Abstract
Respiratory distress in preterm or low birth weight infants is often treated with supplemental oxygen. However, this therapy can disrupt normal lung development and architecture and alter responses to respiratory insults. Similarly, exposure of newborn mice to 100% oxygen during saccular lung development leads to permanent alveolar simplification, and upon challenge with influenza A virus, mice exhibit reduced host resistance. Natural killer (NK) cells are key players in antiviral immunity, and emerging evidence suggest they also help to maintain homeostasis in peripheral tissues, including the lung, by promoting epithelial cell regeneration via IL-22. We tested the hypothesis that adult mice exposed to hyperoxia as neonates have modified NK cell responses to infection. We report here that mice exposed to neonatal hyperoxia had fewer IL-22+ NK cells in their lungs after influenza virus challenge and a parallel increase in IFN-γ+ NK cells. Using reciprocal bone marrow chimeric mice, we show that exposure of either hematopoietic or nonhematopoietic cells was sufficient to increase the severity of infection and to diminish the frequency of IL-22+ NK cells in the infected lung. Overall, our findings suggest that neonatal hyperoxia leads to long-term changes in the reparative vs. cytotoxic nature of NK cells and that this is due in part to intrinsic changes in hematopoietic cells. These differences may contribute to how oxygen alters the host response to respiratory viral infections.
Keywords: respiratory infection, innate immunity, hematopoietic
it is estimated that at least 15 million preterm births occur each year worldwide (51). Being born too soon or at extremely low birth weight is not only a major cause of neonatal mortality but can have long-term health consequences in survivors (50). Postnatal interventions including supplemental oxygen, mechanical ventilation, and steroid treatments have profoundly increased survival of these infants; however, negative consequences are associated with many of these treatments, and improving the health of survivors remains a challenge (28, 45). These infants have an increased risk of developing the chronic lung disease bronchopulmonary dysplasia (BPD) (13, 24, 31). Lungs of infants who died from complications associated with BPD are often simplified with fewer and larger alveoli, attributed in part to abnormal vascular development. (25). Children diagnosed with BPD in infancy often have reduced pulmonary function. Also, school-age children and adolescents who were born too soon, and survived, experience higher rates of severe respiratory infections and more hospitalizations (10, 21, 26, 36). Knowing that 10% of infants are born too soon even in developed countries, it is imperative that we understand the cellular mechanisms that underlie the negative health outcomes later in life.
Mouse models of neonatal supplemental oxygen treatment mimic many of the pathological features described in human epidemiological studies. In mice, high oxygen (hyperoxia) exposure during the saccular stage of lung development (postnatal days 0–4) results in structural changes to the adult lung including larger, simplified alveolar spaces, and these alterations in lung structure and function persist throughout the life of the animal (9, 12, 32, 34). Moreover, upon challenge with influenza A virus, young adult mice that were exposed to hyperoxia as neonates demonstrate an increase in the severity of infection, as measured by greater weight loss, enhanced recruitment of leukocytes, increased epithelial injury, and poorer survival (34, 35). Despite these changes, the overall pulmonary viral burden and kinetics of viral clearance were comparable between adult mice treated briefly with supplemental oxygen at birth and those born into room air. This is consistent with the presence of an intact CD8+ T-cell response to the infection; one of the cell types critically important for clearing primary influenza virus infection (6, 16, 46). This paradox of enhanced morbidity and mortality in the absence of an overt deficit in adaptive immune function raised the question whether there were other aspects of the antiviral response that were persistently altered by early life supplemental oxygen exposure.
Natural killer (NK) cells comprise an innate population that is essential for controlling influenza virus A infection, as elimination of NK cells results in increased mortality (15, 17). Recent evidence also supports a role for NK cells in epithelial cell repair after clearance of influenza virus through the production of cytokine IL-22, suggesting they may be vital throughout the infection rather than only during early stages (19, 29). Based on these factors, we developed the hypothesis that early life exposure to a hyperoxic environment persistently influences NK cell activity. In addition, we sought to identify whether alterations in NK cell responses to infection were due to effects of early life supplemental oxygen treatment on the immune system or if they were attributable entirely to changes in extra-hematopoietic tissues.
MATERIALS AND METHODS
Mice, exposure, and infection.
C57BL/6 mice (CD45.2+) and CD45.1+ congenic C57BL/6 mice were obtained from the National Cancer Institute (National Institutes of Health). Newborn mice were exposed to room air (RA) or 100% oxygen (O2) as previously described (52). Briefly, newborn mice were maintained in 100% O2 for 96 h immediately after birth and then returned to RA. Littermates were maintained in RA. Dams were rotated between RA and 100% oxygen litters every 24 h to minimized oxygen toxicity and control for any maternal effects. Unless otherwise specified, at 8 wk of age, female mice were anesthetized with tribromoethanol (Avertin) and infected with 120 HAU HKx31 (H2N3) influenza A virus intranasally. Based on power analyses and prior experience, a minimum of five mice per group (at a single time point) is sufficient to detect statistically significant differences between means of the two groups. Thus the experimental design for our studies uses more than or equal to six mice per treatment group to provide sufficient statistical power. Each experiment was performed at least twice to ensure reproducibility. All procedures involving laboratory animals and infectious agents were reviewed and approved by the University of Rochester Institutional Animal Care and Use and Institutional Biosafety Committees. The University has accreditation through the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All guidelines from the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals were followed in handling of vertebrate animals.
Cell preparation.
Lung vasculature was perfused with PBS. Lung-derived leukocytes were obtained by mincing lungs and digesting the tissue pieces in 5 ml collagenase media [RPMI 1640 media, containing 5 mM HEPES, 2.5% BSA, 0.7 mg/ml collagenase type II (Worthington Biochemical, Lakewood, NJ), and 30 μg/ml DNase I] and shaking gently at 37°C for 25 min. Lung suspensions were filtered through 70-μm nylon filters, and the remaining pieces were mechanically dissociated using a syringe plunger. Filters were washed with 10 ml RPMI 1640 media, supplemented with 5 mM HEPES, 2.5% BSA. Cells were pelleted, lysed with 150 mM ammonium chloride solution, and washed before counting. Bone marrow was harvested by flushing femurs and tibias with PBS containing 10% FBS. Marrow was pulled up into syringes and expelled three times to create a single cell suspension. Bone marrow cell suspensions were filtered through 70-μm nylon filters. Cells were pelleted and erythrocytes were lysed with ammonium chloride before counting. All cells were enumerated using a TC10 cell counter (Bio-Rad).
Flow cytometry staining.
Single cell suspensions were stained in PBS containing 1% BSA and 0.01% sodium azide (PAB). Nonspecific binding was blocked by preincubation with the anti-Fc receptor antibody CD16/32 for 15 min on ice. The following antibodies were used for extracellular staining: NK1.1 (clone PK136; BD Bioscience, San Jose, CA), CD3 (clone 145-2C11; BD Bioscience), CD11b (clone M1/70; eBioscience, San Diego, CA), KLRG1 (clone 2F1; eBioscience), and IL-23R (clone 753317; R&D Systems, Minneapolis, MN). Antibodies to extracellular antigens were added, and cells were incubated on ice in the dark from 20 to 30 min. Cells were washed twice with 200 μl PAB. For intracellular staining, cells were washed with PAB and fixed with 2% paraformaldehyde. Cells were permeabilized with 0.2% saponin solution containing 3% FBS, and nonspecific binding was blocked with CD16/32 and 50 μg/ml rat IgG. Intracellular levels of cytokines were evaluated with anti-IFN-γ (clone XMG1.2; eBioscience), granzyme B (clone NGZB; eBioscience), and IL-22 (clone 140301; R&D Systems). Cells were incubated with antibodies for 45 min on ice in the dark. Cells were subsequently washed in saponin-containing solution and then in PAB. Flow cytometry was performed on an LSR II flow cytometer (BD Bioscience). Data were analyzed using FlowJo software (Treestar, Ashland, OR).
Bone marrow cell transplantation.
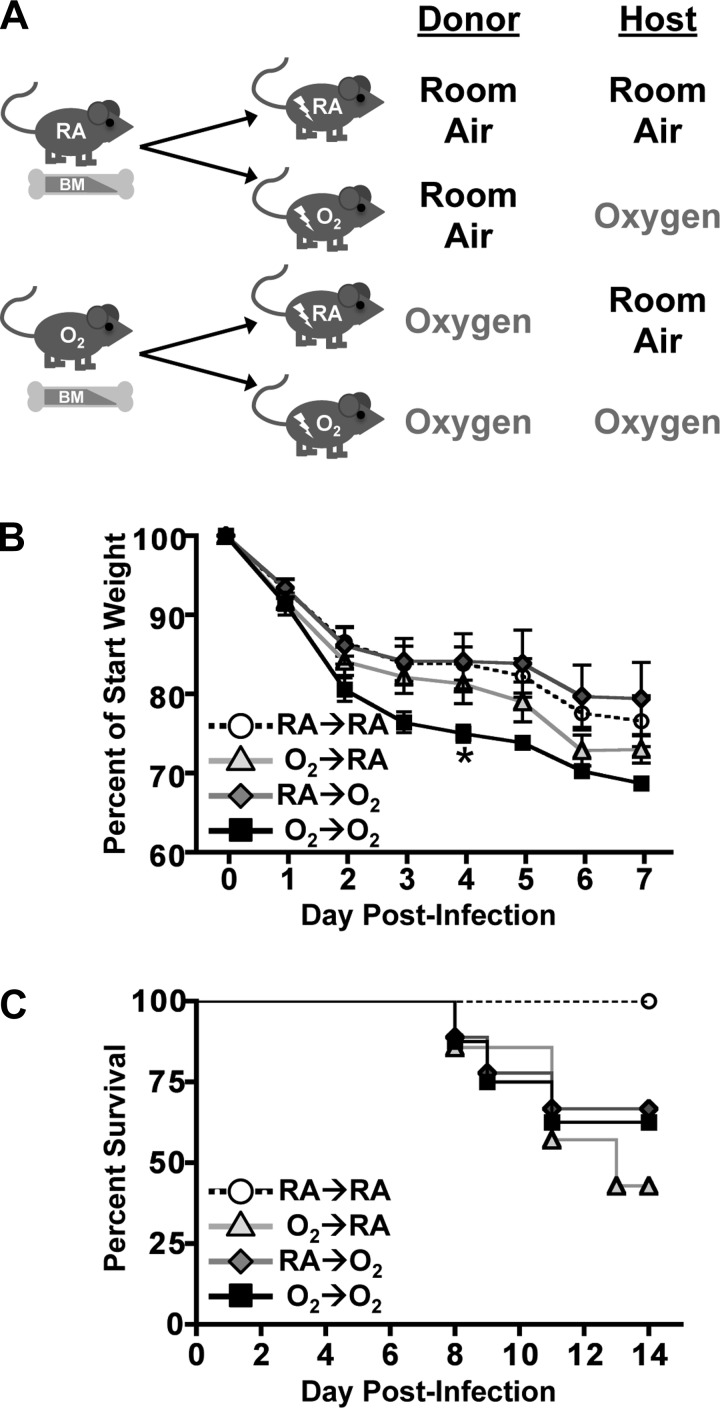
One week before irradiation, young adult C57BL/6 (CD45.2) mice (age 5–7 wk) that were born into RA or O2 were switched to a medicated diet (Modified Isopro w/0.124% Sulfadiazine/0.025% Trimethoprim; TestDiet, St. Louis, MO) to prevent postirradiation infection. Mice were irradiated with two doses of 5.5 Gy, spaced 4 h apart (137Cs Gamma Irradiator, Department of Radiation Oncology, University of Rochester). Bone marrow cells were acquired from CD45.1+ congenic donors that were also exposed neonatally to O2 and RA. Within 6 h of the second dose of irradiation, 2 × 106 bone marrow cells were transferred by intravenous injection into O2- and RA-irradiated hosts. Four groups of mice were created: RA-CD45.1→O2-CD45.2 (RA→O2), O2-CD45.1→RA-CD45.2 (O2→RA), RA-CD45.1→RA-CD45.2 (RA→RA), and O2-CD45.1→O2-CD45.2 (O2→O2). To ensure full immune system reconstitution, mice were allowed to rest for 10 wk before infection with 120 HAU X31 influenza A virus.
Statistical analysis.
All statistical analyses were performed using the JMP Statistics Package (SAS, Cary, NC). For the time-course experiments, a two-way ANOVA was first used to determine overall significance, followed by post hoc Tukey-Kramer honestly significant difference (HSD) tests. Tukey-Kramer HSD or Dunnett's tests were used for analyzing bone marrow chimera experiments. For experiments in which there were only two groups and single point in time was evaluated, a Student's t-test was used. Mean values were considered significantly different as defined by a P ≤ 0.05.
RESULTS
NK cell number and maturation status are maintained after neonatal supplemental oxygen.
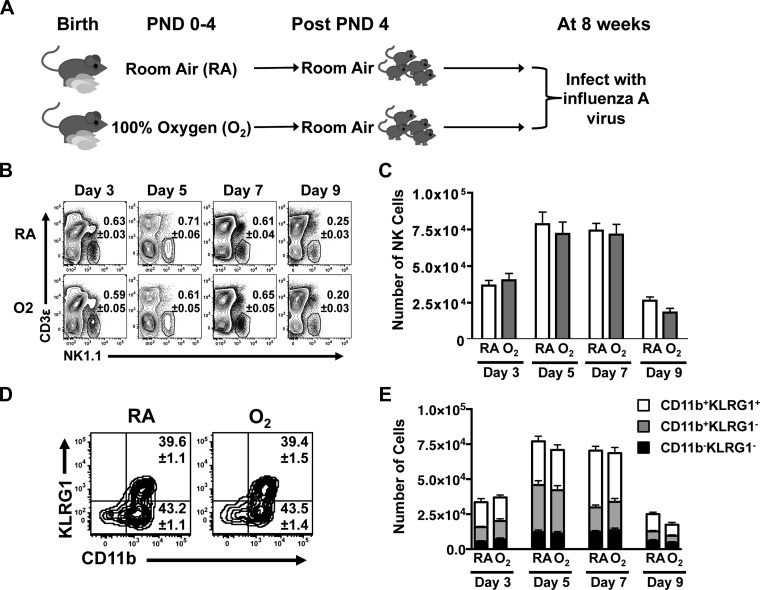
Mice exposed to either 100% oxygen (O2) or RA oxygen at birth, were infected at 8 wk of age with influenza A virus (Fig. 1A), and NK cells in the lung were examined by flow cytometry during the course of infection. Specifically, the percentage (Fig. 1B) and number (Fig. 1C) of NK cells in the lung were measured in O2-exposed adult mice and control (RA-exposed) littermates before infection (day 0) and 3, 5, 7, and 9 days postinfection. In the absence of infection, very few NK cells were found in the lung (data not shown). However, NK cells were detected by postinfection day 3 and their number and percent peaked on days 5 and 7 before declining by day 9. There were no significant differences in the percentage or number of NK cells between the RA and O2 groups, suggesting no overt alteration in the response of the population as a whole (Fig. 1, B and C).
Fig. 1.
Natural killer (NK) cell population is maintained after neonatal oxygen supplementation and viral challenge. A: experimental design and timeline for neonatal exposure influenza A virus infection later in life. B and C: percentage and number of NK cells (NK1.1+CD3−) at days 3, 5, 7, and 9 postinfection, as determined by flow cytometry. D and E: NK cell activation and maturation status defined by CD11b and KLRG1 expression on the cell surface. D: number in the 2 right quadrants of representative dot plots depicts the percentage of CD11b+KLRG1+ (top) and CD11b+KLRG1− (bottom) NK cells on day 5 after infection. Data are shown as means ± SE with 8 mice per group at each point in time. Data are from 1 experiment that is representative of at least 2 independent experiments. PND, postnatal day; RA, room air.
To further investigate whether NK cell maturation or activation was disrupted by early life supplemental oxygen, CD11b and KLGR1 were used to dissect the population. NK cells devoid of both markers are defined as immature NK cells. Expression of CD11b denotes NK cell maturation, whereas upregulation of the inhibitory receptor KLRG1 distinguishes activated NK cells. The percentage of immature, mature, and activated NK cell subsets was unchanged in lungs from the RA and O2 treatment groups at day 5 postinfection (Fig. 1D). There was also no difference in the percentage of these NK cell subsets at the other time points evaluated (data not shown). Infection promoted expansion of the CD11b single-positive NK cells and CD11b KLRG1 double-positive NK cells subsets; however, this increase was comparable in the lungs of adult mice in both groups. (Fig. 1E).
NK cells display persistent functional changes after early life oxygen supplementation.
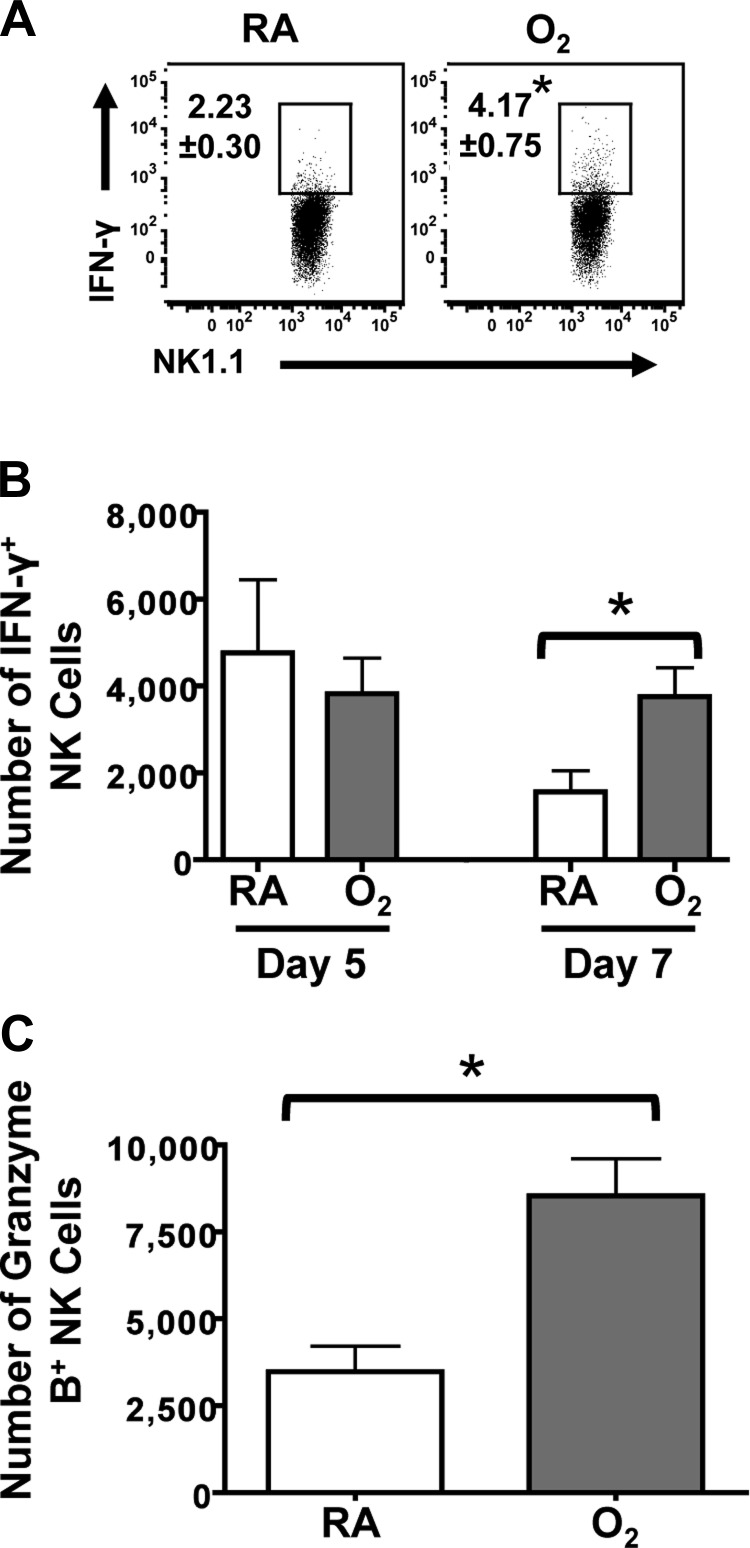
Conventional functions of NK cells include the production of the cytokine interferon-γ (IFN-γ) and the ability to kill infected cells, via release of granzymes and other cytolytic mediators. To evaluate whether early life oxygen supplementation altered these typical NK cell functions, we first compared the percentage and number of IFN-γ+ NK cells from the RA- and O2-exposed groups after infection (Fig. 2, A and B). At day 5 postinfection, the number of IFN-γ+ NK cells was comparable between the O2 and RA groups (Fig. 2B). There was also no difference in the percentage of IFN-γ+ NK cell (data not shown). However, on day 7 postinfection, there were significantly more IFN-γ+ NK cells in mice exposed to supplemental oxygen at birth, compared with RA-exposed controls (Fig. 2, A and B). To examine if another marker of NK cell cytotoxicity was increased at this time point, granzyme B was measured by intracellular staining. Analogous to the two- to threefold increase in the frequency of IFN-γ+ NK cells, there was a significant increase in the number of granzyme B+ NK cells (Fig. 2C). The increase was also observed as the percentage of granzyme B+ NK cells (data not shown).
Fig. 2.
Classical NK cell cytolytic markers are enhanced in mice exposed to supplemental oxygen at birth. A: percentage of IFN-γ+ NK cells directly ex vivo at day 7 postinfection with influenza A virus infection, as measured by flow cytometry. B: number of IFN-γ+ NK cells at days 5 and 7 of influenza A virus infection. C: number of granzyme B+ NK cells ex vivo at day 7 postinfection. Data are shown as means ± SE with 8 mice per group from 1 experiment that is representative of 2 independently performed experiments. *P ≤ 0.05, two-tailed Student's t-test.
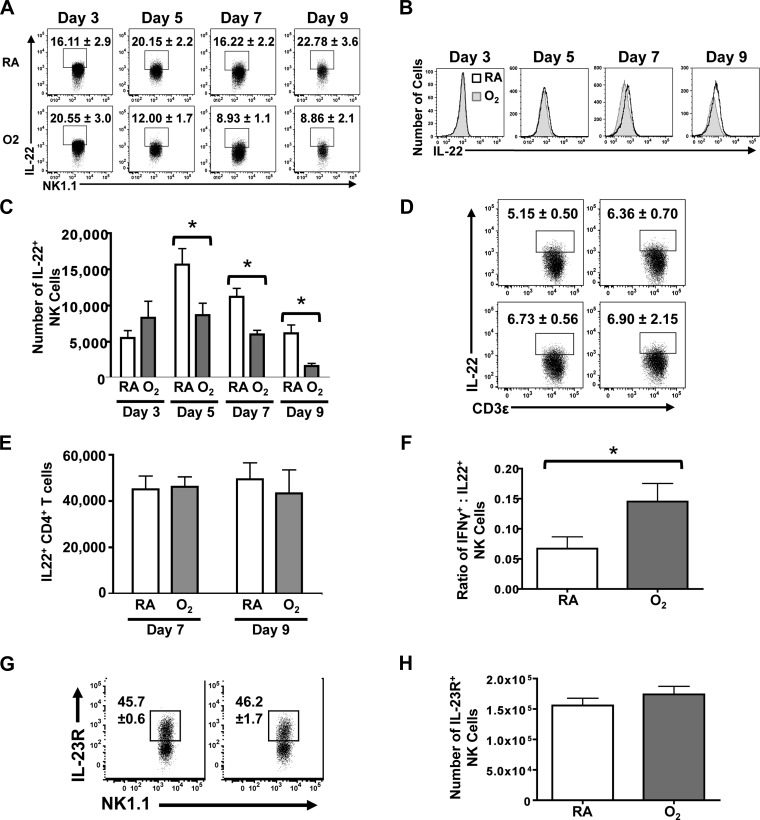
We next sought to determine whether early life O2 supplementation affected other NK cell responses to infection, such as their production of the cytokine IL-22. To this end, NK cells were stained for intracellular IL-22, directly ex vivo, and evaluated by flow cytometry at days 3, 5, 7, and 9 postinfection (Fig. 3, A and B). Three days after infection, the percentage and number of IL22+ NK cells were equivalent between the RA and O2 groups. However, beginning on day 5 postinfection, a lower percentage of NK cells were IL-22+ in lungs from adult mice that had been exposed to supplemental oxygen at birth (Fig. 3A). Moreover, this diminished frequency of IL-22+ NK cells persisted and remained apparent on days 7 and 9 postinfection (Fig. 3A). This does not appear to reflect a decrease in the level of IL-22 produced on a per cell basis but rather reflects fewer NK cells making IL-22, as the mean fluorescence intensity of IL-22 staining is not shifted (Fig. 3B). This is further substantiated by a concurrent reduction in the number of IL-22+ NK cells (Fig. 3C). Since a subset of CD4+ T cells also produces IL-22, we evaluated the percentage and number of IL-22+ CD4+ T cells in the lung at days 7 and 9 after infection. There was no difference in the total number of CD4+ T cells in the lung (data not shown). Moreover, CD4+ T cells in the infected lung did not display any difference in the percentage or number that stained positively for IL-22, suggesting that this may be an effect specific to NK cells (Fig. 3, D and E). To examine whether the observed changes in IFN-γ and IL-22 resulted in a shift in the proportion of cytotoxic to regulatory NK cells, the ratio of these two subsets was calculated (Fig. 3F). Early life exposure to a high oxygen environment gives rise to a persistent skewing in functional subsets toward NK cells with classical cytotoxic features.
Fig. 3.
The cytokine IL-22 is produced by fewer NK cells in lungs of infected mice that received supplemental oxygen as neonates. A: percentage of IL-22+ NK cells at days 3, 5, 7, and 9 postinfection. B: histograms depict the mean fluorescent intensity of IL-22 staining in NK cells from RA- and O2-exposed mice on the indicated days after infection. C: number of IL-22+ NK cells at days 3, 5, 7, and 9 postinfection. D and E: percentage and number of IL-22+ CD4+ T cells was measured at day 7 postinfection with influenza A virus by flow cytometry. F: ratio of IFN-γ+:IL-22+ NK cells at day 7 postinfection. G and H: percentage and number of IL-23R+ NK cells at day 7 postinfection. Data are displayed as means ± SE. There were 8 mice in each treatment group, and 1 experiment that is representative of at least 2 independent experiments is shown. *P ≤ 0.05, two-way ANOVA followed by post hoc Tukey-Kramer honestly significant difference (HSD) test for time-course studies and Student's t-test for single points in time.
The production of IL-22 by NK cells is elicited largely via signaling through the IL-23 receptor (29). Therefore, we examined whether early life oxygen supplementation altered the level of IL-23 receptor on NK cells, which could account for the observed reduction in IL-22+ NK cells. The IL-23 receptor was measured at day 7 postinfection, a point in time that is in the midst of the decreased frequency of IL-22+ NK cells. The frequency and number of NK cells expressing the IL-23 receptor were comparable in infected adult mice that were exposed to high oxygen at birth and RA littermates (Fig. 3, G and H). The level of expression on a per cell basis was also similar, with no differences in mean fluorescence intensity observed between the groups (data not shown). Overall, this suggests that the reduction in IL-22+ NK cells in the infected lung is unlikely due to reduced IL-23 receptor expression levels.
Neonatal oxygen supplementation influences hematopoietic and nonhematopoietic compartments.
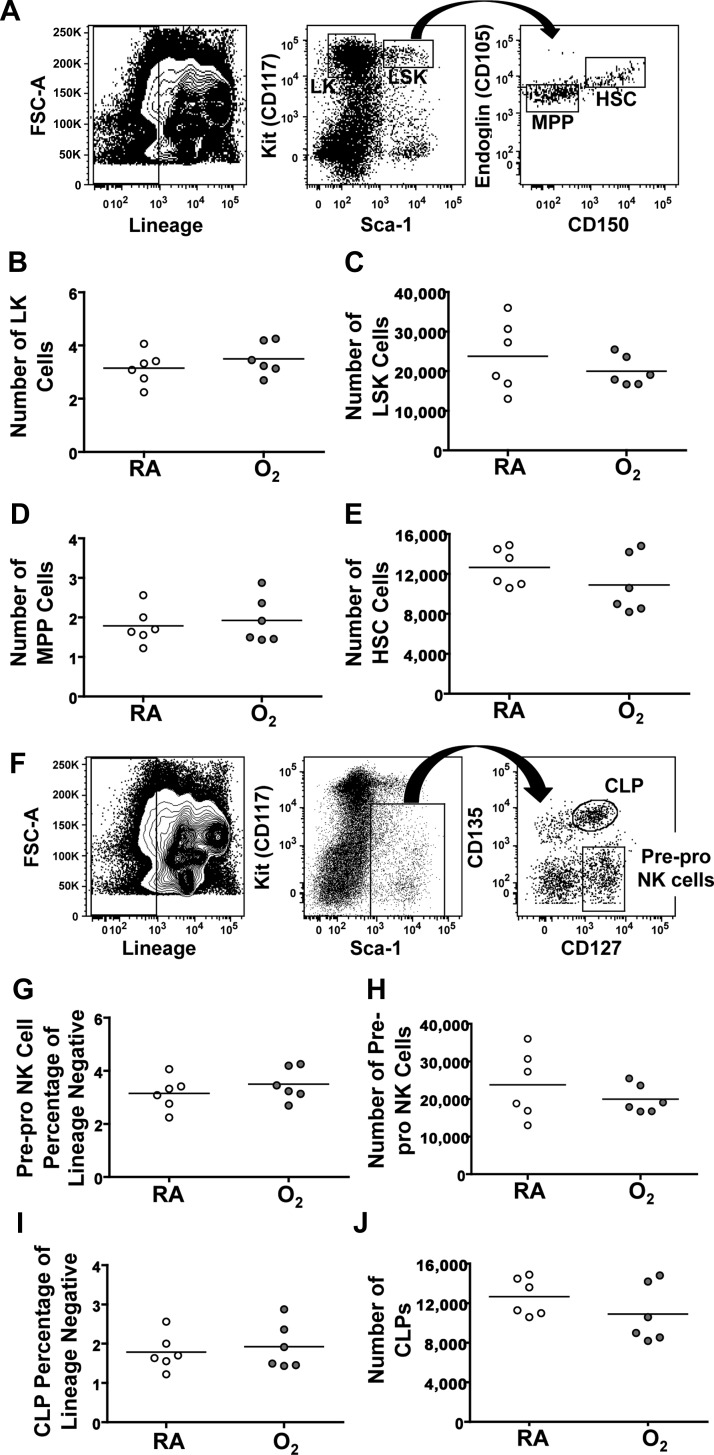
Differences in the response of NK cells to infection could result from O2-mediated effects within bone marrow-derived cells, which renders responses to infection permanently altered. If this were the case, then functional changes in hematopoietic cells would be retained and observed in the infected lung. That is, changes would still be evident when bone marrow cells from mice exposed to neonatal hyperoxia were moved into a new environment. Alternatively, neonatal oxygen supplementation could change the response of NK cells to infection in an indirect manner by causing long-lasting alterations outside of the hematopoietic system (i.e., changes in lung). To distinguish between these two scenarios, reciprocal bone marrow chimeras were created, using congenic mice that express allelic variants of the pan-leukocyte marker CD45. Importantly, before performing bone marrow cell transplantations, hematopoietic progenitor cell populations in the bone marrow from mice exposed to RA and neonatal hyperoxia were compared using flow cytometry. Within the lineage negative population, we observed a comparable number of Kit+Sca-1− cells in mice exposed to neonatal hyperoxia and controls (Fig. 4, A and B). These cells comprise a population of precursors for granulocytes and megakaryocytes, which were also unchanged (data not shown). Likewise, the same number of Kit+Sca-1+ cells was detected in the bone marrow cells from both groups of mice, and further division of this population using Endoglin and CD150 showed no difference in the presence of multipotent progenitors or hematopoietic stem cells (HSC; Fig. 4, A and C–E). Since this study focused on NK cells, further analysis of the common lymphoid progenitor cells as well as pre-pro NK cells was completed (Fig. 4F). In summary, there is no evidence that neonatal oxygen supplementation caused detectable differences in the percentage or number of any bone marrow cell immune progenitor cell populations (Fig. 4, G–J).
Fig. 4.
Bone marrow progenitor populations are maintained after neonatal supplemental oxygen. A: gating strategy for identifying multipotent progenitors (MPP) and hematopoietic stem cells (HSCs). B-E: number of Lin−Kit+ (LK) cells, MPPs, Lin−Sca-1+Kit+ (LSK) cells, and HSCs in the bone marrow of naïve RA and O2 mice. F: gating strategy for analysis of pre-pro NK cells and common lymphoid progenitors (CLPs). G and H: percentage and number or pre-pro NK cells in naïve mice. I and J: percentage and number of CLPs in naïve mice. Data are shown as means ± SE from 6 mice per group. Data are derived from 1 experiment that is representative of 3 independent experiments.
After it was established that neonatal hyperoxia did not explicitly change the presence of bone marrow progenitors, lethally irradiated C57BL/6 (CD45.2) recipient mice that were exposed to hyperoxia or RA at birth were reciprocally reconstituted with bone marrow cells from congenic B6.CD45.1 mice that were similarly exposed to either RA or oxygen. This created the following four groups: RA-CD45.1→O2-CD45.2 (RA→O2), O2-CD45.1→RA-CD45.2 (O2→RA), RA-CD45.1→RA-CD45.2 (RA→RA), and O2-CD45.1→O2-CD45.2 (O2→O2) (Fig. 5A). The RA→RA and O2→O2 groups serve as controls, with the expectation that responses in these groups would mirror effects observed in intact adult mice that were maintained in RA or exposed to four days of supplemental oxygen at birth.
Fig. 5.
Neonatal supplemental oxygen exposure of bone marrow cells or nonhematopoietic cells increased morbidity of mice infected with influenza A virus A: experimental design for reciprocal bone marrow cell transplantations. B and C: weight loss and survival of bone marrow chimeric mice after influenza A virus infection. Data are shown as means ± SE. Data are from 1 experiment that is representative of 2 independent experiments. Weight loss data are from 5 to 6 mice per group (RA→RA n = 5, O2→RA n = 6, RA→O2 n = 6, O2→O2 n = 5). Survival data are from 5–9 mice per group (RA→RA n = 7, O2→RA n = 7, RA→O2 n = 9, O2→O2 n = 8). *P ≤ 0.05, Dunnett's test with RA→RA as the control group.
The four groups of chimeric mice were infected with influenza A virus 10 wk after reconstitution, and morbidity and mortality were monitored. Importantly, all groups of mice evaluated were a comparable weight before infection (data not shown). Similar to nonchimeric adult mice exposed to neonatal hyperoxia, mice in the O2→O2 group lost significantly more weight during the first 7 days of infection and experienced higher mortality than infected mice in the RA→RA group (Fig. 5, B and C). Infected mice in both of the mixed chimera groups (RA→O2 and O2→RA) also displayed lower levels of survival than did mice in the RA→RA group (Fig. 5, B and C). Weight loss and survival in these experiments may seem slightly discordant. However, weight loss during influenza virus infection is not necessarily indicative of disease outcome, as weight loss is often not accurately predictive of survival (47). Overall, these data suggest that exposure to a hyperoxic environment at birth may have local and systemic effects.
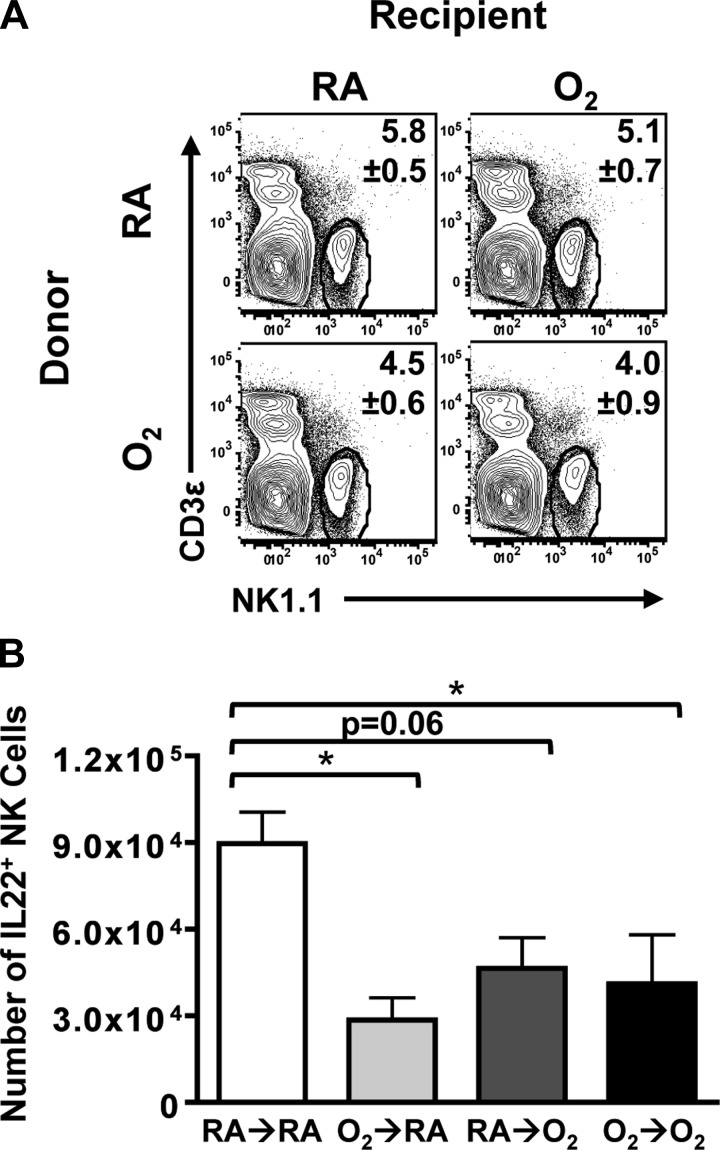
After infection, there was no discernible difference in the percentage (Fig. 6A) and number (data not shown) of CD45.1+ NK cells in the lungs among all four groups. Similar to the difference observed when intact adult mice exposed to O2 and RA mice at birth are infected, O2-exposed hosts that received bone marrow from O2-exposed donors (O2→O2) displayed a significant decrease in the number of IL-22+ NK cells compared with infected RA→RA mice (Fig. 6B). Likewise, O2-exposed hosts that received RA bone marrow cells (RA→O2) exhibited fewer IL22+ NK cells in the infected lung. This is consistent with a role for the altered lung as a factor that influences the altered immune response to infection. Yet, oxygen exposure of the donor bone marrow cells (O2→RA) was also sufficient to diminish the number of IL-22+ NK cells in the infected lung (Fig. 6B). These observations suggest that neonatal oxygen supplementation influences inherent properties of hematopoietic cells as well as changing extra-hematopoietic environments, such as the lung.
Fig. 6.
Decreased number of IL22+ NK cells can be attributed to oxygen exposure of hematopoietic cells or nonhematopoietic cells. A and B: percentage and number of CD45.1+ IL-22+ NK cells in reciprocal bone marrow chimera mice at day 3 postinfection. Data are shown as means ± SE with 5–7 mice per group from 1 experiment that is representative of 2 independent experiments. *P ≤ 0.05, Tukey-Kramer HSD.
DISCUSSION
Supplemental oxygen treatment, along with other clinical interventions, has increased the survival of preterm infants and resulted in a shift in the window of viability to include neonates born as early as 22 wk of gestation (11, 43, 51). Despite this improved survival, being born too soon in conjunction with life-saving medical procedures leads to persistent sequelae, including increased incidence and severity of respiratory infections (41). Therefore, it is critical to gain a clearer understanding of the mechanisms that cause permanent changes. It is well established that neonatal oxygen supplementation changes the lung epithelium; however, it is important to further define the extent to which organ systems outside the lung may be affected (35). Mouse models of neonatal hyperoxia reveal altered responses to respiratory viral infection, potentially mirroring the respiratory morbidity seen in infected children born preterm (26, 34, 36). Prior studies examined the contribution of modified adaptive immune responses to this altered disease outcome and found that CD8+ T cells expanded and differentiated normally in response to infection (16, 34). Similarly, no impairment was observed in CD4+ T-cell responses following infection or after sensitization and challenge with ovalbumin (34, 38). Therefore, this study focused on whether early-life oxygen exposure affected innate immune cells and specifically NK cells, because NK cells are important antiviral mediators, which release cytokines that shape host response to viral infection (7). We interrogated whether neonatal oxygen supplementation altered NK cell accumulation or phenotype in the lungs of mice. Additionally, we determined whether supplemental oxygen at birth could directly act upon the hematopoietic compartment, leading to persistent changes in the response of NK cells upon infection.
In this study, we show that NK cells from adult mice exposed to supplemental oxygen at birth display a propensity toward classical effector NK cell responses, such as expression of IFN-γ and granzyme B. Moreover, the frequency of IL22+ NK cells, which are considered NK cells with a tissue reparative function, is decreased. Previous reports showed that increased mortality after influenza A virus infection was not due to changes in viral titers and viral clearance or defects in the control response. However, in contrast to RA controls, surviving mice that had been exposed to neonatal hyperoxia exhibit pulmonary fibrosis suggesting an altered repair process in the lung (8, 34). IL-22 is critical for promoting growth and regeneration of a healthy epithelium (29, 42). Although it is better studied in the digestive tract, where it maintains the intestinal barrier, IL-22 has recently been shown to play an important role in the lung, as absence of this cytokine leads to extensive fibrosis upon viral infection (19, 29, 53). In addition to pathophysiological consequences of the complete absence of IL-22, decreased IL-22 levels in the lung exacerbate pathology in bleomycin-induced fibrosis (30). Also, despite critical roles of NK cell antiviral function, excessive NK cell cytotoxicity can promote pathology (1). Therefore, the observed decrease in IL22+ NK cells, working in concert with the increase in effector NK cells, could contribute to poorer lung repair processes and to the exacerbated postinfection fibrosis that has been observed in lungs of infected adult mice that had received neonatal oxygen supplementation (8, 9, 34).
In addition to pulmonary health effects, preterm infants who receive oxygen therapy display impeded growth, neurodevelopmental delays, and develop retinopathy of prematurity, indicating that neonatal supplemental oxygen has pathophysiological effects outside of the lung (22, 37, 39, 40). How early life supplemental oxygen affects these systems is not fully understood. Yet, the mechanisms leading to these negative health outcomes likely differ among the affected specific organ systems. There are several possible mechanisms that underlie the observed changes in NK cells. One possibility is that the NK cells themselves are unchanged but that they receive altered signals from other immune cells and from lung epithelial cells. Consistent with this interpretation, NK cells in mice exposed to supplemental oxygen at birth appear to be similar to NK cells in unexposed mice initially. Moreover, no overt differences in hematopoietic lineage precursors were observed. However, as infection progresses, the proportion of NK cell subsets in mice exposed to neonatal oxygen supplementation is altered, such that there is a decrease in IL-22+ NK cells and an increased proportion of IFN-γ+ NK cells. A simple explanation is that NK cells are responding to reduced levels of the cytokines that elicit IL-22 production, such as IL-23; however, our findings thus far do not support this being the critical mechanism driving these outcomes. Rather, there may be a combination of mechanisms at play, resulting in changes in the recruitment, retention, or elimination of NK cell subpopulations in the lung. Alternatively, NK cells in mice exposed to neonatal hyperoxia could have inherently different properties due to reprogramming of this lineage, which leads to altered responses to viral infection. Developmental exposure with other agents has been shown to alter the functional capacity of bone marrow progenitors; therefore, it is possible that NK cell precursors could be modified by supplemental oxygen exposure at birth but that the consequences of are not observed until they are activated by another insult, such as viral infection (2).
Another possible explanation for the results is that NK cells are not inherently changed but that other hematopoietic cells may be affected by neonatal oxygen supplementation. NK cells receive cues from various other cells during immune responses, specifically dendritic cells. Therefore, the idea that systemic inflammation and the release of growth factors or chemokines can induce changes in immune progenitor cell mobilization or motility in the bone marrow, and may even regulate HSC function directly, may be a contributing factor to the observed outcomes (3, 14). As one set of examples, granulocyte colony-stimulating factor (GCSF) and CC chemokine receptor 2 (CCR2) signaling are required for release of neutrophil and monocyte progenitors, respectively (27, 49). Additionally, tumor necrosis factor-α (TNF-α) has been shown to modulate proliferation of HSCs (23). Since neonatal hyperoxia can induce the production of inflammatory mediators including TNF-α and IL-6, it is possible that neonatal hyperoxia could induce systemic changes within the bone marrow compartment that may not cause changes in the number of progenitor cells but may lead to altered programming of progenitor populations and changes in their function (5). High levels of oxygen in conjunction with the local structural changes caused by neonatal oxygen supplementation could also alter the normal commensal environment of the lung. Although the examination of the lung microflora is a relatively new field of study, its role is being characterized in a number of disease states such as cystic fibrosis, allergic airway disease, and bronchopulmonary dysplasia (4, 18, 20, 44). Extensive research is also underway to understand how changes in the microbiota of the gut alter both local and systemic inflammatory conditions, including changes in lung immunity (33, 48). Therefore, it is possible that alterations to the microbiota of the lung and digestive tract could have systemic effects and promote prolonged changes in local immunity.
In summary, this study demonstrates that neonatal oxygen supplementation alters the response of NK cells to respiratory viral infection, such that there is an increased frequency of IFN-γ+ NK cells and fewer IL22+ NK cells in the lung. This work also reveals that early life oxygen exposure affects not only the lung but durably changes NK cells. Developing new therapies for reducing respiratory morbidity in children born preterm will therefore require a better understanding of how early-life oxygen exposures disrupts both lung development and hematopoietic cells involved in innate and adaptive immunity.
GRANTS
This work was supported by National Institutes of Health Research and Training Grants R01-HL-097141, R01-ES-017250, R01-ES-023260, R01-HL-091968, T32-ES-07026, and P30-ES-01247.
DISCLOSURES
The authors of this paper declare no financial or scientific conflicts of interest or any other competing interests.
AUTHOR CONTRIBUTIONS
Author contributions: E.C.R., K.C.M., G.-b.J., and M.Y. performed experiments; E.C.R., K.C.M., and G.-b.J. analyzed data; E.C.R. and B.P.L. interpreted results of experiments; E.C.R. prepared figures; E.C.R. drafted manuscript; E.C.R., M.A.O., and B.P.L. edited and revised manuscript; E.C.R., K.C.M., G.-b.J., M.Y., M.A.O., and B.P.L. approved final version of manuscript; M.A.O. and B.P.L. conception and design of research.
ACKNOWLEDGMENTS
Present address of G.-b. Jin: Department of Preventive Medicine, College of Medicine Yanbian University, Yanji City, Jilin Province, China.
REFERENCES
- 1.Abdul-Careem MF, Mian MF, Yue G, Gillgrass A, Chenoweth MJ, Barra NG, Chew MV, Chan T, Al-Garawi AA, Jordana M, Ashkar AA. Critical role of natural killer cells in lung immunopathology during influenza infection in mice. J Infect Dis 206: 167–177, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Ahrenhoerster LS, Tate ER, Lakatos PA, Wang X, Laiosa MD. Developmental exposure to 2,3,7,8 tetrachlorodibenzo-p-dioxin attenuates capacity of hematopoietic stem cells to undergo lymphocyte differentiation. Toxicol Appl Pharmacol 277: 172–182, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol 32: 57–65, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beeton ML, Maxwell NC, Davies PL, Nuttall D, McGreal E, Chakraborty M, Spiller OB, Kotecha S. Role of pulmonary infection in the development of chronic lung disease of prematurity. Eur Respir J 37: 1424–1430, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Ari J, Makhoul IR, Dorio RJ, Buckley S, Warburton D, Walker SM. Cytokine response during hyperoxia: sequential production of pulmonary tumor necrosis factor and interleukin-6 in neonatal rats. Isr Med Assoc J 2: 365–369, 2000. [PubMed] [Google Scholar]
- 6.Bender BS, Croghan T, Zhang L, Small PA. Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med 175: 1143–1145, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol 17: 189–220, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Buczynski BW, Yee M, Martin KC, Lawrence BP, O'Reilly MA. Neonatal hyperoxia alters the host response to influenza A virus infection in adult mice through multiple pathways. Am J Physiol Lung Cell Mol Physiol 305: L282–L290, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buczynski BW, Yee M, Paige Lawrence B, O'Reilly MA. Lung development and the host response to influenza A virus are altered by different doses of neonatal oxygen in mice. Am J Physiol Lung Cell Mol Physiol 302: L1078–L1087, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chien YH, Tsao PN, Chou HC, Tang JR, Tsou KI. Rehospitalization of extremely-low-birth-weight infants in first 2 years of life. Early Hum Dev 66: 33–40, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR. The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics 106: 659–671, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Dauger S, Ferkdadji L, Saumon G, Vardon G, Peuchmaur M, Gaultier C, Gallego J. Neonatal exposure to 65% oxygen durably impairs lung architecture and breathing pattern in adult mice. Chest 123: 530–538, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Eber E, Zach MS. Long term sequelae of bronchopulmonary dysplasia (chronic lung disease of infancy). Thorax 56: 317–323, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 458: 904–908, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, Zakay-Rones Z, Porgador A, Mandelboim O. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol 7: 517–523, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Giannandrea M, Yee M, O'Reilly MA, Lawrence BP. Memory CD8+ T cells are sufficient to alleviate impaired host resistance to influenza A virus infection caused by neonatal oxygen supplementation. Clin Vaccine Immunol 19: 1432–1441, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasner A, Zurunic A, Meningher T, Lenac Rovis T, Tsukerman P, Bar-On Y, Yamin R, Meyers AF, Mandeboim M, Jonjic S, Mandelboim O. Elucidating the mechanisms of influenza virus recognition by Ncr1. PLoS One 7: e36837, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, Nicod LP, Lloyd CM, Marsland BJ. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med 20: 642–647, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Guo H, Topham DJ. Interleukin-22 (IL-22) production by pulmonary natural killer cells and the potential role of IL-22 during primary influenza virus infection. J Virol 84: 7750–7759, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guss AM, Roeselers G, Newton ILG, Young CR, Klepac-Ceraj V, Lory S, Cavanaugh CM. Phylogenetic and metabolic diversity of bacteria associated with cystic fibrosis. ISME J 5: 20–29, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hack M, Weissman B, Breslau N, Klein N, Borawski-Clark E, Fanaroff AA. Health of very low birth weight children during their first eight years. J Pediatr 122: 887–892, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Hack M. Catch-up growth during childhood among very low-birth-weight children. Arch Pediatr Adolesc Med 150: 1122–1129, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsen SE, Ruscetti FW, Dubois CM, Keller JR. Tumor necrosis factor alpha directly and indirectly regulates hematopoietic progenitor cell proliferation: role of colony-stimulating factor receptor modulation. J Exp Med 175: 1759–1772, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163: 1723–1729, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 46: 641–643, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Kitchen WH, Ford GW, Doyle LW, Rickards AL, Kelly EA. Health and hospital readmissions of very-low-birth-weight and normal-birth-weight children. Am J Dis Child 144: 213–218, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Köhler A, De Filippo K, Hasenberg M, van den Brandt C, Nye E, Hosking MP, Lane TE, Männ L, Ransohoff RM, Hauser AE, Winter O, Schraven B, Geiger H, Hogg N, Gunzer M. G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood 117: 4349–4357, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroon AA, Delriccio V, Tseu I, Kavanagh BP, Post M. Mechanical ventilation-induced apoptosis in newborn rat lung is mediated via FasL/Fas pathway. Am J Physiol Lung Cell Mol Physiol 305: L795–L804, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol 6: 69–82, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang M, Wang J, Chu H, Zhu X, He H, Liu Q, Qiu J, Zhou X, Guan M, Xue Y, Chen X, Zou H. Interleukin-22 inhibits bleomycin-induced pulmonary fibrosis. Mediators Inflamm 2013: 209179, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madurga A, Mizíková I, Ruiz-Camp J, Morty RE. Recent advances in late lung development and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 305: L893–L905, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Mascaretti RS, Mataloun MM, Dolhnikoff M, Rebello CM. Lung morphometry, collagen and elastin content: changes after hyperoxic exposure in preterm rabbits. Clinics (Sao Paulo) 64: 1099–1104, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun 72: 4996–5003, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Reilly MA, Marr SH, Yee M, McGrath-Morrow SA, Lawrence BP. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med 177: 1103–1110, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Reilly MA, Yee M, Buczynski BW, Vitiello PF, Keng PC, Welle SL, Finkelstein JN, Dean DA, Lawrence BP. Neonatal oxygen increases sensitivity to influenza A virus infection in adult mice by suppressing epithelial expression of Ear1. Am J Pathol 181: 441–451, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen O, Herskind AM, Kamper J, Nielsen JP, Kristensen K. Rehospitalization for respiratory syncytial virus infection in infants with extremely low gestational age or birthweight in Denmark. Acta Paediatr 92: 240–242, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Peralta-Carcelen M, Jackson DS, Goran MI, Royal SA, Mayo MS, Nelson KG. Growth of adolescents who were born at extremely low birth weight without major disability. J Pediatr 136: 633–640, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Regal JF, Lawrence BP, Johnson AC, Lojovich SJ, O'Reilly MA. Neonatal oxygen exposure alters airway hyper-responsiveness but not the response to allergen challenge in adult mice. Pediatr Allergy Immunol 25: 180–186, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saugstad OD. Oxygen and retinopathy of prematurity. J Perinatol 26: S46–S50, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz T, Ritter J, Mueller S, Felderhoff-Mueser U, Chew LJ, Gallo V. Cellular changes underlying hyperoxia-induced delay of white matter development. J Neurosci 31: 4327–4344, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith VC, Zupancic J, McCormick MC. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr 144: 799–803, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol 12: 383–390, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O'Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, Watterberg KL, Saha S, Das A, Higgins RD; Eunice Kennedy Shriver National Institute of Child Health, and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126: 443–456, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stressmann FA, Connett GJ, Goss K, Kollamparambil TG, Patel N, Payne MS, Puddy V, Legg J, Bruce KD, Rogers GB. The use of culture-independent tools to characterize bacteria in endo-tracheal aspirates from preterm infants at risk of bronchopulmonary dysplasia. J Perinat Med 38: 333–337, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Thébaud B, Lacaze-Masmonteil T, Watterberg K. Postnatal glucocorticoids in very preterm infants: “the good, the bad, and the ugly”? Pediatrics 107: 413–415, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol 159: 5197–5200, 1997. [PubMed] [Google Scholar]
- 47.Trammell RA, Toth LA. Markers for predicting death as an outcome for mice used in infectious disease research. Comp Med 61: 492–498, 2011. [PMC free article] [PubMed] [Google Scholar]
- 48.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20: 159–166, 2014. [DOI] [PubMed] [Google Scholar]
- 49.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 117: 902–909, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics 115: 997–1003, 2005. [DOI] [PubMed] [Google Scholar]
- 51.World Health Organization. Born too Soon: the Global Action Report on Preterm Birth. Geneva, Switzerland: World Health Organization, 2012. [Google Scholar]
- 52.Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright TW, McGrath-Morrow SA, Maniscalco WM, Finkelstein JN, O'Reilly MA. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol 291: L1101–L1111, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29: 947–957, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]