Abstract
Previous studies have indicated that macrophage phenotype diversity is involved in the progression of renal fibrosis. However, the factors facilitating M1 or M2 phenotypes and the function of these polarized macrophages in kidney injury and fibrosis remain largely unknown. In the present study, we found that macrophages accumulated in the kidney interstitium exhibited mainly as the M1 phenotype at the early stage of unilateral ureter obstruction (UUO). High-mobility group box 1 (HMGB1) protein expressed and released from tubular epithelial cells and interstitial macrophages was essential for the M1 macrophage transition. HMGB1 significantly induced the expression of the M1 marker inducible nitric oxide synthase while decreasing the M2 marker IL-10 in macrophages. Moreover, a glycyrrhizic acid derivative, a blocker of HMGB1 release, reduced UUO-mediated kidney injury and ameliorated UUO-induced renal fibrosis. Interestingly and importantly, UUO caused a low pH value in the urine accumulated in the obstructed ureter, and the acidified urine induced HMGB1 release from tubular epithelial cells and macrophages in vitro. Our data demonstrate that HMGB1 is an essential contributor in facilitating M1 polarization at the early stage of UUO. Inhibition of HMGB1 release may alter macrophage phenotype and contribute to the protection of kidney tissue from injury and fibrosis.
Keywords: high-mobility group box 1 protein, unilateral ureter obstruction, macrophage, phenotype, renal fibrosis
renal fibrosis is an inevitable pathological hallmark for many chronic kidney diseases. Among the diverse causative factors, interstitially accumulated macrophages play an important role in motivating the fibrosis process (23). Recent studies have demonstrated that tissue macrophages have tremendous plasticity in their function and phenotype. Based on their response to environmental stimuli, macrophages can be broadly divided into two categories: classically activated macrophages (M1) and alternatively activated macrophages (M2) (8). M1 activation is associated with tissue destruction and inflammation and is responsible for the upregulation of proinflammatory cytokines and increasing the production of reactive nitrogen species and ROS. Conversely, M2 polarized macrophages dampen the inflammatory process by producing anti-inflammatory factors, such as IL-10 and transforming growth factor-β1 (7). Recent studies have shown some hints of macrophage phenotype diversity in the process of renal fibrosis. For example, depletion of macrophages at an early stage of unilateral ureter obstruction (UUO) reduces kidney injury. Paradoxically, adaptive infusion of macrophage at late stages plays a protective role in tubulointerstitial injury and fibrosis (19, 22). These results imply that during the early stages of UUO, interstitial macrophages may be dominated by the M1 phenotype, whereas in the later stages of UUO, M1 macrophages may transition toward the M2 phenotype. However, macrophage polarization during UUO-induced fibrosis, especially the factors inducing or maintaining the macrophage phenotype transition to M1 or M2, remains largely unknown.
In infectious diseases, pathogen-associated molecular patterns play important role in activating the macrophage shift to the M1 phenotype, whereas in sterile disease, damage-associated molecular patterns (DAMPs) are responsible for initiating the M1 phenotype (1). High-mobility group box 1 (HMGB1) protein is considered to be one of the DAMPs expressed by many cell types, including tubular epithelial cells. When tubular injury occurs, HMGB1 expression is increased and then released from interstitial and epithelial cells (20, 24). A in vitro study (13) has shown that HMGB1 induces macrophages to express proinflammatory cytokines. A glycyrrhizic acid derivative (GA), a HMGB1 inhibitor, can counteract this effect (6, 18, 21). However, it is unclear if HMGB1 can induce the macrophage phenotype transition or influence interstitial fibrosis in UUO kidneys. We hypothesized that increased expression and release of HMGB1 in UUO-injured kidneys induces M1 macrophage polarization and thereby contributes to the development of interstitial fibrosis and that blockade of HMGB1 release or counteracting its function would alleviate the inflammation and fibrosis. Our results showed that HMGB1 expression and release are increased in the kidney at the early stage of UUO and that macrophages accumulated in the kidney interstitium exhibit a M1 phenotype. In vitro experiments confirmed that HMGB1 can induce the M1 macrophage transition. Moreover, we demonstrated that GA can reverse the detrimental M1 macrophage polarization and alleviate the renal fibrosis.
EXPERIMENTAL PROCEDURES
Animals and the UUO mouse model.
Male C57BL6/J mice, weighing 20–25 g at the start of the experiment, were anesthetized with isoflurane inhalation. UUO was achieved by double ligation of the left ureter with 3-0 silk through a left lateral incision. Sham-operated (sham) animals were used as controls. UUO and sham mice were euthanized at 3, 5, 7, 10, or 14 days (n = 5 mice/group) after UUO. Some animals received GA [glycyrrhizic acid monosodium (50 mg/kg body wt), Sigma] through peritoneal injection and were euthanized at 5 or 7 days after UUO (n = 5 mice/group). Urine, blood, and obstructed kidneys were collected and subjected to the experiments described below. For UUO animals, retained urine in the left ureters and pelvis were collected using a 1-ml syringe. For sham animals, metabolic cages were used to collect urine. All procedures were performed according to a protocol approved by the Institutional Animal Care and Use Committee of the University of Georgia.
Histopathological analyses.
One half of obstructed kidney tissue was fixed in buffered 4% paraformaldehyde for 24 h and then embedded in paraffin wax. To assess tubulointerstitial injury and fibrosis, 5-μm sections were stained with a hematoxylin and eosin staining kit (Master Tech Lab) and Masson's Trichrome 2000 Stain Kit (MasterTech Lab), respectively. Tubular injury, characterized by tubular dilation and epithelial desquamation with interstitial expansions, was graded according to the extent of cortical involvement on a scale from 0 to 4 and assessed using a semiquantitative scale (17). Interstitial fibrosis was evaluated by collagen deposition using the point-counting method (10).
Immunofluorescent staining.
Sections of formalin-fixed, parafin-embedded tissue were dewaxed, rehydrated, and antigen retrieved. Sections were incubated overnight with HMGB1 (Cell Signaling) or fibroblast-specific protein-1 (Abcam) monoclonal antibody at 1:100 dilution. The secondary antibody, FITC-conjugated goat anti-rabbit antibody (Life Tech), was applied at 1:1,000 dilution, and immunofluorescence photomicrographs were obtained at ×200 magnification for a 80-ms exposure time. For inducible nitric oxide synthase (iNOS) and F4/80 or IL-10 and F4/80 double immunostaining, rabbit iNOS (Abcam) or IL-10 (Santa Cruz Biotechnology) monoclonal antibody and rat F4/80 monoclonal antibody (Abcam) at 1:100 dilution were applied at the same time, and secondary antibodies included both FITC-conjugated goat anti-rabbit antibody (Life Tech) and TRITC-conjugated goat-anti-rat antibody (Life Tech). For cytoimmunofluorescent staining, macrophages or human proximal tubule cells (HPTCs) were grown on glass coverslips in 24-well culture plates. Immunostaining was performed as previously described (3).
Macrophage isolation and culture.
Bone marrow-derived macrophages (BMMs) were obtained as previously described (13). Briefly, the femur and tibia were dissected in DMEM containing 10% FBS, and bone marrow cells were flushed from femurs and tibias. After red blood cells had been lysed, the remaining cells were counted and plated in a T-25 flask, and 10 ng/ml of macrophage colony-stimulating factor (Sigma) was added. After being cultured overnight, nonadherent cells were collected, washed, and plated in 60-mm petri plates with 10 ng/ml macrophage colony-stimulating factor in DMEM containing 10% FBS. After 7 days, cells were washed, and adherent cells were released and removed with 0.1% EDTA. The resulting BMMs were judged to be >98% pure based on F4/80 staining. For macrophage polarization testing, BMMs were stimulated with HMGB1 (Sigma) or HMGB1 plus GA.
HPTC culture and treatment.
HPTCs were cultured in DMEM-F-12 (Invitrogen) with supplements as previously described (9). HCl (1 N) was used to adjust pH to prepare the acidified medium.
Reverse transcription and quantitative RT-PCR.
Total RNA was extracted using TRIzol reagent (Invitrogen) following the manufacturer's instructions. cDNA was synthesized using 1 μg total RNA and an iScript cDNA Synthesized Kit (Bio-Rad). mRNA expression of the related genes was normalized to cyclophilin. The primer sets used were mouse HMGB1 (forward: 5′-GCTGACAAGGCTCGTTATGAA-3′ and reverse: 5′-CCTTTGATTTTGGGGCGGTA-3′), IL-6 (forward: 5′-AGGATACCACTCCCAACAGACCT-3′ and reverse: 5′-CAAGTGCATCATCGTTGTTCATAC-3′), iNOS (forward: 5′-ACATCGACCCGTCCACAGTAT-3′ and reverse: 5′-CAGAGGGGTAGGCTTGTCTC-3′), IL-1β (forward: 5′-AAATGCCTCGTGCTGTCTGACC-3′ and reverse: 5′-CTGCTTGAGAGGTGCTGATGTACC-3′), IL-10 (forward: 5′-GGTTGCCAAGCCTTATCGGA-3′ and reverse: 5′-ACCTGCTCCACTGCCTTGCT-3′), and cyclophilin (forward: 5′-TGCAGCCATGGTCAACCCC-3′ and reverse: 5′-CCCAAGGGCTCGTCA-3′).
Urine and blood HMGB1 measurements.
HMGB1in urine, serum, and the macrophage supernatant were measured using an ELISA kit (MyBioSource) following the manufacturer's instructions.
Western blot analysis.
Protein was extracted using lysis buffer containing protease inhibitors. Protein concentration was determined using a BCA kit (Pierce). Western blot analysis was carried out as previously described (9).
Statistical analysis.
All data are reported as means ± SD. Data were analyzed using ANOVA followed by a q test using SPSS for Windows (version 10.0). P values of <0.05 were considered as statistically significant difference.
RESULTS
HMGB1 expression and release in UUO kidneys.
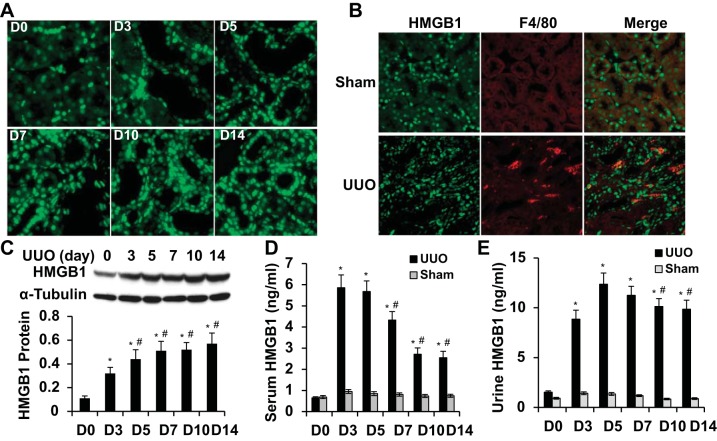
To determine if HMGB1 is involved in tubular injury and interstitial fibrosis, we first examined whether HMGB1 expression or release is altered by UUO. HMGB1 was observed in both tubular and interstitial cells. The HMGB1 expression level was very low in normal mouse kidney tubular cells. After UUO, HMGB1 expression was rapidly and significantly upregulated in tubular cells, especially in damaged tubules (Fig. 1A). HMGB1-expressing interstitial cells appeared to be, at least partially, macrophages (Fig. 1B). HMGB1 protein expression was time dependently upregulated in the kidney by UUO (Fig. 1C). Surgical wounds in the abdominal wall may influence HMGB1 concentration in serum and urine. Thus, we used blood and urine HMGB1 concentrations in sham mice as controls to measure HMGB1 released from injured kidneys. As shown in Fig. 1, D and E, the serum HMGB1 concentration rapidly reached the highest level on 3 days after UUO and gradually decreased after 7 days of UUO. The high level of urine HMGB1 in ligated ureters was observed 3–7 days after UUO and slightly declined after 10 days of UUO. HMGB1 levels in sham mouse serum or urine were not altered. These results indicate that HMGB1 expression was induced by UUO and released from injured kidney tissue.
Fig. 1.
High-mobility group box 1 (HMGB1) expression and release in mouse kidneys with unilateral ureter obstruction (UUO). A: HMGB1 expression in kidney tissue on different days [days 0–14 (D0–D14)] after UUO as indicated. HMGB1 expression was detected by immunostaining. B: HMGB1 expression in macrophage was detected by coimmunostaining of HMGB1 with F4/80 on kidney sections with UUO for 5 days. C: HMGB1 expression was quantified by Western blot analysis and normalized to α-tubulin. n = 5. *P < 0.01 compared with D0; #P < 0.01 compared with D3. D: serum HMGB1 concentration as measured by ELISA. n = 5. *P < 0.01 compared with D0; #P < 0.01 compared with D3 or D5 in UUO groups. E: urine HMGB1 concentration as measured by ELISA. n = 5. *P < 0.01 compared with D0; #P < 0.01 compared with D5.
HMGB1 overexpression contributed to UUO-caused kidney injury.
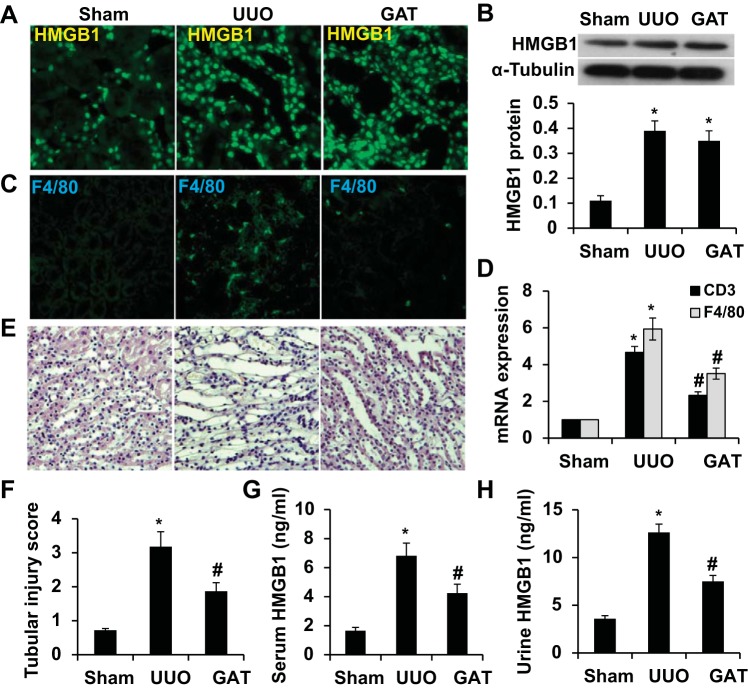
To test if HMGB1 plays a role in the kidney structural damage caused by UUO, we treated UUO mice with GA (HMGB1 inhibitor) via peritoneal injection. A previous study (18) has shown that GA binds to HMGB1 protein and inhibits its cytokine activities. GA has also been reported to inhibit HMGB1-induced inflammation with few side effects (4, 6, 21). We found that although GA treatment did not alter HMGB1 expression in kidney tissue (Fig. 2, A and B), it attenuated UUO-induced interstitial inflammation, as shown by the reduction of F4/80-expressing macrophages and CD3-expressing T cells (Fig. 2, C and D). GA treatment also ameliorated the kidney structural damage (Fig. 2, E and F). Since GA did not affect HMGB1 expression, we tested if GA affected HMGB1 release. As shown in Fig. 2, G and H, HMGB1 concentrations in mouse serum and urine were significantly increased on day 7 after UUO. However, GA treatment significantly blocked the release of HMGB1 from the kidney to blood and urine. These data suggest that HMGB1 released to the interstitium or urine contributed to the UUO-induced damage.
Fig. 2.
Blockade of HMGB1 release attenuated UUO-induced kidney structural damage. Mice received either saline or glycyrrhizic acid monosodium (GA) treatment (GAT) as described in experimental procedures and then underwent sham operation (sham) or UUO for 7 days. A: HMGB1 expression was detected by immunostaining. B: HMGB1 protein expression was detected by Western blot (top) and quantified by normalization to α-tubulin expression (bottom). C: macrophage infiltration in the interstitium was detected by immunostaining of F4/80. D: quantitative analysis of the expression of T cell marker CD3 and macrophage marker F4/80 by quantitative RT-PCR. E and F: kidney structure (E) and injury score (F) were assessed by hematoxylin and eosin staining. G: serum HMGB1 concentration was measured by ELISA. H: urine HMGB1 concentration was measured by ELISA. n = 5. *P < 0.01 compared with the sham group; #P < 0.01 compared with the UUO group.
HMGB1 release caused kidney fibrosis.
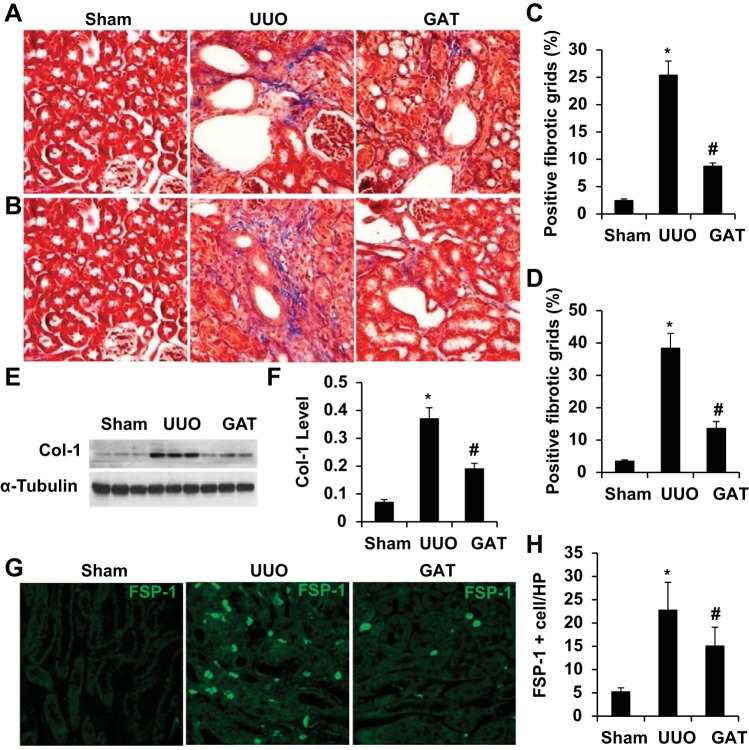
UUO is known to cause kidney interstitial fibrosis, as shown by collagen deposition in kidney tissue (Fig. 3A). However, blockade of HMGB1 release by GA significantly blocked the injury-induced collagen deposition in kidneys with UUO for 7 and 14 days (Fig. 3, A–D) and collagen type I expression (Fig. 3, E and F), indicating that HMGB1 played an important role in the development of renal fibrosis in UUO kidneys. Since collagen is produced by myofibroblasts during the fibrogenic process, we tested if HMGB1 is involved in myofibroblast activation. As shown in Fig. 3, G and H, UUO significantly induced myofibroblast activation, as shown by the increased number of fibroblast-specific protein-1-positive cells. However, GA treatment reduced the number of myofibroblasts, demonstrating that HMGB1 contributes to the fibrogenic process by activating myofibroblasts in the kidney interstitium.
Fig. 3.
Blockade of HMGB1 release attenuated UUO-induced fibrosis. Mice received saline or GAT as described in experimental procedures and then underwent sham operation or UUO for 7 or 14 days. A and B: collagen deposition was detected by Masson's staining on kidney sections with UUO for 7 days (A) and 14 days (B). C and D: kidney fibrosis at 7 days (C) or 14 days (D) after UUO was measured using the point-counting method with the point-counting grid points arranged in a square pattern. Percentages of the positive fibrotic grids are shown. E and F: collagen type I (Col-1) expression was detected by Western blot (E) and quantified by normalization to α-tubulin expression (F). G: collagen-producing myofibroblasts were detected by immunostaining of fibroblast-specific protein (FSP)-1. H: quantification of myofibroblasts in the interstitium by counting FSP-1-positive cells in each group. n = 5. *P < 0.01 compared with the sham group; #P < 0.01 compared with the UUO group.
HMGB1 modulated the macrophage phenotype in the kidney with UUO.
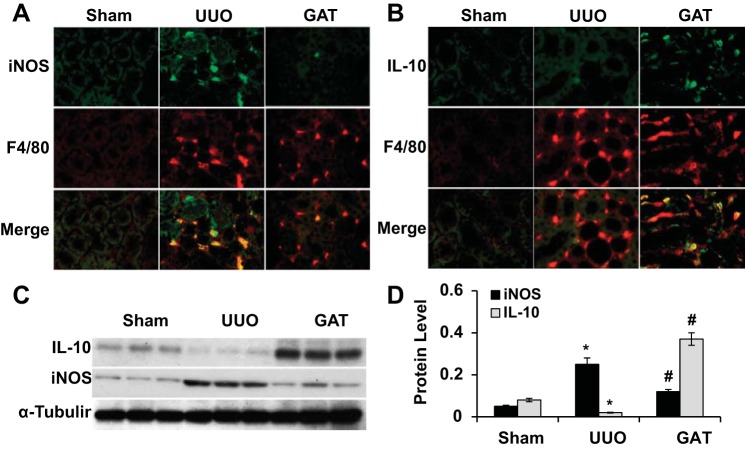
It is known that UUO triggers macrophage infiltration in kidney tissue after injury. To determine which macrophage phenotype is present in the UUO kidney, we used iNOS to identify the M1 phenotype and IL-10 to identify the M2 phenotype, respectively, and used F4/80, a macrophage marker, to stain macrophages. We found that at the early stage of injury (5 days of UUO), M1 but not M2 macrophages were accumulated in the kidney (Fig. 4A). GA treatment, however, diminished the presence of M1 macrophages (Fig. 4, A, C, and D). Interestingly, GA treatment resulted in a large number of M2 macrophages appearing in the kidney with the early stage of injury (Fig. 4, B–D). Since no M2 macrophage activation was observed at this stage of UUO (Fig. 4B), the M2 macrophages that appeared with GA treatment were likely to be converted from M1 macrophages, i.e., blockade of HMGB1 release by GA caused a M1-to-M2 macrophage transition in UUO kidneys.
Fig. 4.
Effect of HMGB1 on macrophage phenotype in mouse kidney with UUO. Mice received saline or GAT as described in experimental procedures and then underwent sham operation or UUO for 5 days. A: M1 macrophages were immunostained with inducible nitric oxide synthase (iNOS) and costained with macrophage marker F4/80. GAT blocked the accumulation of M1 macrophages. B: M2 macrophages were immunostained with IL-10 and costained with F4/80. GAT induced the accumulation of M2 macrophages in UUO kidneys. C and D: iNOS and IL-10 expression were examined by Western blot (C) and quantified by normalization to α-tubulin (D). n = 5. *P < 0.01 compared with the sham group; #P < 0.01 compared with the UUO group for iNOS and IL-10.
HMGB1 played a role in macrophage polarization.
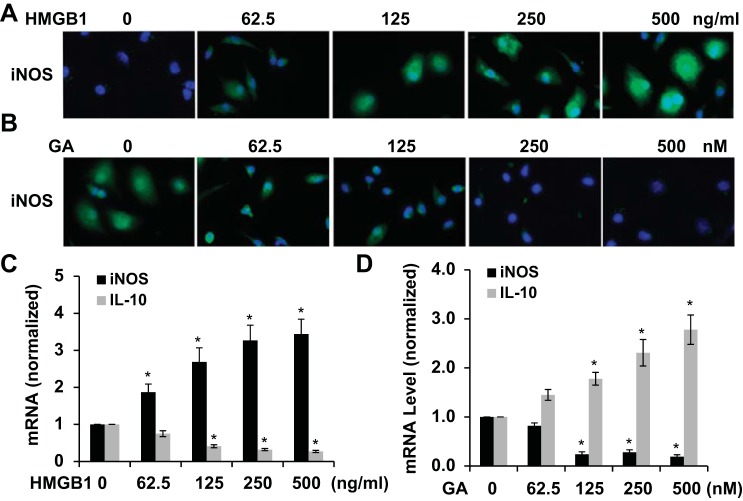
To further determine the role of HMGB1 in macrophage polarization, we directly tested if HMGB1 induces M1 marker expression in BMMs in vitro. As shown in Fig. 5A, HMGB1 stimulation induced expression of the M1 marker iNOS in a dose-dependent manner. GA treatment, however, dose dependently inhibited HMGB1 function (Fig. 5B). Importantly, HMGB1 upregulated iNOS mRNA expression while downregulating IL-10 expression, suggesting that HMGB1 suppressed M2 macrophage activation while inducing the M1 phenotype (Fig. 5C). GA treatment reversed these effects of HMGB1 (Fig. 5D). These data demonstrate that HMGB1 can directly induce macrophage polarization to the M1 phenotype.
Fig. 5.
Effect of HMGB1 on macrophage polarization. A: HMGB1 increased iNOS expression in macrophages in a dose-dependent manner. B: GA dose dependently attenuated HMGB1-induced iNOS expression in macrophages. C: HMGB1 induced iNOS while inhibiting IL-10 mRNA expression in macrophages in a dose-dependent manner. D: GA abrogated HMGB1-induced iNOS mRNA expression and increased IL-10 mRNA expression. n = 3. *P < 0.05 compared with untreated cells (0 ng/ml or 0 nM) in each corresponding group.
Acidified environment induced HMGB1 expression and release in macrophages and HPTCs.
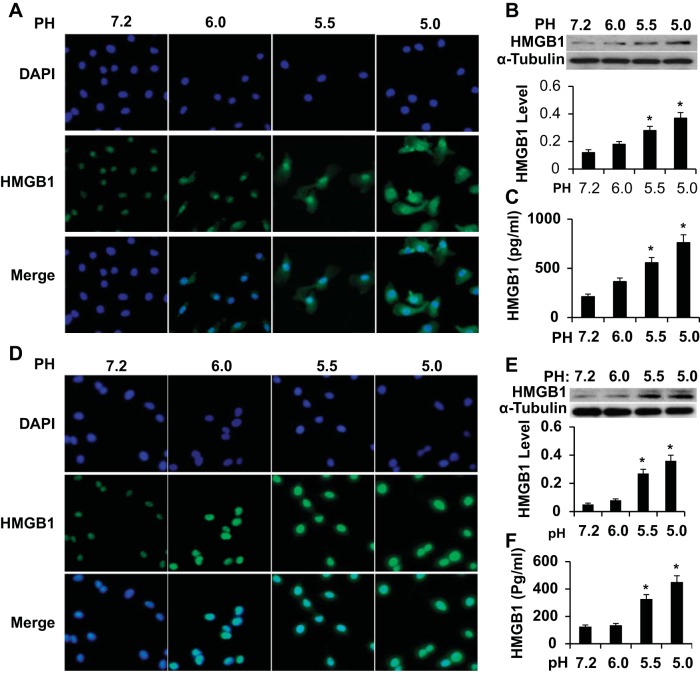
It is clear that HMGB1 expression and release are critical for macrophage M1 polarization, kidney structural damage, and fibrosis. However, it is unknown what factor is responsible for HMGB1 expression and release in the kidney. Interestingly, urine retained in the left ureter after ligation had a mean pH value of 5.52 (data not shown), which caused kidney tissues to be directly exposed to acidified urine along with tubular lumen damage. Thus, we hypothesized that the acidic environment may alter HMGB1 expression/release in tubular epithelial cells and macrophages. Indeed, when macrophages and HPTCs were cultured in a low-pH environment, the acidified medium significantly increased the expression and release of HMGB1 in both macrophages (Fig. 6, A–C) and HPTCs (Fig. 6, D–F). It appeared that the lower the pH, the more expression and release of HMGB1. In addition, as the medium pH decreased, HMGB1 translocation from the nuclei to cytoplasm was observed (Fig. 6A). These results suggest that the acidic environment may be one of the factors causing the increased expression and release of HMGB1 in kidney tissue after UUO.
Fig. 6.
HMGB1 expression and release in cultured macrophage and human proximal tubule cells (HPTCs). A and D: HMGB1 expression and translocation from the nuclei to cytoplasm induced by acidified medium in macrophages (A) and HPTCs (D) as shown by immunostaining. DAPI, 4′,6-diamidino-2-phenylindole. B and E: Western blot analysis of HMGB1 expression in macrophages (B) and HPTCs (E) treated with acidified medium. HMGB1 levels were normalized to α-tubulin in each corresponding group. C and F: HMGB1 secretion to the supernatant of cultured macrophages (C) and HPTCs (F) as measured by ELISA. n = 3. *P < 0.01 compared with the physiological pH environment (pH 7.2) in each group.
DISCUSSION
The role of macrophages in chronic kidney disease is well established (23). A recent study (1) has indicated the divergent role of macrophage polarization. M1 and M2 macrophages are observed at different stages of injury in the mouse acute ischemia-reperfusion model (13), and infusion of in vitro-induced M2 macrophages has shown protection against kidney injury in murine adriamycinnephrosis (16). Our present study provided novel information about the role of the M1 phenotype in an early stage of chronic disease, i.e., UUO-induced fibrosis. We found that interstitially accumulated macrophages exhibit the M1 phenotype, which is critical for the onset of interstitial fibrosis. HMGB1 released in UUO kidneys plays a critical role in the M1 phenotype formation because inhibition of HMGB1 release blocks UUO-induced fibrosis and the M1 phenotype. Moreover, HMGB1 can directly induce the M1 phenotype in vitro, supporting that HMGB1 is one of the culprits for the M1 phenotype at the early stage of UUO.
In addition to HMGB1, other DAMPs may also be involved in M1 macrophage formation in the UUO kidney. Kidney-specific DAMPs, such as crystals and uromodulinare, are released after renal tubular damage (2). These DAMPs have been shown to trigger innate immunity by activating Toll-like receptors, purinergic receptors, or the Nod-like receptory family, pyrin domain-containing 3 inflammasome (2). Although our study does not address the molecular mechanism underlying HMGB1 function in the activating M1 phenotype, HMGB1 is likely to act synergistically with other DAMPS to mediate the initial inflammatory response in kidneys with UUO damage.
HMGB1 is highly induced in both tubular epithelial cells and macrophages by UUO. Moreover, UUO causes HMGB1 release from the kidney to serum and urine. UUO-mediated HMGB1 release may be attributed to different sources. Since UUO damages the kidney structure, especially renal tubules, some HMGB1 in serum and urine may come from dead epithelial cells. The cell death or necroptosis may also cause the release of other DAMPS. Interestingly, UUO reduces the pH of urine accumulated in the obstructed ureter, and the acidic environment is able to induce HMGB1 release from tubular epithelial cells and macrophages in vitro, suggesting that acidic stress in the kidney contributes to the release of HMGB1. Although this phenomenon has not yet confirmed in vivo, our finding may be clinically important because metabolic acidosis is commonly associated with chronic kidney diseases (11, 12). This acidosis may cause the release of HMGB1 or other DAMPS in the kidney of patients with chronic kidney diseases, leading to fibrogenesis, which would be an interesting subject for future study.
GA appears to be an effective agent to halt UUO-induced fibrosis. Although GA inhibits HMGB1 release, its renal protection may not only be due to its activity on HMGB1 because GA also inhibits other molecules involved in renal fibrosis. One of these molecules is phospholipase A2. Phospholipase A2 is known to be involved in UUO-mediated kidney injury (5). In addition, GA inhibits 11-β-hydroxysteroid dehydrogenase type 2, whose expression is reduced in the obstructed kidney and is likely to have a protective role in kidney injury (14). Therefore, the mechanism underlying GA function in lessening UUO-induced fibrosis is likely to be sophisticated, involving modulation of activities of multiple proteins, including HMGB1, phospholipase A2, etc.
There are two limitations in the present study. First, the UUO model is not an ideal model for chronic kidney disease because it is not a usual cause of adult human renal disease. In addition, the UUO mouse exhibits normal creatinine without proteinuria and hypertension. Second, the causal role of the GA-mediated reduction of HMBG1 release in renal protection and the role of acidic stress in HMGB1 release have not been established in vivo. Despite these limitations, our study provides evidence that macrophages accumulated in the kidney interstitium at the early stage of UUO display M1 polarization, the elevated HMGB1 at this stage appears to be one of the key factors facilitating the M1 phenotype, and GA is a potential agent useful for reducing tubular injury and subsequent fibrosis.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-123302, HL-119053, and HL-107526 and by National Natural Science Foundation of China Grant 81328002.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.T. and S.-Y.C. conception and design of research; S.T., L.Z., J.T., X.G., and K.D. performed experiments; S.T. analyzed data; S.T. and S.-Y.C. interpreted results of experiments; S.T. prepared figures; S.T. drafted manuscript; S.T., L.Z., and S.-Y.C. edited and revised manuscript; S.T., L.Z., J.T., X.G., K.D., and S.-Y.C. approved final version of manuscript.
REFERENCES
- 1.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotype determine progression or resolution of renal inflammation and fibrosis. Kidney Int 80: 915–925, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Anders HJ, Schaefer L. Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25: 1387–1400, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, Crawford M, Day RM, Briones VR, Leader JE, Jose PA, Lechleider RJ. RhoA modulates Smad signaling during transforming growth factor-β-induced smooth muscle differentiation. J Biol Chem 281: 1765–1770, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du D, Yan J, Ren J, Lv H, Li Y, Xu S, Wang Y, Ma S, Qu J, Tang W, Hu Z, Yu S. Synthesis, biological evaluation, and molecular modeling of glycyrrhizin derivatives as potent high-mobility group box-1 inhibitors with anti-heart-failure activity in vivo. J Med Chem 56: 97–108, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Fukuzaki A, Morrissey J, Klahr S. Enhanced glomerular phospholipase activity in the obstructed kidney. Int Urol Nephrol 27: 783–790, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Gong G, Xiang L, Yuan L, Hu L, Wu W, Cai L, Yin L, Dong H. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLos One 9: e89450, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 32: 593–604, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Huang WY, Li ZG, Rus H, Wang X, Jose PA, Chen SY. RGC-32 mediates transforming growth factor-β-induced epithelial-mesenchymal transition in human renal proximal tubular cells. J Biol Chem 284: 9426–9432, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isaka Y, Tsujie M, Ando Y, Nakamura H, Kaneda Y, Imai E, Hori M.. Transforminggrowthfactor-β1 antisense oligodeoxynucleotides block interstitial fibrosis in unilateral ureteral obstruction. Kidney Int 58: 1885–1892, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Kopple JD, Kalantar-Zadeh K, Mehrotra R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int Suppl 95: S21–S27, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kidney Dis 45: 978–993, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Wang W, Norregaard R, Knepper MA, Nielsen S, Frøkiaer J. Altered expression of epithelial sodium channel in rats with bilateral or unilateral ureteral obstruction. Am J Physiol Renal Physiol 293: F333–F341, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Mariappan N, Megyesi J, Shank B, Kannan K, Theus S, Price PM, Duffield JS, Portilla D. Proximal tubule PPARα attenuates renal fibrosis and inflammation caused by unilateral ureteral obstruction. Am J Physiol Renal Physiol 305: F618–F627, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu J, Cao Q, Zheng D, Sun Y, Wang C, Yu X, Wang Y, Lee VW, Zheng G, Tan TK, Wang X, Alexander SI, Harris DC, Wang Y. Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney Int 84: 745–755, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Mizuno S, Matsumoto K, Nakamura T. Hepatocyte growth factor suppresses interstitial fibrosis in a mouse model of obstructive nephropathy. Kidney Int 59: 1304–1314, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G, Bianchi ME. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol 14: 431–441, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Nishida M, Hamaoka K. Macrophage phenotype and renal fibrosis in obstructive nephropathy. Nephron Exp Nephrol 110: e31–e36, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Rabadi MM, Ghaly T, Goligorksy MS, Ratliff BB. HMGB1 in renal ischemic injury. Am J Physiol Renal Physiol 303: F873–F885, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Q, Wang F, Li W, Li W, Hu YC, Li S, Zhu JH, Zhou M, Hang CH. Glycyrrhizic acid confers neuroprotection after subarachnoid hemorrhage via inhibition of high mobility group box-1 protein: a hypothesis for novel therapy of subarachnoid hemorrhage. Med Hypotheses 81: 681–685, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Tampe D, Zeisberg M. Potential approaches to reverse or repair renal fibrosis. Nat Rev Nephrol 10: 226–237, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Harris DC. Macrophages in renal disease. J Am Soc Nephrol 22: 21–27, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Wu H, Ma J, Wang P. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol 21: 1878–1890, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]