Abstract
We have shown recently that glucose-dependent insulinotropic polypeptide (GIP), but not glucagon-like peptide 1 (GLP-1) augments H+ peptide cotransporter (PepT1)-mediated peptide absorption in murine jejunum. While we observed that inhibiting cAMP production decreased this augmentation of PepT1 activity by GIP, it was unclear whether PKA and/or other regulators of cAMP signaling pathway(s) were involved. This study utilized tritiated glycyl-sarcosine [3H-glycyl-sarcosine (Gly-Sar), a relatively nonhydrolyzable dipeptide] uptake to measure PepT1 activity in CDX2-transfected IEC-6 (IEC-6/CDX2) cells, an absorptive intestinal epithelial cell model. Similar to our earlier observations with mouse jejunum, GIP but not GLP-1 augmented Gly-Sar uptake (control vs. +GIP: 154 ± 22 vs. 454 ± 39 pmol/mg protein; P < 0.001) in IEC-6/CDX2 cells. Rp-cAMP (a PKA inhibitor) and wortmannin [phosophoinositide-3-kinase (PI3K) inhibitor] pretreatment completely blocked, whereas neither calphostin C (a potent PKC inhibitor) nor BAPTA (an intracellular Ca2+ chelator) pretreatment affected the GIP-augmented Gly-Sar uptake in IEC-6/CDX2 cells. The downstream metabolites Epac (control vs. Epac agonist: 287 ± 22 vs. 711 ± 80 pmol/mg protein) and AKT (control vs. AKT inhibitor: 720 ± 50 vs. 75 ± 19 pmol/mg protein) were shown to be involved in GIP-augmented PepT1 activity as well. Western blot analyses revealed that both GIP and Epac agonist pretreatment enhance the PepT1 expression on the apical membranes, which is completely blocked by wortmannin in IEC-6/CDX2 cells. These observations demonstrate that both cAMP and PI3K signaling pathways augment GIP-induced peptide uptake through Epac and AKT-mediated pathways in intestinal epithelial cells, respectively. In addition, these observations also indicate that both Epac and AKT-mediated signaling pathways increase apical membrane expression of PepT1 in intestinal absorptive epithelial cells.
Keywords: IEC-6 cells, CDX2 expression, Intestinal absorptive epithelial cells, dipeptide uptake Gly-Sar uptake.
the proton (H+)-peptide cotransporter (PepT1) is a member of the larger family of peptide transporters that is found primarily in the kidney and in absorptive epithelial cells in the small intestine (12). PepT1 located on the brush border membranes of villus cells mediates di- and tripeptide absorption in the small intestine (2). PepT1-mediated peptide absorption driven by H+ gradient is coupled to Na:H exchange isoform-3 (NHE3) that recycles H+ across brush border membranes in intestine (34). PepT1 has been shown to be regulated by hormones such as insulin, thyroxin, and leptins that influence energy balance, food consumption, and appetite (5, 13, 27, 28, 54). In recent studies, we have shown that glucose-dependent insulinotropic polypeptide (GIP), but not glucagon-like peptide-1 (GLP-1), stimulates glucose and dipeptide absorption by increasing SGLT1 and PepT1 activities, respectively, in the murine jejunum (10, 52). Postprandially, the duodenal neuroendocrine cells secrete the incretin hormones GIP (from mucosal K cells) and GLP-1 (from L cells) into the portal circulation (62, 64). Both GIP and GLP-1 stimulate insulin secretion and play important roles in blood glucose homeostasis (7). In addition to stimulating insulin secretion, GIP also directly activates SGLT1- and PepT1-mediated glucose and dipeptide in murine jejunum, respectively (10, 52).
The cAMP, Ca2+-inositol phosphate, phosophoinositide-3-kinase (PI3K), and Ca2+-calmodulin-dependent pathways are the major signaling pathways that regulate biological processes. Of these four pathways, the cAMP and the Ca2+-inositol phosphate pathways have been shown to regulate a wide array of nutrient transporters (20, 30, 65), whereas PI3K- and Ca2+-calmodulin-regulated pathways have been shown to regulate the nutrient transporters only to a lesser extent in the epithelial cells (26, 31, 46). Activation of GIP receptor (GIPR) has been shown to release cAMP and PI3K in various cell types (14, 16, 18, 36). GIPR splice variants expressed in different tissues including pancreatic β-cells have been shown to be regulated by distinct signaling pathways (24, 38, 66). In our recent studies, there is a possibility of multiple GIPRs expressed on the basolateral membranes of mouse jejunal villus cells and, upon stimulation of GIPR, enhanced PepT1-mediated peptide absorption in a cAMP-dependent manner (10). However, it is not known which signaling pathway(s) augment the PepT1-mediated peptide absorption in intestinal epithelial cells. The aim of the present study was to validate a cellular model of a differentiated, peptide-absorbing intestinal epithelium and to use 3H-glycyl-sarcosine (Gly-Sar; a nonhydrolyzable peptide) to study the intracellular signaling pathways that participate in the augmentation of PepT1 by GIP.
METHODS
Cell culture and transfection.
A rat intestinal crypt-like epithelial cell line (IEC-6) was transfected with the homeobox protein gene CDX2 (Origene), a transcription factor that induces epithelial cell differentiation (44, 45). In brief, IEC-6 cells were grown to 80% confluence and transfected with the CDX2 plasmid (1 μg) by use of Lipofectamine (5 μl). Three days following CDX2 transfection, G418 (400 μg/l) was added to select for stably CDX2-transfected IEC-6 (IEC-6/CDX2) cells. CDX2 expression was verified by PCR analysis of RNA isolated from IEC-6/CDX2 cells by use of primers supplied with the plasmid (Origene, data not shown). The IEC-6/CDX2 cells grown in 75 ml flasks with DMEM medium supplemented with 10% FBS and insulin (50 pmol/l) at 37°C with 10% CO2 atmosphere were split every 2–3 days. IEC-6/CDX2 cells grown to 80% confluence in flasks were transferred to Matrigel-coated Transwell plates (Corning). IEC-6/CDX2 cells were grown for 3–5 days postconfluent to form a differentiated/polarized monolayer on the Transwell plates and used for Western blot analyses and peptide uptake studies.
Western blot analyses were performed to determine whether the 3–5 days postconfluent polarized IEC-6/CDX2 monolayers express features of villus cells of intestinal epithelium. GIPR, GLP-1 receptor (GLP-1R), and PepT1 specific proteins were enhanced and/or primarily expressed in IEC-6/CDX2 cells compared with that in IEC-6/wild-type (IEC-6/WT) cells (Fig. 1). Similar expression levels of E-cadherin, an adhesion transmembrane protein indicates that the enhanced and/or primary expression of GIPR, GLP-1R and PepT1 specific proteins seen is specific to IEC-6/CDX2 cells (Fig. 1). GIPR and GLP-1R specific antibodies used for Western analyses are widely used to detect these receptors in several other tissues (6, 22, 43, 63). In addition to Western blot analyses, we also measured H+-gradient-driven 3H-Gly-Sar uptake in IEC-6/CDX2 and IEC-6/WT cells. As shown in Fig. 2, IEC-6/CDX2 cells exhibited severalfold higher rates of Gly-Sar uptake compared with IEC-6/WT cells. These observations establish that IEC-6/CDX2 cells exhibit features of differentiated villus-like cells with mature functional nutrient transport processes. As such, further characterization of the regulation of PepT1 activity by GIP was pursued in IEC-6/CDX2 cells.
Fig. 1.
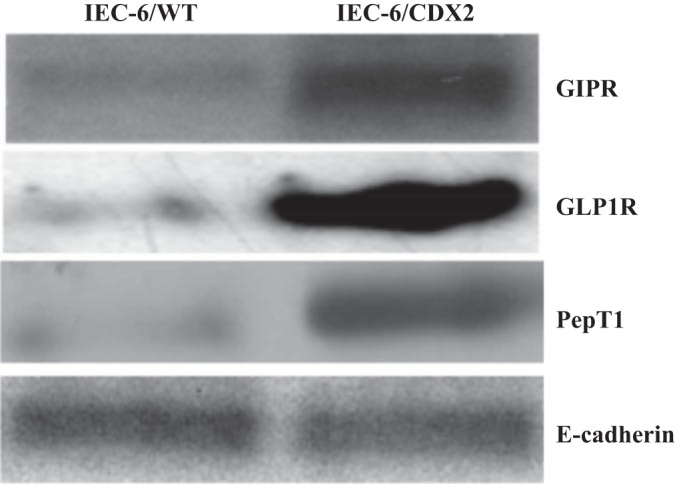
Western blot analyses of IEC-6/wild-type (IEC-6/WT) and CDX2-transfected IEC-6 (IEC-6/CDX2) cell homogenates. Homogenates (30 μg) of IEC-6/WT and IEC-6/CDX2 cells grown to 3–5 days postconfluence in Transwell plates resolved on PAGE gel electrophoresis were transferred to nitrocellulose membranes. The blots were probed with glucose-dependent insulinotropic polypeptide (GIP) receptor (GIPR), glucagon-like peptide-1 (GLP1) receptor (GLP1R), H+ peptide cotransporter (PepT1), and E-cadherin specific primary antibodies and developed as described in methods.
Fig. 2.
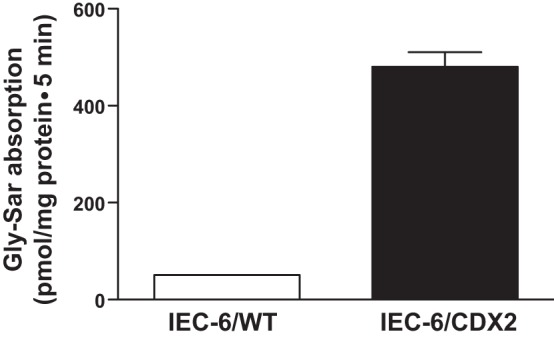
Proton (H+)-gradient-driven glycyl-sarcosine (Gly-Sar) uptake as a measure of H+ peptide cotransporter (PepT1) in IEC-6/WT and IEC-6/CDX2 cells. 3H-Gly-Sar absorption was measured for 5 min in 3–5 days postconfluent IEC-6/WT and IEC-6/CDX2 cells grown on Transwell plates. Gly-Sar absorption was measured both in the presence (mucosal vs. serosal pH: 6.5 vs. 7.5) and in the absence (mucosal vs. serosal pH: 7.5 vs. 7.5) of a pH gradient. Absolute Gly-Sar uptake values presented were calculated by subtracting uptake with apical pH 7.5 from that of uptake in apical pH 6.5. Results presented represent means ± SE from triplicate assays from four different cultures. P < 0.02 compared with control.
Uptake studies.
Gly-Sar (100 μM) uptake was measured in both IEC-6/CDX2 and IEC-6/WT monolayers grown 3–5 days postconfluence on Transwell plates. The monolayers were washed and incubated in Leibovitz L-15 medium supplemented with FBS (10%) and insulin (50 pmol/l) at 37°C for 1 h. Subsequently, both the mucosal and serosal sides were washed briefly and incubated for 5 min in Na-HEPES buffer (in mM: 130 NaCl, 4.5 KC1, 1.2 KH2PO4, 1.0 MgSO4, 1.25 CaC12, 20 HEPES, pH 7.4) at room temperature. Uptake was initiated by replacing mucosal medium with uptake medium (400 μl). Uptake medium contained 3H-Gly-Sar (100 μM) and either Na-HEPES (pH 7.4) or Na-HEPES (pH 6.0) buffer. Following a 5-min incubation period, uptake was arrested with 1 ml ice-cold stop solution (uptake buffer without radioactive tracer), after which the cells were lysed in 500 μl 1 N NaOH. The cell lysates were used for protein assay (10 μl) and 3H-Gly-Sar radioactivity measurement in a scintillation counter (Beckman Instruments). 3H-Gly-Sar uptake was expressed as picomoles per milligram protein.
Drug treatments.
The IEC-6/CDX2 and IEC-6/WT cells were grown in Transwell plates for 3–5 days postconfluence washed and incubated in Leibovitz L-15 medium at 37°C for 15 min. Either GIP or GLP-1 (0.1 μM each, Bachem) was added to the basolateral medium (i.e., lower chamber of the Transwell plate) and incubated for an additional 45 min. Based on previous studies, Gly-Sar uptake experiments had either Rp-cAMP (50 mM; adenosine 3′5′-cyclic-monophorothioate, RP isomer triethyl ammonium salt; Calbiochem (19, 57), calphostin C (200 nM) (35, 49); BAPTA (5 mM) (3, 19) or wortmannin (200 nM) (11, 55) inhibitors added either alone or simultaneously with GIP to IEC6-CDX2 monolayers. The effects of 8-pCPT-2′-O-Me-cAMP [an Epac (exchange protein directly activated by cAMP) agonist; Tocris Biosciences] (17, 32) and 8Br-cAMP (a PKA agonist; Calbiochem) (8, 9) were also examined and compared with GIP-treated cells in Gly-Sar uptake studies.
Biotinylation and Western blotting.
Apical membrane from IEC-6/WT and IEC-6/CDX2 cells was isolated by using a protein biotinylation kit per the manufacturer's instructions (Pierce). Briefly, IEC-6/WT and IEC-6/CDX2 cell monolayers grown for 3–5 days postconfluence were washed and incubated (both upper and lower chambers) in Leibovitz L-15 medium for 1 h at 37°C. Fifteen minutes following initiation of incubation in Leibovitz L-15 medium, GIP was added to the basolateral chamber and was incubated for an additional 45 min with AKT inhibitor-15 (AKT antagonist). Various agonists were also added in the absence of GIP including an 8-pCPT-2′-O-Me-cAMP (Epac agonist) and 8Br-cAMP (a PKA agonist). Biotinylation solution was placed in the upper (apical) chamber of the Transwell plate and incubated on a shaker. Following 30-min incubation at room temperature, the reaction was stopped and the cells were scraped into RIPA buffer (Boston BioProducts). Protein concentration was measured by the Bradford method. Proteins (100 μg) were resolved by use of 20%-gradient premade agarose gels and Accugene Tris-glycine-SDS running buffer (Lonza) and were transferred by use of a Transfer buffer (Boston BioProducts) containing 20% methanol onto nitrocellulose membranes (Bio-Rad). The blots were probed with anti-PepT1, anti-GIPR, anti-GLP1R, or anti-E-cadherin antibodies (1:1,000; Santa Cruz) and the appropriate HRP-secondary antibody (1:5,000; Santa Cruz). SuperSignal West Pico Chemiluminescent Substrate kits (Pierce) were used to expose the blots on HyBlot CL film (Denville). E-cadherin used as an internal loading control.
Statistical analyses.
The results presented represent means ± SE of triplicate assays from four different experiments. Statistical analyses were performed by unpaired or paired Student's t-test by use of GraphPad analysis software. A P < 0.05 is considered statistically significant.
RESULTS
Initial studies established that IEC-6/CDX2 cells grown on Transwell plates exhibited characteristics of differentiated intestinal absorptive epithelium with enhanced GIPR, GLP1R, and PepT1 specific protein expression and increased rates of Gly-Sar uptake (Figs. 1 and 2). Thus all further experiments were performed to determine whether incretin hormones applied to the basolateral side of IEC-6/CDX2 monolayers would increase Gly-Sar uptake across the apical membrane. As shown in Fig. 3, GIP enhanced the Gly-Sar absorption by nearly 2.5-fold in IEC-6/CDX2 cells (control vs. GLP1: 154 ± 22 vs. 454 ± 45 pmol/mg protein; P < 0.001). In contrast, GLP1 did not significantly alter the Gly-Sar absorption in IEC-6/CDX2 cells (control vs. GLP1: 154 ± 22 vs. 144 ± 25 pmol/mg protein). These observations establish that GIP, but not GLP1, augment Gly-Sar absorption in intestinal epithelial cells.
Fig. 3.
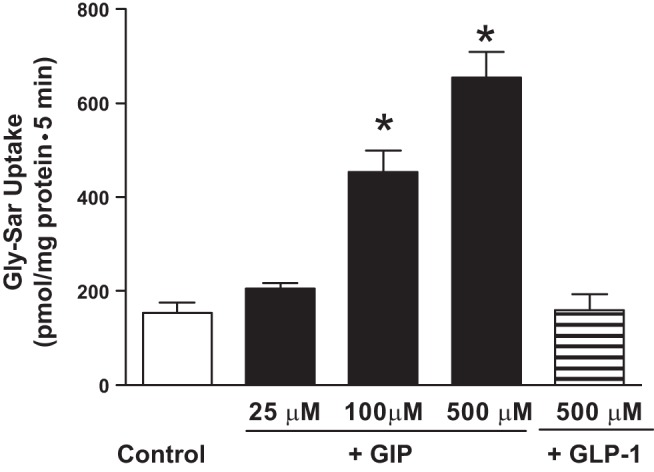
Effect of incretin hormones on H+-Gly-Sar cotransport in IEC-6/CDX2 cells. Gly-Sar absorption was measured for 5 min in 3–5 days postconfluent IEC-6/CDX2 cells grown on Transwell plates. Gly-Sar absorption was measured both in the presence (mucosal vs. serosal pH: 6.5 vs. 7.5) and in the absence (mucosal vs. serosal pH: 7.5 vs. 7.5) of a pH gradient. Absolute values represent H+-gradient-driven Gly-Sar absorption calculated by subtracting uptake measured in the presence apical pH 7.5 from that of apical pH 6.5 (Control). Gly-Sar absorption was also measured in the presence of serosal GIP in a dose-dependent manner (25, 100, and 500 μM) or GLP-1 (0.5 μM). GIP did not significantly alter Gly-Sar absorption in the absence of pH gradient (data not shown). Results presented represent means ± SE triplicate assays from 4 different experiments. *P < 0.05 compared with control.
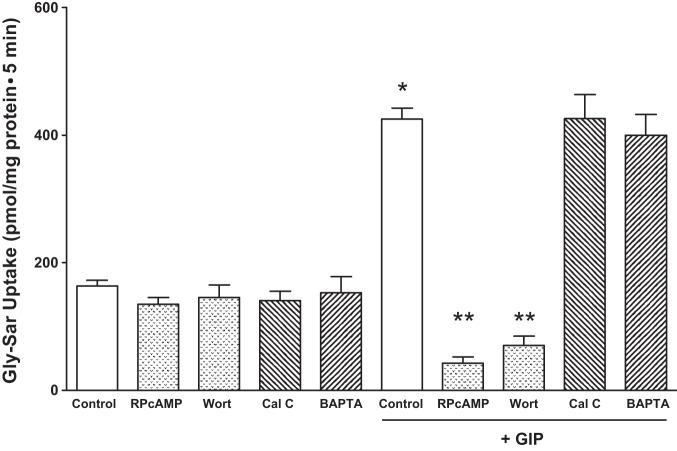
To determine which of the four major intracellular signaling pathways (i.e., PKA, PKC, PI3K, and Ca2+-calmodulin-dependent pathways) regulate the GIP enhanced PepT1 activity, the effect of Rp-cAMP (a competitive inhibitor of cAMP action), calphostin C (a potent PKC inhibitor), BAPTA (an intracellular Ca2+ chelator), calmodulin (BAPTA), and PI3K (wortmannin) pathways were assessed for their effect on GIP-augmented PepT1 activity. As shown in Fig. 4, GIP-stimulated PepT1 activity was inhibited nearly 80% by Rp-cAMP (425 ± 44 vs. 52 ± 20 pmol/mg protein; P < 0.001) and wortmannin (425 ± 39 vs. 70 ± 30 pmol/mg protein; P < 0.001), whereas calphostin C and BAPTA did not significantly affect the PepT1 activity. Inhibitors without GIP treatment had no significant effect on PepT1 transport. These observations indicate that cAMP- and PI3K-mediated, but not PKC- and calmodulin-mediated, signaling pathways regulate the GIP augmented PepT1 activity in intestinal epithelial cells. Rp-cAMP, an analog of cAMP, and BAPTA, a Ca2+ chelator are specific for their targets, but calphostin C can inhibit PKA and PKG at higher concentrations. Nevertheless, the cAMP-mediated signaling occurs through both the Epac and PKA (21). Experiments were, therefore, designed to distinguish whether the GIP mediated the PepT1 activation through Epac and/or PKA. In this study, the effect of 8-pCPT-2′-O-Me-cAMP-AM (a selective Epac agonist) and 8Br-cAMP (a selective PKA agonist) were examined on PepT1 activity. Since neither Epac nor PKA specific inhibitors were available, we used Epac and PKA specific activators in this study (25, 60). As shown in Fig. 5A, similar to GIP, the Epac activator 8-pCPT-2′-O-Me-cAMP also activated PepT1 activity. 8-pCPT-2′-O-Me-cAMP exhibited concentration-dependent activation of PepT1 activity. Although the PepT1 activity was not significantly activated by 2 nM, 20 nM 8-pCPT-2′-O-Me-cAMP activated the PepT1 activity more than that of activation by 0.1 mM GIP (GIP vs. 20 nM 8-pCPT-2′-O-Me-cAMP: 630 ± 78 vs. 701 ± 18 pmol/mg protein). The PepT1 activity was activated 10-fold by 40 nM 8-pCPT-2′-O-Me-cAMP (Fig. 5A). In contrast, the PKA activator 8-Br-cAMP did not activate the PepT1 activity (Fig. 5B). These observations suggest that GIP may regulate the PepT1 activity through Epac signaling pathway.
Fig. 4.
Effect of second messenger pathway inhibitors on GIP-augmented H+-Gly-Sar cotransport in IEC-6/CDX2 cells. GIP-augmented, H+-gradient-driven Gly-Sar absorption was measured and calculated for 5 min in 3–5 days postconfluent IEC-6/CDX2 cells grown on Transwell plates. GIP-augmented, H+-gradient-driven Gly-Sar absorption was also measured in the presence of Rp-cAMP (50 nM), wortmannin (Wort, 200 nM), calphostin (Cal C, 200 nM), or BAPTA (5 mM). Rp-cAMP, wortmannin, calphostin, and BAPTA were added individually to both chambers in the presence of serosal GIP. Cells treated with inhibitors in the absence of GIP were not significantly different than control. Results presented represent means ± SE of triplicate assays from 4 different experiments. *P < 0.05 compared with normal control; **P < 0.05 compared with GIP control.
Fig. 5.
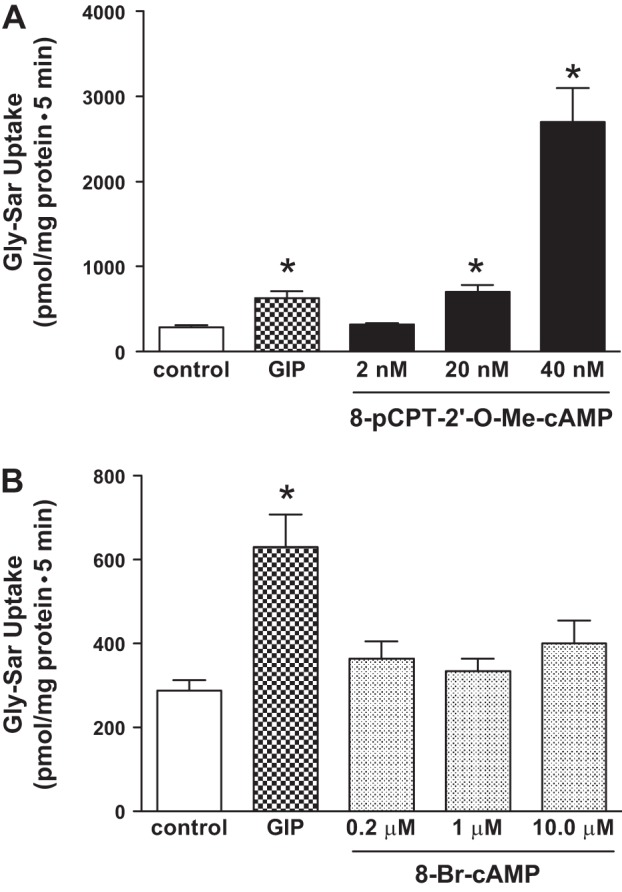
Effect of cAMP metabolite inhibitors on GIP-augmented H+-Gly-Sar cotransport in IEC-6/CDX2 cells. GIP-augmented, H+-gradient-driven Gly-Sar absorption was measured and calculated for 5 min in 3–5 days postconfluent CDX2/IEC-6 cells grown on Transwell plates. GIP-augmented, H+-gradient-driven Gly-Sar absorption was also measured when Epac (A) and PKA (B) agonists at various concentrations were added separately to both chambers in the presence of serosal GIP. Results presented represent means ± SE of triplicate assays from 4 different experiments. *P < 0.05 compared with normal control.
Experiments were also performed to identify whether, in addition to Epac, the PI3K-dependent pathway has any role in PepT1 activity. Since wortmannin can also inhibit other pathways such as the polo-like kinase 1 (PLK1), further examination of the PI3K was performed. PI3K pathways include activation of AKT and downstream pathways that regulate cellular metabolism. As shown in Fig. 6, AKT inhibitor-15 inhibited the Gly-Sar absorption in a dose-dependent manner. Taken altogether, our results indicate that Epac and AKT, but not PKA, are involved in the signaling of GIP-dependent augmentation of PepT1 activity in IEC-6-CDX2 cells.
Fig. 6.
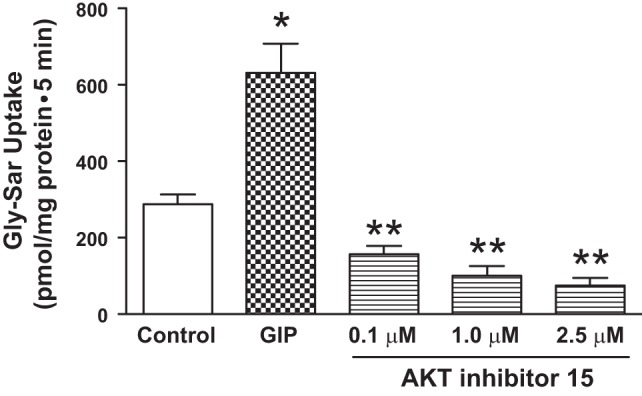
Effect of PI3K metabolite inhibitors on GIP-augmented H+-Gly-Sar cotransport in IEC-6/CDX2 cells. GIP-augmented, H+-gradient-driven Gly-Sar absorption was measured and calculated for 5 min in 3–5 days postconfluent IEC-6/CDX2 cells grown on Transwell plates. GIP-augmented, H+-gradient-driven Gly-Sar absorption was also measured in the presence of an AKT antagonist added simultaneously to both chambers in the presence of serosal GIP. Results presented represent means ± SE of triplicate assays from 4 different experiments. *P < 0.05 compared with normal control; **P < 0.05 compared with GIP control. E-cadherin was used as the loading control.
To determine the manner by which cAMP and PI3K signaling pathways increase PepT1 activity in the presence of GIP, surface biotinylation experiments were performed on IEC-6-CDX2 monolayers to assess changes in expression of PepT1 at the apical border. As shown in Fig. 7A, basolateral GIP increased PepT1 expression at the apical border of IEC-6-CDX2 cell monolayers. Furthermore, we found that Epac but not PKA agonists increase the apical expression of PepT1. AKT inhibitor 15 also showed a decrease apical expression of PepT1 as shown in Fig. 7B.
Fig. 7.
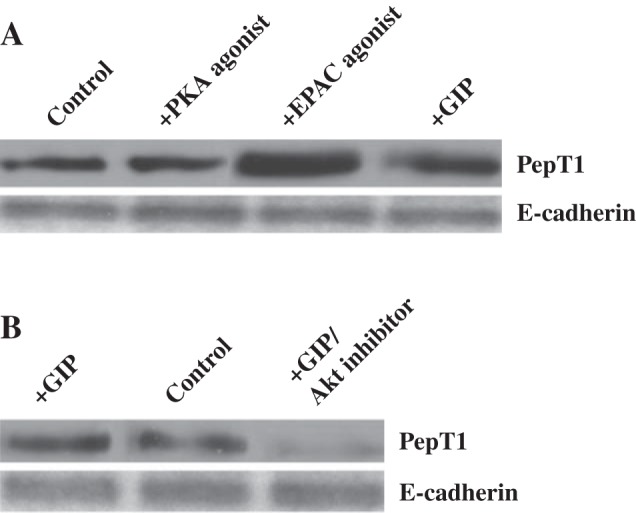
Effect of GIP, Epac, and AKT on apical PepT1 specific protein expression in apical membranes of IEC-6/CDX2 confluent monolayers. Anti-PepT1 antibody detects a 70 kDa protein in untreated IEC-6/CDX2 monolayer homogenates (A) as well as monolayers treated separately with Epac agonist, PKA agonists as well as GIP (B). Anti-PepT1 antibody detects a 70-kDa protein in untreated IEC-6/CDX2 monolayer homogenates as well as treated monolayers treated with GIP in the presence and the absence of AKT inhibitor. GIP Western blots presented represent typical blots selected from 3 different IEC-6-CDX2 preparations. E-cadherin was used as the loading control.
DISCUSSION
In previous studies we showed that GIP regulates both SGLT1-mediated glucose absorption and PepT1-mediated peptide absorption in mouse jejunum in a cAMP-dependent manner (10, 52). However, the details of the cAMP-dependent signaling pathways involved in jejunal nutrient transport remain incompletely understood. Thus we initiated studies in a model of absorptive intestinal epithelium to study the signaling mechanisms involved in the augmentation of PepT1 activity by GIP. Our results demonstrate that GIPR activation regulates PepT1 by activating cAMP and PI3K-dependent pathways. We also found that downstream intermediates of PKA signaling pathways, notably Akt and Epac, increase Gly-Sar absorption by enhancing PepT1 expression in the apical membranes of IEC-6/CDX2 cells.
Since native IEC-6 cells only minimally transport nutrients, making analysis difficult, IEC-6 cells transfected with the CDX2 gene were used to produce cells that better resemble the function of villus cells in the jejunum (44). CDX2 is a transcription factor that facilitates development, differentiation, and maintenance of intestinal epithelial cells (50, 51). CDX2 transfected IEC-6 cells possess features of more differentiated villus cells with greater expression of nutrient transporters and an extensive brush border (44). In the present study, we validated the IEC-6/CDX2 cell line as an appropriate model for the study of incretin-regulation of nutrient transport by demonstrating the presence and function of GIP and GLP-1 receptors as well as PepT1.
Expression of GIPRs has been shown in intestinal tissue (59) but only recently localized by our group to the basolateral membrane of villus cells (10). Although GIP receptors exist in other cell types in the intestine to regulate motility and other functions (7), PepT1 expression on the basolateral membrane (1) suggests that GIP directly regulates PepT1 activity through a second messenger pathway. PepT1 activity itself has been shown to be sensitive to cAMP release (42) and to the PI3K pathway (46). GIPRs utilize cAMP pathways to stimulate insulin secretion from pancreatic β cells (18, 66) and calcium-dependent as well as PI3K pathways have been described in other tissues (36, 66).
As such, it was necessary for us to investigate each of the major second messenger pathways in the augmentation of PepT1 activity by GIP. The two most prominent second messenger pathways involve the cAMP and the calcium/inositol phosphate pathways. The cAMP pathway includes protein kinase A, an important intermediate that primarily regulates transcription and trafficking of channels and transporters like the cystic fibrosis transmembrane regulator (CFTR) and NHE3 by hormone action and other agents (39, 42, 65). GIP appears to exert its effects specifically through Epac (30, 65).
The calcium/inositol phosphate pathway leads to the activation of a number of isoforms of PKC, some of which are calcium dependent (61). Although not all PKC isoforms are inhibited, calphostin C blocks calcium-dependent isoforms known to regulate intestinal Na:H and Cl:HCO3 exchangers including PKCs δ and ϵ (20, 29, 41, 48). However, in the present study, calphostin C had no effect on PepT1 activity. Calcium-calmodulin pathways also regulate an array of intestinal transporters (26, 31, 46). Calmodulin kinase regulates SGLT1 and riboflavin transport as well as sodium and chloride absorption (4, 15, 47). BAPTA, an intracellular calcium chelator, also failed to decrease GIP augmentation of PepT1 activity, thus eliminating participation of the calmodulin pathway and making the PKC pathway less likely.
For the cAMP messenger pathways there are several possible intermediates. The signaling could be activating PKA or Epac, and both pathways regulate intestinal epithelial transporters (23, 30, 40). In this study, Epac was shown to increase PepT1 activity while PKA did not. GIP therefore in part increases PepT1 activity through the cAMP pathway via Epac rather that PKA. Epac is increasingly recognized as a key pathway for the regulation of intestinal nutrient transport.
The PI3K pathway is also utilized by hormones to regulate nutrient transporters in the intestine including PepT1 (3, 26, 46, 58). AKT, a downstream intermediate of the PI3K pathway, stimulates growth and differentiation in the intestine and other tissues and is also a central metabolite in cell apoptosis, cell cycle regulation, and growth (37, 53). AKT is known to activate other intestinal and renal nutrient transporters (33, 40). GLP-1 and GIP activate the PI3K/AKT pathway to promote insulin secretion and the growth of pancreatic β cells (7). We found that inhibiting AKT also blocks GIP-augmented PepT1 activity and indicates that the PI3K pathway regulates the PepT1 protein.
An explanation for the existence of dual pathways of regulation may be that GIPR splice variants can act via different pathways simultaneously. Variants or other isoforms of the GIPR have been detected in other tissues (24, 66). Indeed, in our previous studies, Western blots of jejunal tissue revealed a number of bands for the GIPR, but whether these represented splice variants has not been determined (10); if this is the case, the presence of multiple regulatory pathways might be expected (10, 24, 66).
Having elucidated the major signaling pathways involved in the GIP-mediated PepT1 response, it is also important to determine how GIP alters PepT1 activity via downstream metabolites. Epac and AKT, major metabolites of the cAMP and PI3K pathways, respectively, were shown to be important in GIP augmentation of PepT1 activity.
Since the response is relatively rapid, a change in transcriptional activity is unlikely and thus there are two remaining possibilities: More PepT1 protein is trafficked to the apical membrane and/or the substrate turnover rate is increased. To examine these possibilities, Western blots were performed by use of biotinylation to isolate PepT1 proteins on the cell surface. Trafficking and insertion of PepT1 protein into the apical membrane appear to be the major mechanism by which GIPR signaling increases PepT1 activity.
This is the first study in which two pathways appear to simultaneously regulate GIP activation in intestinal cells. There are several possibilities to explain this finding. First, there may be two entirely different pathways: the cAMP and the PI3 pathways may be distinct and activated by the same GIP receptor isoform. Second, there have been many reports of multiple transcripts of GIPR in other cells indicating that multiple isoforms could activate different pathways. Finally, there may actually not be two distinct pathways but a single pathway that overlaps to augment PepT1 activity. It appears that the PI3K pathway is necessary to augment PepT1 activity. Some reports indicate that the cAMP release activates the PI3 pathway whereas others show they are independent (56, 58). Further studies will be necessary to clarify further these redundant signaling mechanisms.
When one consumes a meal, as nutrients are absorbed, GIP is released by K cells in the jejunum. GIP then performs at least two functions. First it enters the bloodstream to activate its receptors in pancreatic β cells to stimulate insulin secretion to maintain proper glucose levels in the blood. Secondly, on the basis of our own research, the secreted GIP feeds back to stimulate absorption of glucose and dipeptides. GIP receptors have been found on villus cells and in the current study have now been shown to directly regulate dipeptide absorption. PepT1 activity would be elevated in the fed state when GIP is released and at its highest levels. For the same reason it would be reduced in the fasted state when GIP would be at its lowest level.
In conclusion, this is the first study of its kind to examine how GIP augments the absorption of dipeptides in an intestinal epithelium. GIP via GIPR activates two signaling pathways, the cAMP pathway through Epac and the PI3K pathway through Akt, to increase the trafficking of PepT1 proteins to the apical membrane. The result of this series of events would be to increase the absorption of dipeptides and tripeptides into the circulation upon feeding.
GRANTS
Support from the Boston University Clinical and Translational Science Institute grant UL1-TR000157 was used for this project and also grant support for V. M. Rajendran by NIH/NIDDK DK-018777.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.D.C., V.M.R., and J.H.S. conception and design of research; S.D.C. performed experiments; S.D.C. and V.M.R. analyzed data; S.D.C., V.M.R., and J.H.S. interpreted results of experiments; S.D.C. and V.M.R. prepared figures; S.D.C. and V.M.R. drafted manuscript; S.D.C., V.M.R., J.H.S., and S.K.S. edited and revised manuscript; S.D.C., V.M.R., J.H.S., and S.K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Barbara Corkey for insights in the preparation of this manuscript.
REFERENCES
- 1.Adibi SA. Regulation of expression of the intestinal oligopeptide transporter (Pept-1) in health and disease. Am J Physiol Gastrointest Liver Physiol 285: G779–G788, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Alpers DH. Digestion and absorption of carbohydrates and proteins. In: Physiology of the Gastrointestinal Tract (3rd ed.), edited by Johnson L. New York: Raven, 1994, p. 1723–1750. [Google Scholar]
- 3.Alrefai WA, Saksena S, Tyagi S, Gill RK, Ramaswamy K, Dudeja PK. Taurodeoxycholate modulates apical Cl−/OH− exchange activity in Caco2 cells. Dig Dis Sci 52: 1270–1278, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Amador P, Marca MC, Garcia-Herrera J, Lostao MP, Guillen N, de la Osada J, Rodriguez-Yoldi MJ. Lipopolysaccharide induces inhibition of galactose intestinal transport in rabbits in vitro. Cell Physiol Biochem 22: 715–724, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Ashida K, Katsura T, Motohashi H, Saito H, Inui K. Thyroid hormone regulates the activity and expression of the peptide transporter PEPT1 in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 282: G617–G623, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Boylan MO, Jepeal LI, Wolfe MM. Sp1/Sp3 binding is associated with cell-specific expression of the glucose-dependent insulinotropic polypeptide receptor gene. Am J Physiol Endocrinol Metab 290: E1287–E1295, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 17: 819–837, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Carranza ML, Rousselot M, Chibalin AV, Bertorello AM, Favre H, Feraille E. Protein kinase A induces recruitment of active Na+,K+-ATPase units to the plasma membrane of rat proximal convoluted tubule cells. J Physiol 511: 235–243, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow YW, Wang HL. Functional modulation of P2X2 receptors by cyclic AMP-dependent protein kinase. J Neurochem 70: 2606–2612, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Coon S, Schwartz JH, Rajendran V, Jepeal L, Singh SK. Glucose-dependent insulinotropic polypeptide regulates dipeptide absorption in mouse jejunum. Am J Physiol Gastrointest Liver Physiol 305: G678–G684, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui XL, Schlesier AM, Fisher EL, Cerqueira C, Ferraris RP. Fructose-induced increases in neonatal rat intestinal fructose transport involve the PI3K/Akt signaling pathway. Am J Physiol Gastrointest Liver Physiol 288: G1310–G1320, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflügers Arch 447: 610–618, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Das UN. Obesity: genes, brain, gut, and environment. Nutrition 26: 459–473, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Ding WG, Gromada J. Protein kinase A-dependent stimulation of exocytosis in mouse pancreatic beta-cells by glucose-dependent insulinotropic polypeptide. Diabetes 46: 615–621, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Donowitz M, Wicks J, Madara JL, Sharp GW. Studies on role of calmodulin in Ca2+ regulation of rabbit ileal Na and Cl transport. Am J Physiol Gastrointest Liver Physiol 248: G726–G740, 1985. [DOI] [PubMed] [Google Scholar]
- 16.Drucker DJ, Campos R, Reynolds R, Stobie K, Brubaker PL. The rat glucagon gene is regulated by a protein kinase A-dependent pathway in pancreatic islet cells. Endocrinology 128: 394–400, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4: 901–906, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Furman B, Ong WK, Pyne NJ. Cyclic AMP signaling in pancreatic islets. Adv Exp Med Biol 654: 281–304, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Gentili C, Morelli S, Boland R, de Boland AR. Parathyroid hormone activation of map kinase in rat duodenal cells is mediated by 3′,5′-cyclic AMP and Ca2+. Biochim Biophys Acta 1540: 201–212, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, Turner JR, Ramaswamy K, Dudeja PK. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKC alpha in human intestinal epithelial cells. Gastroenterology 128: 962–974, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50: 355–375, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Gromada J, Brock B, Schmitz O, Rorsman P. Glucagon-like peptide-1: regulation of insulin secretion and therapeutic potential. Basic Clin Pharmacol Toxicol 95: 252–262, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Halm ST, Zhang J, Halm DR. β-Adrenergic activation of electrogenic K+ and Cl− secretion in guinea pig distal colonic epithelium proceeds via separate cAMP signaling pathways. Am J Physiol Gastrointest Liver Physiol 299: G81–G95, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada N, Yamada Y, Tsukiyama K, Yamada C, Nakamura Y, Mukai E, Hamasaki A, Liu X, Toyoda K, Seino Y, Inagaki N. A novel GIP receptor splice variant influences GIP sensitivity of pancreatic β-cells in obese mice. Am J Physiol Endocrinol Metab 294: E61–E68, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Hei YJ, MacDonell KL, McNeill JH, Diamond J. Lack of correlation between activation of cyclic AMP-dependent protein kinase and inhibition of contraction of rat vas deferens by cyclic AMP analogs. Mol Pharmacol 39: 233–238, 1991. [PubMed] [Google Scholar]
- 26.Helliwell PA, Rumsby MG, Kellett GL. Intestinal sugar absorption is regulated by phosphorylation and turnover of protein kinase C βII mediated by phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent pathways. J Biol Chem 278: 28644–28650, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Hindlet P, Bado A, Farinotti R, Buyse M. Long-term effect of leptin on H+-coupled peptide cotransporter 1 activity and expression in vivo: evidence in leptin-deficient mice. J Pharmacol Exp Ther 323: 192–201, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Hindlet P, Bado A, Kamenicky P, Delomenie C, Bourasset F, Nazaret C, Farinotti R, Buyse M. Reduced intestinal absorption of dipeptides via PepT1 in mice with diet-induced obesity is associated with leptin receptor down-regulation. J Biol Chem 284: 6801–6808, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodges K, Gill R, Ramaswamy K, Dudeja PK, Hecht G. Rapid activation of Na+/H+ exchange by EPEC is PKC mediated. Am J Physiol Gastrointest Liver Physiol 291: G959–G968, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Hoque KM, Woodward OM, van Rossum DB, Zachos NC, Chen L, Leung GP, Guggino WB, Guggino SE, Tse CM. Epac1 mediates protein kinase A-independent mechanism of forskolin-activated intestinal chloride secretion. J Gen Physiol 135: 43–58, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanda H, Tamori Y, Shinoda H, Yoshikawa M, Sakaue M, Udagawa J, Otani H, Tashiro F, Miyazaki J, Kasuga M. Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J Clin Invest 115: 291–301, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science 282: 2275–2279, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Kempe DS, Siraskar G, Frohlich H, Umbach AT, Stubs M, Weiss F, Ackermann TF, Volkl H, Birnbaum MJ, Pearce D, Foller M, Lang F. Regulation of renal tubular glucose reabsorption by Akt2/PKBβ. Am J Physiol Renal Physiol 298: F1113–F1117, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy DJ, Leibach FH, Ganapathy V, Thwaites DT. Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity. Pflügers Arch 445: 139–146, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Khanal RC, Peters TM, Smith NM, Nemere I. Membrane receptor-initiated signaling in 1,25(OH)2D3-stimulated calcium uptake in intestinal epithelial cells. J Cell Biochem 105: 1109–1116, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Kubota A, Yamada Y, Yasuda K, Someya Y, Ihara Y, Kagimoto S, Watanabe R, Kuroe A, Ishida H, Seino Y. Gastric inhibitory polypeptide activates MAP kinase through the wortmannin-sensitive and -insensitive pathways. Biochem Biophys Res Commun 235: 171–175, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Lavine JA, Attie AD. Gastrointestinal hormones and the regulation of β-cell mass. Ann NY Acad Sci 1212: 41–58, 2010. [DOI] [PubMed] [Google Scholar]
- 38.McIntosh CH, Wheeler MB, Gelling RW, Brown JC, Pederson RA. GIP receptors and signal-transduction mechanisms. Acta Physiol Scand 157: 361–365, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Muanprasat C, Chatsudthipong V. Cholera: pathophysiology and emerging therapeutic targets. Future Med Chem 5: 781–798, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Murtazina R, Kovbasnjuk O, Zachos NC, Li X, Chen Y, Hubbard A, Hogema BM, Steplock D, Seidler U, Hoque KM, Tse CM, De Jonge HR, Weinman EJ, Donowitz M. Tissue-specific regulation of sodium/proton exchanger isoform 3 activity in Na+/H+ exchanger regulatory factor 1 (NHERF1) null mice. cAMP inhibition is differentially dependent on NHERF1 and exchange protein directly activated by cAMP in ileum versus proximal tubule. J Biol Chem 282: 25141–25151, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Muthusamy S, Shukla S, Amin MR, Cheng M, Orenuga T, Dudeja PK, Malakooti J. PKCδ-dependent activation of ERK1/2 leads to upregulation of the human NHE2 transcriptional activity in intestinal epithelial cell line C2BBe1. Am J Physiol Gastrointest Liver Physiol 302: G317–G325, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nduati V, Yan Y, Dalmasso G, Driss A, Sitaraman S, Merlin D. Leptin transcriptionally enhances peptide transporter (hPepT1) expression and activity via the cAMP-response element-binding protein and Cdx2 transcription factors. J Biol Chem 282: 1359–1373, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Qureshi SA, Rios Candelore M, Xie D, Yang X, Tota LM, Ding VD, Li Z, Bansal A, Miller C, Cohen SM, Jiang G, Brady E, Saperstein R, Duffy JL, Tata JR, Chapman KT, Moller DE, Zhang BB. A novel glucagon receptor antagonist inhibits glucagon-mediated biological effects. Diabetes 53: 3267–3273, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Rao JN, Li J, Li L, Bass BL, Wang JY. Differentiated intestinal epithelial cells exhibit increased migration through polyamines and myosin II. Am J Physiol Gastrointest Liver Physiol 277: G1149–G1158, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Rao JN, Liu L, Zou T, Marasa BS, Boneva D, Wang SR, Malone DL, Turner DJ, Wang JY. Polyamines are required for phospholipase C-γ1 expression promoting intestinal epithelial restitution after wounding. Am J Physiol Gastrointest Liver Physiol 292: G335–G343, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Rexhepaj R, Rotte A, Pasham V, Gu S, Kempe DS, Lang F. PI3 kinase and PDK1 in the regulation of the electrogenic intestinal dipeptide transport. Cell Physiol Biochem 25: 715–722, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Said HM, Ortiz A, Moyer MP, Yanagawa N. Riboflavin uptake by human-derived colonic epithelial NCM460 cells. Am J Physiol Cell Physiol 278: C270–C276, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Saksena S, Gill RK, Syed IA, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Inhibition of apical Cl−/OH− exchange activity in Caco-2 cells by phorbol esters is mediated by PKCϵ. Am J Physiol Cell Physiol 283: C1492–C1500, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Saksena S, Gill RK, Tyagi S, Alrefai WA, Sarwar Z, Ramaswamy K, Dudeja PK. Involvement of c-Src and protein kinase Cδ in the inhibition of Cl−/OH− exchange activity in Caco-2 cells by serotonin. J Biol Chem 280: 11859–11868, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Salari K, Spulak ME, Cuff J, Forster AD, Giacomini CP, Huang S, Ko ME, Lin AY, van de Rijn M, Pollack JR. CDX2 is an amplified lineage-survival oncogene in colorectal cancer. Proc Natl Acad Sci USA 109: E3196–E3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silberg DG, Swain GP, Suh ER, Traber PG. Cdx1 and Cdx2 expression during intestinal development. Gastroenterology 119: 961–971, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Singh SK, Bartoo AC, Krishnan S, Boylan MO, Schwartz JH, Wolfe MM. Glucose-dependent insulinotropic polypeptide (GIP) stimulates transepithelial glucose transport. Obesity (Silver Spring) 16: 2412–2416, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Song DH, Getty-Kaushik L, Tseng E, Simon J, Corkey BE, Wolfe MM. Glucose-dependent insulinotropic polypeptide enhances adipocyte development and glucose uptake in part through Akt activation. Gastroenterology 133: 1796–1805, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thamotharan M, Bawani SZ, Zhou X, Adibi SA. Hormonal regulation of oligopeptide transporter pept-1 in a human intestinal cell line. Am J Physiol Cell Physiol 276: C821–C826, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Tuo B, Wen G, Wang X, Xu J, Xie R, Liu X, Dong H. Estrogen potentiates prostaglandin E2-stimulated duodenal mucosal HCO3− secretion in mice. Am J Physiol Endocrinol Metab 303: E111–E121, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Tuo B, Wen G, Zhang Y, Liu X, Wang X, Dong H. Involvement of phosphatidylinositol 3-kinase in cAMP- and cGMP-induced duodenal epithelial CFTR activation in mice. Am J Physiol Cell Physiol 297: C503–C515, 2009. [DOI] [PubMed] [Google Scholar]
- 57.Tuo BG, Sellers Z, Paulus P, Barrett KE, Isenberg JI. 5-HT induces duodenal mucosal bicarbonate secretion via cAMP- and Ca2+-dependent signaling pathways and 5-HT4 receptors in mice. Am J Physiol Gastrointest Liver Physiol 286: G444–G451, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Tuo BG, Wen GR, Seidler U. Phosphatidylinositol 3-kinase is involved in prostaglandin E2-mediated murine duodenal bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol 293: G279–G287, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 133: 2861–2870, 1993. [DOI] [PubMed] [Google Scholar]
- 60.Vliem MJ, Ponsioen B, Schwede F, Pannekoek WJ, Riedl J, Kooistra MR, Jalink K, Genieser HG, Bos JL, Rehmann H. 8-pCPT-2′-O-Me-cAMP-AM: an improved Epac-selective cAMP analogue. Chembiochem 9: 2052–2054, 2008. [DOI] [PubMed] [Google Scholar]
- 61.Webb BL, Hirst SJ, Giembycz MA. Protein kinase C isoenzymes: a review of their structure, regulation and role in regulating airways smooth muscle tone and mitogenesis. Br J Pharmacol 130: 1433–1452, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu T, Rayner CK, Jones K, Horowitz M. Dietary effects on incretin hormone secretion. Vitam Horm 84: 81–110, 2011. [DOI] [PubMed] [Google Scholar]
- 63.Yamada Y, Seino Y. Physiology of GIP—a lesson from GIP receptor knockout mice. Horm Metab Res 36: 771–774, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Yip RG, Wolfe MM. GIP biology and fat metabolism. Life Sci 66: 91–103, 2000. [DOI] [PubMed] [Google Scholar]
- 65.Yun CH, Oh S, Zizak M, Steplock D, Tsao S, Tse CM, Weinman EJ, Donowitz M. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA 94: 3010–3015, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong Q, Bollag RJ, Dransfield DT, Gasalla-Herraiz J, Ding KH, Min L, Isales CM. Glucose-dependent insulinotropic peptide signaling pathways in endothelial cells. Peptides 21: 1427–1432, 2000. [DOI] [PubMed] [Google Scholar]