic networks. J Neurosci 32: 4042–4048, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci 7: 335–336, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem 268: 15,174–15,179, 1993. [PubMed] [Google Scholar]
- 24.Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J. Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. J Comp Neurol 518: 6–24, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol 31: 61–78, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci 18: 10128–10135, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guard DB, Swartz TD, Ritter RC, Burns GA, Covasa M. NMDA NR2 receptors participate in CCK-induced reduction of food intake and hindbrain neuronal activation. Am J Physiol Regul Integr Comp Physiol 296: R37–R44, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Guthmann A, Herbert H. Expression of N-methyl-d-aspartate receptor subunits in the rat parabrachial and Kolliker-Fuse nuclei and in selected pontomedullary brainstem nuclei. J Comp Neurol 415: 501–517, 1999. [PubMed] [Google Scholar]
- 29.Hermann GE, Van Meter MJ, Rood JC, Rogers RC. Proteinase-activated receptors in the nucleus of the solitary tract: evidence for glial-neural interactions in autonomic control of the stomach. J Neurosci 29: 9292–9300, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hrabetova S, Serrano P, Blace N, Tse HW, Skifter DA, Jane DE, Monaghan DT, Sacktor TC. Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J Neurosci 20: RC81, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Wang H, Pickel VM. Rostrocaudal variation in targeting of N-methyl-d-aspartate and mu-opioid receptors in the rat medial nucleus of the solitary tract. J Comp Neurol 421: 400–411, 2000. [PubMed] [Google Scholar]
- 32.Hung CY, Covasa M, Ritter RC, Burns GA. Hindbrain administration of NMDA receptor antagonist AP-5 increases food intake in the rat. Am J Physiol Regul Integr Comp Physiol 290: R642–R651, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Kanterewicz BI, Urban NN, McMahon DB, Norman ED, Giffen LJ, Favata MF, Scherle PA, Trzskos JM, Barrionuevo G, Klann E. The extracellular signal-regulated kinase cascade is required for NMDA receptor-independent LTP in area CA1 but not area CA3 of the hippocampus. J Neurosci 20: 3057–3066, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457: 213–235, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Nomura S, Mizuno N. Binding of the isolectin I-B4 from Griffonia simplicifolia to the general visceral afferents in the vagus nerve: a light- and electron-microscope study in the rat. Neurosci Lett 222: 53–56, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci 23: 7143–7154, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martineau M. Gliotransmission: focus on exocytotic release of l-glutamate and d-serine from astrocytes. Biochem Soc Trans 41: 1557–1561, 2013. [DOI] [PubMed] [Google Scholar]
- 38.McDougal DH, Hermann GE, Rogers RC. Vagal afferent stimulation activates astrocytes in the nucleus of the solitary tract via AMPA receptors: evidence of an atypical neural-glial interaction in the brainstem. J Neurosci 31: 14,037–14,045, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menegon A, Bonanomi D, Albertinazzi C, Lotti F, Ferrari G, Kao HT, Benfenati F, Baldelli P, Valtorta F. Protein kinase A-mediated synapsin I phosphorylation is a central modulator of Ca2+-dependent synaptic activity. J Neurosci 26: 11670–11681, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8: 1298–1308, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Palkovits M, Mezey E, Eskay RL. Proopiomelanocortin-derived peptides (ACTH/β-endorphin/α-MSH) in brainstem baroreceptor areas of the rat. Brain Res 436: 323–338, 1987. [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Elsevier, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci 28: 11731–11740, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson J, Cruz MT, Majumdar U, Lewin A, Kingsbury KA, Dezfuli G, Vicini S, Verbalis JG, Dretchen KL, Gillis RA, Sahibzada N. Melanocortin signaling in the brainstem influences vagal outflow to the stomach. J Neurosci 33: 13286–13299, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol Behav 81: 249–273, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213: 451–452, 1981. [DOI] [PubMed] [Google Scholar]
- 47.Shigemoto R, Ohishi H, Nakanishi S, Mizuno N. Expression of the mRNA for the rat NMDA receptor (NMDAR1) in the sensory and autonomic ganglion neurons. Neurosci Lett 144: 229–232, 1992. [DOI] [PubMed] [Google Scholar]
- 48.Singru PS, Wittmann G, Farkas E, Zseli G, Fekete C, Lechan RM. Refeeding-activated glutamatergic neurons in the hypothalamic paraventricular nucleus (PVN) mediate effects of melanocortin signaling in the nucleus tractus solitarius (NTS). Endocrinology 153: 3804–3814, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci 9: 501–510, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology 150: 5351–5361, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell 152: 612–619, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutton GM, Duos B, Patterson LM, Berthoud HR. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology 146: 3739–3747, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Sutton GM, Patterson LM, Berthoud HR. Extracellular signal-regulated kinase 1/2 signaling pathway in solitary nucleus mediates cholecystokinin-induced suppression of food intake in rats. J Neurosci 24: 10240–10247, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14: 311–317, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Treece BR, Covasa M, Ritter RC, Burns GA. Delay in meal termination follows blockade of N-methyl-d-aspartate receptors in the dorsal hindbrain. Brain Res 810: 34–40, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J 279: 12–19, 2012. [DOI] [PubMed] [Google Scholar]
- 57.Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, Berthoud HR, Travagli RA. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci 28: 4957–4966, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang M, Wong AH, Liu F. Interactions between NMDA and dopamine receptors: a potential therapeutic target. Brain Res 1476: 154–163, 2012. [DOI] [PubMed] [Google Scholar]
- 59.Wenzel A, Villa M, Mohler H, Benke D. Developmental and regional expression of NMDA receptor subtypes containing the NR2D subunit in rat brain. J Neurochem 66: 1240–1248, 1996. [DOI] [PubMed] [Google Scholar]
- 60.Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology 141: 1332–1337, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Wright J, Campos C, Herzog T, Covasa M, Czaja K, Ritter RC. Reduction of food intake by cholecystokinin requires activation of hindbrain NMDA-type glutamate receptors. Am J Physiol Regul Integr Comp Physiol 301: R448–R455, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang K, Jackson MF, MacDonald JF. Recent progress in understanding subtype-specific regulation of NMDA receptors by G protein-coupled receptors (GPCRs). Int J Mol Sci 15: 3003–3024, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao H, Peters JH, Zhu M, Page SJ, Ritter RC, Appleyard SM. Frequency-dependent facilitation of synaptic throughput via postsynaptic NMDA receptors in the nucleus of the solitary tract. J Physiol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol 289: R247–R258, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Zheng H, Patterson LM, Rhodes CJ, Louis GW, Skibicka KP, Grill HJ, Myers MG Jr, Berthoud HR. A potential role for hypothalamomedullary POMC projections in leptin-induced suppression of food intake. Am J Physiol Regul Integr Comp Physiol 298: R720–R728, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]