Background: Mitochondrial function is dependent on mitochondrial fission and fusion dynamics.
Results: Calpain-mediated degradation of MFN2 is responsible for glutamate-induced mitochondrial dysfunction and neuronal death in spinal cord motor neurons.
Conclusion: Calpain-mediated MFN2 degradation is a novel mechanism regulating mitochondrial fusion during glutamate excitotoxicity.
Significance: MFN2 might be a novel therapeutic target against glutamate excitotoxicity in motor neurons.
Keywords: Calpain, Excitotoxicity, Glutamate, Mitochondria, Neurodegeneration, MFN2, Glutamate Excitotoxicity, Mitochondrial Dynamics, Motor Neuron
Abstract
Mitochondrial dysfunction plays a central role in glutamate-evoked neuronal excitotoxicity, and mitochondrial fission/fusion dynamics are essential for mitochondrial morphology and function. Here, we establish a novel mechanistic linker among glutamate excitotoxicity, mitochondrial dynamics, and mitochondrial dysfunction in spinal cord motor neurons. Ca2+-dependent activation of the cysteine protease calpain in response to glutamate results in the degradation of a key mitochondrial outer membrane fusion regulator, mitofusin 2 (MFN2), and leads to MFN2-mediated mitochondrial fragmentation preceding glutamate-induced neuronal death. MFN2 deficiency impairs mitochondrial function, induces motor neuronal death, and renders motor neurons vulnerable to glutamate excitotoxicity. Conversely, MFN2 overexpression blocks glutamate-induced mitochondrial fragmentation, mitochondrial dysfunction, and/or neuronal death in spinal cord motor neurons both in vitro and in mice. The inhibition of calpain activation also alleviates glutamate-induced excitotoxicity of mitochondria and neurons. Overall, these results suggest that glutamate excitotoxicity causes mitochondrial dysfunction by impairing mitochondrial dynamics via calpain-mediated MFN2 degradation in motor neurons and thus present a molecular mechanism coupling glutamate excitotoxicity and mitochondrial dysfunction.
Introduction
l-Glutamate is the major excitatory neurotransmitter in the central nervous system (1). Excessive stimulation or overactivation of neurons by glutamate causes neuronal death, a pathological process referred to as glutamate excitotoxicity (2, 3). It has been proposed that glutamate excitotoxicity is an important pathological mechanism in a wide range of neurological disorders, including cerebral ischemia, stroke, brain trauma, and even neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS),3 Parkinson disease, and Huntington disease (4–6). The detailed mechanisms underlying glutamate excitotoxicity-induced neuronal death are not fully elucidated. However, mitochondrial dysfunction, including mitochondrial membrane potential (MMP) reduction and ROS overproduction, was reported as a primary event in glutamate-induced neurotoxicity (7, 8).
Mitochondria are highly dynamic organelles that undergo continuous fission/fusion events that play a critical role in maintaining mitochondrial homeostasis (9). Unopposed fission leads to fragmentation whereas unopposed fusion causes elongation, both of which could impair mitochondrial function (10, 11). Mitochondrial fission and fusion events are tightly regulated by mitochondrial fission and fusion proteins as follows: dynamin-like protein 1 (DLP1, also referred to as DRP1) and its recruiting factors on mitochondria such as FIS1, MFF, MID49, and MID51 for fission (12); and mitofusin 1 (MFN1), mitofusin 2 (MFN2), and optic atrophy protein 1 (OPA1) for fusion (13). In addition to regulating mitochondrial morphology, mitochondrial fission and fusion processes are essential for the maintenance of various aspects of mitochondrial function (14). Thus, not surprisingly, emerging studies suggest the altered balance in mitochondrial fission and fusion might be a potential mechanism leading to mitochondrial dysfunction in neurological disorders (15–17).
Around 40–75% of ALS cases demonstrated defects in glutamate transport and elevated extracellular glutamate levels in spinal cord, and abundant evidence suggests an important role of glutamate excitotoxicity in motor neuron degeneration (18–20). Because mitochondrial dysfunction was reported as a primary event in glutamate-induced neurotoxicity, and mitochondrial function is highly dependent on mitochondrial dynamics, here we sought to investigate whether and how mitochondrial dynamics play a role in glutamate-induced mitochondrial dysfunction and neuronal death in spinal cord motor neurons both in vitro and in mice.
MATERIALS AND METHODS
Embryonic Primary Motor/Cortical Neuron Isolation, Culture, and Transfection
MFN2 floxed male and female mice were the kind gifts of Dr. David Chan (California Institute of Technology). MFN2 transgenic male and female mice were generated by pronuclear injection of the Thy1-MFN2 transgene into C57BL/6N fertilized eggs using the murine Thy-1.2 genomic expression cassette (gift of Dr. Philip C. Wong, Johns Hopkins University). Timed pregnant Sprague-Dawley female rats (Harlan or Charles River) or C57BL/6N female mice were sacrificed following the protocol approved by the Institutional Animal Care and Use Committee (IACUC) at Case Western Reserve University. Primary motor neurons were isolated from E13 to E15 female rat/E12 to E14 female mouse embryos as we described before (21). Primary cortical neurons were isolated from E18 female rats as described previously (22) but with some modifications.
Expression Vectors, Antibodies, Chemicals, and Measurements
MitoDsRed2 (Clontech), Case12 construct (Evrogen, Moscow, Russia), GFP-Cre (Addgene, Cambridge, MA), and GFP/Myc-tagged MFN2 (gift of Dr. Margaret T. Fuller, Stanford University) were obtained. MitoDsRed2/Cre dual promoter regular construct was generated by replacing the neomycin/kanamycin resistance gene in MitoDsRed2 construct with sequence of recombinase Cre with nuclear localization signal (NLS-Cre from GFP-Cre construct). MitoDsRed2/Cre dual promoter lentiviral construct was generated by cloning mitoDsRed2 into pLVX-Puro (Clontech) and replacing puromycin resistance gene with NLS-Cre, whereas the Cre lentiviral construct was generated by cloning NLS-Cre into pLVX-Puro. Lentivirus were generated using the third generation packaging system (packaging plasmid, pMDLg/pRRE and pRSV-Rev; envelope plasmid, pMD2.G from Addgene, Cambridge MA) as described previously (23). The primary antibodies used included the following: mouse anti-VDAC1/rabbit anti-Mff/Spectrin (Abcam, Cambridge, MA); rabbit anti-calpain 1, calpain 2, and cleaved caspase3 (Cell Signaling, Danvers, MA); rabbit anti-Mfn1/mouse anti-Mfn2 (Santa Cruz Biotechnology, Dallas, TX); mouse anti-HB9 (DSHB, Iowa City, IA); mouse anti-glial fibrillary acidic protein (Invitrogen); rabbit anti-Iba1 (Wako, Richmond, VA); mouse anti-DLP1/OPA1/Tom20 (BD Biosciences); and mouse anti-actin/rabbit anti-MAP2 (Millipore, Billerica, MA). Glutamate (Sigma) and MK-801/calpeptin/BAPTA-AM/benzyloxycarbonyl-VAD-fluoromethyl ketone (Tocris, Minneapolis, MN) were also obtained. ATP levels were measured by the ATP colorimetric/fluorometric assay kit (Biovision, Milpitas, CA). The ROS level and mitochondrial membrane potential were measured as described before (24). Real time measurement of oxygen consumption rate (OCR) in live cultured motor neurons with optimal seeding density (60,000 cells/well) was measured using the Seahorse XF24 Analyzer (Seahorse Bioscience, North Billerica, MA) according to the manufacturer's instruction. ATP synthase inhibitor oligomycin (1 μm), uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone (4 μm), and complex I inhibitors antimycin A (1 μm) and rotenone (1 μm) were injected at the indicated times. After measurements, cells were lysed, and OCR data were normalized by total protein. Cell death and viability was measured by the cytotoxicity detection kit (LDH; Roche Applied Science) or the cell proliferation kit (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Roche Applied Science). Neuronal viability propidium iodide assay in positively transfected neurons was performed as we described (25). Neurons with propidium iodide-positive nuclei and/or obvious fragmented nuclear/neurites were counted as nonviable neurons, whereas neurons without propidium iodide-positive nuclei and clear nuclear contour/neurites were counted as viable neurons.
Mitochondrial Isolation and Western Blot Analysis
Crude and purified mitochondria were isolated as described before (21). Purified mitochondria from cells were lysed with 1× cell lysis buffer (Cell Signaling, Danvers, MA) plus 1 mm PMSF (Sigma) and protease inhibitor mixture (Sigma). Equal amounts of total protein extract were resolved by SDS-PAGE and transferred to Immobilon-P (Millipore, Billerica, MA). Following blocking with 10% nonfat dry milk, primary and secondary antibodies were applied as described previously (24), and the blots were developed with Immobilon Western Chemiluminescent HRP substrate (Millipore, Billerica, MA).
Animal Surgery and Glutamate Infusion
Mouse surgery/procedures were performed according to the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) at Case Western Reserve University. One day before implantation, mini-osmotic pumps (model 2001, Alzet, Cupertino, CA; flow rate of 1 μl/h) and brain infusion cannulae attached with 2-cm catheter tubes (brain infusion kit 3, Alzet, Cupertino, CA) were filled with artificial cerebrospinal fluid (aCSF) (124 mm NaCl, 25 mm NaHCO3, 10 mm d-glucose, 2.5 mm KCl, 1 mm MgCl2, 2 mm CaCl2, and 1 mm NaH2PO4, adjusted to pH 7.2–7.4 using NaOH) or aCSF containing 10 mm glutamate followed by pump incubation in phosphate-buffered saline (PBS) at 37 °C overnight according to the manufacturer's instructions. For stereotaxic surgery, male mice were anesthetized with avertin and placed on a stereotaxic frame. A small incision was first made to expose skull and bregma, and both the catheter and the connected mini-osmotic pump were implanted subcutaneously. A hole was drilled in the skull (relative to bregma, anterior-posterior −0.2 mm; medial-lateral, 1 mm), and the cannula was positioned 2 mm above the lateral ventricle. Another two holes were drilled by the edge of cannula, and self-tapping bone screws (MD-1310, BASi, West Lafayette, IN) were screwed into the holes. The cannulae and screws were finally secured by cement. 7 days after surgery, male mice were deeply anesthetized with avertin and transcardially perfused with cold PBS, and the spinal cord/brain were collected for further analysis.
Immunocytochemistry and Immunofluorescence of Mouse Spinal Cord
To investigate the mitochondrial morphology in motor neurons in male mice, we also developed an optimized immunofluorescence-based imaging technique that could directly visualize individual filamentous mitochondria in paraffin-embedded buffered formalin-fixed spinal cord tissues using specific antibodies against mitochondrial protein Tom20 or VDAC1. Taken briefly, deparaffinized and re-hydrated tissue sections were washed briefly three times with distilled H2O and placed in 1× antigen decloaker (Biocare, Concord, CA). The sections were then subjected to antigen retrieval under pressure using Biocare's Decloaking Chamber by heating to 125 °C for 10 s and cooling to 90 °C for 30 s followed by heating to 22 p.s.i. at 128 °C and cooling to 0 p.s.i. at 94 °C. After the temperature decreased to 30 °C, the sections were gradually rinsed with distilled H2O five times. The sections were then blocked with 10% normal goat serum (NGS) (Sigma) for 30 min at room temperature and incubated with primary antibodies in PBS containing 1% NGS overnight at 4 °C. After three washes with PBS, the sections were incubated in 10% NGS for 10 min and then with Alexa Fluor-conjugated secondary antibody (Invitrogen) (1:300) for 2 h at room temperature in the dark. Finally, the sections were rinsed three times with PBS, stained with DAPI, washed again with PBS three times, and mounted with Fluoromount-G mounting medium (Southern Biotech, Birmingham, AL).
Electron Microscopy of Mouse Spinal Cord
Male mice were transcardially perfused with EM fixative (quarter-strength Karnovsky, 1.25% DMSO mixture) for 5 min, and the spinal cords were removed quickly and placed in EM fixative solution for an additional 15 min at room temperature followed by additional fixation in fresh EM fixative for another 2 h at room temperature. After washing, tissue blocks were postfixed in 1% osmium, 1.25% ferrocyanide mixture for two changes of solution of 1 h each (total postfixation time is 2 h) at room temperature. Then the specimens were rinsed and soaked overnight in acidified 0.5% uranyl acetate. After another wash, the blocks were dehydrated in ascending concentrations of ethanol, passed through propylene oxide, and embedded in Poly/Bed 812 embedding resin (Polysciences, Warrington, PA). Thin sections were sequentially stained with 2% acidified uranyl acetate followed by Sato's triple lead staining as modified by Hanaichi et al. (26) and examined in an FEI Tecnai T12 electron microscope equipped with a Gatan single tilt holder and a Gatan US4000 4kx4k CCD camera (Gatan, Pleasanton, CA).
In Vitro Calpain Cleavage Assay
100 μg of purified mitochondria or 1 μg of recombinant Mfn2 protein (Origene, Rockville, MD) were incubated with purified human erythrocyte μ-calpain (calpain-I) (Biovision, Milpitas, CA) at 30 °C for 30 min according to the manufacturer's instructions. For inhibition, calpain-I was preincubated with PMSF or calpeptin for 5 min at 30 °C. The reaction was stopped by the addition of SDS sample buffer containing 62.5 mm Tris-HCl, 4% SDS, 10% glycerol, 50 mm DTT, and 0.1% bromphenol blue (pH 6.8). The samples were boiled at 100 °C for 10 min. 1/6 of reaction volume was loaded into SDS-polyacrylamide gel for Western blot.
Time-lapse Imaging and Confocal Microscopy
For time-lapse imaging, neurons were seeded in 35-mm dishes and transfected/co-transfected with mito-DsRed2, GFP-Mfn2, and Case12. 48 h after transfection, neurons were placed in a well equipped environmental chamber with controlled CO2 content, humidity, and temperature and imaged at 37 °C by an inverted Leica DMI6000 fluorescence microscope (Leica, Buffalo Grove, IL) (controlled through Leica LAS AF 3 software) with a 20X/0.7NA Plan Apochromat dry objective. During time-lapse imaging, frames were captured every 1 min for at least 6 h without phototoxicity or photobleaching. For confocal microscopy, neurons cultured on coverslips were fixed and stained as we described previously (24). All other fluorescence images were captured at room temperature with a Zeiss LSM 510 inverted laser-scanning confocal fluorescence microscope (controlled through Zeiss LSM 510 confocal software, Zeiss, Peabody, MA) equipped with a C-Apochromat 40×/1.2-watt water objective or αPlan-Fluar 100×/1.45 oil objective as described previously (24). Confocal images of far-red fluorescence were collected using 633 nm excitation light from a HeNe laser and a 650-nm long pass filter; images of red fluorescence were collected using 543-nm excitation light from an argon laser and a 560-nm long pass filter; and green fluorescence images were collected using 488 nm excitation light from an argon laser and a 500–550-nm bandpass barrier filter.
Image Analysis
Morphology of mitochondria was quantified as we described previously (21). All single plane or series of z-stacks of raw images were background-corrected, linearly contrast-optimized, applied with a 7 × 7-nm “top hat” filter, subjected to a 3 × 3-nm median filter, and then thresholded to generate binary images by ImageJ. Most mitochondria were well separated in binary images, and large clusters of mitochondria were excluded automatically. All binary images were either directly analyzed by ImageJ, or assembled into three-dimensional volumes and quantified by Imaris 7.2 (Bitplane, South Windsor, CT).
RESULTS
Glutamate Induces Mitochondrial Fragmentation in Spinal Cord Motor Neurons
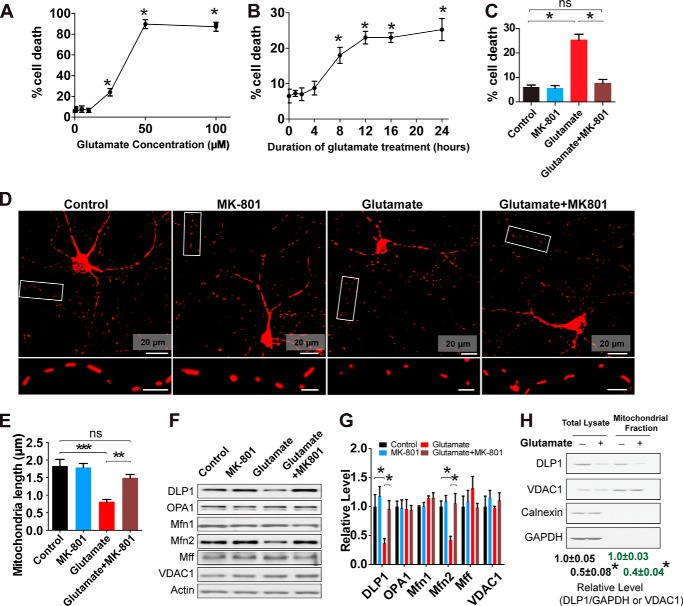
We first investigated the effect of glutamate exposure on mitochondrial morphology in cultured rat primary embryonic spinal cord motor neurons. Consistent with previous studies (27), glutamate treatment caused a dose- and time-dependent increase of cell death in motor neurons, which could be blocked by glutamate receptor blocker MK-801 (Fig. 1, A–C). To examine the potential effect of glutamate on mitochondrial morphology, primary motor neurons (DIV5) were first transfected with mitoDsRed2 and then treated with a sublethal dose of glutamate (25 μm) for 24 h at DIV7. Treatment with glutamate resulted in significant mitochondrial fragmentation (Fig. 1, D and E). In accordance with previous studies (28, 29), the glutamate-induced mitochondrial fragmentation as well as neuronal death were completely prevented by MK-801 (Fig. 1, D and E).
FIGURE 1.
Glutamate induces mitochondrial fragmentation and reduction of DLP1 and MFN2 in primary spinal cord motor neurons. Primary rat spinal cord motor neurons (DIV7) were treated with or without 25 μm glutamate and/or glutamate receptor antagonist MK-801 for 24 h. To label mitochondria, neurons were transfected with mitoDsRed2 2 days before glutamate treatment. A, cell death determined by LDH release assay in primary rat spinal cord motor (DIV7) treated with different concentrations of glutamate for 24 h. B, cell death determined by LDH release assay in primary rat spinal cord motor neurons (DIV7) treated with 25 μm glutamate for different time points. C, cell death determined by LDH release assay in primary rat spinal cord motor neurons (DIV7) co-treated with 25 μm glutamate and/or glutamate receptor antagonist MK-801 for 24 h. Representative confocal images (D) and quantification (E) of mitochondrial length in mitoDsRed2 positively transfected primary rat spinal cord motor neurons (DIV8). Inset scale bar, 5 μm. More than 50 neurons were analyzed per group. Representative immunoblot (F) and quantification analysis (G) of the expression of mitochondrial fusion protein MFN2 (relative to actin) are shown. Equal protein amounts (10 μg) were loaded and confirmed by actin. H, representative immunoblot and quantification of the expression of DLP1 in mitochondria isolated from neurons (DIV7) treated with glutamate for 24 h. Equal protein amounts (20 μg) were loaded and confirmed by VDAC1. Cytosolic marker GAPDH and endoplasmic reticulum marker calnexin confirmed the purity of prepared mitochondrial fraction. All experiments were repeated more than four times. Data are means ± S.E. Statistics: one-way analysis of variance (ANOVA) was followed by Tukey's multiple comparison test. A and B, *, p < 0.05, compared with control neurons without treatment. For other panels, *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, nonsignificant.
We further sought to determine whether glutamate-induced mitochondrial fragmentation was due to changes in the expression of mitochondrial fission and fusion proteins in motor neurons. Interestingly, glutamate treatment caused a significant and specific decrease of the expression of DLP1 and Mfn2 in motor neurons, although it had no effect on the expression of OPA1, Mfn1, and Mff (Fig. 1, F and G). As expected, the glutamate-induced DLP1/Mfn2 reduction could be completely blocked by MK-801. DLP1 is a cytosolic protein, and its translocation from cytosol to mitochondria mediates mitochondrial fission (30). We therefore measured the level of DLP1 in mitochondrial fraction and also found a similar and significant decrease of mitochondrial DLP1 in glutamate-treated neurons (Fig. 1H).
MFN2 Deficiency Elicits Mitochondrial and Neuronal Toxicity in Spinal Cord Motor Neurons
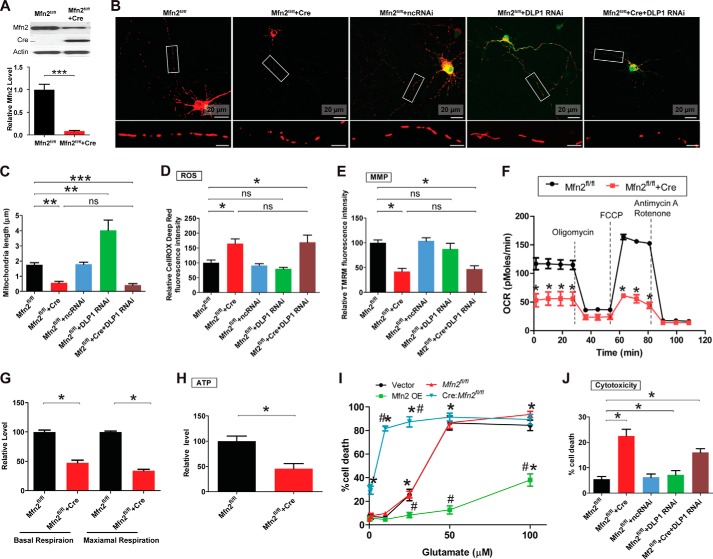
Because both DLP1 and MFN2 were reduced by glutamate treatment, we next examined the impact of DLP1 and MFN2 deficiency on mitochondrial dynamics and mitochondrial/neuronal function in in vitro cultured primary mouse spinal cord motor neurons. To measure the effect of MFN2 deficiency on mitochondrial morphology, we used mouse primary motor neurons (Mfn2fl/fl neurons) isolated from Mfn2 floxed mice (31). Mfn2fl/fl motor neurons (DIV4) were transfected with dual promoter constructs encoding both mitoDsRed2 and Cre to genetically label mitochondria and Mfn2 knock-out genes simultaneously. Cre-mediated ablation of Mfn2 in Mfn2fl/fl motor neurons was confirmed by Western blot (Fig. 2A). As expected, Mfn2fl/fl motor neurons positively transfected with constructs encoding mitoDsRed2 only demonstrated normal tubular mitochondrial morphology with an average length of around 1.8 μm, whereas Mfn2fl/fl neurons positively transfected with constructs encoding both Cre and MitoDsRed2 showed significant reduction of the average mitochondrial length, suggestive of enhanced mitochondrial fragmentation (Fig. 2, B and C). To investigate the effect of DLP1 deficiency on mitochondrial morphology, primary motor neurons (DIV4) were co-transfected with mitoDsRed2 and a GFP-tagged RNAi construct targeting DLP1 as we described before (24). The down-regulation of DLP1 by RNAi in spinal cord motor neurons resulted in a significant increase of mitochondrial length (Fig. 2, B and C). Mfn2fl/fl motor neurons were also co-transfected with DLP1 RNAi construct and the dual promoter construct encoding both mitoDsRed2 and Cre to reduce the expression of both DLP1 and MFN2, in a condition mimicking mitochondrial dynamic changes in neurons treated with glutamate. As expected, mitochondria in spinal cord motor neurons remained dominantly fragmented when the expressions of DLP1 and MFN2 were both reduced (Fig. 2, B and C).
FIGURE 2.
MFN2 deficiency impairs mitochondrial dynamics and function in primary spinal cord motor neurons. To generate MFN2 deficiency neurons, primary mouse motor neurons (Mfn2fl/fl neurons) isolated from Mfn2 floxed mice were transfected with Cre only, GFP-Cre, or mitoDsRed2-Cre or were infected with lentivirus encoding mitoDsRed2 and Cre. Neurons were also transfected/co-transfected with GFP-tagged miR RNAi construct to knock down DLP1 at a 9:1 ratio (Cre construct/GFP-RNAi construct, the 9:1 ratio enables that >99% GFP-positive neurons are also Cre-positive). A, representative immunoblot and quantification analysis confirming the reduction of MFN2 in Tg motor neurons by Cre expression. Representative confocal images (B) and quantification (C) of mitochondrial length in mitoDsRed2 positively transfected primary mouse spinal cord motor neurons (DIV8) as shown. Red, mito-DsRed2. More than 50 neurons were analyzed in per group. Measurement of intracellular levels of ROS (D), MMP (E), OCR (F and G), ATP (H), and neuronal death by LDH assays (I) are shown. J, quantification of neuronal death by propidium iodide assays in positively transfected neurons 3 days after transfection. More than 200 neurons were analyzed per group. All experiments were repeated three times. Data are means ± S.E. Statistics: one-way ANOVA followed by Tukey's multiple comparison test. *, p < 0.05; **, p < 0.01; ***, p < 0.001. FCCP, carbonyl cyanide p-trifluoromethoxyphenylhydrazone; ns, nonsignificant.
Because mitochondrial function is highly dependent on mitochondrial morphology, intracellular levels of ROS and MMP were next measured. Compared with control neurons, motor neurons with MFN2 deficiency or motor neurons with both MFN2 and DLP1 deficiency demonstrated a comparably significant increase of ROS and decrease of MMP, whereas motor neurons with DLP1 deficiency showed unchanged ROS and MMP (Fig. 2, D and E). The high infection efficiency (>95%) of motor neurons with lentivirus encoding Cre enabled the performance of bulk measurement of the effect of MFN2 deficiency on other mitochondrial functions such as OCR and ATP production. As expected, MFN2 deficiency caused significant reduction of basal/maximal OCR and ATP levels (Fig. 2, F–H). In addition to the change of mitochondrial function, it was noted that MFN2-deficient motor neurons demonstrated significantly increased basal neuronal death (Fig. 2, I and J). On the contrary, DLP1 RNAi had no significant effect on neuronal viability.
MFN2 Overexpression Prevents Glutamate-induced Mitochondrial Dysfunction and Motor Neuron Death in Vitro
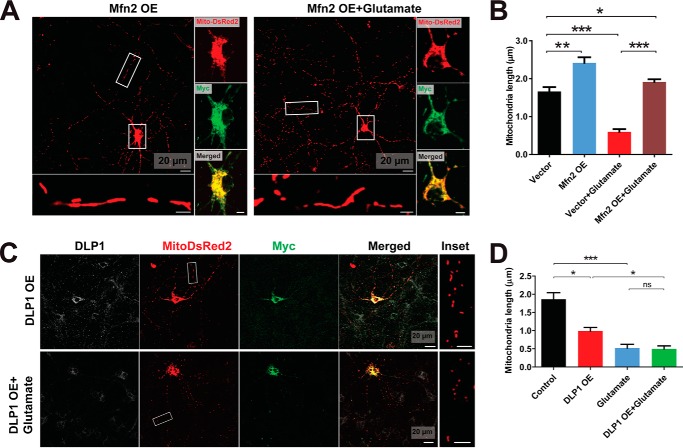
To determine the specific role of DLP1 and MFN2 in glutamate-induced mitochondrial fragmentation, rat primary motor neurons were co-transfected with Myc-tagged DLP1 or MFN2 together with mitoDsRed2 and treated with glutamate 2 days after transfection. Overexpression of DLP1 or MFN2 was confirmed by Myc staining (Fig. 3, A and C). In the absence of glutamate treatment, MFN2 overexpression resulted in mitochondrial elongation, whereas DLP1 overexpression caused mitochondrial fragmentation (Fig. 3, A–D). Strikingly, no glutamate-induced mitochondrial fragmentation was found in neurons overexpressing MFN2, indicating that MFN2 reduction is critical for glutamate-induced mitochondrial fragmentation. Moreover, DLP1 overexpression did not rescue or exacerbate glutamate-induced mitochondrial fragmentation.
FIGURE 3.
MFN2 but not DLP1 overexpression (OE) prevents glutamate-induced mitochondrial fragmentation in primary spinal cord motor neurons. To overexpress MFN2, primary rat spinal cord motor neurons (DIV5) were co-transfected with Myc-tagged MFN2 and mitoDsRed2 or transfected with MitoDsRed2 only and treated with/without 25 μm glutamate at DIV7 for 24 h. Representative confocal images (A) and quantification (B) of mitochondrial length in mitoDsRed2 positively transfected primary rat spinal cord motor neurons overexpressing MFN2 (DIV8) are shown. Primary rat motor neurons were also transfected with mitoDsRed2 and Myc-tagged DLP1 at DIV5 and treated with 25 μm glutamate at DIV7 for 24 h. Neurons were double-stained with specific antibodies against Myc and DLP1. Representative confocal images (C) and quantification (D) of mitochondrial length in mitoDsRed2 positively transfected primary spinal cord motor neurons overexpressing DLP1 (DIV8). Red, mito-DsRed2; green, Myc; white, DLP1. More than 50 neurons were analyzed in per group. All experiments were repeated more than four times. Data are means ± S.E. Statistics: one-way ANOVA followed by Tukey's multiple comparison test. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, nonsignificant.
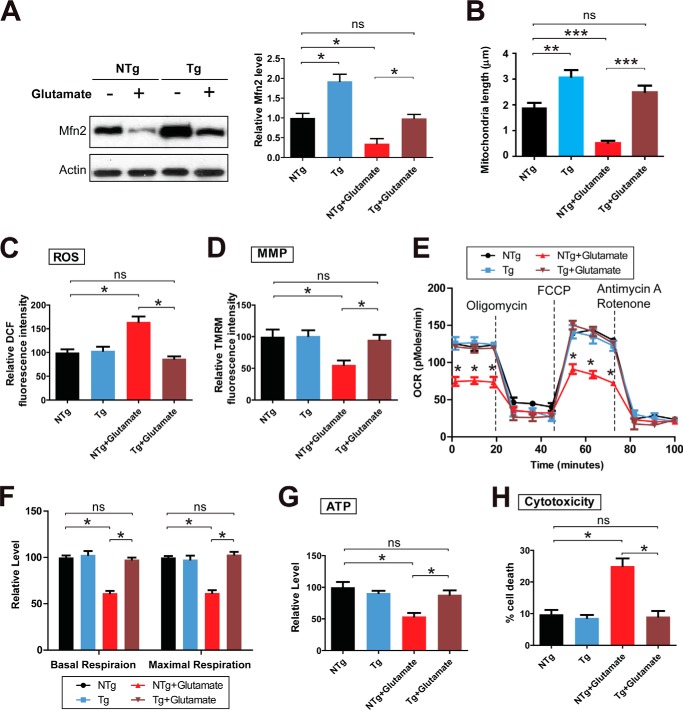
To determine the functional importance of Mfn2-depdendent impairment of mitochondrial dynamics in glutamate-induced mitochondrial dysfunction, we used the mouse primary spinal cord motor neurons isolated from transgene-positive embryos (Tg neurons) and transgene-negative embryos (NTg neurons) of transgenic mice overexpressing human MFN2 in neurons under the control of the mouse Thy1 gene promoter (32), which allows bulk measurement of many mitochondrial function parameters. The overexpression of MFN2 in Tg neurons was confirmed by Western blot (Fig. 4A). Compared with untreated control NTg neurons, NTg neurons exposed to glutamate demonstrated significant reduction of MFN2 (Fig. 4A), mitochondrial fragmentation (Fig. 4B), increased ROS, decreased MMP, decreased basal/maximal OCR, and decreased ATP (Fig. 4, C–G). Although glutamate also caused a relative decrease of MFN2 in Tg neurons, the Mfn2 level after treatment was comparable to controlling NTg neurons. Interestingly, along with the near complete blockage of glutamate-induced mitochondrial fragmentation, MFN2 Tg neurons in the presence of glutamate demonstrated comparable ROS, MMP, basal/maximal OCR, and ATP levels to untreated control neurons, strongly suggesting that MFN2-mediated mitochondrial fragmentation plays an essential role in glutamate-induced mitochondrial dysfunction.
FIGURE 4.
Protection from glutamate-induced mitochondrial dysfunction and neuronal death by MFN2 in primary spinal cord motor neurons. Primary mouse spinal cord motor neurons (DIV7) isolated from Tg neurons and NTg neurons of transgenic mice overexpressing human MFN2 were treated with or without 25 μm glutamate for 24 h. A, representative immunoblot and quantification analysis confirming the overexpression of MFN2 (relative to actin) in Tg motor neurons are shown. Equal protein amounts (10 μg) were loaded and confirmed by actin. Measurement of mitochondrial length by mitoDsRed2 labeling (B) and intracellular levels of ROS (C), MMP (D), OCR (E and F), ATP (G), and neuronal death by LDH assays (H). All experiments were repeated three times. Data are means ± S.E. Statistics: one-way ANOVA followed by Tukey's multiple comparison test. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, nonsignificant.
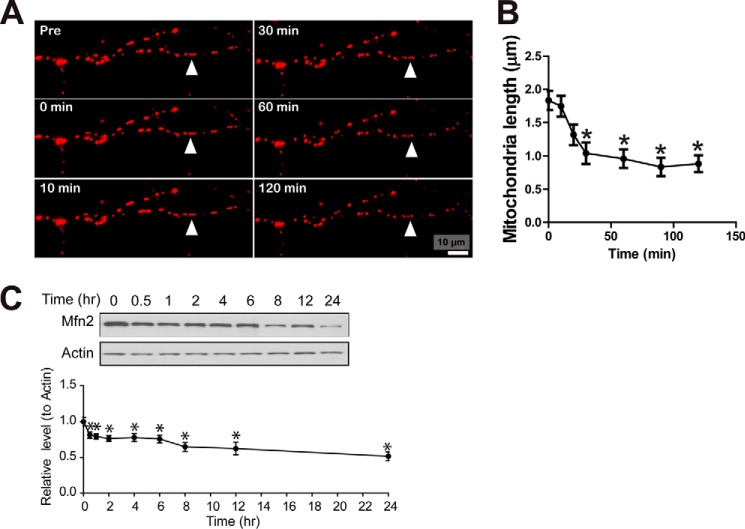
To investigate whether glutamate-induced mitochondrial fragmentation was downstream or upstream of neuronal death, we next measured neuronal viability in neurons with manipulated MFN2 expression after glutamate treatment. MFN2-deficient cells (i.e. Cre:Mfn2fl/fl neurons) demonstrated significantly higher vulnerability to glutamate treatment (Fig. 2I). On the contrary, MFN2 overexpressing motor neurons were resistant to glutamate-induced cell death (Figs. 2I and 4H). These data suggested that mitochondrial fragmentation is upstream of glutamate-induced cell death. Indeed, a detailed time lapse study demonstrated that mitochondrial length and MFN2 expression were decreased within 30 min following glutamate treatment (Fig. 5, A–C), although significant neuronal death was only observed 8 h later (Fig. 1B).
FIGURE 5.
Time course study of mitochondrial morphology and MFN2 expression during glutamate excitotoxicity. A, representative time- lapse pictures of motor neuron positively transfected with mitoDsRed2 in the presence of 25 μm glutamate. B, quantification of mitochondrial length in live cultured cells treated with 25 μm glutamate. At least 50 cells were analyzed. C, representative immunoblot and quantification analysis of the expression of MFN2 in motor neurons at different time points after 25 μm glutamate treatment. Equal protein amounts (10 μg) were loaded and confirmed by actin. Data are means ± S.E. Statistics: one-way ANOVA followed by Tukey's multiple comparison test. All experiments were repeated three times. *, p < 0.05, compared with untreated rat motor neurons transfected with empty vectors (vector control).
MFN2 Overexpression Protects Mitochondria and Motor Neurons from Glutamate Excitotoxicity in Vivo
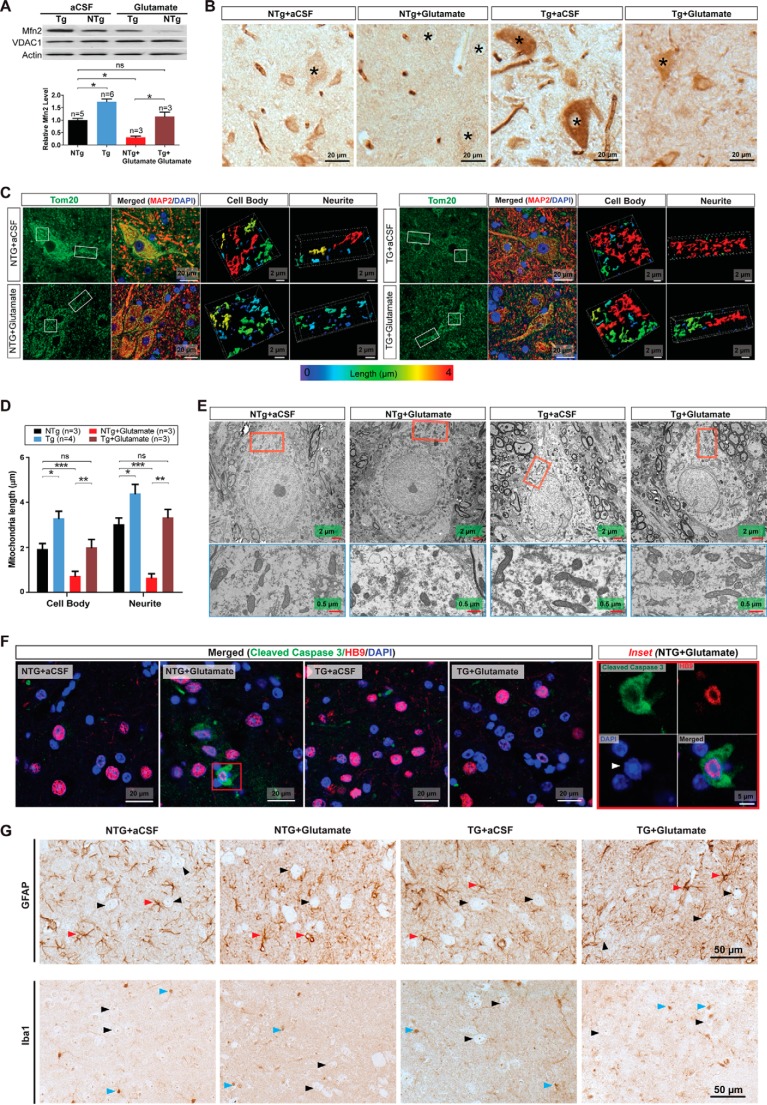
Because glutamate causes MFN2-dependent mitochondrial fragmentation and neuronal death in vitro, we further sought to determine whether these events happen in spinal cord motor neurons in vivo. Nontransgenic control mice (NTg mice) or transgenic mice overexpressing MFN2 (Tg mice) received continuous administration of glutamate into the left lateral ventricle via osmotic mini-pumps. According to previous studies (33–35), a low dose of glutamate (10 mm at a flow rate of 1 μl/h) was employed to minimize side effects. 7 days of continuous glutamate infusion did not cause the death of mice or histological abnormalities in brain, spinal cord, and muscle (data not shown). The overexpression of MFN2 in spinal cords of Tg mice was confirmed by Western blot (Fig. 6A) and immunocytochemistry (Fig. 6B). Compared with untreated or aCSF-infused control NTg mice, NTg mice exposed to low dose glutamate demonstrated significant reduction of MFN2 in spinal cords (Fig. 5, A and B). Similar to what we observed in in vitro cultured Tg motor neurons, the MFN2 level is also reduced by glutamate infusion in Tg mice spinal cords but to a level comparable with control NTg mice without glutamate infusion.
FIGURE 6.
Protection from glutamate excitotoxicity on mitochondria and motor neurons by MFN2 in mice in vivo. Glutamate dissolved in aCSF at a concentration of 10 mm was delivered into the lateral ventricle of 4–6-month-old NTg mice and transgenic mice overexpressing MFN2 (Tg) by mini-osmotic pumps (1 μl/h flow rate). Mice were sacrificed 7 days after continuous infusion. A, representative immunoblot and quantification of MFN2 (relative to actin) in total spinal cord homogenates from control mice (without surgery or infused with aCSF only (n = 5 or 6 mice/group) or age-matched mice infused with glutamate (n = 3 mice/group). Equal protein amounts (20 μg) were loaded and confirmed by actin. VDAC1 was used as a mitochondrial specific marker. B, representative immunocytochemistry of MFN2 in lumbar spinal cords. Asterisk denotes representative MFN2 staining in motor neurons. C, immunostaining of TOM20 (green) in the lumbar spinal cords. Nucleus was stained by DAPI, and neuronal shape was stained by MAP2. Left two panels show large field of view of mitochondria in motor neurons, and the right panel shows magnified area illustrating representative three-dimensional images of mitochondria (from Cell body and Neurite insets denoted by white line boxes). Color map at the bottom provides information on mitochondrial length using a color-coded spectrum. D, quantification of mitochondrial length (maximum ellipsoid axis length) based on three-dimensional images. n = 3 or 4 mice/group. More than 20 neurons were imaged per mouse. E, representative EM micrographs of mitochondria in spinal cord motor neurons from NTg and Tg mice with/without glutamate infusion. Lower panels are magnified areas illustrating representative ultrastructural morphology of mitochondria. F, representative immunostaining of cleaved caspase 3 (green)-positive motor neurons in the lumbar spinal cords. Nucleus was stained by DAPI. Arrowhead points to deformed nucleus. G, representative immunostaining of astrocytic marker glial fibrillary acidic protein (GFAP) and microglial marker Iba1 in the lumbar spinal cords. Black arrowhead points to motor neurons; red arrowhead points to astrocytes; and blue arrowhead points to microglia. n = 3–6 mice/group. Data are means ± S.E. Statistics: one-way ANOVA followed by Tukey's multiple comparison test. *, p < 0.05; **, p < 0.01, ***, p < 0.001; ns, nonsignificant.
Because of the relatively large size of spinal cord motor neurons and the use of the specific antibody against the outer mitochondrial protein TOM20, individual filamentous mitochondria could be discriminated and imaged by immunofluorescence confocal microscopy (Fig. 6C). Unlike neurons cultured in vitro, mitochondria in neurons in vivo are not in single focal plane. Thus, the reconstructed three-dimensional images were used to measure mitochondrial morphology in spinal cord motor neurons in vivo. As shown in single plane and reconstructed three-dimensional images, mitochondria of spinal cord motor neurons from normal NTg subjects demonstrated typical tubular network-like morphologies similar to what we observed in in vitro cultured mouse primary motor neurons and mouse spinal cord motor neurons in vivo (21), and glutamate caused significant mitochondrial fragmentation (Fig. 6, C and D). Importantly, glutamate-induced mitochondrial fragmentation was completely blocked in MFN2 Tg mice. Consistent with confocal microscopic results, electron microscopic analysis confirmed the significant protective effect of Mfn2 overexpression against glutamate-induced mitochondrial fragmentation in spinal cord motor neurons in Tg mice (Fig. 6E).
We also determined the effects of glutamate-induced neuronal death in spinal cord by double immunostaining using motor neuronal marker HB9 and cleaved caspase-3, the central executive molecule implicated in excitotoxic neuronal death in vivo (36) and the ALS mouse model (37). Glutamate infusion led to a significantly increased number of cleaved caspase-3-positive motor neurons in the spinal cords of NTg mice, which also exhibited clumping or fragmented nuclei (7.5 ± 1.57% of total) (Fig. 6F). In contrast, Tg mice infused with glutamate did not show any cleaved caspase-3-positive motor neurons in the spinal cord, indicating the complete blockage of glutamate-induced neuronal death by MFN2 overexpression. There were no cleaved caspase-3 positively stained neurons observed in the brain of either genotypes exposed to continuous infusion of glutamate (data not shown). Although non-neuronal cells such as astrocytes and microglia have been reported to affect the survival of motor neuron in mice (38–41), no increase of astrocytes or microglia was observed in either NTg or Tg spinal cords after glutamate infusion (Fig. 6G).
Calpain Cleaves MFN2 in Spinal Cord Motor Neurons Following Glutamate Exposure
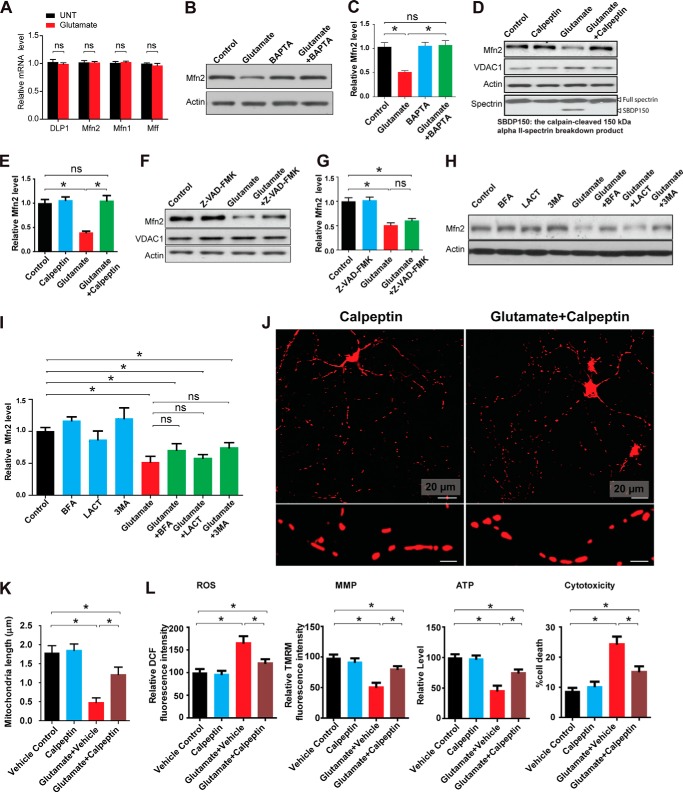
Given the critical role of MFN2 reduction in glutamate-induced mitochondrial fragmentation and dysfunction, we sought to determine the mechanism by which glutamate caused MFN2 reduction in spinal cord motor neurons. Real time PCR revealed no changes in the mRNA levels of MFN2 in neurons treated with glutamate (Fig. 7A), suggesting that glutamate causes MFN2 reduction likely through post-translational protein degradation. Because glutamate exposure resulted in increase of intracellular Ca2+ (42, 43), we first examined the effect of calcium on glutamate-induced MFN2 reduction by treating neurons with a cell-permeable calcium chelator, BAPTA-AM. BAPTA-AM totally prevented the glutamate-induced MFN2 reduction, suggesting the dependence of MFN2 degradation on calcium signaling (Fig. 7, B and C). Following glutamate receptor activation, calpain is the major neuronal cysteine protease activated by calcium and is critical for neuronal function (44). We confirmed that calpain was activated by glutamate in motor neurons as evidenced by the appearance of the 150-kDa αII-spectrin breakdown product (SBDP150) (Fig. 7D), a calpain cleavage product that has been widely used as a direct measurement of calpain activity in neurons (45). To determine whether calpain is involved in MFN2 reduction, motor neurons at DIV7 were co-treated with glutamate and calpeptin, a cell-permeable calpain-specific inhibitor. Calpeptin completely prevented both glutamate-induced calpain activation and MFN2 reduction (Fig. 7, D and E), suggesting the involvement of calpain in glutamate-induced MFN2 degradation. Not surprisingly, along with the prevention of MFN2 degradation, the calpain inhibitor calpeptin resulted in significant inhibition of glutamate-induced mitochondrial fragmentation (Fig. 7, J and K), the increase of ROS, the reduction of MMP/ATP production, and neuronal death (Fig. 7L).
FIGURE 7.
Degradation of MFN2 by calpain in response to glutamate challenge in primary spinal cord motor neurons. A, motor neurons (DIV7) were treated with 25 μm glutamate for 24 h, and mRNA levels of mitochondrial dynamic proteins were assayed by real time PCR. Representative immunoblot and quantification analysis of the expression of MFN2 (relative to actin) in rat spinal cord motor neurons (DIV7) treated with or without 25 μm glutamate and/or 2.5 μm intracellular calcium chelator BAPTA-AM (B and C), 20 μm calpain inhibitor calpeptin (D and E), and 20 μm pan-caspase inhibitor Z-VAD-FMK (F and G) for 24 h or 5 mm macroautophagy inhibitor 100 nm autophagy inhibitor bafilomycin A1 (BFA), 5 μm proteasome inhibitor lactacystin (LACT), and 3-methyladenine (3MA) (H and I) for 8 h. Equal protein amounts (10 μg) were loaded and confirmed by actin. VDAC1 was used as a mitochondrial specific marker. Representative confocal images (J) and quantification (K) of mitochondrial length in mitoDsRed2 positively transfected primary rat spinal cord motor neurons (DIV8) treated with or without 25 μm glutamate and/or calpain inhibitor calpeptin for 24 h. To label mitochondria, neurons were transfected with mitoDsRed2 2 days before treatment. Red, mito-DsRed2. More than 50 neurons were analyzed in per group. L, measurement of intracellular levels of ROS, MMP, ATP, and neuronal death by LDH assays. All experiments were repeated three times. Data are means ± S.E. Statistics: one-way ANOVA followed by Tukey's multiple comparison test. *, p < 0.05; ns, nonsignificant.
Although caspases were reportedly activated late after glutamate treatment and caspase inhibitors could alleviate glutamate-induced neuronal death (46), the caspase pan inhibitor Z-VAD-FMK had a slight but not significant effect on glutamate-induced MFN2 degradation (Fig. 7, F and G). Furthermore, proteasome inhibitor lactacystin, autophagy inhibitor bafilomycin A1, and macroautophagy inhibitor 3-methyladenine also failed to significantly block glutamate-induced MFN2 degradation (Fig. 7, H and I).
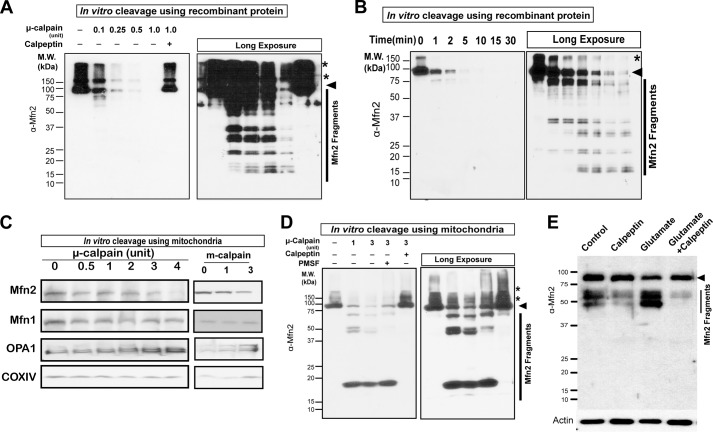
To prove the direct cleavage of MFN2 by calpain, we performed in vitro calpain cleavage assays using recombinant human MFN2 purified from HEK293 cells. There are two major forms of calpains, i.e. μ-calpain (calpain 1) and m-calpain (calpain 2) (47). m-Calpain and/or μ-calpain caused both a dose-dependent and time-dependent decrease of recombinant full-length MFN2 and a concurrent increase of many lower molecular mass (<75 kDa) fragments (Fig. 8, A and B), protein degradation of which could be completely blocked by calpeptin. To investigate the cleavage of MFN2 by calpain in physiologically relevant conditions, we took advantage of the purified mitochondria isolated from mouse brain. The purified mitochondria were incubated with calpain in the presence/absence of calpeptin or the broad spectrum serine protease inhibitor, phenylmethylsulfonyl fluoride (PMSF), followed by immunoblot using antibodies again MFN2 (immunogen spanning last 50 residues). MFN2, but not other mitochondrial fission and fusion proteins such as MFN1 and OPA1, was decreased by calpain in a dose-dependent manner that was effectively prevented by calpeptin but not PMSF (Fig. 8, C and D). Interestingly, significantly fewer fragments were observed using purified mitochondria compared with cleavage using recombinant MFN2. Consistent with in vitro experiments showing calpain-derived MFN2 fragments, examination of neuronal lysates showed the presence of MFN2 fragments ranging from 3 to 75 kDa in control neurons, which accumulated significantly after glutamate treatment and could be greatly suppressed by calpeptin (Fig. 8E).
FIGURE 8.
Degradation of MFN2 by calpain in vitro. A, representative immunoblot of recombinant human MFN2 in the presence of different units of μ-calpain for 30 min at 30 °C. Cleavage of recombinant of MFN2 could be totally inhibited by calpain inhibitor calpeptin. Arrowheads denote MFN2, and asterisks denotes dimerized/polymerized MFN2. B, representative immunoblot of recombinant human MFN2 in the presence of 0.5 units of μ-calpain for different time points at 30 °C. C, representative immunoblot of MFN2, MFN1, OPA1, and COXIV in purified mitochondria isolated from mouse cortex after incubation with/without μ-calpain and m-calpain at 30 °C for 30 min. D, representative immunoblot of MFN2 in purified mitochondria isolated from mouse cortex after incubation with/without μ-calpain and/or calpeptin/PMSF at 30 °C for 30 min. All experiments were repeated at least three times. E, representative picture of MFN2 fragments in neurons after 24 h of glutamate treatment. Arrow denotes MFN2. All experiments were repeated more than three times.
DISCUSSION
Previous studies demonstrated mitochondrial dysfunction as a primary event in excitatory neurons, yet how glutamate induces mitochondrial dysfunction is not clear. Here, we report that glutamate induced significant mitochondrial fragmentation that mediates glutamate-induced mitochondrial dysfunction in spinal cord motor neurons both in vitro and in vivo. This is likely driven by the glutamate-induced decrease in MFN2 expression because loss of MFN2 caused similar mitochondrial dysfunction and motor neuronal death, and overexpression of MFN2 almost completely alleviates glutamate-induced mitochondrial dysfunction and motor neuronal death. Mechanistically, we found that glutamate caused MFN2 reduction through calpain-dependent cleavage of MFN2. To our best knowledge, these studies for the first time not only provide a mechanism explaining how glutamate induces mitochondrial dysfunction but also present a novel pathway describing how mitochondrial dynamics are regulated in response to stress.
Normal mitochondrial morphology is regulated by the delicate balance of fission and fusion processes (48). Glutamate-induced mitochondrial fragmentation observed in this study suggested a tipped balance of these processes toward excessive fission that can be due to either enhanced mitochondrial fission, reduced mitochondrial fusion, or both. The level of mitochondrial fusion protein MFN2 and mitochondrial fission protein DLP1 were both reduced, suggesting the possible impairment of both fission and fusion. Despite the fact that decreased expression of DLP1 caused mitochondrial elongation and decreased expression of MFN2 led to mitochondrial fragmentation, mitochondria became predominantly fragmented when the expression of DLP1 and MFN2 were both decreased in motor neurons, which is consistent with our prior observation in cortical neurons (49). Further functional measurements showed that DLP1 RNAi had no significant effect on mitochondrial dysfunction caused by MFN2 in motor neurons. Therefore, our data suggest that MFN2 reduction supersedes DLP1 reduction on the regulation of mitochondrial morphology and mitochondrial function. This is somewhat expected because MFN2 is a predominantly mitochondrially localized protein, whereas DLP1 mainly resides in cytosol and is demonstrated to be involved in other cellular functions such as endoplasmic reticulum distribution (50) and vesicle membrane dynamics (51). Nevertheless, because MFN2 but not DLP1 deficiency was sufficient to cause mitochondrial fragmentation/dysfunction and neuronal death, similar to glutamate-induced changes, mitochondrial fragmentation and hence mitochondrial dysfunction during glutamate excitotoxicity appeared to be primarily caused by the impairment of mitochondrial fusion via MFN2 reduction. It is still unclear why DLP1 is reduced and what may be the functional consequence of DLP1 reduction. Of interest, DLP1 interacts with Bax, which may play a critical role in apoptosis (52, 53), and inactivation of DLP1 renders cells more resistant to stresses such as ROS (54). It is possible that DLP1 reduction may be an adaptation or defense mechanism against glutamate-induced cell death.
Calpain overactivation through glutamate receptor-mediated intracellular calcium influx has been regarded as the major cause of glutamate-induced dying-back axonopathy and neuronal death, but the mechanism is still unclear (55–57). Unlike the most recent study demonstrating the late stage reduction of MFN2 during glutamate excitotoxicity in cortical neurons (58), our detailed time lapse study in motor neurons demonstrated that, concurrent with mitochondrial fragmentation, MFN2 was decreased rapidly following glutamate treatment. This discrepancy could be due to either different responses to or sensitivity of motor and cortical neurons to glutamate excitotoxicity. MFN2 reduction and mitochondrial fragmentation well preceded neuronal death during glutamate excitotoxicity, suggesting glutamate-induced mitochondrial fragmentation was unlikely a secondary effect of glutamate-evoked neurotoxicity. In support of this notion, overexpression of MFN2 significantly inhibited neuronal death caused by glutamate treatment. Therefore, MFN2-mediated mitochondrial fragmentation is a prerequisite for glutamate-evoked motor neuronal death. We further demonstrated that calpain was responsible for MFN2 degradation following glutamate exposure. Because the inhibition of calpain could also effectively block glutamate-induced mitochondrial fragmentation, mitochondrial dysfunction, and neuronal death, our data suggest that calpain and MFN2 act in a linear pathway, with calpain-induced MFN2 degradation impairing mitochondrial fusion to cause mitochondrial dysfunction and neuronal death during glutamate challenge. Indeed, MFN2 ablation alone was sufficient to induce motor neuronal death, further suggesting the critical role of MFN2-mediated mitochondrial fragmentation during glutamate excitotoxicity. It is still unclear how MFN2-dependent mitochondrial fragmentation leads to mitochondrial dysfunction and neuronal death. Previous studies showed that mitochondrial fission/fusion proteins are involved in the assembly of respiratory complexes (59, 60). Therefore, glutamate-induced mitochondrial fragmentation probably impairs mitochondrial function and causes subsequent neuronal death through the regulation of the mitochondrial respiratory complex assembly. Nevertheless, the exact underlying mechanism(s) still remains to be determined.
We demonstrated that calpain was responsible for MFN2 degradation following glutamate exposure and calpain-cleaved MFN2 in vitro. Compared with recombinant MFN2, MFN2 in mitochondria was more resistant to calpain and generated less calpain-derived fragments. Because recombinant MFN2 was purified from HEK293 cells, it had similar post-translational modifications as MFN2 in mitochondria. So, the relative resistance of MFN2 in mitochondria to calpain was probably due to either the binding of other proteins/factors or the protein conformation that inhibited or masked potential calpain cleavage sites. Our in vitro cleavage assay using isolated mitochondria found that neither MFN1 nor OPA1 in mitochondria could be cleaved by calpain that could efficiently cleave MFN2. This is consistent with our finding that levels of full-length OPA1 and MFN1 remained unchanged in neurons exposed to glutamate.
Although caspases were reported to be activated late after glutamate treatment (46) and caspase inhibitors could alleviate glutamate-induced neuronal death, we found that caspases were not involved in glutamate-induced MFN2 degradation. A previous study showed that MFN2 could be degraded through the ubiquitin-proteasome system during apoptosis (61). However, similar to caspases, the inhibition of either proteasome or the autophagy/lysosomal degradation pathway failed to prevent MFN2 degradation in response to glutamate treatment in motor neurons. The most recent study demonstrated that MFN2 was regulated at the transcriptional level at late stage during glutamate excitotoxicity in cortical neurons. However, we did not observe significant changes in the mRNA level of MFN2 and other fission/fusion proteins during glutamate excitotoxicity in motor neurons. Calpain is the major protease reported to be activated following glutamate challenge, and indeed, glutamate-induced MFN2 reduction could be completely blocked by calpain inhibition. Thus, our study suggests that the calpain-mediated MFN2 reduction might be the major pathway responsible for MFN2 degradation during glutamate excitotoxicity at least in motor neurons.
The inhibition of calpain, however, did not completely block glutamate-induced mitochondrial fragmentation and dysfunction, suggesting the possible existence of other pathways contributing to mitochondrial abnormalities during glutamate excitotoxicity. For example, MIRO-1 has been reported to regulate mitochondrial dynamics in response to calcium signaling (62, 63). MIRO-1 is a calcium-binding GTPase, the activity of which could be directly regulated by calcium. It is possible that the inhibition of calpain has no effect on mitochondrial fragmentation and dysfunction mediated by MIRO-1 or other proteins following glutamate-evoked calcium signaling. Nevertheless, overexpression of MFN2 could completely prevent glutamate-induced mitochondrial fragmentation and dysfunction, indicating that MFN2 might compensate for the effect of other pathway(s). In addition to MFN2, calpain has other targets such as CAIN/CABIN1 (64), collapsin response mediator proteins (55), and SRC protein kinase (65) during glutamate-induced excitotoxicity. Considering the involvement of mitochondria in almost all forms of cell death (66) and cellular processes, future studies are expected to address the interplay between MFN2 and other calpain targets in response to glutamate excitotoxicity.
Taken together, this study presents how calpain-mediated MFN2 degradation may act as the novel mechanistic linker among glutamate excitotoxicity, mitochondrial dynamic abnormalities, mitochondrial dysfunction, and neuronal death in spinal cord motor neurons. As our understanding of the important role of mitochondrial dynamics in pathophysiology continues to expand, this study implicates that this newly identified MFN2 degradation pathway through calpain activation might be a potential novel therapeutic target worthy of validation in disease models that show either elevated glutamate and/or calpain activation.
This work was supported, in whole or in part, by National Institutes of Health Grants R03AG044680, R21NS085747, and R01NS089604. This work was also supported by Alzheimer's Association Grant NIRG-11-204281 and China Scholarship Council (to F. Z.).
This article was selected as a Paper of the Week.
- ALS
- amyotrophic lateral sclerosis
- MMP
- mitochondrial membrane potential
- ROS
- reactive oxygen species
- LDH
- lactate dehydrogenase
- OCR
- oxygen consumption rate
- BAPTA-AM
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)
- aCSF
- artificial cerebrospinal fluid
- ANOVA
- analysis of variance
- NLS
- nuclear localization signal
- NGS
- normal goat serum
- DIV
- days in vitro
- Tg
- t transgene-positive
- NTg
- transgene-negative.
REFERENCES
- 1. Sommer B., Keinänen K., Verdoorn T. A., Wisden W., Burnashev N., Herb A., Köhler M., Takagi T., Sakmann B., Seeburg P. H. (1990) Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science 249, 1580–1585 [DOI] [PubMed] [Google Scholar]
- 2. Olney J. W. (1969) Brain lesions obesity and other disturbances in mice treated with monosodium glutamate. Science 164, 719–721 [DOI] [PubMed] [Google Scholar]
- 3. Olney J. W., Sharpe L. G. (1969) Brain lesions in an infant rhesus monkey treated with monosodium glutamate. Science 166, 386–388 [DOI] [PubMed] [Google Scholar]
- 4. Choi D. W. (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1, 623–634 [DOI] [PubMed] [Google Scholar]
- 5. Lau A., Tymianski M. (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 460, 525–542 [DOI] [PubMed] [Google Scholar]
- 6. Mehta A., Prabhakar M., Kumar P., Deshmukh R., Sharma P. L. (2013) Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 698, 6–18 [DOI] [PubMed] [Google Scholar]
- 7. Reynolds I. J., Hastings T. G. (1995) Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J. Neurosci. 15, 3318–3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schinder A. F., Olson E. C., Spitzer N. C., Montal M. (1996) Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J. Neurosci. 16, 6125–6133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nunnari J., Marshall W. F., Straight A., Murray A., Sedat J. W., Walter P. (1997) Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Mol. Biol. Cell 8, 1233–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bleazard W., McCaffery J. M., King E. J., Bale S., Mozdy A., Tieu Q., Nunnari J., Shaw J. M. (1999) The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat. Cell Biol. 1, 298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sesaki H., Jensen R. E. (1999) Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J. Cell Biol. 147, 699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Losón O. C., Song Z., Chen H., Chan D. C. (2013) Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell 24, 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Detmer S. A., Chan D. C. (2007) Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 8, 870–879 [DOI] [PubMed] [Google Scholar]
- 14. Picard M., Shirihai O. S., Gentil B. J., Burelle Y. (2013) Mitochondrial morphology transitions and functions: implications for retrograde signaling? Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R393–R406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi P., Gal J., Kwinter D. M., Liu X., Zhu H. (2010) Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochim. Biophys. Acta 1802, 45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Su B., Wang X., Zheng L., Perry G., Smith M. A., Zhu X. (2010) Abnormal mitochondrial dynamics and neurodegenerative diseases. Biochim. Biophys. Acta 1802, 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Archer S. L. (2013) Mitochondrial dynamics–mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 369, 2236–2251 [DOI] [PubMed] [Google Scholar]
- 18. Rothstein J. D. (1995) Excitotoxic mechanisms in the pathogenesis of amyotrophic lateral sclerosis. Adv. Neurol. 68, 7–20 [PubMed] [Google Scholar]
- 19. Bruijn L. I., Miller T. M., Cleveland D. W. (2004) Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 27, 723–749 [DOI] [PubMed] [Google Scholar]
- 20. Jaiswal M. K. (2013) Calcium, mitochondria, and the pathogenesis of ALS: the good, the bad, and the ugly. Front. Cell. Neurosci. 7, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang W., Li L., Lin W. L., Dickson D. W., Petrucelli L., Zhang T., Wang X. (2013) The ALS disease-associated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons. Hum. Mol. Genet. 22, 4706–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaech S., Banker G. (2006) Culturing hippocampal neurons. Nat. Protoc. 1, 2406–2415 [DOI] [PubMed] [Google Scholar]
- 23. Tiscornia G., Singer O., Verma I. M. (2006) Production and purification of lentiviral vectors. Nat. Protoc. 1, 241–245 [DOI] [PubMed] [Google Scholar]
- 24. Wang X., Su B., Lee H. G., Li X., Perry G., Smith M. A., Zhu X. (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J. Neurosci. 29, 9090–9103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X., Yan M. H., Fujioka H., Liu J., Wilson-Delfosse A., Chen S. G., Perry G., Casadesus G., Zhu X. (2012) LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum. Mol. Genet. 21, 1931–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanaichi T., Sato T., Iwamoto T., Malavasi-Yamashiro J., Hoshino M., Mizuno N. (1986) A stable lead by modification of Sato's method. J. Electron Microsc. 35, 304–306 [PubMed] [Google Scholar]
- 27. Vincent A. M., Mobley B. C., Hiller A., Feldman E. L. (2004) IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiol. Dis. 16, 407–416 [DOI] [PubMed] [Google Scholar]
- 28. Ackerley S., Grierson A. J., Brownlees J., Thornhill P., Anderton B. H., Leigh P. N., Shaw C. E., Miller C. C. (2000) Glutamate slows axonal transport of neurofilaments in transfected neurons. J. Cell Biol. 150, 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Donnelly C. J., Zhang P. W., Pham J. T., Haeusler A. R., Heusler A. R., Mistry N. A., Vidensky S., Daley E. L., Poth E. M., Hoover B., Fines D. M., Maragakis N., Tienari P. J., Petrucelli L., Traynor B. J., Wang J., Rigo F., Bennett C. F., Blackshaw S., Sattler R., Rothstein J. D. (2013) RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron 80, 415–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoon Y., Pitts K. R., Dahan S., McNiven M. A. (1998) A novel dynamin-like protein associates with cytoplasmic vesicles and tubules of the endoplasmic reticulum in mammalian cells. J. Cell Biol. 140, 779–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen H., McCaffery J. M., Chan D. C. (2007) Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130, 548–562 [DOI] [PubMed] [Google Scholar]
- 32. Caroni P. (1997) Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J. Neurosci. Methods 71, 3–9 [DOI] [PubMed] [Google Scholar]
- 33. Hawkins R. A., DeJoseph M. R., Hawkins P. A. (1995) Regional brain glutamate transport in rats at normal and raised concentrations of circulating glutamate. Cell Tissue Res. 281, 207–214 [DOI] [PubMed] [Google Scholar]
- 34. Fujisawa H., Landolt H., Bullock R. (1996) Patterns of increased glucose use following extracellular infusion of glutamate: an autoradiographic study. J. Neurotrauma 13, 245–254 [DOI] [PubMed] [Google Scholar]
- 35. Hirata A., Nakamura R., Kwak S., Nagata N., Kamakura K. (1997) AMPA receptor-mediated slow neuronal death in the rat spinal cord induced by long-term blockade of glutamate transporters with THA. Brain Res. 771, 37–44 [DOI] [PubMed] [Google Scholar]
- 36. Namura S., Zhu J., Fink K., Endres M., Srinivasan A., Tomaselli K. J., Yuan J., Moskowitz M. A. (1998) Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J. Neurosci. 18, 3659–3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaspar B. K., Lladó J., Sherkat N., Rothstein J. D., Gage F. H. (2003) Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science 301, 839–842 [DOI] [PubMed] [Google Scholar]
- 38. Hall E. D., Oostveen J. A., Gurney M. E. (1998) Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia 23, 249–256 [DOI] [PubMed] [Google Scholar]
- 39. Zhao W., Xie W., Le W., Beers D. R., He Y., Henkel J. S., Simpson E. P., Yen A. A., Xiao Q., Appel S. H. (2004) Activated microglia initiate motor neuron injury by a nitric oxide and glutamate-mediated mechanism. J. Neuropathol. Exp. Neurol. 63, 964–977 [DOI] [PubMed] [Google Scholar]
- 40. Sargsyan S. A., Monk P. N., Shaw P. J. (2005) Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia 51, 241–253 [DOI] [PubMed] [Google Scholar]
- 41. Yamanaka K., Chun S. J., Boillee S., Fujimori-Tonou N., Yamashita H., Gutmann D. H., Takahashi R., Misawa H., Cleveland D. W. (2008) Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 11, 251–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manev H., Favaron M., Guidotti A., Costa E. (1989) Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol. Pharmacol. 36, 106–112 [PubMed] [Google Scholar]
- 43. Randall R. D., Thayer S. A. (1992) Glutamate-induced calcium transient triggers delayed calcium overload and neurotoxicity in rat hippocampal neurons. J. Neurosci. 12, 1882–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doshi S., Lynch D. R. (2009) Calpain and the glutamatergic synapse. Front. Biosci. 1, 466–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Czogalla A., Sikorski A. F. (2005) Spectrin and calpain: a ‘target’ and a ‘sniper’ in the pathology of neuronal cells. Cell. Mol. Life Sci. 62, 1913–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Du Y. S., Bales K. R., Dodel R. C., HamiltonByrd E., Horn J. W., Czilli D. L., Simmons L. K., Ni B. H., Paul S. M. (1997) Activation of a caspase 3-related cysteine protease is required for glutamate-mediated apoptosis of cultured cerebellar granule neurons. P. Natl. Acad. Sci. U.S.A. 94, 11657–11662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goll D. E., Thompson V. F., Li H., Wei W., Cong J. (2003) The calpain system. Physiol. Rev. 83, 731–801 [DOI] [PubMed] [Google Scholar]
- 48. Chan D. C. (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125, 1241–1252 [DOI] [PubMed] [Google Scholar]
- 49. Wang X., Su B., Siedlak S. L., Moreira P. I., Fujioka H., Wang Y., Casadesus G., Zhu X. (2008) Amyloid-β overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl. Acad. Sci. U.S.A. 105, 19318–19323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pitts K. R., Yoon Y., Krueger E. W., McNiven M. A. (1999) The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol. Biol. Cell 10, 4403–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li H., Alavian K. N., Lazrove E., Mehta N., Jones A., Zhang P., Licznerski P., Graham M., Uo T., Guo J., Rahner C., Duman R. S., Morrison R. S., Jonas E. A. (2013) A Bcl-xL-Drp1 complex regulates synaptic vesicle membrane dynamics during endocytosis. Nat. Cell Biol. 15, 773–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frank S., Gaume B., Bergmann-Leitner E. S., Leitner W. W., Robert E. G., Catez F., Smith C. L., Youle R. J. (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 1, 515–525 [DOI] [PubMed] [Google Scholar]
- 53. Karbowski M., Lee Y. J., Gaume B., Jeong S. Y., Frank S., Nechushtan A., Santel A., Fuller M., Smith C. L., Youle R. J. (2002) Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159, 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yu T., Robotham J. L., Yoon Y. (2006) Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. U.S.A. 103, 2653–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hou S. T., Jiang S. X., Desbois A., Huang D., Kelly J., Tessier L., Karchewski L., Kappler J. (2006) Calpain-cleaved collapsin response mediator protein-3 induces neuronal death after glutamate toxicity and cerebral ischemia. J. Neurosci. 26, 2241–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bano D., Young K. W., Guerin C. J., Lefeuvre R., Rothwell N. J., Naldini L., Rizzuto R., Carafoli E., Nicotera P. (2005) Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell 120, 275–285 [DOI] [PubMed] [Google Scholar]
- 57. Hou S. T., MacManus J. P. (2002) Molecular mechanisms of cerebral ischemia-induced neuronal death. Int. Rev. Cytol. 221, 93–148 [DOI] [PubMed] [Google Scholar]
- 58. Martorell-Riera A., Segarra-Mondejar M., Muñoz J. P., Ginet V., Olloquequi J., Pérez-Clausell J., Palacín M., Reina M., Puyal J., Zorzano A., Soriano F. X. (2014) Mfn2 downregulation in excitotoxicity causes mitochondrial dysfunction and delayed neuronal death. EMBO J. 33, 2388–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu W., Acín-Peréz R., Geghman K. D., Manfredi G., Lu B., Li C. (2011) Pink1 regulates the oxidative phosphorylation machinery via mitochondrial fission. Proc. Natl. Acad. Sci. U.S.A. 108, 12920–12924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cogliati S., Frezza C., Soriano M. E., Varanita T., Quintana-Cabrera R., Corrado M., Cipolat S., Costa V., Casarin A., Gomes L. C., Perales-Clemente E., Salviati L., Fernandez-Silva P., Enriquez J. A., Scorrano L. (2013) Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155, 160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leboucher G. P., Tsai Y. C., Yang M., Shaw K. C., Zhou M., Veenstra T. D., Glickman M. H., Weissman A. M. (2012) Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis. Mol. Cell 47, 547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Macaskill A. F., Rinholm J. E., Twelvetrees A. E., Arancibia-Carcamo I. L., Muir J., Fransson A., Aspenstrom P., Attwell D., Kittler J. T. (2009) Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61, 541–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang X., Schwarz T. L. (2009) The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell 136, 163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim M. J., Jo D. G., Hong G. S., Kim B. J., Lai M., Cho D. H., Kim K. W., Bandyopadhyay A., Hong Y. M., Kim D. H., Cho C., Liu J. O., Snyder S. H., Jung Y. K. (2002) Calpain-dependent cleavage of cain/cabin1 activates calcineurin to mediate calcium-triggered cell death. Proc. Natl. Acad. Sci. U.S.A. 99, 9870–9875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hossain M. I., Roulston C. L., Kamaruddin M. A., Chu P. W., Ng D. C., Dusting G. J., Bjorge J. D., Williamson N. A., Fujita D. J., Cheung S. N., Chan T. O., Hill A. F., Cheng H. C. (2013) A truncated fragment of Src protein kinase generated by calpain-mediated cleavage is a mediator of neuronal death in excitotoxicity. J. Biol. Chem. 288, 9696–9709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kroemer G., Galluzzi L., Vandenabeele P., Abrams J., Alnemri E. S., Baehrecke E. H., Blagosklonny M. V., El-Deiry W. S., Golstein P., Green D. R., Hengartner M., Knight R. A., Kumar S., Lipton S. A., Malorni W., Nuñez G., Peter M. E., Tschopp J., Yuan J., Piacentini M., Zhivotovsky B., Melino G., and Nomenclature Committee on Cell Death 2009 (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]