Abstract
While there is an increased prevalence of stroke at altitude in individuals who are considered to be low risk for thrombotic events, it is uncertain how venous thrombi reach the brain. The patent foramen ovale (PFO) is a recruitable intracardiac shunt between the right and left atrium. We aimed to determine whether body position and oxygen tension affect blood flow through the PFO in healthy adults. We hypothesized that hypoxia and body positions that promote right atrial filling would independently recruit the PFO. Subjects with a PFO (n = 11) performed 11 trials, combining four different fractions of inhaled oxygen (FiO2) (1.0, 0.21, 0.15, and 0.10) and three positions (upright, supine, and 45° head down), with the exception of FiO2 = 0.10, while 45° head down. After 5 min in each position, breathing the prescribed oxygen tension, saline bubbles were injected into an antecubital vein and a four-chamber echocardiogram was obtained to evaluate PFO recruitment. We observed a high incidence of PFO recruitment in all conditions, with increased recruitment in response to severe hypoxia and some contribution of body position at moderate levels of hypoxia. We suspect that increased pulmonary vascular pressure, secondary to hypoxia-induced pulmonary vasoconstriction, increased right atrial pressure enough to recruit the PFO. Additionally, we hypothesize that the minor increase in breathing resistance that was added by the mouthpiece, used during experimental trials, affected intrathoracic pressure and venous return sufficiently to recruit the PFO.
Keywords: patent foramen ovale, hypoxia, altitude, stroke, airway resistance
the foramen ovale is a right-to-left fetal communication that allows blood to be shunted between the right and left atria in utero (3, 4). After birth, decreases in right heart pressure result in reversal of the atrial pressure gradient, favoring higher left atrial pressure and causing functional closure of the patent foramen ovale (PFO). At some future time point, fibrous adhesions can form between the septum secundum and the atrial septum, resulting in anatomical closure of the PFO (3, 4). However, autopsy and echocardiographic studies estimate that anatomical closure remains incomplete in 20–50% of the population, with the incidence of recruitable PFO decreasing as a function of age (15, 16, 28).
Although the majority of individuals with a PFO are asymptomatic—and most cardiologists regard the PFO as a normal cardiac variant—the PFO may be an important contributor to neuropathology. Recruitment of the PFO provides a means for venous thrombi, air, fat, or infectious materials to bypass the lung capillary filter, enter the systemic circulation, and embolize the brain. The prevalence of PFO in cryptogenic stroke patients is higher than both stroke patients with known etiology and stroke-free control patients (2, 25, 26, 31, 42). Migraines are a known risk factor for cryptogenic stroke, and a 3.5-fold higher prevalence of PFO exists in patients with migraine and aura than that observed in the general population (5) (17). The speculated etiology of migraine and aura is localized cerebral depolarization caused by small venous emboli or chemical substances that have gained access to the cerebral circulation (13, 21, 33, 44). Supporting this mechanism is a 15-fold higher prevalence of subclinical brain lesions in migraine and aura patients than the general population (21). Emboli gaining access to the arterial circulation through the PFO may explain the high prevalence of both cryptogenic stroke and migraine and aura occurring in individuals with a PFO.
In individuals less than 55 yr old, the prevalence of stroke is higher at high altitude than at sea level (6, 11, 37). These individuals are typically at low risk for stroke associated with atherosclerosis and other known stroke risk factors, suggesting that altitude exposure itself is a primary contributor to stroke (45). Hypoxia-induced dehydration and polycythemia may contribute to venous thrombi development at altitude (10, 20), but it is unknown whether intravascular pressure shifts during hypoxia modulate PFO recruitment and facilitate embolization. Increased pulmonary vascular pressure, secondary to hypoxia-induced pulmonary vasoconstriction, may raise right atrial pressure enough to increase the frequency of PFO recruitment (1, 38). Thus, we speculate that recruitment of the PFO under hypoxic conditions may provide a route for venous thrombi to traverse the heart and embolize the brain, potentially explaining the increased risk of stroke at altitude.
We hypothesized that hypoxia would increase the frequency of PFO recruitment in normal, healthy humans with a previously discovered PFO. Considering changes in body position affect right atrial preload (6, 11, 37), we additionally hypothesized that body positions promoting right atrial filling may also affect the frequency of PFO recruitment, individually or in combination with hypoxia. Foramen ovale recruitment was evaluated using saline contrast echocardiography in 11 adults while breathing four different fractions of inhaled oxygen (FiO2; 1.0, 0.21, 0.15, and 0.10) in three different body positions (upright, supine, and 45° head-down tilt).
METHODS
Subjects.
Eleven healthy, nonsmoking adults were recruited from another study conducted by our laboratory that excluded participants for the presence of PFO. Exclusion criteria for this study included current pregnancy or breastfeeding, previous diagnosis of cardiopulmonary disease, intra-atrial or ventricular defect, intrapulmonary arteriovenous malformation or other significant cardiac anomaly, daily medication use other than hormonal birth control, and any neurological or motor deficit that would prevent participation. The study received approval from the University of Wisconsin School of Medicine and Public Health's Institutional Review Board. Each subject gave written, informed consent prior to participating.
Saline contrast echocardiogram.
Four of eleven participants were studied on the same day their PFO was identified. The remaining seven participants returned to the laboratory after the discovery of their PFO for study on a subsequent day. In all participants, the presence of a PFO was reevaluated immediately prior to the experimental protocol.
Saline contrast echocardiography, with and without a Valsalva maneuver, was used to identify the PFO. A 22-gauge catheter was inserted in an antecubital vein and externally connected to two, three-way stopcocks attached in series. A 10-ml syringe was attached to each stopcock. Bubbles were created by manually flushing 4 ml of sterile saline and 1 ml of air between the two syringes. The Valsalva maneuver was standardized, such that each participant wore nose clips and exhaled against an occluded mouthpiece to generate +40 cmH2O mouth pressure, coinciding with the injection of agitated saline contrast. After 10–15 s, pressure was released and the transeptal passage of bubbles was assessed. A saline contrast echocardiogram without Valsalva was performed to ensure that participants did not have atrial or ventricular septal defects, or resting intrapulmonary arteriovenous malformations.
Experimental protocol.
Subjects performed trials combining four different FiO2 levels (1.0, 0.21, 0.15, 0.10, balance N2) with three body positions (upright, supine, and 45° head down tilt) in a randomized order, except head-down tilt with FiO2 = 0.10 was not performed, since preliminary trials showed that this oxygen-position combination often resulted in SpO2 dropping to <70%. Therefore, this yielded 11 independent trials for the assessment of PFO recruitment. Subjects breathed through a low-resistance mouthpiece fitted to a two-way nonrebreathing valve (model 2700; Hans Rudolph, Shawnee, KS). A gas blender combining 100% O2 and N2 was used to create the desired FiO2, which was validated with a commercially available oxygen analyzer (MiniOx, Ohio Medical Corp, Gurnee, IL), and ventilation and mixed expired CO2 and O2 were continuously sampled (Ultima PFX, Medgraphics, St. Paul, MN). Peripheral oxygen saturation (SpO2) was continuously assessed by finger sensor pulse oximetry (Nexfin, BMEYE, Amsterdam, The Netherlands), and heart rate was continuously measured by a three-lead ECG.
After 5 min in each condition, an agitated saline contrast injection was performed. An apical four-chamber echocardiogram was acquired, and the transeptal passage of contrast was evaluated (GE Vivid Ultrasound, Tirat Carmel, Israel). The foramen ovale was considered patent when contrast appeared in the left atrium within three cardiac cycles following complete opacification of the right atrium (43). Only considering the first three cardiac cycles allowed for the exclusion of bubbles that may have passed through inducible intrapulmonary arteriovenous anastomoses. These pathways are transiently recruitable by hypoxia and allow for the right-to-left passage of bubbles in ≥5 cardiac cycles (7, 24, 27).
Between trials, participants rested for 5 min in the supine position, breathing room air without a mouthpiece. The tricuspid regurgitation peak velocity was then measured in the left lateral position to estimate pulmonary artery systolic pressure using the modified Bernoulli equation (19, 32, 46). This was done to ensure that pulmonary artery systolic pressure, which may have been elevated in response to hypoxia, was normal before the commencement of a new trial. The experimental positions did not allow for accurate transthoracic echocardiographic assessment of the left ventricular outflow tract and the tricuspid regurgitation peak velocity. Therefore, these were only evaluated while participants rested between trials and not during the trials.
The magnitude of foramen ovale patency in response to Valsalva was scored according to the degree of contrast passage from right atrium to left as [0 = no bubbles in left heart; 1 = passage of 1–3 bubbles; 2 = passage of 4–12 bubbles; 3 = >12 bubbles entering the left ventricle as a bolus; 4 = >12 bubbles filling the left ventricle, with a density less than the right ventricle; 5 = >12 bubbles filling the left ventricle, with a density equal to the right ventricle] (33). All scores were based on the single cardiac cycle with the greatest density of contrast, out of the first three cardiac cycles following contrast appearance in the right heart. A licensed cardiac and vascular sonographer, blinded to the study's hypothesis and the experimental conditions, independently scored the echocardiograms.
Data analysis.
Descriptive and physiological data are presented as means ± SD. A multilevel generalized linear mixed effects model (version 9.3, SAS Institute, Cary NC) was used to analyze the association between position, FiO2, and PFO response (defined as an opening of the PFO). The main effects of position and breathing level, as well as the interaction between position and breathing, on the frequency of PFO recruitment was evaluated. Pairwise comparisons between position, FiO2, and the interaction between position and FiO2 were performed using both unadjusted (for multiple comparisons) and adjusted analysis (Tukey's honestly significant difference method for multiple comparisons). All P values are two-sided, and P < 0.05 indicates statistical significance.
RESULTS
Anthropometric data and initial PFO evaluation.
The anthropometric characteristics of the participants are shown in Table 1. Two individuals (10 and 11) with a Valsalva-induced PFO score of 1 from our earlier evaluation did not have PFO recruitment in response to the Valsalva performed on the day of the experiment (score = 0) (Table 2), or in response to any of the experimental trials. However, because they had a previously proven PFO, observed in our laboratory, their data are included in our data set.
Table 1.
Anthropometric characteristics
| Characteristic | Value |
|---|---|
| Age, yr | 26 ± 10 |
| Sex | Male, n = 4; Female, n = 7 |
| Height, cm | 170.0 ± 6.1 |
| Weight, kg | 64.7 ± 13.1 |
| Body mass index (kg/m2) | 22.5 ± 5.4 |
Table 2.
Baseline PFO score
| Subject | Current Study | Previous Studies |
|---|---|---|
| 1 | 4 | ND |
| 2 | 4 | ND |
| 3 | 3 | ND |
| 4 | 2 | ND |
| 5 | 5 | ND |
| 6 | 2 | 2 |
| 7 | 1 | 1 |
| 8 | 4 | 4 |
| 9 | 4 | 4 |
| 10 | 0 | 1 |
| 11 | 0 | 1 |
A 0–5 scale quantifying the spatial distribution and density of bubbles in left heart was used to score Valsalva-induced patent foramen ovale (PFO) recruitment. ND dentoes no data and pertains to the individuals who were studied on the day their PFO was identified (subjects 1–5). Previous scores are included for participants who had a PFO evaluated in one of our previous studies (subjects 6–11). See materials and methods.
Ventilation, heart rate, and SpO2.
Ventilation trended toward being inversely correlated with FiO2. As expected, heart rate was independently affected by both position (P < 0.001) and FiO2 (P < 0.001), and SpO2 (P < 0.001) was affected by FiO2 (P < 0.001) but not position (P = 0.70) (Table 3).
Table 3.
Cardiorespiratory data and oxygen saturation
| Position FiO2 | V̇e, l/min | Heart Rate, bpm | SpO2, % |
|---|---|---|---|
| Upright | |||
| 1.00 | 11.4 ± 1.9 | 64 ± 15 | 100 ± 1 |
| 0.21 | 12.6 ± 2.4 | 67 ± 9 | 98 ± 1 |
| 0.15 | 12.8 ± 1.5 | 70 ± 10 | 90 ± 1 |
| 0.10 | 14.3 ± 1.6 | 81 ± 12 | 73 ± 1 |
| Supine | |||
| 1.00 | 11.2 ± 1.0 | 56 ± 10 | 100 ± 1 |
| 0.21 | 10.0 ± 1.2 | 54 ± 8 | 98 ± 1 |
| 0.15 | 10.9 ± 2.1 | 62 ± 9 | 91 ± 3 |
| 0.10 | 12.4 ± 1.2 | 75 ± 9 | 71 ± 9 |
| Head-down tilt | |||
| 1.00 | 10.1 ± 0.7 | 56 ± 8 | 100 ± 1 |
| 0.21 | 11.0 ± 0.9 | 60 ± 8 | 99 ± 1 |
| 0.15 | 11.6 ± 2.7 | 62 ± 7 | 88 ± 8 |
Data are expressed as means ± SD. Data are collected during experimental trials. FiO2, fraction inspired oxygen; V̇e, exhaled minute ventilation BTPS, body temperature and pressure, saturated; bpm, beats per minute; SpO2, peripheral oxygen saturation.
Main and interaction effects of position and FiO2 on PFO recruitment.
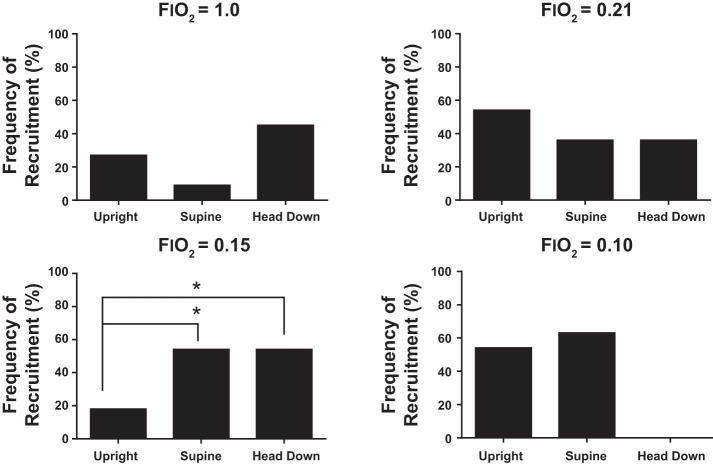
There was a high occurrence of PFO recruitment in response to all trials. There was a significant main effect of FiO2 on the frequency of PFO recruitment (P = 0.02), where an FiO2 = 0.10 had greater incidences of PFO recruitment than FiO2 = 1.0 (P < 0.01) (Fig. 1). There was no effect of position alone on the frequency of PFO recruitment (P = 0.30) (Fig. 1). At an FiO2 of 0.15, there was a combined effect of position and O2 level, where greater occurrences of PFO recruitment were observed in the supine position compared with the upright position (P < 0.05) (Fig. 2). Pairwise comparisons within each FiO2 category and within each position category are shown in Table 4.
Fig. 1.
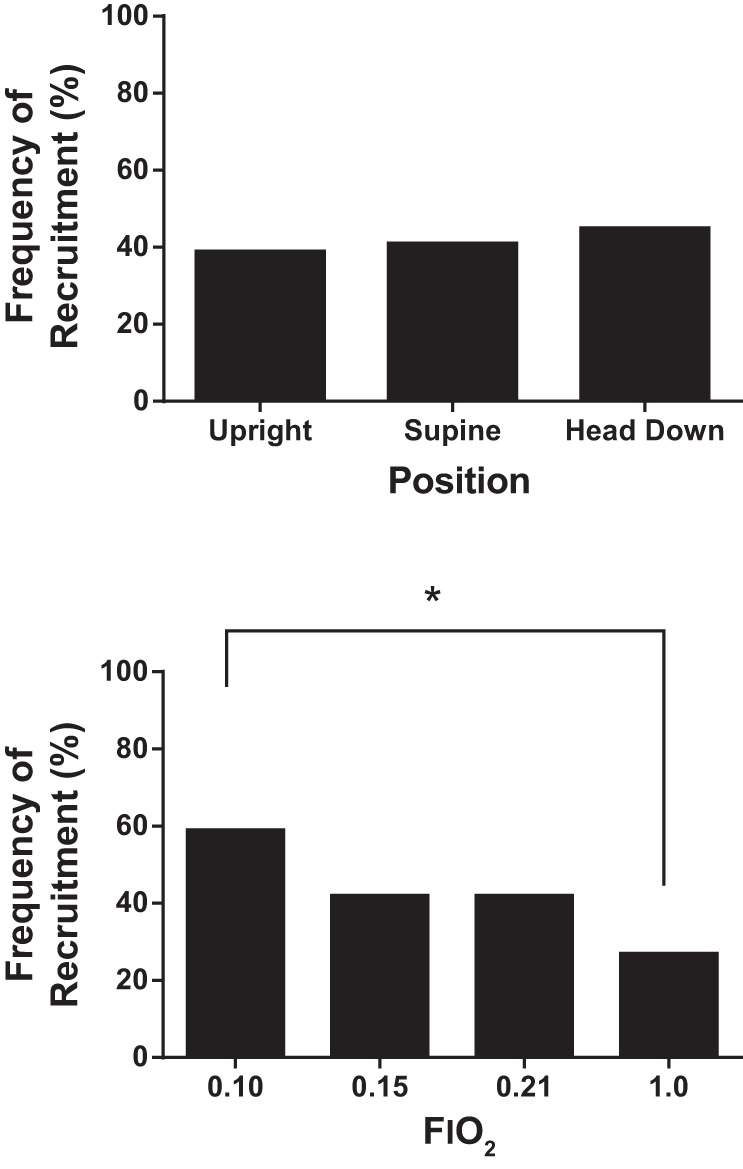
Overall frequency of patent foramen ovale (PFO) recruitment in response to position and FiO2. There was no effect of position alone on PFO recruitment. There was a main effect of FiO2 on PFO recruitment. Specifically, the frequency of recruitment during FiO2 trials of 1.0 was significantly less than the frequency of recruitment during FiO2 trials of 0.10 (*P < 0.01).
Fig. 2.
Interaction of position and FiO2 on PFO recruitment. At FiO2 = 0.15, a higher frequency of PFO recruitment was observed in the supine and 45° head-down positions, compared with the upright position (*P < 0.05).
Table 4.
Pairwise comparisons within each FiO2 category
| FiO2 | Position Comparisons | P Value |
|---|---|---|
| 0.15 | HDT vs. supine | >0.999 |
| 0.15* | HDT vs. upright | 0.047 |
| 0.15* | supine vs. upright | 0.047 |
| 0.21 | HDT vs. supine | 0.998 |
| 0.21 | HDT vs. upright | 0.415 |
| 0.21 | supine vs. upright | 0.439 |
| 1.00 | HDT vs. supine | 0.051 |
| 1.00 | HDT vs. upright | 0.401 |
| 1.00 | supine vs. upright | 0.332 |
P values adjusted for multiple comparisons. HDT, head-down tilt position.
P < 0.05.
Initial PFO evaluation and frequency of PFO recruitment.
As a post hoc analysis, low (score 0–2) vs. high (score 3–5) bubble passage in response to the initial Valsalva-induced PFO evaluation was correlated with frequency of recruitment during experimental trials (Mann-Whitney nonparametric U test) and trended toward, but was not significant (P = 0.051) (Fig. 3). Data are reported as the average of the absolute bubble scores. Pairwise comparisons within each position category are shown in Table 5.
Fig. 3.
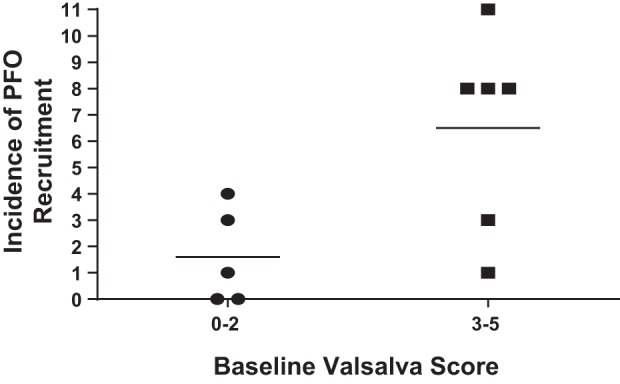
Initial PFO evaluation and frequency of PFO recruitment. There was trend toward an increased frequency of PFO recruitment in individuals with a higher baseline, Valsalva-induced PFO score (P = 0.051).
Table 5.
Pairwise comparisons within each position category
| Position | FiO2 Comparisons | P value |
|---|---|---|
| HDT | 0.15 vs. 0.21 | 0.401 |
| HDT | 0.15 vs. 1.00 | 0.795 |
| HDT | 0.21 vs. 1.00 | 0.769 |
| Supine | 0.15 vs. 0.21 | 0.431 |
| Supine* | 0.15 vs. 1.00 | 0.018 |
| Supine | 0.21 vs. 1.00 | 0.123 |
| Upright* | 0.15 vs. 0.21 | 0.045 |
| Upright | 0.15 vs. 1.00 | 0.717 |
| Upright | 0.21 vs. 1.00 | 0.153 |
P values adjusted for multiple comparisons. HDT, head-down tilt position.
Significant difference, P < 0.05.
DISCUSSION
We were motivated to perform this study by the increased prevalence of stroke at altitude, particularly in individuals who are considered to be at low risk for thrombotic events (i.e., <55 years) (17–19). We observed a high incidence of PFO recruitment in all conditions, with increased recruitment in response to severe hypoxia, and some contribution of body position at moderate levels of hypoxia.
Overall high frequency of PFO recruitment.
During screening, no participant had a right-to-left intracardiac shunt without a Valsalva. Unexpectedly, 45% of participants recruited their PFO while supine, during normoxic quiet breathing through the mouthpiece. We identify two differences between the experimental condition and our screening: 1) a difference in position (supine vs. left lateral) and 2) the addition of the mouthpiece, which provides a small amount of extrathoracic airway resistance.
The mouthpiece used during trials increased breathing resistance by 1.5 cmH2O·l−1·s. While this increase is minimal, the slight elevation in resistance may have been sufficient to affect intrathoracic pressure and cause cyclic elevations in venous return, thus causing PFO recruitment. Indeed, during normal breathing, fluctuations in venous return are respiratory cycle-dependent (42). During inspiration, intrathoracic pressure becomes negative, widening the pressure gradient between the right atrium and peripheral veins and facilitating venous return. The drop in intrathoracic pressure causes expansion of the right heart and vena cava, further facilitating venous return. Additionally, right heart expansion may stretch the atrial septum and facilitate ease of PFO recruitment (42).
Augmented venous return, compounded with a direct effect of position on the atrial septum may explain why we observed the highest frequency of PFO recruitment in response to the upright position in normoxia. A decrease in preload caused by the gravitational effects of being upright can place additional stretch on the atrial septum (39, 47). Patients with platypnoa-orthodeoxia syndrome experience right-to-left shunting through the PFO while upright that can be alleviated by reclining supine (29, 36, 39, 47). While the etiology of this condition is often multifactorial, one contributing factor is that the additional stretch placed on the atrial septum when upright causes venous return to preferentially flow toward the atrial septal wall (39, 47). This can cause PFO recruitment in the absence of an elevated mean right atrial pressure (18, 29). Enhanced atrial stretch induced by the upright position combined with the effects of breathing resistance on right heart expansion may similarly direct venous return toward the foramen ovale, facilitating PFO recruitment under our experimental conditions.
Despite it being a plausible mechanism, whether breathing resistance plays a causal role in PFO recruitment remains unknown, but it could have important implications for specific populations. Divers who have had decompression illness and individuals susceptible to high-altitude pulmonary edema have a higher prevalence of PFO (1, 40). Interestingly, both populations commonly experience increased breathing resistance. The regulator of self-contained underwater breathing apparatus increases both inspiratory and expiratory resistance (41), and individuals at altitude commonly adopt pursed lip breathing, which serves to improve gas exchange at the expense of increased expiratory resistance (30). It is possible that breathing resistance may play a role in pathophysiology of decompression illness and high-altitude pulmonary edema.
Enhanced PFO recruitment during hypoxic conditions.
We found enhanced PFO recruitment in response to severe hypoxia (FiO2 = 0.10). We saw no effect of position at FiO2 = 0.10, but we were unable to study individuals breathing at FiO2 = 0.10, while in the head-down position. Therefore, it is unknown whether a synergistic effect of hypoxia and increased right atrial filling, while in the head-down position would have had a significant effect on PFO recruitment. Additionally, we observed a combined effect of hypoxia and position during FiO2 = 0.15 trials, where both the supine and head-down positions had greater frequencies of PFO recruitment than the upright position. We speculate that the increase in PFO recruitment during our hypoxic conditions was largely a result of hypoxic pulmonary vasoconstriction, increasing pulmonary vascular resistance and, consequently, pulmonary artery and right heart pressures. Fractions of inspired oxygen of 0.10 and 0.15 are equivalent to Po2 values at 15,000 and 8,000 feet altitude, respectively. Thus, our findings may have implications for high-altitude travelers and inhabitants with a PFO, as well as aircraft passengers, since cabin pressure is equivalent to 8,000 feet (9). Considering air travel is an independent risk factor for deep vein thrombosis, cause for concern may be warranted for aircraft passengers with a PFO who are on long flights (8, 9, 23).
However, it is important to note that our findings were observed at sea level, with normobaric hypoxia. Recruitment of the PFO may be different when hypoxia occurs in the context of low barometric pressure, potentially by altering airway and pulmonary vascular resistance. Hypobaric hypoxia elicits a more profound arterial hypoxemia than normobaric hypoxia (34), presumably inducing a stronger hypoxic pulmonary vasoconstrictor response. Indeed, pulmonary artery pressure is higher at 3,810 m altitude (12,500 feet) compared with normobaric hypoxia of the same PiO2 (14), consistent with a stronger hypoxic pulmonary vasoconstrictor response. Thus, it is possible that our results underestimate the extent PFO recruitment occurs in response to hypobaric hypoxia. Alternatively, gas viscosity is lower at lower barometric pressures, which would decrease airway resistance (12), potentially decreasing the frequency of PFO recruitment. Future studies in hypobaric conditions are needed to address the relative importance of these factors.
Day-to-day variability in PFO recruitment.
We took care to rigorously standardize the methodology for conducting Valsalva-induced saline contrast echocardiography. The mouth pressure (+40 cmH2O) and the length of the breath hold (15 s) was consistent in all participants, and we took care to note bowing of the atrial septum after release of the Valsalva. Surprisingly, two individuals with a previously discovered PFO via Valsalva did not have PFO recruitment on the day of study. The reason for this finding is not clear. The prevalence of PFO decreases with age (16), and although only 4 to 11 mo passed between the previous and current study, it is possible that complete anatomical closure of the PFO occurred in these individuals. Another explanation is that variations in central venous pressure affect PFO recruitment. Variations in central venous pressure would presumably affect the pressure gradient that is created during a Valsalva maneuver. The high intrathoracic pressure generated during the breath-hold phase of a Valsalva maneuver impedes venous return, thus creating a pressure gradient between the falling right atrial pressure and venous pressure proximal to the impedance (22). Upon release of the Valsalva, this gradient drives venous return and raises right atrial pressure. Lower baseline values of central venous pressure would attenuate the pressure gradient; thus, the driving force for venous return upon Valsalva release. Therefore, daily variations in hydration status or other factors affecting central venous pressure may produce false-negative PFO evaluation results. Additionally, a correlation exists between PFO size and pathological events. Patients with neurological events, particularly multiple cerebrovascular events, have significantly larger PFOs than controls (35). We investigated whether low (score 0–2) vs. high (score 3–5) bubble passage during the initial Valsalva-induced PFO evaluation was correlated with frequency of recruitment during experimental trials. Although not statistically significant (P = 0.051), there was a trend of lower incidences of PFO recruitment occurring in individuals with low bubble passage in response to Valsalva.
Applicability and limitations of our findings.
While there is an increased risk of stroke at altitude, it remains debatable how venous thrombi get to the brain. We do not know whether the PFO is a contributing factor or a risk modifier for stroke at altitude, but our results show an enhanced frequency of PFO recruitment during severe hypoxia and that body position affects the frequency of PFO recruitment with moderate hypoxia. We suspect that increased pulmonary vascular pressure, secondary to hypoxia-induced pulmonary vasoconstriction, raises right atrial pressure sufficiently to increase the frequency of PFO recruitment. Although there was an effect of FiO2 on PFO recruitment, there was a high frequency of PFO recruitment in response to all trials. We hypothesize that this was due to the minor increase in respiratory resistance provided by the mouthpiece. Nonetheless, this condition was imposed on all participants during all trials, suggesting that the effects of hypoxia and body position on PFO recruitment exist in addition to any effect of respiratory resistance. Furthermore, whether a high Valsalva PFO score translates to higher blood flow across the PFO at altitude, and whether PFO closure is warranted in these individuals, remains an important question worthy of further study.
Conclusions.
We show that severe hypoxia affects PFO recruitment and that positions promoting right atrial filling affect PFO recruitment during moderate hypoxia. These findings may suggest a possible mechanism to explain the incidence of ischemic neurological events at altitude in otherwise low-risk individuals. Furthermore, this study suggests that a minimal amount of respiratory load may affect PFO recruitment.
GRANTS
This work was funded by grants from the National Institutes of Health (5R01HL-086897 and 5T32HL-007654).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.L.M., K.R.B., D.F.P., M.W.E., and M.L.B. conception and design of research; K.L.M., A.G.B., N.H., K.R.B., D.F.P., and M.L.B. performed experiments; K.L.M., A.G.B., N.H., K.R.B., and M.L.B. analyzed data; K.L.M., A.G.B., N.H., K.R.B., D.F.P., M.W.E., and M.L.B. interpreted results of experiments; K.L.M. and M.L.B. prepared figures; K.L.M. and M.L.B. drafted manuscript; K.L.M., A.G.B., N.H., K.R.B., D.F.P., M.W.E., and M.L.B. edited and revised manuscript; K.L.M., A.G.B., N.H., K.R.B., D.F.P., M.W.E., and M.L.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Jens C. Eickhoff for assistance with statistical analysis, Dr. William G. Schrage for the use of lab space and equipment, and John W. Harrell for technical assistance.
REFERENCES
- 1.Allemann Y, Hutter D, Lipp E, Sartori C, Duplain H, Egli M, Cook S, Scherrer U, Seiler C. Patent foramen ovale and high-altitude pulmonary edema. JAMA 296: 2954–2958, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke incidental or pathogenic? Stroke 40: 2349–2355, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson RH, Brown NA, Webb S. Development and structure of the atrial septum. Heart 88: 104–110, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson RH, Webb S, Brown NA. Clinical anatomy of the atrial septum with reference to its developmental components. Clin Anat 12: 362–374, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Azarbal B, Tobis J, Suh W, Chan V, Dao C, Caster R. Association of interatrial shunts and migraine headaches. Impact of transcatheter closure. J Am Coll Cardiol 45: 489–492, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Basnyat B, Wu TY, Gertsch JH. Neurological conditions at altitude that fall outside the usual definition of altitude sickness. High Alt Med Biol 5: 171–179, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Bates M, Fulmer B, Farrell E, Drezdon A, Pegelow D, Conhaim R, Eldridge M. Hypoxia recruits intrapulmonary arteriovenous pathways in intact rats but not isolated rat lungs. J Appl Physiol (1985) 112: 1915–1920, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belcaro G, Geroulakos G, Nicolaides AN, Myers KA, Winford M. Venous thromboembolism from air travel. The LONFLIT study. Angiology 52: 369–374, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Brundrett G. Comfort and health in commercial aircraft: a literature review. J R Soc Promot Health 121: 29–37, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Clarke C. Acute mountain sickness: medical problems associated with acute and subacute exposure to hypobaric hypoxia. Postgrad Med J 82: 748–753, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson J, Heath D, Gosney J, Williams D. Altitude-related deaths in 7 trekkers in the Himalayas. Thorax 38: 646–656, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drazen JM, Loring SH, Ingram RH Jr. Distribution of pulmonary resistance: effects of gas density, viscosity, and flow rate. J Appl Physiol 41: 388–395, 1976. [DOI] [PubMed] [Google Scholar]
- 13.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 17: 439–447, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Eldridge MW, Podolsky A, Richardson RS, Johnson DH, Knight DR, Johnson EC, Hopkins SR, Michimata H, Grassi B, Feiner J, Kurdak SS, Bickler PE, Wagner PD, Severinghaus JW. Pulmonary hemodynamic response to exercise in subjects with prior high-altitude pulmonary edema. J Appl Physiol (1985) 81: 911–921, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Gerriets T, Tetzlaff K, Liceni T, Schafer C, Rosengarten B, Kopiske G, Algermissen C, Struck N, Kaps M. Arteriovenous bubbles following cold water sport dives: Relation to right-to-left shunting. Neurology 55: 1741–1743, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 59: 17–20, 1984. [DOI] [PubMed] [Google Scholar]
- 17.Hara H, Virmani R, Ladich E, Mackey-Bojack S, Titus J, Reisman M, Gray W, Nakamura M, Mooney M, Poulose A, Schwartz RS. Patent foramen ovale: Current pathology, pathophysiology, and clinical status. J Am Coll Cardiol 46: 1768–1776, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Hegland DD, Kunz GA, Harrison JK, Wang A. Clinical problem-solving. A hole in the argument. N Engl J Med 353: 2385–2390, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Himelman RB, Stulbarg M, Kircher B, Lee E, Kee L, Dean NC, Golden J, Wolfe CL, Schiller NB. Noninvasive evaluation of pulmonary artery pressure during exercise by saline-enhanced Doppler echocardiography in chronic pulmonary disease. Circulation 79: 863–871, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Jha SK, Anand AC, Sharma V, Kumar N, Adya CM. Stroke at high altitude: Indian experience. High Alt Med Biol 3: 21–27, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Kruit MC, van Buchem MA, Hofman PAM, Bakkers JTN, Terwindt GM, Ferrari MD, Launer LJ. Migraine as a risk factor for subclinical brain lesions. JAMA 291: 427–434, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Lam YY, Yu CM, Zhang Q, Yan BP, Yip GW. Enhanced detection of patent foramen ovale by systematic transthoracic saline contrast echocardiography. Int J Cardiol 152: 24–27, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Lapostolle F, Surget V, Borron SW, Desmaizieres M, Sordelet D, Lapandry C, Cupa M, Adnet F. Severe pulmonary embolism associated with air travel. N Engl J Med 345: 779–783, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Laurie SS, Yang X, Elliott JE, Beasley KM, Lovering AT. Hypoxia-induced intrapulmonary arteriovenous shunting at rest in healthy humans. J Appl Physiol 110: 1502, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, Drobinski G, Thomas D, Grosgogeat Y. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 318: 1148–1152, 1988. [DOI] [PubMed] [Google Scholar]
- 26.Lopez MF, Sarracino DA, Vogelsang M, Sutton JN, Athanas M, Krastins B, Garces A, Prakash A, Peterman S, Demirjian Z, Inglessis-Azuaje I, Feeney K, Elia M, McMullin D, Dec GW, Palacios I, Lo EH, Buonanno F, Ning MM. Heart-brain signaling in patent foramen ovale-related stroke: differential plasma proteomic expression patterns revealed with a 2-pass liquid chromatography-tandem mass spectrometry discovery workflow. J Investig Med 60: 1122–1130, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS, Eldridge MW. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol 104: 1418–1425, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Lovering AT, Stickland MK, Amann M, Murphy JC, Hokanson JS, Eldridge MW. The effects of patent foramen ovale (PFO) on pulmonary gas exchange during incremental exercise. FASEB J 22: 1175.–16., 2008. [Google Scholar]
- 29.Marini C, Miniati M, Ambrosino N, Formichi B, Tonelli L, Di Ricco G, Michelassi C, Gill S, Spadoni I. Dyspnoea and hypoxaemia after lung surgery: the role of interatrial right-to-left shunt. Eur Respir J 28: 174–181, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Nield MA, Hoo GWS, Roper JM, Santiago S. Efficacy of pursed-lips breathing. A breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil 27: 237–244, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Overell JR, Bone I, Lees KR. The association of interatrial septal abnormalities and stroke: A meta-analysis of case control studies. Neurology 54: A466–A466, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Rudski LG, Lai WW, Afilalo J, Hua LQ, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23: 685–713, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Sandler M. Migraine pulmonary disease. Lancet 299: 618–619, 1972. [DOI] [PubMed] [Google Scholar]
- 34.Savourey G, Launay JC, Besnard Y, Guinet A, Travers S. Normo- and hypobaric hypoxia: are there any physiological differences? Eur J Appl Physiol 89: 122–126, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Schuchlenz HW, Weihs W, Horner S, Quehenberger F. The association between the diameter of a patent foramen ovale and the risk of embolic cerebrovascular events. Am J Med 109: 456–462, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Seward JB, Hayes DL, Smith HC, Williams DE, Rosenow EC, Reeder GS, Piehler JM, Tajik AJ. Platypnea-orthodeoxia. Clinical profile, diagnostic workup, management, and report of 7 cases. Mayo Clin Proc 59: 221–231, 1984. [DOI] [PubMed] [Google Scholar]
- 37.Song SY, Asaji T, Tanizaki Y, Fujimaki T, Matsutani M, Okeda R. Cerebral thrombosis at altitude—its pathogenesis and the problems of prevention and treatment. Aviat Space Environ Med 57: 71–76, 1986. [PubMed] [Google Scholar]
- 38.Swenson ER. Hypoxic pulmonary vasoconstriction. High Alt Med Biol 14: 101–110, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Toffart AC, Bouvaist H, Feral V, Blin D, Pison C. Hypoxemia-orthodeoxia related to patent foramen ovale without pulmonary hypertension. Heart Lung 37: 385–389, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Torti SR, Billinger M, Schwerzmann M, Vogel R, Zbinden R, Windecker S, Seiler C. Risk of decompression illness among 230 divers in relation to the presence and size of patent foramen ovate. Eur Heart J 25: 1014–1020, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Warkander DE, Nagasawa GK, Lundgren CEG. Effects of inspiratory and expiratory resistance in divers' breathing apparatus. Undersea Hyperb Med 28: 63–73, 2001. [PubMed] [Google Scholar]
- 42.Webster MWI, Smith HJ, Sharpe DN, Chancellor AM, Swift DL, Bass NM, Glasgow GL. Patent foramen ovale in young stroke patients. Lancet 2: 11–12, 1988. [DOI] [PubMed] [Google Scholar]
- 43.Weyman AE. Principles and Practice of Eechocardiography. Philadelphia, PA: Lea & Febiger, 1994, p. xvii, 1335 p. [Google Scholar]
- 44.Wilmshurst PT, Nightingale S, Walsh KP, Morrison WL. Effect on migraine of closure of cardiac right-to-left shunts to prevent recurrence of decompression illness or stroke or for haemodynamic reasons. Lancet 356: 1648–1651, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Wilson JA. Performance and training standards for endovascular ischemic stroke treatment. J Neurointerv Surg 1: 153–153, 2009. [DOI] [PubMed] [Google Scholar]
- 46.Yock PG, Naasz C, Schnittger I, Popp RL. Doppler tricuspid and pulmonic regurgitation in normals. Is it real? Circulation 70: 40–40, 1984. [Google Scholar]
- 47.Zavalloni D, Lisignoli V, Barbaro C, Mennuni M, Tosi P, Marcheselli S, Presbitero P. Platypnoea-orthodeoxia syndrome secondary to patent foramen ovate (PFO): a challenging subset for PFO percutaneous closure. Heart Lung Circ 22: 642–646, 2013. [DOI] [PubMed] [Google Scholar]