Background: Prionoid propagation requires cell internalization of aggregated polypeptides.
Results: Aggregates of different sequence are internalized through different endocytic pathways. Only phagocytosed aggregates (>1 μm) elicit an HSF1-dependent proteostatic response.
Conclusion: Proteostatic response upon aggregate internalization differs markedly depending on the sequence.
Significance: The characterization of mechanisms of cell penetration is fundamental for the understanding of aggregate transmission in disease.
Keywords: Aggresome, Amyloid, Molecular Chaperone, Peptide Transport, Protein Aggregation, Internalization, Peptide, Prionoid
Abstract
Recently, a number of aggregation disease polypeptides have been shown to spread from cell to cell, thereby displaying prionoid behavior. Studying aggregate internalization, however, is often hampered by the complex kinetics of the aggregation process, resulting in the concomitant uptake of aggregates of different sizes by competing mechanisms, which makes it difficult to isolate pathway-specific responses to aggregates. We designed synthetic aggregating peptides bearing different aggregation propensities with the aim of producing modes of uptake that are sufficiently distinct to differentially analyze the cellular response to internalization. We found that small acidic aggregates (≤500 nm in diameter) were taken up by nonspecific endocytosis as part of the fluid phase and traveled through the endosomal compartment to lysosomes. By contrast, bigger basic aggregates (>1 μm) were taken up through a mechanism dependent on cytoskeletal reorganization and membrane remodeling with the morphological hallmarks of phagocytosis. Importantly, the properties of these aggregates determined not only the mechanism of internalization but also the involvement of the proteostatic machinery (the assembly of interconnected networks that control the biogenesis, folding, trafficking, and degradation of proteins) in the process; whereas the internalization of small acidic aggregates is HSF1-independent, the uptake of larger basic aggregates was HSF1-dependent, requiring Hsp70. Our results show that the biophysical properties of aggregates determine both their mechanism of internalization and proteostatic response. It remains to be seen whether these differences in cellular response contribute to the particular role of specific aggregated proteins in disease.
Introduction
Recently, it has been demonstrated that several disease-associated aggregates, including human (1–3) and yeast prions (4), Aβ (5), Tau (6), α-synuclein (7), SOD1 (8), and PolyQ (9), can cross cellular membranes and spread aggregation from cell to cell (10). This has led to the notion that all of these proteins potentially possess a certain degree of prionoid behavior (8, 11, 12). Despite these reports, the mechanism by which this process takes place remains obscure because the transmission of a protein or aggregate from the cytosol of one cell to the cytosol of a neighboring cell requires the crossing of both cellular membranes. The existence of cell membrane translocation mechanisms has been proposed for some amyloids, such as nanotubules for prions (3) or membrane diffusion by an unknown mechanism for Aβ40 (13, 14) and α-synuclein (15), although it is now widely accepted that aggregate transmission can also occur through a combination of exocytosis, endocytosis, and endosomal escape (16).
In accordance with this hypothesis, several mechanisms of endocytosis and exocytosis have been postulated for the most common amyloids. Exocytosis by conventional exosomes, as a result of the fusion of multivesicular bodies with the plasma membrane, has been reported for monomeric Aβ (17), α-synuclein (18–20), PrpSc (2, 21), and Tau (22) in neuroblastoma cell lines. Other unconventional exocytosis mechanisms have been described for PrP (23) and α-synuclein (19). Endocytosis of monomeric Aβ (13, 14, 24–26) and α-synuclein (15, 27–29) and endocytosis of the fibrillar and oligomeric states of some amyloids have also been reported. For instance, fibrilar Aβ can be cleared from the medium by microglia and astroglia (30–32), whereas oligomeric Aβ can be taken up by neuroblastoma SH-SY5Y cells (33). The internalization of PrpSc aggregates has been reported in murine and human neuroblastoma cell lines and mouse fibroblasts, whereby heparan sulfates and lipid rafts turned out to be involved (1, 34–37). SOD1 aggregates are internalized by macropinocytosis by N2a cells, a neuroblastoma cell line (8), whereas Tau aggregates were taken up by HEK-293 cells and neuroblastoma cell lines (6, 38, 39). Currently, it is not known whether different amyloids share common pathways of internalization. In addition, different pathways of internalization have been described for the monomeric and fibrillar forms of α-synuclein (15) and Aβ (13, 32), demonstrating that the aggregation status could also determine different routes of internalization. Finally, interaction of the native protein with natural partners could also determine specific handling by a specific subset of cells, as occurs during the intracellular production of Aβ (40, 41).
Here our aim is to investigate whether the biophysical properties of an aggregating polypeptide sequence affect the way in which it is recognized and processed by the cell. Because several competing uptake mechanisms have been described previously, our objective here was to design synthetic aggregating peptides with a strong bias toward a particular mode of uptake, which would illustrate how biophysical properties affect uptake and would allow the investigation of pathway-specific cellular responses to aggregates. It is accepted that a size threshold determines the choice of the endocytic pathway that will be used for the uptake of different extracellular bodies. Whereas particles below 0.5 μm in diameter could be internalized through clathrin, caveolin, or general pinocytosis, particles of a bigger diameter will require the activation of a macropinocytic or phagocytic process (42). To this purpose, we have compared the internalization of two synthetic peptides with different aggregation propensities resulting in aggregate particles of different sizes. We found that aggregates of both peptides are efficiently internalized by non-specialist cells in culture. Further, aggregate size not only determines the mechanism of uptake but also modulates the involvement of the proteostasis machinery in the process. Whereas large aggregates with a diameter greater than 0.5 μm were taken up by phagocytosis in an HSF1 (heat shock factor 1)-dependent manner, smaller aggregates were internalized through fluid phase endocytosis in an HSF1-independent manner.
Our work demonstrates that aggregate uptake is an inherent activity of mammalian cells. It also shows that biophysical parameters that affect the aggregation propensity and particle size determine the mode of uptake as well as the proteostatic response to aggregates; whereas larger aggregates are detected by the proteostatic machinery and actively internalized, smaller aggregates remain largely undetected and enter the cell in a nonspecific manner.
EXPERIMENTAL PROCEDURES
Peptides and Reagents
Peptides PepL (sequence, RPILTIITLE RGSRRPILTI ITLE; Tango score, 1273.17), PepS (sequence, DMISYAGMDP PDMISYAGMD; Tango score, 10.44), Inf12 (sequence, RLIQLIVSRP PRLIQLIVSR; Tango score, 532.08), and Inf36 (sequence, RGVSILNLRP PRGVSILNLR; Tango score, 29.36) were custom synthesized by JPT at a purity of >95% as determined by HPLC. Lyophilized peptide powder was resuspended in DMSO to 2 mm concentration. This DMSO stock solution was diluted to working solutions in PBS or cell culture medium ranging from 2 to 20 μm, as indicated in each experiment. Dynamic light scattering analysis was performed in a DynaPro Plate Reader II (Wyatt Technology) equipped with a 830-nm wavelength laser, and Dynamics software (Wyatt Technology) was used to analyze the data. The antibody against the extracellular region of membrane Hsp70, cmHSP70.1, was a kind donation of Prof. Dr. Gabriele Multhoff. The inhibitors dynasore hydrate, 5-(N-ethyl-N-isopropyl)amiloride (EIPA),2 cytochalasin D, methyl-β-cyclodextrin (MβCD), mevinolin, rapamycin, and chlorpromazine hydrochloride were purchased from Sigma-Aldrich; KRIBB11 was obtained from Merck; VER155008 was from Tocris Bioscience; and geldanamycin was from Invivogen. Dextran (Mr 10,000) conjugated to Texas Red was purchased from Invitrogen. Purified Hsp70 was obtained from ENZO Life Sciences. Before cell culture incubations, storage solution was substituted by PBS in a Zeba Spin desalting column 7K (Thermo Scientific).
Cell Culture, Transfections, and Peptide Incubations
The HEK-293 cell line was cultured in DMEM supplemented with 10% (v/v) FCS (Invitrogen), penicillin (100 units/ml), and streptomycin (100 units/ml). Proliferating cell cultures were maintained in a 5% CO2 humidified incubator at 37 °C. Transfections were performed with FuGENE HD (Promega) according to the manufacturer's recommendations. Cellular expression of RFP fusions of proteins Rab5, Rab7, and Lamp1 was achieved by the use of baculovirus-based BacMam 2.0 probes (Invitrogen). GFP-LC3 expression was performed by transduction of cells with a Lentibrite lentiviral biosensor following the manufacturer's recommendations (Merck Millipore). For peptide treatments of culture cells, 2 mm peptide stock solutions in DMSO were diluted to a 20 μm concentration in serum-free DMEM/F-12 medium (Invitrogen) to allow the formation of aggregates before addition to the cells. Cells in culture were always at 90% confluence when peptide was added to the medium. For 20 μm incubations, cells were incubated for 1 h in the peptide solution and then washed and incubated for different periods of time in complete cell culture medium. For 5 and 2 μm incubations, 20 μm peptide solutions were added to the cell culture medium as 4× or 10× concentrates, respectively, and no further wash steps were performed unless otherwise indicated.
Immunofluorescence Staining and in Vivo Confocal Microscopy
Cell imaging was performed in vivo unless otherwise indicated, using an inverted microscope (Nikon Eclipse TE2000-S) equipped with a confocal microscopy imaging system (Nikon Eclipse C1). For time lapse experiments, a Nikon A1R Eclipse Ti was used. cmHsp70.1 staining was performed in vivo as follows. After overnight incubation in complete cell culture medium containing 5 μm peptide or 10 μm geldanamycin, cells were first blocked in a solution of 20% goat serum and 0.2% Tween 20 in PBS for 10 min and incubated immediately afterward for 1 h in 1:100 or 1:1000 antibody dilutions in PBS. Cells were then washed in PBS three times before being fixed in 4% paraformaldehyde in PBS. After fixation, cells were washed three times in PBS and mounted in ProLong gold antifade reagent with DAPI (Invitrogen). High content analysis was performed in vivo in an IN cell analyzer 2000 (GE Healthcare). The software IN Cell Developer was used for the quantification of the different structures. Internalized aggregates were differentiated from extracellular membrane-attached aggregates by means of their higher fluorescence intensity. Small peripheral endolysosomes were distinguished by their smaller size.
Transmission Electron Microscopy
For peptide aggregate solution analysis, 20 μm peptide suspensions in PBS were adhered onto carbon-coated copper grids and stained in a solution of 2% uranyl acetate for 5 min. After five rounds of washing in ultrapure water, grids were analyzed in a JEM-1400 transmission electron microscope. Cell samples were grown on Aclar and incubated with peptide as described above. At given time points, they were fixed overnight at 4 °C in 0.1 m sodium cacodylate buffer containing 2.5% glutaraldehyde. After washing, they were fixed additionally for 2 h at 4 °C in 1% osmium tetroxide, rinsed with distilled water, and dehydrated through a graded ethanol series. During the dehydration steps, they were stained in 3% uranyl acetate, 70% ethanol for 30 min at 4 °C. After the last step in 100% ethanol, samples were washed in propylene oxide and embedded in epoxy resin (epoxy-embedding kit, Fluke Analytical). After polymerization, 50-nm slices were obtained and transferred to carbon-coated copper grids. Grids were subsequently poststained for 10 min in 3% uranyl acetate/water and for 5 min in a lead citrate solution (Reynolds' formulation). After extensive washes in water, grids were air-dried and analyzed in a JEM-1400 transmission electron microscope.
Microarrays
Cells were incubated with the different peptides as indicated above. After 24 h of incubation, total RNAs were extracted using an RNeasy minikit (QIAgen). RNA concentration and purity were determined spectrophotometrically using the Nanodrop 2000 spectrophotometer (Thermo Scientific), and RNA integrity was assessed using a Bioanalyzer 2100 (Agilent, Santa Clara, CA). Per sample, an amount of 100 ng of total RNA added to bacterial RNA transcript positive controls (Affymetrix) was amplified and labeled using the GeneChip 3′ IVT express kit (Affymetrix). All steps were carried out according to the manufacturer's protocol (Affymetrix). A mixture of purified and fragmented biotinylated RNA and hybridization controls (Affymetrix) was hybridized on Affymetrix GeneChip® PrimeViewTM human gene expression arrays, followed by staining and washing in a GeneChip® fluidics station 450 (Affymetrix) according to the manufacturer's procedures. To assess the raw probe signal intensities, chips were scanned using a GeneChip® scanner 3000 (Affymetrix). Raw data were processed all together with the RMA algorithm (43) and subsequently subjected to a two-factor analysis of variance.
RESULTS
Synthetic Aggregation-prone Peptides with Low and High Aggregation Propensities form Aggregate Pools of Largely Non-overlapping Size Distributions in Vitro
Most aggregating peptides and proteins form aggregates ranging from soluble oligomers to large insoluble inclusions. Moreover, the size distribution of these aggregates evolves over time, which makes it difficult to isolate aggregates of a specific size range in solution. In order to partially circumvent this difficulty, we used TANGO (44), an algorithm to predict protein aggregation, to select two peptide sequences with either low or high aggregation propensities with the aim of generating two aggregate populations with non-overlapping (or minimally overlapping) size distributions over sufficient time to study the cellular internalization of these peptides.
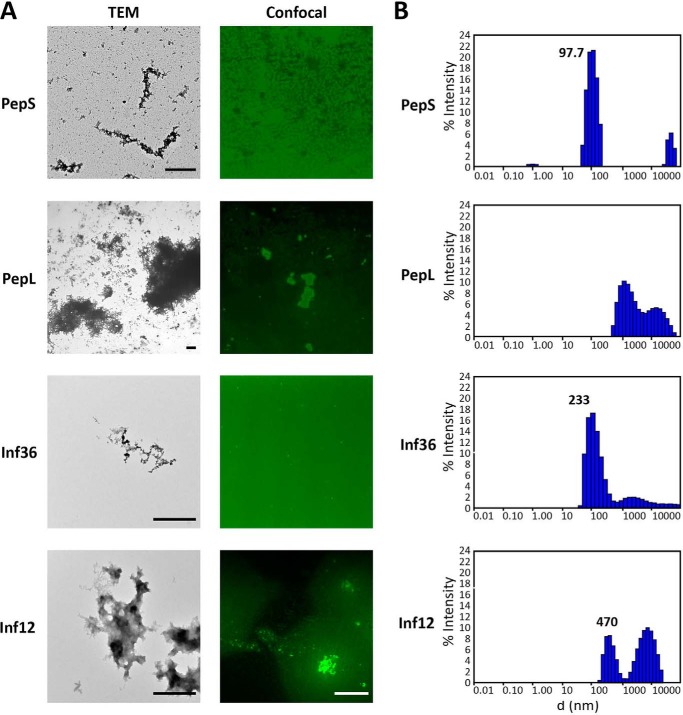
A peptide with low aggregation propensity and negative charge, referred to as PepS (for small amino acid sequence DMISYAGMDPPDMISYAGMD; Tango score, 10.44; pI = 3.3) (Table 1), was derived from the VEGFR2 (vascular-endothelial growth factor receptor 2) protein sequence. When put in solution in PBS at a concentration of 20 μm, amorphous aggregates of different sizes were observed by electron and confocal microscopy (Fig. 1A). Although particles above 1 μm were occasionally observed, confocal images and dynamic light scattering indicated that most of the peptide molecules were in a monomeric or oligomeric status (0.5-nm diameter) or in aggregates with a size distribution around 100 nm (Fig. 1B). A prolonged incubation for over a month at 37 °C with shaking at 1000 rpm did not increase the maximum size of the aggregates, although the amount of low molecular weight aggregates decreased in favor of the formation of aggregates of an approximate diameter of 500 nm (data not shown).
TABLE 1.
Sequence, aggregation propensity and isoelectric point of the peptides used throughout this study
Amino acids were colored according to the properties of their side chains: blue, positively charge; red, negatively charged; green, aliphatic; gray, polar; purple, aromatic; orange, glycines; black, prolines.
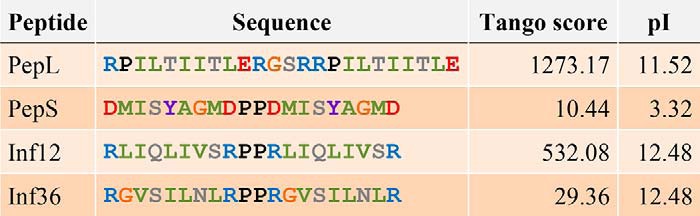
FIGURE 1.
Size analysis of PepL and PepS. A, microscopic observation of the peptide solutions. Left panels, electron microscopy. 20 μm solutions in PBS of FITC-conjugated peptides were negatively stained with uranyl acetate for TEM analysis. Scale bar, 1 μm. Right panels, confocal microscopy. Peptides conjugated to DyLight 488 were resuspended in PBS to 20 μm and observed at the confocal microscope. Scale bar, 10 μm. B, dynamic light scattering analysis of the peptide solutions. Size distribution of the aggregates present in 20 μm solutions in PBS of FITC-conjugated peptides were obtained by differential light scattering. The distributions were obtained by adjustment to a cumulant fit of the autocorrelation curves of 50 measurements of 5 s/sample. d, diameter.
The sequence of the highly aggregating positively charged peptide, referred to as PepL (for large amino acid sequence RPILTIITLERGSRRPILTIITLE; Tango score, 1280; pI = 11.5) (Table 1), consists of a tandem repeat of an aggregation-prone sequence of the p53 DNA binding domain (45). Analysis by electron and confocal microscopy of a 20 μm solution of this peptide in PBS showed, as for PepS, a heterogeneous population of amorphous aggregates of different sizes, but, contrary to PepS, confocal analysis of PepL solutions showed an enrichment in aggregates that typically exceeded 1 μm in diameter (Fig. 1A), although a population of aggregates of smaller size was also present (Fig. 1A). Dynamic light scattering analysis confirmed that these solutions are mainly composed of aggregates well over 1 μm in diameter (Fig. 1B).
We therefore managed to select two aggregating peptide sequences displaying very different charge and size distributions. Importantly, although the size distributions of PepS and PepL evolved over time, they remain distinct, with PepS peptides never exceeding a maximum size of 500 nm, whereas PepL immediately formed aggregates larger than 1 μm.
PepL Aggregates Are Fragmented on the Cell Surface Prior to Internalization
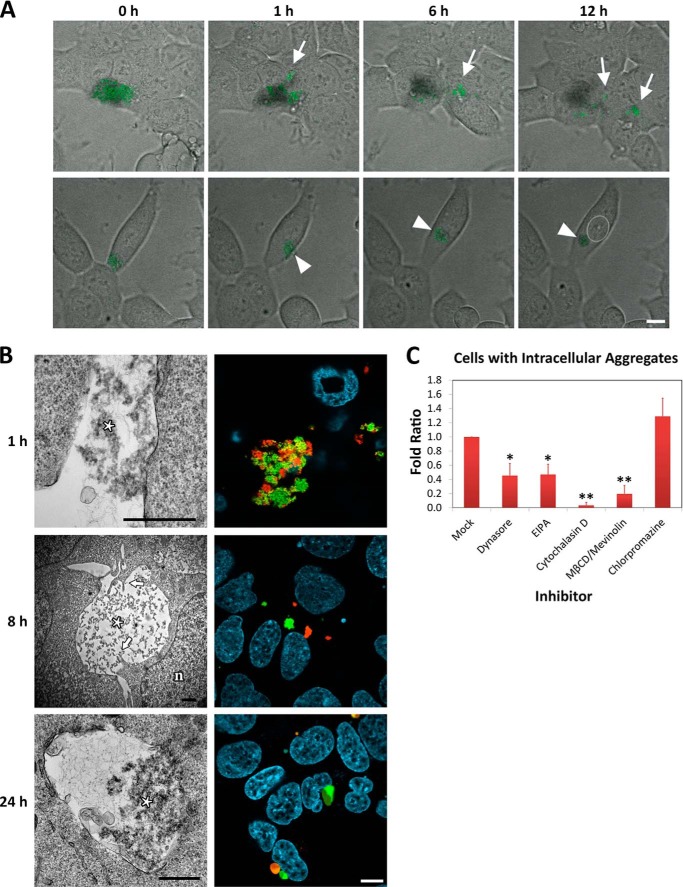
PepL was added to the culture medium of HEK-293 cells at a concentration of 20 μm. After a 1-h incubation, association of the aggregates with the cell membrane could be detected after a medium change to wash away unbound aggregates (Fig. 2A). Time lapse microscopy revealed that this association was not just deposition of the aggregates on the cell membrane but rather a dynamic interaction between peptide aggregates and the cell membrane. Two main processes were observed in this interaction. On the one hand, the biggest aggregate particles were fragmented to smaller particles (Fig. 2A (top panels, arrows) and supplemental Video 1). On the other hand, aggregates were able to move along cells and even migrate from them to neighboring cells (Fig. 2A, top panels, arrows). In some cases, aggregates contacting the periphery of a cell moved toward perinuclear regions of it, where they were engulfed (Fig. 2A (bottom panels, arrowhead) and supplemental Video 2). Confocal analysis in living cells confirmed that aggregate internalization was associated with fragmentation of large aggregates into smaller aggregates rather than disaggregation into monomeric/single peptides. To visualize this, we first prepared two separate solutions of aggregates, each labeled with a different fluorophore (DyLight 488 and DyLight 550) and subsequently mixed these together immediately before adding them to the cells. Upon mixing, both aggregates further matured into heterogeneous aggregates containing both labels (Fig. 2B, 1 h). Rather than forming vesicles containing these heterogeneous aggregates, the internalized aggregates were generally labeled with only one of the fluorophores. Thus, upon contact with the cell membrane, aggregates are broken down to smaller units corresponding to premixing aggregate nuclei rather than being disaggregated (Fig. 2B, 8 h).
FIGURE 2.
Internalization of PepL. A, time lapse imaging of peptide aggregates in contact with cell membranes. HEK-293 cells were incubated in medium containing 20 μm PepL-DyLight 488 and observed in vivo at the confocal microscope. Peptide is shown in green over bright field images. Top panels, fragmentation of aggregate conglomerates and intercellular movement of aggregates. Bottom panels, aggregate movement from the periphery to perinuclear areas. A white dotted line was drawn around the nucleus for clarity. Scale bar, 10 μm. B, internalization of PepL aggregates by confocal microscopy and TEM. Left panels, HEK-293 cells were incubated in culture medium containing 20 μm PepL. At the indicated time points, cells were fixed in glutaraldehyde and processed for TEM. Membrane interaction is shown in the top panel (1 h), engulfment is shown in the middle panel (8 h), and an “enlarged” vesicle is shown in the bottom panel (24 h). Aggregated material is marked with an asterisk. Arrows indicate cellular protrusions. Nuclei are indicated with an n. Scale bar, 1 μm. Right panels, HEK-293 cells were exposed to conglomerates of aggregates formed by DyLight 488 (green)- and DyLight 550 (red)-conjugated PepL (see text for details) and observed by confocal microscopy. Nuclei were stained with Hoechst (cyan). Scale bar, 10 μm. C, selective chemical inhibition of endocytic pathways. HEK-293 cells were incubated in medium containing 5 μm PepL-DyLight 488 in the absence (mock) or presence of the inhibitors dynasore (10 μm), EIPA (100 μm), cytochalasin D (1 μm), and MβCD (10 mm), followed by 10 μm mevinolin and 15 μm chlorpromazine. The number of cells containing internalized aggregates was quantified by high content analysis in vivo after 24 h of incubation. The percentage of cells with aggregates with respect to the total was calculated for each condition and represented as the -fold ratio with respect to untreated cells. Error bars, S.D. of three independent experiments performed in duplicate. Statistical significance after analysis of variance with Tukey's post-test is indicated as follows: α ≤ 0.01 (*) and α ≤ 0.001 (**).
PepL Is Internalized by Phagocytic Processes
The irregular morphology of the vesicles formed after internalization of the aggregates suggests phagocytic internalization. Following the process by TEM further corroborated this idea, showing contacts between aggregates and membrane, protrusions reaching over the surface of the aggregates and final engulfment (Fig. 2B, left panels). To corroborate this, a set of inhibitors of different endocytic pathways was used to better define the pathway implicated in aggregate internalization. To this end, high content analysis was performed, quantifying an average of 2000 cells/sample for the presence and number of endocytic particles, which were discerned depending on their different size and fluorescence intensity (see “Experimental Procedures”). In our assay conditions, only a percentage of cells ranging from 10 to 40% takes up an average of one aggregate per cell. Therefore, the percentage of cells containing one aggregate in the population rather than the number of aggregates internalized per cell was used as measure of peptide uptake. This percentage was reduced by inhibitors like dynasore (inhibitor of dynamin-driven endocytosis), cytochalasin D (inhibitor of actin cytoskeleton reorganization), and EIPA (inhibitor of the Na+/H+ pump) and acute treatment of the cells with MβCD (a membrane cholesterol depletor), followed by chronic inhibition of cholesterol synthesis with mevinolin (Fig. 2C). All of these inhibitors, although not specific for phagocytosis, have been described previously as phagocytosis inhibitors (46–49). On the other hand, uptake of the aggregates was not inhibited by a clathrin-mediated endocytosis inhibitor, such as chlorpromazine (Fig. 2C). Taking together the morphological and pharmacological data, the internalization of large aggregates formed by PepL is most likely due to phagocytic uptake.
PepL Disaggregation in Endosomes Is Associated with Endosomal Swelling
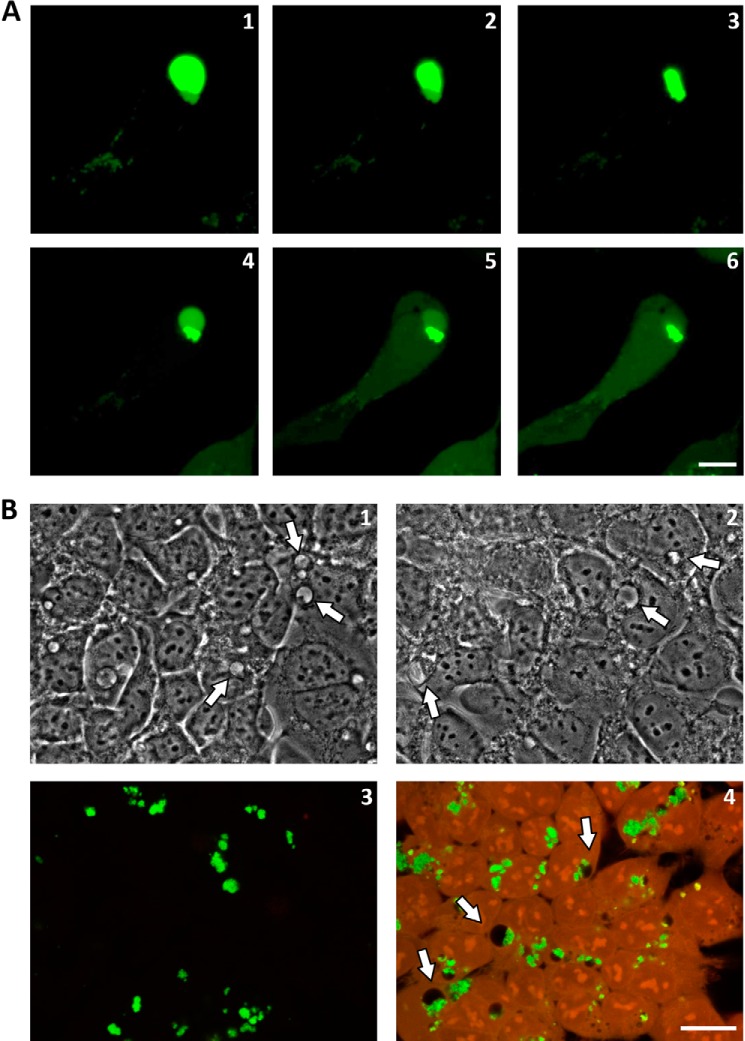
Once internalized, the particles formed vesicles of irregular size with increased fluorescence levels (Fig. 2B, 8 h). As the internalization progressed, the endosomal vesicles expanded and increased their fluorescence intensity, probably due to fluorophore dequenching. Both facts suggest that peptide disaggregation into soluble monomeric or oligomeric peptides occurs in these vesicles (Fig. 2B, 24 h). The resulting enlarged endosomes are non-homogenous. Their fluorescence intensity depicts an aggregated part of irregular shape at one pole surrounded by a rounded envelope of soluble material (Fig. 2B, 24 h). TEM analysis confirms this arrangement; the micrographs clearly show aggregated material at one pole of the vesicle surrounded by smaller soluble peptide particles, which is again indicative of disaggregation activity (Fig. 2B, 24 h). Interestingly, these enlarged endosomal vesicles were particularly sensitive to photodisruption because illumination with the confocal laser made the vesicles burst in a typically two-phase event. In a first movement, illumination resulted in membrane contraction probably due to photo-oxidative membrane damage (Fig. 3A (panels 1–3) and supplemental Video 3). This was then followed by vesicle dilatation until a final burst liberated its contents into the cytosol (Fig. 3A (panels 4–6) and supplemental Video 3). These enlarged endosomal vesicles could also be observed by bright field microscopy with non-labeled peptide, excluding the possibility that vesicle swelling is a fluorophore-associated artifact (Fig. 3B, panel 1, arrows). The structure remained morphologically identical after formaldehyde fixation when observed by bright field microscopy (Fig. 3B, panel 2, arrows). However, after chemical fixation and permeabilization with detergents, only the aggregated part of the enlarged endosomal vesicles remained, resembling cytoplasmic inclusions of irregular shape by fluorescent microscopy (Fig. 3B, panel 3). Only under conditions of high background autofluorescence (e.g. when using glutaraldehyde fixation) was the structure of the enlarged vesicle fully depicted because the empty vesicular part appeared black in contrast to the surrounding fixed cellular cytoplasmic content (Fig. 3B, panel 4, arrows).
FIGURE 3.
Morphological analysis of enlarged vesicles. A, an HEK-293 cell bearing an enlarged vesicle containing a PepL aggregate was illuminated constantly with the confocal laser (argon, 488 nm) for 15 min. Morphological changes in the vesicle were followed by time lapse confocal microscopy: 30 s (1), 3 min (2), 9 min (3), 13 min (4), 14 min (5), and 15 min (6). B, fixation artifacts. HEK-293 cells were incubated for 24 h with PepL-DyLight 488 aggregates and imaged by bright field microscopy in vivo (1), bright field after fixation in 4% formaldehyde for 20 min (2), and confocal microscopy after fixation in 4% formaldehyde (3) or 2.5% glutaraldehyde (4), followed by permeabilization in 0.1% Triton X-100. Green, PepL; red, autofluorescence. Enlarged vesicles are indicated by arrows. Scale bar, 10 μm.
PepL Aggregates Are Trafficked into the Endolysosomal Pathway
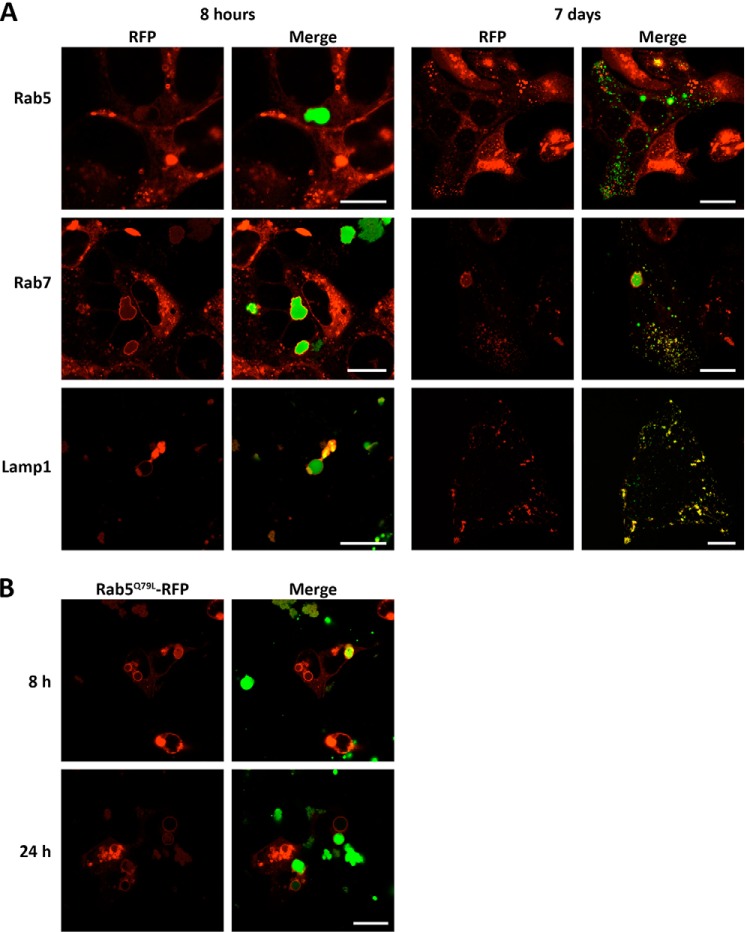
To characterize the vesicular trafficking of the aggregates, HEK-293 cells were transfected with expression vectors of several fluorescently labeled endocytosis markers before being exposed to the aggregates. We observed that ruffled vesicles and enlarged endosomal vesicles were positive for Rab7 staining and weakly positive for Rab5 staining (Fig. 4A, left panels), indicating that these compartments acquire late endosome properties rather quickly. Both ruffled and enlarged vesicles also stained for the lysosomal marker Lamp1, indicating that fusion with lysosomes or late endosomes already took place (Fig. 4A, bottom left panels). After 8 h of incubation, relatively small peripheral rounded vesicles containing the peptide were detected in the cells. These vesicles did not co-localize with the marker Rab5, but they did with markers Rab7 and Lamp1 (Fig. 4A, right panels). Because the culture medium was refreshed after the first hour of incubation, these vesicles are more likely to be due to the distribution of material contained in ruffled and enlarged vesicles into peripheral endolysosomes rather than to fluid phase endocytosis of soluble peptide still present in the extracellular solution.
FIGURE 4.
Endocytic pathways of peptide PepL internalization. A, endocytic markers. HEK-293 cells expressing the RFP-fusion proteins (red) indicated were incubated in medium containing 20 μm PepL-DyLight 488 (green). The internalization process was followed by in vivo confocal imaging at the indicated time points. B, early endosome function inhibition. Cells expressing the constitutively active mutant Rab5Q79L were incubated in culture medium containing 20 μm PepL-DyLight 488 and imaged as in A. Scale bar for A and B, 10 μm.
Despite Rab5 being just weakly visible in the membranes of the vesicles, its function is necessary for the progression of the peptides through the endosomal compartment. In fact, the expression of a constitutively active mutant of this protein (Rab5Q79L) prevented the distribution of the peptide to the peripheral endolysosomes (Fig. 4B).
PepS Is Trafficked to the Endolysosomal Pathway by Fluid Phase Endocytosis
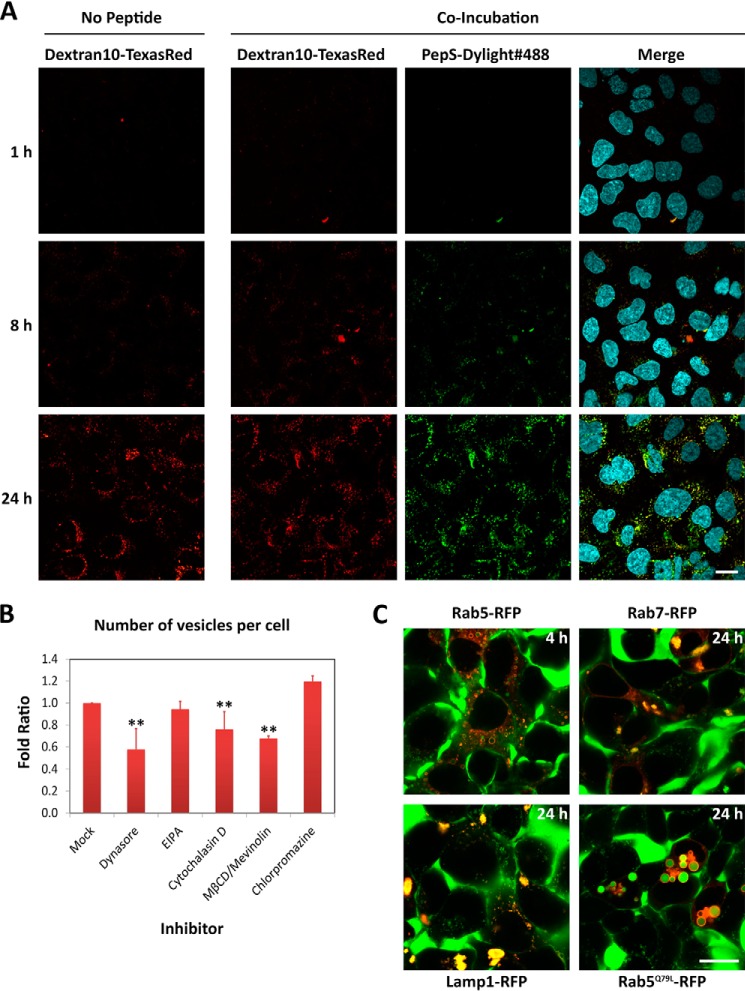
The pathway of internalization of peptide PepS was different from that of PepL from both a morphological and a functional perspective. First, contrary to PepL, confocal imaging did not detect any association of PepS with the cell membrane (Fig. 5A). Further, the uptake of PepS was similar in time and morphology to the internalization of a fluid phase marker (Texas Red-Dextran10), suggesting a mechanism of fluid phase endocytosis (Fig. 5A). Thus, the internalization of this peptide takes place through the formation of vesicles of a diameter ranging from 0.5 to 1 μm that increase progressively in fluorescence intensity over time, indicating peptide accumulation (Fig. 5A). The effect of specific endocytic inhibitors was also in agreement with fluid phase endocytosis because only partial inhibition of this process could be achieved. Through high content microscopy analysis, this decrease in the uptake can be quantified as a reduction of the number of detectable intracellular vesicles. Dynasore is the inhibitor with the biggest impact, reducing the intracellular number of vesicles to 50% in treated cells (Fig. 5B). The macropinocytosis inhibitor EIPA has a marginal impact on the number of vesicles detected, indicating that most of the peptide is internalized through other pathways. Inhibition of the cytoskeleton dynamics by cytochalasin D showed a decrease in the number of vesicles of about 25% (Fig. 5B). This could be due to a decrease in the phagocytosis or macropinocytosis of the particles of bigger size present in the solution. Finally, chlorpromazine showed an increase in peptide uptake both in number and size of the vesicles (Fig. 5B), which suggests a compensation effect on endocytic uptake through clathrin-independent endocytosis, such as pinocytosis and macropinocytosis. Overall, the partial and compensatory effects caused by the inhibitors indicate that the peptide was internalized as bulk in the fluid phase during constitutive endocytosis and not as a result of specific membrane recognition, signaling, and uptake.
FIGURE 5.
Endocytic pathways of peptide PepS internalization. A, Dextran10 co-incubation. HEK cells were incubated in medium containing 20 μm Dextran10-Texas Red (red) in the presence or absence of 20 μm PepS-DyLight 488 (green). The internalization process was followed by in vivo confocal imaging at the indicated time points. Medium was removed just before imaging at each time point to remove background fluorescence. Scale bar, 20 μm. B, selective chemical inhibition of endocytic pathways. HEK-293 cells were incubated in medium containing 5 μm PepS-DyLight 488 in the absence (mock) or presence of the indicated inhibitors at the concentrations indicated in Fig. 2C. The number of fluorescent vesicles per cell after 24 h of incubation was quantified by high content analysis in vivo and represented as the -fold ratio with respect to untreated cells. Error bars, S.D. of three independent experiments performed in duplicate. Statistical significance is indicated as in Fig. 2C. **, α ≤ 0.001. C, endocytic markers. Cells expressing the indicated RFP fusion proteins (red) were incubated in medium containing 20 μm PepS-DyLight 488 and imaged at the indicated time points by in vivo confocal microscopy. Scale bar, 20 μm. EIPA.
The use of the endocytosis markers described earlier showed that vesicles that accumulate peptide were positive for Rab7 and Lamp1 (Fig. 5C). Rab5-positive vesicles were spotted as well, more abundantly at short incubation times (Fig. 5C). Overexpression of the constitutively active mutant Rab5Q79L induced an arrest of the internalized PepS in early endosomes (Fig. 5C), as shown before for PepL. Together, this indicates that both PepS and PepL trafficking converge toward endolysosomal pathways.
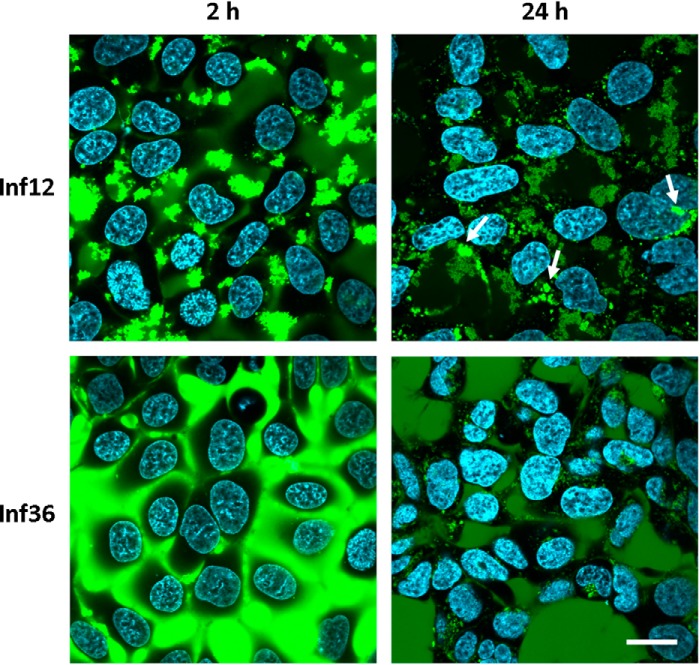
PepL and PepS differ in several biophysical parameters, such as pI, hydrophobicity, and aggregation propensity, which can modify their mechanism of interaction with membranes. It has been described that electrostatic interactions play an important role in protein-membrane interactions, and therefore the difference in net charge of the peptides could influence the difference in uptake mechanism. To rule out this possibility, we studied the cellular uptake of another set of peptides that have the same charge and similar mean hydrophobicity but only differ in their aggregation propensity. These peptides, called Inf12 and Inf36, are derived from aggregating stretches found in the basic polymerases 1 and 2 (PB1 and PB2) of the influenza A virus (Inf36, RGVSILNLRP PRGVSILNLR; Inf12, RLIQLIVSRP PRLIQLIVSR) (Table 1). Having very different Tango scores (29 and 532, respectively) they form small aggregates (79% of particles in solution are about 200 nm) and larger aggregates (64% of aggregates are around 10 μm), respectively (Fig. 1). Corroborating our previous results, the highly aggregating peptide Inf12 was internalized through the formation of large cytosolic inclusions as observed for peptide PepL (Fig. 6, top panels, arrows). On the other hand, Inf36, the least aggregating peptide, was taken up through a mechanism resembling fluid phase endocytosis (Fig. 6, bottom panels), with gradual accumulation of peptide over time in relatively small intracellular compartments, as observed for PepS. Although this additional experiment does not resolve the particular biophysical factors determining aggregate uptake, these results suggest that aggregate size plays an important modulating role in the mode of aggregate uptake by cells.
FIGURE 6.
Internalization of peptides Inf12 and Inf36. HEK-293 cells were incubated in medium containing a 5 μm concentration of peptide Inf12-DyLight 488 or Inf36-DyLight 488. The internalization was followed by in vivo confocal microscopy. Images were taken at the indicated time points. Arrows, intracellular inclusions. Scale bar, 10 μm.
PepL but Not PepS Internalization Requires Hsp70 and Is HSF1-dependent
Because phagocytosis is a specific and active process, our results suggested that extracellular aggregates were specifically recognized as cargo and internalized upon intracellular signaling. We therefore analyzed the role of the protein quality control system during aggregate internalization. The protein quality control system consists of chaperones, which assist protein folding and trafficking, and the degradation machinery, composed mainly of the proteasome and autophagy systems working independently or in collaboration with ubiquitin ligases (50–52). In addition, dedicated transcription factors promote the expression of the necessary protein quality control system components upon proteotoxic stimuli. Among these transcription factors, heat shock factor 1 (HSF1) has a prominent role (53).
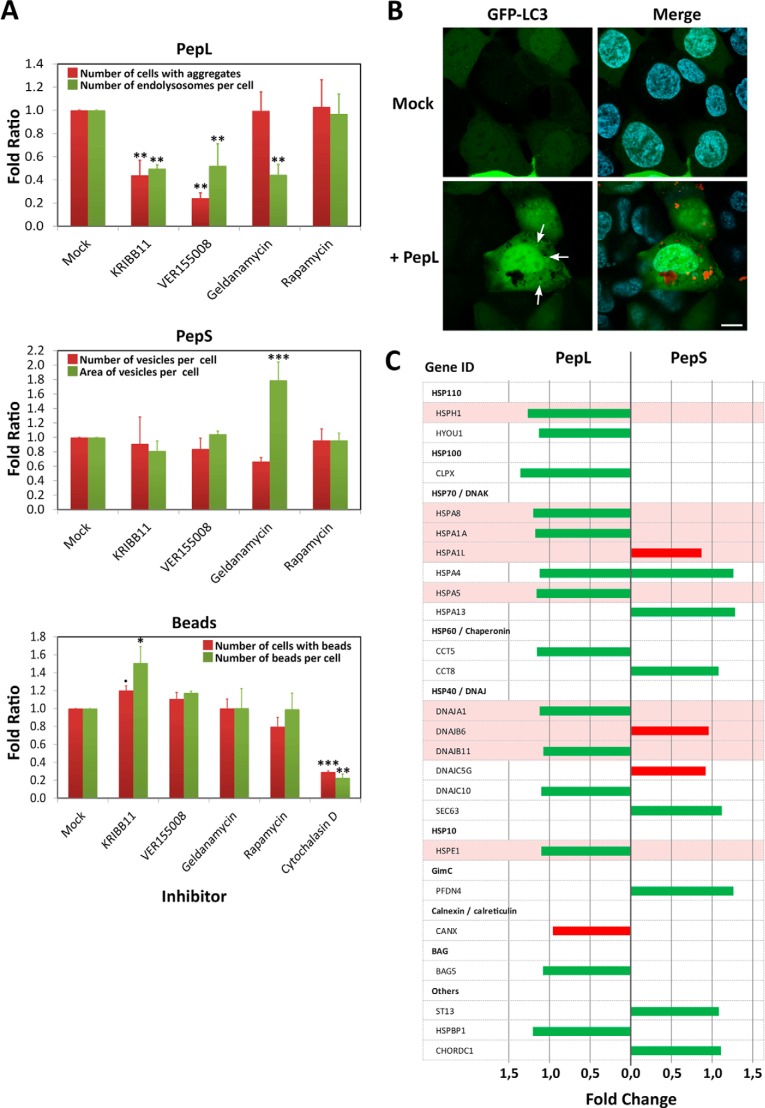
HEK-293 cells were treated before and during incubation in medium containing aggregating peptides with inhibitors of the chaperones Hsp70 (VER155008) and Hsp90 (geldanamycin), a specific inhibitor of HSF1 (KRIBB11) and an autophagy stimulator (rapamycin). Treatment with VER155008 decreased the number of cells containing internalized PepL to 20% with respect to the untreated controls, indicating a prominent role for Hsp70 in PepL aggregate uptake (Fig. 7A). Consistent with the above result, inhibition of HSF1 produced a similar inhibition pattern (Fig. 7A), showing that the uptake of large extracellular aggregates requires a proteostatic response. Contrary to PepL, the uptake of PepS was not affected by VER155008 or KRIBB11 because the use of these inhibitors did not decrease the amount of peptide being internalized by cells (Fig. 7A). This indicates that fluid phase endocytosis of small aggregates is nonspecific and HSF1-independent. As a control for the specificity of the Hsp70 inhibitors in aggregate uptake, phagocytosis of polystyrene beads of 3 μm in diameter was tested in the presence of the aforementioned inhibitors. Bead uptake was efficiently blocked by endocytosis inhibitors, such as cytochalasin D, but, on the contrary, none of the protein quality control system inhibitors tested, including KRIBB11 and VER155008, had negative effects on bead internalization (Fig. 7A), which argues for a specific role of Hsp70 in the uptake or endosomal trafficking of extracellular aggregates rather than a general role in phagocytosis or macropinocytosis of any type of particle.
FIGURE 7.
Role of the protein quality control system in the internalization of aggregating peptides. A, selective chemical inhibition of various agents of the protein quality control system. HEK-293 cells were incubated in medium containing 5 μm concentrations of peptides PepL-DyLight 488 or PepS-DyLight 488 or in a 1% suspension of polystyrene microspheres (3-μm diameter Fluoresbrite microparticles, Polysciences, Inc.) in the absence (mock) or presence of the following inhibitors: 10 μm KRIBB11, 40 μm VER155008, 10 μm geldanamycin, 100 nm rapamycin, and 1 μm cytochalasin D. Different quantifications of size and number of the different vesicle types and beads were performed by high content analysis in vivo after 24 h of incubation. Error bars, S.D. of three independent experiments performed in duplicate. Statistical significance is indicated as in Fig. 2C: α ≤ 0.05 (●); α ≤ 0.01 (*); α ≤ 0.001 (**); α = 0 (***). B, LC3 localization. HEK-293 cells expressing a fluorescent fusion protein GFP-LC3 (green) were incubated in medium containing 5 μm PepL-DyLight 550 (red) and analyzed by in vivo confocal microscopy. Images were captured after an overnight incubation. C, expression levels of several representative members of the quality control system after incubation with aggregating peptides. HEK-293 cells were incubated for 24 h in medium containing 5 μm PepL or PepS. Cells were then lysed, and total mRNAs were extracted, purified, and hybridized in an expression profile microarray (Affymetrix Human PrimeView). Up-regulated genes (green bars) and down-regulated genes (red bars) are indicated for each of the peptides. Expression levels are the average of three independent experimental replicates and are represented as the -fold change with respect to untreated controls.
Geldanamycin treatment did not reduce the number of cells internalizing PepL aggregates or the number of internalized aggregates per cell (not shown), although the number of peripheral endolysosomes per cell decreased (Fig. 7A). Because the number of cells with internalized aggregates is the same as in untreated cells, the reduction in the number of endolysosomes can only be explained by a role of Hsp90 in endosomal trafficking between early endosomes and lysosomes (54). Geldanamycin also affected the endosomal trafficking of PepS. In this case, we observed an increase in the average endosome size associated with a 40% decrease in the number of peptide-containing vesicles (Fig. 7A).
Finally, autophagy has been implicated in the degradation of intracellular aggregates through chaperone-mediated mechanisms (51). We examined the role of autophagy in the cellular trafficking of internalized aggregates using rapamycin. After incubation of cells with PepL, we did not observe any change in the uptake efficiency or the number or size of the intracellular compartments containing the peptides in rapamycin-treated cells (Fig. 7A). To further study the role of autophagy, we assayed the internalization of PepL in cells transduced with a GFP-LC3 expression vector that allows visualization of autophagosomes. The expression of this protein generated a cellular diffuse staining with the occasional presence of autophagosomes appearing as bright small vesicles (Fig. 7B, arrows). When cells expressing this GFP-LC3 protein were incubated with peptide PepL, no increase in the number of autophagosomes or colocalization between LC3 and the vesicles containing the peptides was detected (Fig. 7B). We therefore concluded that a role of autophagy in the processing of extracellular aggregates is unlikely.
Gene Expression Analysis Reveals a Proteostatic Response upon PepL Uptake
The fact that KRIBB11 had an inhibitory effect on aggregate uptake suggests a proteostatic response of cells challenged by large aggregates. We analyzed the cellular response to the presence of aggregates by mRNA microarray analysis at different time points of internalization (8 and 24 h after incubation). Changes in expression levels were evaluated using analysis of variance with a significance threshold of p < 0.01. Overall, PepL internalization had a larger effect than PepS on the gene expression profile of HEK-293 cells, both in the number of affected genes (626 versus 377 after 24 h) and the magnitude of the changes (maximum -fold change of 2.4 versus 1.5). Interestingly, there is only little overlap in gene expression changes induced by PepS and PepL internalization (4.5% after 8 h, 4% after 24 h). Looking at proteostatic changes in particular again confirmed a larger effect of PepL internalization. Notably, we observed the up-regulation of the Hsp70 family of chaperones and their co-chaperones of the Hsp40 family upon PepL but not PepS incubation (Fig. 7C). In accordance with this, we also observed the up-regulation of the chaperone Hsp110 (HSPH1) that has recently been described as part of the cytosolic machinery responsible for the disaggregation of peptide aggregates (55–57). The other two main elements of the disaggregating complex, Hsp40 (DNAJA1) and Hsp70 (HSPA1A), were also up-regulated in cells incubated with PepL (Fig. 7C). Many of these chaperones have been described as targets of HSF1 (Fig. 7C, highlighted). Although biologically relevant, given the effect of HSF1 inhibition on aggregate uptake, the -fold changes we found in response to aggregate uptake were low in comparison with the changes reported previously after heat shock and HSF1 activation, suggesting that PepL internalization induces a more subtle and less acute proteostatic response than thermal stress (58, 59).
Hsp70 Inhibits PepL Internalization by Blocking Membrane Interactions
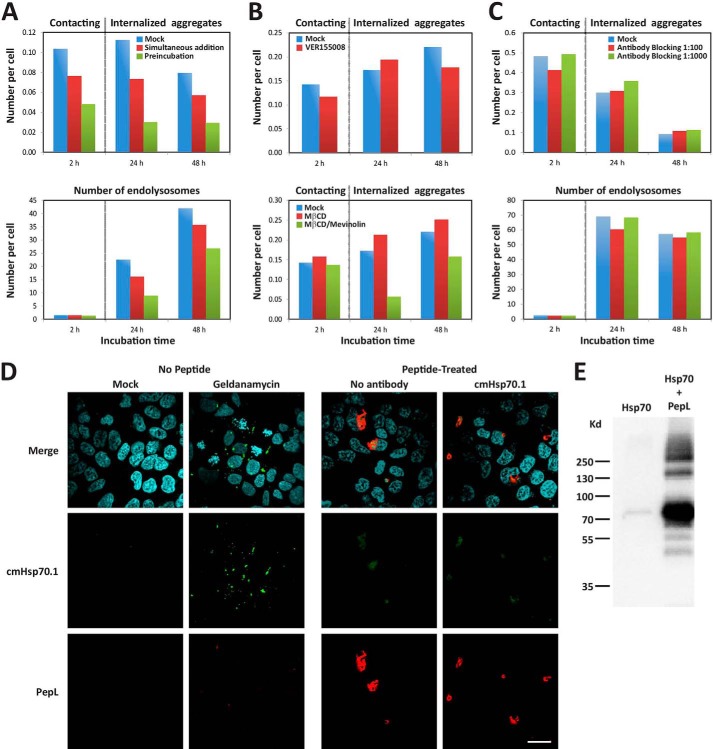
To study the role of Hsp70 in aggregate uptake, we first explored a possible extracellular activity of Hsp70. Preincubation of PepL with Hsp70 for 1 h before adding it to the cells reduced the number of internalized aggregates to 20% after 24 h of incubation (Fig. 8A). Importantly, the inhibitory effect could already be detected in the number of aggregates attached to the cell membranes within the first 2 h of incubation (Fig. 8A, 2 h). This decreased affinity for cell membranes is therefore the cause of the decreased internalization. The subsequent trafficking of the aggregates after engulfment and their distribution to endolysosomes remained unaltered compared with control cells. This went along with a decreasing number of internalized aggregates after 48 h and an increasing number of endolysosomes as the result of aggregates being processed and targeted for lysosomal degradation (Fig. 8A). Importantly, the preincubation of PepL aggregates with Hsp70 inhibited their attachment and internalization more efficiently than the simultaneous addition of aggregates and Hsp70 (Fig. 8A). This indicates that Hsp70 can alter the affinity of aggregates for the cell membrane by binding to areas on the aggregate that are required for their recognition rather than by affecting cellular endocytic activity through the modulation of cell signaling pathways or the modification of components of the cell membrane. This view is confirmed by the co-immunoprecipitation of Hsp70 with PepL (Fig. 8E).
FIGURE 8.
Role of Hsp70 in the internalization of PepL aggregates. A, extracellular addition of Hsp70 protein. A mixed solution of 6 μm PepL and 1.2 μm Hsp70 in PBS was incubated at 37 °C for 1 h and then added to the culture medium of HEK-293 cells at 90% confluence to a final concentration of 2 μm PepL and 400 nm Hsp70 (green bars, Preincubation). Alternatively, a PepL/Hsp70 solution at the same concentration was added to cells without any previous incubation (red bars, Simultaneous addition). As a negative control, a solution containing only 6 μm PepL was added to the cell culture medium (blue bars, Mock). To measure the amount of peptide attached to the cell membranes, the solution containing the peptide was removed after 1 h of incubation, and cells were washed twice with complete medium. The number of aggregates that remained attached to cell membranes was then quantified by high content analysis (2 h time point). 24 and 48 h after peptide addition, the number of internalized aggregates (top) and endolysosomes (bottom) was also quantified by high content analysis. A dotted vertical gray line separates the time points where extracellular aggregates were quantified from time points showing intracellular aggregates. B, effect of Hsp70 inhibition and cholesterol depletion on aggregate membrane attachment. HEK-293 cells were incubated in medium containing 5 μm PepL-DyLight 488 in the absence (mock) or presence of the indicated inhibitors. Top, after a 1-h incubation in the absence or presence of 40 μm VER155008, medium was removed, and cells were washed twice in complete cell culture medium and incubated without inhibitor for the indicated time periods. Bottom, after a 1-h incubation in 10 mm MβCD, cells were washed twice in complete medium and incubated in medium containing 10 μm mevinolin (MβCD/Mevinolin) or in the absence of inhibitors (Mock and MβCD). After an additional 24 h, mevinolin was removed by two medium washes, and cells were incubated for 24 h more (48 h time point). The number of attached extracellular and internalized aggregates was quantified as indicated in A. C, Hsp70 blocking antibodies. cmHsp70.1 antibody was diluted in the culture medium of HEK-293 cells to the indicated concentrations and incubated for 1 h. A solution of PepL was then added to the culture medium to a final concentration of 5 μm. After a 1-h incubation, medium was removed, and cells were washed twice and incubated in complete cell culture medium for the indicated times. The number of attached extracellular and internalized aggregates was quantified as indicated in A. D, membrane Hsp70 staining. HEK-293 cells were either treated with 10 μm geldanamycin or 5 μm peptide PepL-DyLight 550 (red) or left untreated. After an overnight incubation, cells were stained for immunofluorescence with antibody cmHsp70.1-Cy2 (green) and fixed. In samples incubated with aggregates, peptide autofluorescence is responsible for the green signal observed in both antibody-treated and untreated cell samples. Scale bar, 20 μm. E, co-immunoprecipitation of PepL and Hsp70. Biotinylated PepL and purified Hsp70 protein were incubated overnight at 4 °C. The peptide was then precipitated with streptavidin-coupled beads. After SDS-PAGE and Western blot using an anti-Hsp70 antibody, co-precipitation of Hsp70 was detected in the presence of the peptide (right lane). Nonspecific precipitation was not observed in the absence of peptide (left lane), confirming the specific binding between Hsp70 and the peptide.
Although we observed a strong blockage of aggregate uptake by inhibiting the ATPase activity of Hsp70 with VER155008 (Fig. 7A), aggregate attachment was not affected by treatment with this inhibitor because it did not decrease the number of PepL aggregates attached to cell membranes as compared with the untreated controls after 2 h of incubation (Fig. 8B, top). This implies that Hsp70 must be implicated in another step of peptide internalization, for which Hsp70 chaperoning and ATPase activity are required. Blocking this step did not prevent the aggregates from attaching to the membrane because they remained associated and could be internalized upon removal of the inhibitor (Fig. 8B, top, 24 and 48 h time points). Comparably, membranes depleted of cholesterol by MβCD treatment showed the same affinity for PepL as the untreated controls (Fig. 8B, bottom, 2 h). Following MβCD removal, the subsequent uptake of the aggregates that were already attached to the membrane was prevented by the continuous inhibition of cholesterol synthesis by mevinolin for 24 h (Fig. 8B, bottom). Removal of this inhibitor allowed the cells to restart peptide uptake after 24 h, so these cells presented an amount of internalized aggregates similar to that of untreated controls (Fig. 8B, bottom, 48 h time point). This implies that cholesterol-enriched lipid membrane rafts are not required for aggregate association to the membrane, although they may be important in the intracellular signaling leading to their internalization.
To investigate why blocking PepL with soluble Hsp70 would impede recognition, we next tested whether membrane Hsp70 could be working as a receptor for the aggregates. We therefore blocked the extracellular exposed region of the membrane-inserted Hsp70 with a specific antibody (60). As shown in Fig. 8C, a 1-h preincubation of HEK-293 cells with this antibody did not prevent attachment, internalization, or processing of PepL aggregates in treated cells and resulted in a number of extracellular attached aggregates, internalized aggregates, and endolysosomes comparable with the number detected in untreated controls (Fig. 8C). In addition, we could not detect the presence of membrane Hsp70 in peptide-treated or control cells by confocal microscopy (Fig. 8D, right panels). By contrast, treatment of the cells with geldanamycin induced the translocation of Hsp70 to membrane patches resembling those described previously (61) (Fig. 8D, left panels). This result seems to rule out the possibility of direct interaction between Hsp70 and peptide aggregates on the cellular membrane.
DISCUSSION
We have described two pathways of entry of aggregating peptides in human cultured cells: fluid phase uptake of small aggregates and the internalization of large aggregates by phagocytosis, both of which are channeled into the endolysosomal system. Based on our experimental data, we propose that these two pathways occur by default in cells for the uptake of a given peptide in solution and are not mutually exclusive. Therefore, a mixed solution containing both monomeric or oligomeric soluble peptide and large aggregates of several μm in diameter will be taken up by cells using both mechanisms. Importantly, the fluid phase uptake that we described is active for peptides whose sequences are not specifically attached to membranes. In cases where specific recognition of a peptide occurred, cells may use different endocytic mechanisms, such as clathrin, caveolin, or membrane translocation, that would speed up their internalization with respect to this fluid phase uptake. Membrane association has been described previously in different models. For instance, charge-dependent affinity for heparan sulfates is common in several cell-penetrating peptides and amyloids (25, 34, 62). In the case of aggregating peptides, several amyloids have been proposed to be recognized by other membrane receptors, mostly of the TLR and scavenger families (26–31). In addition, it has been shown that amyloid conformation could determine the membrane adhesion of small oligomers (63). Our results indicate that strongly aggregating peptides could reach a size that favors interaction with cell membranes regardless of their amyloid or amorphous organization, although the nature of this interaction remains to be investigated.
The internalization of soluble amyloidogenic species has been described previously in cell culture for α-synuclein (15, 28) and both in cell culture and in vivo for Aβ (14, 24) either by specific uptake through receptor-mediated endocytosis or passive diffusion across the cell membrane. In the case of Aβ, the internalization of soluble species has been demonstrated to promote maturation into larger aggregates due to the acidic pH and increased concentration generated in the lysosomes. It is difficult to infer whether in vivo intracellular accumulation could be achieved only by nonspecific intake during constitutive endocytosis. However, lysosomal accumulation of Aβ relies on a very slow rate of endocytosis together with a slow degradation rate (24), which are characteristics common to the mechanism described here. It is therefore possible that nonspecific fluid phase endocytosis could contribute to the internalization of aggregates in vivo.
Phagocytosis of extracellular aggregates by specialized cells is a documented feature of amyloid diseases. Microglia and astrocytes have been reported as fundamental in the clearance of Aβ plaques both in vivo and in cell culture (64, 65). Despite professional phagocytes being responsible for this function in vivo, it must be considered that most of the non-professional phagocytic cell lines are capable of phagocytosis. Therefore it cannot be excluded that, in circumstances of increasing amyloid deposition in the extracellular space, non-professional phagocytic cell lines also internalize aggregated material, in the same way that they do here when aggregates are directly decanted on their membranes in cell culture. In this regard, it must be considered that HEK-293 cells in our experiments required a minimum of 8 h to complete engulfment of the phagocytized particles, whereas Aβ-activated microglia can internalize microspheres in just 30 min (66). This is probably a reflection of the lack or low abundance of specific receptors in the cell membrane responsible for the recognition of the aggregating species, such as Toll-like, scavenger, complement, and Fc receptors.
PepL internalization by phagocytosis is strongly decreased by chemical inhibition of Hsp70 function, which did not affect the internalization of the smaller PepS aggregates by fluid phase internalization. Moreover, exposure to the bigger basic PepL aggregates induced an up-regulation of the Hsp70 expression level, whereas this remained unaffected in cells treated with the smaller acidic PepS aggregates. Both results argue in favor of a role for Hsp70 in the internalization of aggregates larger than 1 μm. Several possible functions of Hsp70 could explain these results. First, Hsp70 could be part of a chaperone-receptor complex on the cell membrane with adaptor roles between aggregate recognition and cell signaling. On the other hand, Hsp70 could be needed for its chaperoning action on components of the phagocytic machinery in a way analogous to its chaperone activity on clathrin (67). Finally, cytosolic Hsp70 could be regulating signal transduction pathways activated upon aggregate recognition. In any case, its function must be specific for aggregate recognition because inhibition of Hsp70 activity did not alter the efficiency of the uptake of polystyrene beads. In this context, it must also be considered that Hsp70 is part of the disaggregating complex formed together with Hsp40 and Hsp110 to promote disaggregation of intracellular aggregates. More importantly, the inhibition of Hsp70 activity with VER155008 halts the function of the whole disaggregating complex, so a possible dependence of aggregate uptake on disaggregase activity cannot be ruled out (55). It is relevant to point out that the morphological changes in the vesicles containing internalized aggregates, which are mostly vesicle growth to accommodate soluble material, are consistent with an intravesicular disaggregase activity. Regardless of the mechanism Hsp70 is implicated in, exposure to a high concentration of extracellular aggregates might sequester Hsp70 function, eliciting a compensatory chaperone response at the transcriptional level. This response is probably mediated by HSF1 because its specific chemical inhibition also partially inhibited aggregate uptake in our experiments.
Finally, several lines of work have demonstrated an important neuroprotective role for extracellular Hsp70 and other chaperones, such as clusterin, against the toxicity of several amyloids (68–70). This protective action has been attributed to the refolding and aggregation-inhibiting activity of the chaperone and to the activation of phagocytic cell types through membrane receptors to which Hsp70 binds directly (71, 72). In a complementary way, we have demonstrated here that extracellular Hsp70 can inhibit the interaction of peptide aggregates with cellular membranes without changing their aggregation status, which may have a large impact on the toxicity and extracellular clearance of aggregates from the intercellular space. In particular, because contact of extracellular Hsp70 with Toll-like receptors has been shown to activate phagocytosis by macrophages and microglia (71, 72), the inhibition of membrane interactions of aggregates with non-specialist cells might be an added advantage.
In conclusion, the results presented here show that cellular responses to extracellular aggregating peptides vary greatly depending on the biophysical properties of the aggregates, including aggregation propensity and aggregate size and charge. Whereas aggregates exceeding a diameter of 1 μm need specific membrane recognition and phagocytosis to enter the intracellular endosomal compartment of the cells, smaller aggregates are internalized nonspecifically through fluid phase endocytosis. Importantly, large aggregates require aggregate-specific Hsp70 chaperone activity to be internalized and trigger a chaperone response in the cells. Further studies will be necessary to evaluate the actual role of Hsp70 in aggregate recognition and uptake, which could represent a link for the transmission of extracellular proteostatic stresses into intracellular heat shock responses.
Acknowledgments
The VIB Nucleomics Core performed the microarray expression profile analysis. We thank Bart de Strooper and Iryna Benilova for useful comments.
This work was supported in part by VIB, University of Leuven, Grant GOA/11/009 (to W. A.), the Funds for Scientific Research Flanders (FWO), the Flanders Institute for Science and Technology (IWT), Federal Office for Scientific Affairs of Belgium (Belspo) Grant IUAP P7/16, and Hercules Foundation Grants AKUL/09/037 and AKUL/11/30.

This article contains supplemental Videos 1–3.
- EIPA
- 5-(N-ethyl-N-isopropyl)amiloride
- MβCD
- methyl-β-cyclodextrin
- TEM
- transmission electron microscopy.
REFERENCES
- 1. Magalhães A. C., Baron G. S., Lee K. S., Steele-Mortimer O., Dorward D., Prado M. A., Caughey B. (2005) Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. J. Neurosci. 25, 5207–5216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fevrier B., Vilette D., Archer F., Loew D., Faigle W., Vidal M., Laude H., Raposo G. (2004) Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. U.S.A. 101, 9683–9688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gousset K., Schiff E., Langevin C., Marijanovic Z., Caputo A., Browman D. T., Chenouard N., de Chaumont F., Martino A., Enninga J., Olivo-Marin J.-C., Männel D., Zurzolo C. (2009) Prions hijack tunnelling nanotubes for intercellular spread. Nat. Cell Biol. 11, 328–336 [DOI] [PubMed] [Google Scholar]
- 4. Hofmann J. P., Denner P., Nussbaum-Krammer C., Kuhn P.-H., Suhre M. H., Scheibel T., Lichtenthaler S. F., Schätzl H. M., Bano D., Vorberg I. M. (2013) Cell-to-cell propagation of infectious cytosolic protein aggregates. Proc. Natl. Acad. Sci. U.S.A. 110, 5951–5956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meyer-Luehmann M., Coomaraswamy J., Bolmont T., Kaeser S., Schaefer C., Kilger E., Neuenschwander A., Abramowski D., Frey P., Jaton A. L., Vigouret J.-M., Paganetti P., Walsh D. M., Mathews P. M., Ghiso J., Staufenbiel M., Walker L. C., Jucker M. (2006) Exogenous Induction of Cerebral β-Amyloidogenesis Is Governed by Agent and Host. Science 313, 1781–1784 [DOI] [PubMed] [Google Scholar]
- 6. Frost B., Jacks R. L., Diamond M. I. (2009) Propagation of Tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 284, 12845–12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desplats P., Lee H.-J., Bae E.-J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., Lee S.-J. (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl. Acad. Sci. U.S.A. 106, 13010–13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Münch C., O'Brien J., Bertolotti A. (2011) Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc. Natl. Acad. Sci. U.S.A. 108, 3548–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ren P.-H., Lauckner J. E., Kachirskaia I., Heuser J. E., Melki R., Kopito R. R. (2009) Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat. Cell Biol. 11, 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brundin P., Melki R., Kopito R. (2010) Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat. Rev. Mol. Cell Biol. 11, 301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frost B., Diamond M. I. (2010) Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 11, 155–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goedert M., Clavaguera F., Tolnay M. (2010) The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 33, 317–325 [DOI] [PubMed] [Google Scholar]
- 13. Omtri R. S., Davidson M. W., Arumugam B., Poduslo J. F., Kandimalla K. K. (2012) Differences in the cellular uptake and intracellular itineraries of amyloid β proteins 40 and 42: ramifications for the Alzheimer's drug discovery. Mol. Pharm. 9, 1887–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kandimalla K. K., Scott O. G., Fulzele S., Davidson M. W., Poduslo J. F. (2009) Mechanism of neuronal versus endothelial cell uptake of Alzheimer's disease amyloid β protein. PLoS One 4, e4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee H.-J., Suk J.-E., Bae E.-J., Lee J.-H., Paik S. R., Lee S.-J. (2008) Assembly-dependent endocytosis and clearance of extracellular α-synuclein. Int. J. Biochem. Cell Biol. 40, 1835–1849 [DOI] [PubMed] [Google Scholar]
- 16. Lee S.-J., Desplats P., Sigurdson C., Tsigelny I., Masliah E. (2010) Cell-to-cell transmission of non-prion protein aggregates. Nat. Rev. Neurol. 6, 702–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rajendran L., Honsho M., Zahn T. R., Keller P., Geiger K. D., Verkade P., Simons K. (2006) Alzheimer's disease β-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. U.S.A. 103, 11172–11177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Emmanouilidou E., Melachroinou K., Roumeliotis T., Garbis S. D., Ntzouni M., Margaritis L. H., Stefanis L., Vekrellis K. (2010) Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 30, 6838–6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Danzer K. M., Kranich L. R., Ruf W. P., Cagsal-Getkin O., Winslow A. R., Zhu L., Vanderburg C. R., McLean P. J. (2012) Exosomal cell-to-cell transmission of α synuclein oligomers. Mol. Neurodegener. 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alvarez-Erviti L., Seow Y., Schapira A. H., Gardiner C., Sargent I. L., Wood M. J. A., Cooper J. M. (2011) Lysosomal dysfunction increases exosome-mediated α-synuclein release and transmission. Neurobiol. Dis. 42, 360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vella L. J., Sharples R. A., Lawson V. A., Masters C. L., Cappai R., Hill A. F. (2007) Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J. Pathol. 211, 582–590 [DOI] [PubMed] [Google Scholar]
- 22. Saman S., Kim W., Raya M., Visnick Y., Miro S., Saman S., Jackson B., McKee A. C., Alvarez V. E., Lee N. C. Y., Hall G. F. (2012) Exosome-associated Tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 287, 3842–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pooler A. M., Phillips E. C., Lau D. H. W., Noble W., Hanger D. P. (2013) Physiological release of endogenous Tau is stimulated by neuronal activity. EMBO Rep. 14, 389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu X., Crick S. L., Bu G., Frieden C., Pappu R. V., Lee J. M. (2009) Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-β peptide. Proc. Natl. Acad. Sci. U.S.A. 106, 20324–20329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanekiyo T., Zhang J., Liu Q., Liu C.-C., Zhang L., Bu G. (2011) Heparan sulphate proteoglycan and the low-density lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-β uptake. J. Neurosci. 31, 1644–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh T. D., Park S.-Y., Bae J. S., Yun Y., Bae Y.-C., Park R.-W., Kim I.-S. (2010) MEGF10 functions as a receptor for the uptake of amyloid-β. FEBS Lett. 584, 3936–3942 [DOI] [PubMed] [Google Scholar]
- 27. Sung J. Y. (2001) Induction of neuronal cell death by Rab5A-dependent endocytosis of α-synuclein. J. Biol. Chem. 276, 27441–27448 [DOI] [PubMed] [Google Scholar]
- 28. Park J.-Y., Kim K. S., Lee S.-B., Ryu J.-S., Chung K. C., Choo Y.-K., Jou I., Kim J., Park S. M. (2009) On the mechanism of internalization of α-synuclein into microglia: roles of ganglioside GM1 and lipid raft. J. Neurochem. 110, 400–411 [DOI] [PubMed] [Google Scholar]
- 29. Fellner L., Irschick R., Schanda K., Reindl M., Klimaschewski L., Poewe W., Wenning G. K., Stefanova N. (2013) Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia 61, 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakamura K., Ohya W., Funakoshi H., Sakaguchi G., Kato A., Takeda M., Kudo T., Nakamura T. (2006) Possible role of scavenger receptor SRCL in the clearance of amyloid-β in Alzheimer's disease. J. Neurosci. Res. 84, 874–890 [DOI] [PubMed] [Google Scholar]
- 31. Song M., Jin J., Lim J.-E., Kou J., Pattanayak A., Rehman J. A., Kim H.-D., Tahara K., Lalonde R., Fukuchi K. (2011) TLR4 mutation reduces microglial activation, increases Aβ deposits and exacerbates cognitive deficits in a mouse model of Alzheimer's disease. J. Neuroinflammation 8, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mandrekar S., Jiang Q., Lee C. Y. D., Koenigsknecht-Talboo J., Holtzman D. M., Landreth G. E. (2009) Microglia mediate the clearance of soluble Aβ through fluid phase macropinocytosis. J. Neurosci. 29, 4252–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soura V., Stewart-Parker M., Williams T. L., Ratnayaka A., Atherton J., Gorringe K., Tuffin J., Darwent E., Rambaran R., Klein W., Lacor P., Staras K., Thorpe J., Serpell L. C. (2012) Visualization of co-localization in Abeta42-administered neuroblastoma cells reveals lysosome damage and autophagosome accumulation related to cell death. Biochem. J. 441, 579–590 [DOI] [PubMed] [Google Scholar]
- 34. Horonchik L., Tzaban S., Ben-Zaken O., Yedidia Y., Rouvinski A., Papy-Garcia D., Barritault D., Vlodavsky I., Taraboulos A. (2005) Heparan sulfate is a cellular receptor for purified infectious prions. J. Biol. Chem. 280, 17062–17067 [DOI] [PubMed] [Google Scholar]
- 35. Kiachopoulos S., Heske J., Tatzelt J., Winklhofer K. F. (2004) Misfolding of the prion protein at the plasma membrane induces endocytosis, intracellular retention and degradation. Traffic 5, 426–436 [DOI] [PubMed] [Google Scholar]
- 36. Luo K., Li S., Xie M., Wu D., Wang W., Chen R., Huang L., Huang T., Pang D., Xiao G. (2010) Real-time visualization of prion transport in single live cells using quantum dots. Biochem. Biophys. Res. Commun. 394, 493–497 [DOI] [PubMed] [Google Scholar]
- 37. Avrahami D., Dayan-Amouyal Y., Tal S., Mincberg M., Davis C., Abramsky O., Gabizon R. (2008) Virus-induced alterations of membrane lipids affect the incorporation of PrPSc into cells. J. Neurosci. Res. 86, 2753–2762 [DOI] [PubMed] [Google Scholar]
- 38. Santa-Maria I., Varghese M., Ksiezak-Reding H., Dzhun A., Wang J., Pasinetti G. M. (2012) Paired helical filaments from Alzheimer disease brain induce intracellular accumulation of Tau protein in aggresomes. J. Biol. Chem. 287, 20522–20533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kfoury N., Holmes B. B., Jiang H., Holtzman D. M., Diamond M. I. (2012) Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem. 287, 19440–19451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bayer T. A., Wirths O. (2010) Intracellular accumulation of amyloid-β: a predictor for synaptic dysfunction and neuron loss in Alzheimer's disease. Front. Aging Neurosci. 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. LaFerla F. M., Green K. N., Oddo S. (2007) Intracellular amyloid-β in Alzheimer's disease. Nat. Rev. Neurosci. 8, 499–509 [DOI] [PubMed] [Google Scholar]
- 42. Flannagan R. S., Jaumouillé V., Grinstein S. (2012) The cell biology of phagocytosis. Annu. Rev. Pathol. 7, 61–98 [DOI] [PubMed] [Google Scholar]
- 43. Irizarry R. A., Hobbs B., Collin F., Beazer-Barclay Y. D., Antonellis K. J., Scherf U., Speed T. P. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 [DOI] [PubMed] [Google Scholar]
- 44. Fernandez-Escamilla A.-M., Rousseau F., Schymkowitz J., Serrano L. (2004) Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat. Biotechnol. 22, 1302–1306 [DOI] [PubMed] [Google Scholar]
- 45. Xu J., Reumers J., Couceiro J. R., De Smet F., Gallardo R., Rudyak S., Cornelis A., Rozenski J., Zwolinska A., Marine J.-C., Lambrechts D., Suh Y.-A., Rousseau F., Schymkowitz J. (2011) Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat. Chem. Biol. 7, 285–295 [DOI] [PubMed] [Google Scholar]
- 46. Otsuka A., Abe T., Watanabe M., Yagisawa H., Takei K., Yamada H. (2009) Dynamin 2 is required for actin assembly in phagocytosis in Sertoli cells. Biochem. Biophys. Res. Commun. 378, 478–482 [DOI] [PubMed] [Google Scholar]
- 47. Ivanov A. I. (2008) Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol. Biol. 440, 15–33 [DOI] [PubMed] [Google Scholar]
- 48. Nagao G., Ishii K., Hirota K., Makino K., Terada H. (2010) Role of lipid rafts in phagocytic uptake of polystyrene latex microspheres by macrophages. Anticancer Res. 30, 3167–3176 [PubMed] [Google Scholar]
- 49. Magenau A., Benzing C., Proschogo N., Don A. S., Hejazi L., Karunakaran D., Jessup W., Gaus K. (2011) Phagocytosis of IgG-coated polystyrene beads by macrophages induces and requires high membrane order. Traffic 12, 1730–1743 [DOI] [PubMed] [Google Scholar]
- 50. McClellan A. J., Tam S., Kaganovich D., Frydman J. (2005) Protein quality control: chaperones culling corrupt conformations. Nat. Cell Biol. 7, 736–741 [DOI] [PubMed] [Google Scholar]
- 51. Lamark T., Johansen T. (2012) Aggrephagy: selective disposal of protein aggregates by macroautophagy. Int. J. Cell Biol. 2012, 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morimoto R. I. (2008) Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 22, 1427–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Akerfelt M., Morimoto R. I., Sistonen L. (2010) Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11, 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cortese K., Howes M. T., Lundmark R., Tagliatti E., Bagnato P., Petrelli A., Bono M., McMahon H. T., Parton R. G., Tacchetti C. (2013) The HSP90 inhibitor geldanamycin perturbs endosomal structure and drives recycling ErbB2 and transferrin to modified MVBs/lysosomal compartments. Mol. Biol. Cell 24, 129–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shorter J. (2011) The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS One 6, e26319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rampelt H., Kirstein-Miles J., Nillegoda N. B., Chi K., Scholz S. R., Morimoto R. I., Bukau B. (2012) Metazoan Hsp70 machines use Hsp110 to power protein disaggregation. EMBO J. 31, 4221–4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mattoo R. U. H., Sharma S. K., Priya S., Finka A., Goloubinoff P. (2013) Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates. J. Biol. Chem. 288, 21399–21411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Trinklein N. D., Murray J. I., Hartman S. J., Botstein D., Myers R. M. (2004) The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol. Biol. Cell 15, 1254–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Laramie J. M., Chung T. P., Brownstein B., Stormo G. D., Cobb J. P. (2008) Transcriptional profiles of human epithelial cells in response to heat: computational evidence for novel heat shock proteins. Shock 29, 623–630 [DOI] [PubMed] [Google Scholar]
- 60. Gehrmann M., Marienhagen J., Eichholtz-Wirth H., Fritz E., Ellwart J., Jäättelä M., Zilch T., Multhoff G. (2005) Dual function of membrane-bound heat shock protein 70 (Hsp70), Bag-4, and Hsp40: protection against radiation-induced effects and target structure for natural killer cells. Cell Death Differ. 12, 38–51 [DOI] [PubMed] [Google Scholar]
- 61. Vega V. L., Rodríguez-Silva M., Frey T., Gehrmann M., Diaz J. C., Steinem C., Multhoff G., Arispe N., De Maio A. (2008) Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J. Immunol. 180, 4299–4307 [DOI] [PubMed] [Google Scholar]
- 62. Poon G. M. K., Gariépy J. (2007) Cell-surface proteoglycans as molecular portals for cationic peptide and polymer entry into cells. Biochem. Soc. Trans. 35, 788–793 [DOI] [PubMed] [Google Scholar]
- 63. Trevino R. S., Lauckner J. E., Sourigues Y., Pearce M. M., Bousset L., Melki R., Kopito R. R. (2012) Fibrillar structure and charge determine the interaction of polyglutamine protein aggregates with the cell surface. J. Biol. Chem. 287, 29722–29728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee C. Y. D., Landreth G. E. (2010) The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 117, 949–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Paresce D. M., Ghosh R. N., Maxfield F. R. (1996) Microglial cells internalize aggregates of the Alzheimer's disease amyloid β-protein via a scavenger receptor. Neuron 17, 553–565 [DOI] [PubMed] [Google Scholar]
- 66. Koenigsknecht-Talboo J., Landreth G. E. (2005) Microglial phagocytosis induced by fibrillar β-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci. 25, 8240–8249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sousa R., Lafer E. M. (2006) Keep the traffic moving: mechanism of the Hsp70 motor. Traffic 7, 1596–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Danzer K. M., Ruf W. P., Putcha P., Joyner D., Hashimoto T., Glabe C., Hyman B. T., McLean P. J. (2011) Heat-shock protein 70 modulates toxic extracellular α-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J. 25, 326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Novoselova T. V., Margulis B. A., Novoselov S. S., Sapozhnikov A. M., van der Spuy J., Cheetham M. E., Guzhova I. V. (2005) Treatment with extracellular HSP70/HSC70 protein can reduce polyglutamine toxicity and aggregation. J. Neurochem. 94, 597–606 [DOI] [PubMed] [Google Scholar]
- 70. Yerbury J. J., Wilson M. R. (2010) Extracellular chaperones modulate the effects of Alzheimer's patient cerebrospinal fluid on Aβ1–42 toxicity and uptake. Cell Stress Chaperones 15, 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kakimura J.-I., Kitamura Y., Takata K., Umeki M., Suzuki S., Shibagaki K., Taniguchi T., Nomura Y., Gebicke-Haerter P. J., Smith M. A., Perry G., Shimohama S. (2002) Microglial activation and amyloid-β clearance induced by exogenous heat-shock proteins. FASEB J. 16, 601–603 [DOI] [PubMed] [Google Scholar]
- 72. Wang R., Town T., Gokarn V., Flavell R. A., Chandawarkar R. Y. (2006) HSP70 enhances macrophage phagocytosis by interaction with lipid raft-associated TLR-7 and upregulating p38 MAPK and PI3K pathways. J. Surg. Res. 136, 58–69 [DOI] [PubMed] [Google Scholar]