Background: Resistance to 5-fluorouracil leads to the failure of chemotherapy for colorectal cancer.
Results: Suppressing TrpC5 expression decreased nuclear β-catenin accumulation, reduced the induction of ABCB1, and reversed 5-fluorouracil resistance.
Conclusion: TrpC5 is essential in ABCB1 induction and drug resistance in human colorectal cancer cells.
Significance: These findings may help develop a novel target for overcoming resistance to chemotherapy in colorectal cancer.
Keywords: ABC Transporter, Beta-Catenin (B-catenin), Chemoresistance, Colorectal Cancer, Transient Receptor Potential Channels (TRP Channels)
Abstract
5-Fluorouracil (5-Fu) is commonly used in the chemotherapy of colorectal cancer (CRC), but resistance to 5-Fu occurs in most cases, allowing cancer progression. Suppressing ABCB1 (ATP-binding cassette, subfamily B, member 1), which is a pump overproduced in cancer cells to export cytotoxic drugs, is an attractive strategy to overcome drug resistance. In the present study, transient receptor potential channel TrpC5 was found to be overproduced at the mRNA and protein levels together with ABCB1 in 5-Fu-resistant human CRC HCT-8 (HCT-8/5-Fu) and LoVo (LoVo/5-Fu) cells. More nuclear-stabilized β-catenin accumulation was found in HCT-8/5-Fu and LoVo/5-Fu cells than in HCT-8 and LoVo cells. Suppressing TrpC5 expression with TrpC5-specific siRNA inhibited the canonical Wnt/β-catenin signal pathway, reduced the induction of ABCB1, weakened the ABCB1 efflux pump, and caused a remarkable reversal of 5-Fu resistance in HCT-8/5-Fu and LoVo/5-Fu cells. On the contrary, enforcing TrpC5 expression resulted in an activated Wnt/β-catenin signal pathway and up-regulation of ABCB1. Taken together, we demonstrated an essential role of TrpC5 in ABCB1 induction and drug resistance in human CRC cells via promoting nuclear β-catenin accumulation.
Introduction
Colorectal cancer (CRC)4 is a major cause of mortality and morbidity throughout the world (1), the third most common malignant tumor, and the fifth cause of cancer-related death in China. 5-Fluorouracil (5-Fu) is one of the most commonly used cytotoxic drugs in the chemotherapy of CRC. Resistance to 5-Fu occurs in most cases, and this results in cancer progression. High expression of ABCB1 (ATP-binding cassette, subfamily B, member 1) is the most important mechanism in drug resistance and has been considered as a target for its reversal (2). Although many ABCB1 modulators have been developed to inhibit its activity, most are either too toxic or induce intolerable pharmacokinetic interactions (2).
Intracellular Ca2+ ([Ca2+]i) is one of the crucial signals that modulate cellular functions, and some members of the Ca2+-permeable transient receptor potential canonical (TrpC) family of channel proteins are known to play roles in cancer pathogenesis (3, 4). For example, TrpC1, TrpC3, and TrpC6 play roles in tumor cell proliferation. TrpC1 and TrpC3 are involved in the proliferation of breast and ovarian cancer cells, respectively (5, 6), whereas TrpC6 is involved in the proliferation of liver, prostate, breast, and brain tumor cells (7–10). The roles of these channels in cancer may involve changes in [Ca2+]i (3). Previously, we found that TrpC5-mediated Ca2+ entry stimulates ABCB1 overproduction in drug-resistant human breast cancer cells, and inhibiting/suppressing TrpC5 reverses the drug resistance through the TrpC5-NFATC3-Ca2+-ABCB1 signal pathway (11). However, to date there is still no report on TrpC5 channel involvement in drug resistance and/or ABCB1 production in human CRC cells. The detailed mechanism by which TrpC5 regulates ABCB1 expression also deserves exploration.
Activation of the Wnt/Ca2+ pathway results in the mobilization of [Ca2+]i (12) and was recently found to prompt the nuclear accumulation of β-catenin (13), followed by activation of the transcription of T-cell factor/lymphoid enhancer factor-responsive genes (14), including ABCB1 (15). Therefore, we designed experiments to explore the possible roles of TrpC5 in regulating the nuclear translocation of β-catenin and ABCB1 expression in the context of drug resistance.
EXPERIMENTAL PROCEDURES
Cells and Cell Culture
The human CRC cell line HCT-8 and 5-Fu-resistant HCT-8 cells (HCT-8/5-Fu) were from Keygen Biotech Co. Ltd (Nanjing, China). The human CRC cell line LoVo was from the Cell Resource Center of Shanghai Institutes for Biological Sciences, Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). 5-Fu-resistant LoVo cells (LoVo/5-Fu) were obtained by treating LoVo cells with stepwise increasing concentrations of 5-Fu over 6 months. HCT-8 and LoVo cells were cultured in RMPI 1640 supplemented with 10% (v/v) inactivated fetal calf serum, penicillin (100 units/ml), and streptomycin (100 μg/ml). HCT-8/5-Fu cells were cultured in RMPI 1640 supplemented with 15 mg/liter 5-Fu, and LoVo/5-Fu cells were cultured in RMPI 1640 supplemented with 5 mg/liter 5-Fu.
Antibodies, siRNA, and Reagents
The primary antibodies anti-TrpC5 (ab63151), anti-ABCB1 (ab10333), anti-β-catenin (ab6301), and anti-cyclin D1 (ab40754) were from Abcam Biotechnology (Cambridge, MA), anti-histone (AH433), and anti-β-actin (AA128) were from Beyotime Biotechnology (Nantong, China). The secondary antibodies goat anti-rabbit IgG (A0277) and goat anti-mouse IgG (A0286) were from Beyotime Biotechnology. RIPA (P0013B) and the nuclear and cytoplasmic protein extraction kit (P0027) were from Beyotime Biotechnology. TRIzol (10296–010), Lipofectamine 2000 (11668-019), fluorophore-labeled donkey anti-rabbit IgG antibody (A11374), and fluorophore-labeled donkey anti-mouse IgG antibody (A-21202) were from Invitrogen. TrpC5-siRNA was from Invitrogen (BLOCK-iT in vivo siRNA; sense strand, CCA AUG GAC UGA ACC AGC UUU ACU U, and antisense strand, AAG UAA AGC UGG UUC AGU CCA UUG G), and TrpC5-shRNA was from Santa Cruz Biotechnology (Dallas, TX) (sc-42670-V; sc-42670-VA, sense strand, GAA CCA GCU UUA CUU CUA UTT, and antisense strand, AUA GAA GUA AAG CUG GUU CTT; sc-42670-VB, sense strand, CUA CCA UGU UUG GAA CAU ATT, and antisense strand, UAU GUU CCA AAC AUG GUA GTT; sc-42670-VC, sense strand, CCA UCU UUG UUG CCA UUC ATT, and antisense strand, UGA AUG GCA ACA AAG AUG GTT). DMSO, MTT, lanthanum (La3+), rhodamine 123, verapamil, LiCl, and XAV939 were from Sigma. T5E3, a TrpC5-specific blocking antibody, was raised in rabbits as described in our previous study (11). 5-Fu was from Jinyao Amino Acid Co. Ltd (Tianjin, China). The pCI-TrpC5 plasmid was constructed in our laboratory (16).
Cell Transfection
Cells in the logarithmic growth stage were seeded on culture dishes. On reaching 50–70% confluence, HCT-8/5-Fu and LoVo/5-Fu cells were treated with TrpC5-siRNA (HCT-8/5-Fu/RNAi and LoVo/5-Fu/RNAi) (scrambled siRNA as control, HCT-8/5-Fu/Scrambled, and LoVo/5-Fu/Scrambled) or T5E3 (20 μg/ml, overnight) (HCT-8/5-Fu/T5E3 and LoVo/5-Fu/T5E3) (preimmune IgG as control, HCT-8/5-Fu/pre IgG, and LoVo/5-Fu/pre IgG). Expression of TrpC5 and ABCB1 was assessed by real time PCR, Western blot, and immunofluorescent staining. XAV939 (an inhibitor of the canonical Wnt/β-catenin signal pathway) and LiCl (an activator of the canonical Wnt/β-catenin signal pathway) were used in this study. HCT-8/5-Fu cells were treated with XAV939 (10 μm, overnight) (HCT-8/5-Fu/XAV939) (DMSO as control, HCT-8/5-Fu/DMSO), and HCT-8 cells were treated with LiCl (20 mm, 6 h) (HCT-8/LiCl). Expression of Wnt signaling target genes was assessed by Western blot and immunofluorescent staining. HCT-8 cells were transiently transfected with the pCI-TrpC5 plasmid using Lipofectamine 2000 according to the manufacturer's instructions (HCT-8/TrpC5) (pCI as control, HCT-8/pCI).
Immunofluorescent Staining
Cells were seeded in culture dishes. When they reached 40–60% confluence, the cells were fixed in 4% paraformaldehyde. The primary antibodies anti-TrpC5 (1:1000), anti-ABCB1 (1:10), and anti-β-catenin (1:200) were used to detect expression of the TrpC5, ABCB1, and β-catenin proteins. The cells were visualized under a confocal laser scanning microscope (Leica TCS SP8, Wetzlar, Germany).
Western Blot
Whole cell protein was obtained using RIPA containing 1 mm PMSF. Nuclear protein was isolated using a nuclear and cytoplasmic protein extraction kit. The same quantity of total protein was loaded onto each lane. The proteins were electrophoresed on 8% polyacrylamide gel containing 0.1% SDS. The resolved proteins were semi-dry transferred to PVDF membrane. The primary antibodies anti-TrpC5 (1:500), anti-ABCB1 (1:500), anti-β-catenin (1:1000), and anti-cyclin D1 (1:200) were used to detect the expression of the proteins of interest. β-Actin and histone were the internal references. The antigen-antibody complexes were visualized by an enhanced chemiluminescent reaction. Protein bands were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Real Time PCR
Total RNA was extracted from cells using TRIzol. cDNA was synthesized from RNA with oligo(dT) as primer and AMV reverse transcriptase. Ten microliters of 2× Sybrgreen PCR master mix, 0.4 μl of 50× 6-carboxy-X-rhodamine, 0.4 μl of forward and reverse primers, 2 μl of cDNA template, and 6.8 μl of double-distilled H2O made up the quantitative PCR amplification system. The primer pairs are listed in Table 1. Samples were assayed in triplicate. The relative mRNA levels of TrpC5 and ABCB1 were compared using the delta-delta cycle threshold (ΔΔCT) method (17). The β-actin amplicon was used as internal control for normalization.
TABLE 1.
Real time PCR primers
| Gene | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) |
|---|---|---|
| β-Actin | GCCCTTGCTCCTTCCACTATC | CCGGACTCTTCGTACTCATCCT |
| TrpC5 | CCACCAGCTATCAGATAAGG | CGAAACAAGCCACTTATACC |
| ABCB1 | ATGCCTTCATCGAGTCACTG | TAACAAGGGCACGAGCTATG |
Rhodamine 123 Efflux Assay
Confocal microscopy and flow cytometry (FACS) were used to evaluate the efflux activity of ABCB1. For indirect measurement of rhodamine 123 efflux from cells, HCT-8, HCT-8/5-Fu/Scrambled, and HCT-8/5-Fu/RNAi cells were seeded in culture dishes. When the cells grew to 30–50% confluence, the medium was replaced with RMPI 1640 supplemented with 1 μm rhodamine 123. After 30 min of culture at 37 °C, the cells were fixed with 4.0% paraformaldehyde, washed twice with PBS, and visualized under a confocal laser scanning microscope (Leica TCS SP8). The rhodamine 123 signal was excited by 488-nm laser light, and emission was captured at 530 nm. Each field of cells was photographed to calculate the relative fluorescence intensity (RFI). For FACS analysis, HCT-8, HCT-8/5-Fu/Scrambled, and HCT-8/5-Fu/RNAi cells were resuspended in fresh culture medium containing rhodamine 123 (final concentration, 1 μm). After incubation at 37 °C in the dark for 30 min, the cells were resuspended in cold PBS and analyzed by FACS using a flow cytometer (BD FACSCalibur). Data analysis was carried out with FLOWJO 7.6 software. At least three experiments were performed with each cell type.
[Ca2+]i Measurement
La3+ is known to potentiate TrpC5 activity but inhibit many other Ca2+-permeable channels (11, 18). We used a Ca2+-sensitive molecular construct, GECO1.2 (19). Normal physiological saline solution contained 140 mm NaCl, 5 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 10 mm glucose, and 5 mm Hepes (pH 7.4) (15). GECO1.2 fluorescence signals were measured at room temperature using an Olympus fluorescence imaging system. Changes in [Ca2+]i were displayed as the fluorescence intensity relative to the value before La3+ application (100 μm, F1/F0). Genistein (50 μm) was included in the bath solution to potentiate TrpC5.
MTT Assay
The cytotoxic effect of 5-Fu was evaluated at 48 h with the MTT assay. Cells (1 × 104cells/200 μl) were seeded in 96-well plates and placed in an incubator at 37 °C for 12 h. The medium was then replaced with medium containing different concentrations of 5-Fu. After 48 h, the medium was discarded, and each well received 200 μl of fresh medium containing 5 mg/ml MTT for 4 h. DMSO (150 μl) was added to each well, and then the absorbance was determined at 490 nm.
Statistical Analysis
The results are presented as means ± S.E. Statistical significance was determined by Student's t test. A value of p < 0.05 was considered statistically significant.
RESULTS
Up-regulated Expression of TrpC5 and ABCB1 in 5-Fu Chemoresistant Human CRC Cells
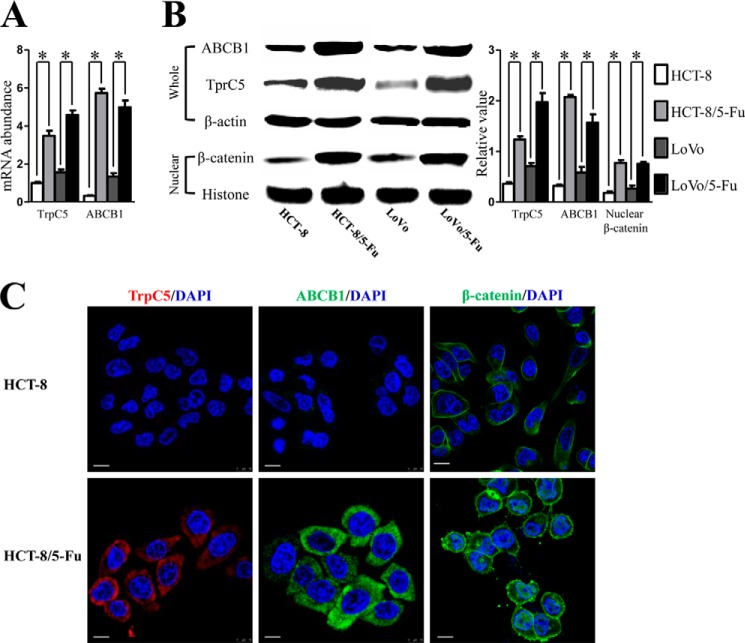
Real time PCR, Western blot, and immunostaining were analyzed to determine TrpC5 and ABCB1 expression at the mRNA and protein levels in HCT-8, HCT-8/5-Fu, LoVo, and LoVo/5-Fu cells. A much higher expression of both TrpC5 and ABCB1 at the mRNA and protein levels was found in HCT-8/5-Fu and LoVo/5-Fu cells, whereas only a low level of TrpC5 and ABCB1 expression was detected in their parental lines HCT-8 and LoVo (Fig. 1, A–C). Immunostaining showed that TrpC5 and ABCB1 were mostly localized near the cell surface (Fig. 1C).
FIGURE 1.
A and B, real time PCR (A) and Western blot (B) showed much higher expression of both TrpC5 and ABCB1 at the mRNA and protein levels in HCT-8/5-Fu and LoVo/5-Fu cells than in their parental lines (HCT-8 and LoVo cells). *, p < 0.05 (Student's t test). B, Western blot showed more nuclear-stabilized β-catenin accumulation in HCT-8/5-Fu and LoVo/5-Fu cells than in HCT-8 and LoVo cells. *, p < 0.05, (Student's t test). C, immunofluorescent staining showed higher expression of TrpC5 and ABCB1 proteins and more nuclear-stabilized β-catenin accumulation in HCT-8/5-Fu cells than in HCT-8 cells. TrpC5 and ABCB1 proteins were mostly localized near the cell surface. Scale bars, 20 μm.
Expression of TrpC5 Was Required for the Increase of [Ca2+]i in HCT-8/5-Fu and LoVo/5-Fu Cells
Application of La3+ (100 μm) elicited a rise in [Ca2+]i in HCT-8/5-Fu and LoVo/5-Fu but not in HCT-8 and LoVo cells. The La3+-elicited [Ca2+]i rise in HCT-8/5-Fu and LoVo/5-Fu cells was inhibited by T5E3 (Fig. 2, A and B), a TrpC5-specific blocking antibody, indicating the involvement of TrpC5 in the process.
FIGURE 2.
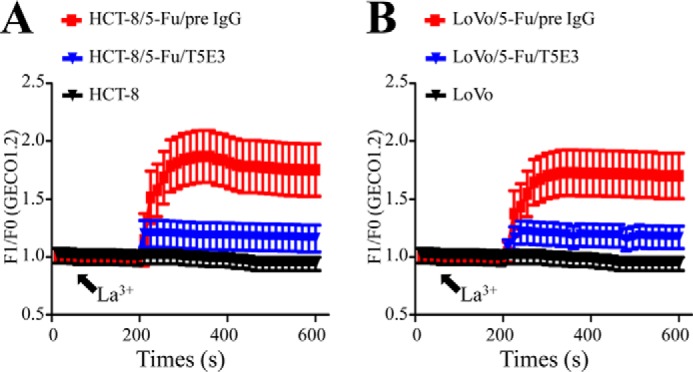
A and B, [Ca2+]i measurements using GECO1.2 showed that the TrpC5 in HCT-8/5-Fu (A) and LoVo/5-Fu (B) cells was functional. Shown are the time courses of [Ca2+]i changes in response to La3+ (100 μm). Application of La3+ elicited a rise in [Ca2+]i in HCT-8/5-Fu (A) and LoVo/5-Fu (B) but not in HCT-8 and LoVo cells. The La3+-elicited [Ca2+]i rise in HCT-8/5-Fu and LoVo/5-Fu cells was inhibited by T5E3, a TrpC5-specific blocking antibody. pre IgG, preimmune IgG.
Suppression of TrpC5 Inhibited ABCB1 Induction, Weakened ABCB1 Efflux Pump Activity, and Reversed 5-Fu Resistance in HCT-8/5-Fu and LoVo/5-Fu Cells
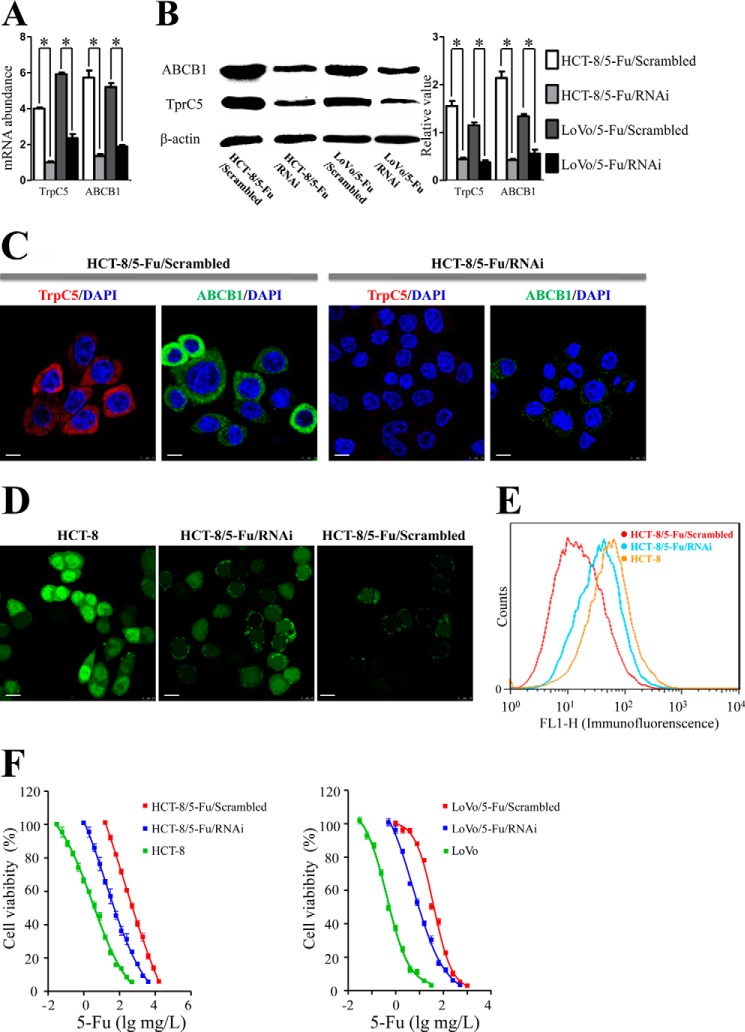
We then explored the possible role of TrpC5 in controlling ABCB1 expression in HCT-8/5-Fu and LoVo/5-Fu cells using TrpC5-specific siRNA. Real time PCR showed that not only the TrpC5 mRNA level decreased, but also the ABCB1 mRNA level was significantly down-regulated compared with scrambled siRNA in both HCT-8/5-Fu and LoVo/5-Fu cells (Fig. 3A). These results were validated by Western blot (Fig. 3B) and immunostaining (Fig. 3C). TrpC5-siRNA caused a reduction in ABCB1 protein expression compared with scrambled siRNA in HCT-8/5-Fu and LoVo/5-Fu cells.
FIGURE 3.
A–C, real time PCR (A), Western blot (B), and immunostaining (C) showed that both TrpC5 and ABCB1 expression at the mRNA and protein levels were significantly down-regulated in HCT-8/5-Fu/RNAi and LoVo/5-Fu/RNAi cells compared with scrambled siRNA in HCT-8/5-Fu and LoVo/5-Fu cells. *, p < 0. 05 (Student's t test). D, confocal laser scanning showed that the fluorescence intensity in HCT-8/5-Fu/Scrambled cells was lower than in HCT-8 cells and increased after inhibition of TrpC5 with siRNA (green fluorescence indicates rhodamine 123, an ABCB1 substrate). E, FACS analysis showed low accumulation of rhodamine 123 immunofluorescence in HCT-8/5-Fu/Scrambled cells compared with HCT-8 cells, and this increased after inhibition of TrpC5 with siRNA. F, MTT assay showed that HCT-8/5-Fu/Scrambled and LoVo/5-Fu/Scrambled cells were much more resistant to 5-Fu-induced cell death than HCT-8 and LoVo/5-Fu cells. Administration of TrpC5-siRNA caused a remarkable reversal of 5-Fu resistance. *, p < 0.05 (Student's t test). Scale bars, 20 μm. Scrambled, scrambled siRNA.
ABCB1 efflux pump activity was measured as accumulation of fluorescence of the ABCB1 substrate rhodamine 123. Confocal laser scanning showed that the fluorescence intensity in HCT-8/5-Fu/Scrambled cells was lower than in HCT-8 cells and increased after inhibition of TrpC5 with siRNA (Fig. 3D). FACS analysis gave similar results (Fig. 3E).
We next explored the possibility that inhibiting TrpC5 reverses the resistance to 5-Fu in HCT-8/5-Fu cells. The MTT assay showed that HCT-8/5-Fu/Scrambled cells were much more resistant to 5-Fu-induced cell death than HCT-8 cells. The half-maximal inhibitory concentrations of 5-Fu (5-Fu IC50) in HCT-8/5-Fu/Scrambled and HCT-8 cells were 186.6 mg/liter (95% confidence interval (CI): 39.6–878.3 mg/liter) and 3.1 mg/liter (95% CI: 2.2–4.3 mg/liter). Verapamil, a classic ABCB1 inhibitor, was used as the positive control. Treatment with 5-Fu in combination with verapamil (10 μm) increased the chemosensitivity of HCT-8/5-Fu cells to 5-Fu significantly, with the 5-Fu IC50 decreased to 31.13 mg/liter (95% CI: 27.85–34.79 mg/liter). Similarly, administration of TrpC5-siRNA caused a remarkable reversal of 5-Fu resistance in HCT-8/5-Fu cells, with the 5-Fu IC50 decreased to 17.54 mg/liter (95% CI: 5.77–53.31 mg/liter) (Fig. 3F). Similar results were obtained in experiments in LoVo and LoVo/5-Fu cells (Fig. 3F). The 5-Fu IC50 of LoVo/5-Fu cells (38.68 mg/liter, 95% CI: 34.60–43.25 mg/liter) was significantly higher than LoVo cells (0.42 mg/liter, 95% CI: 0.36–0.50 mg/liter) and dramatically decreased to 5.45 mg/liter (95% CI: 3.91–7.60 mg/liter) in LoVo/5-Fu/RNAi cells (Fig. 3F).
Translocation of β-Catenin Is Involved in the Regulation of ABCB1 by TrpC5
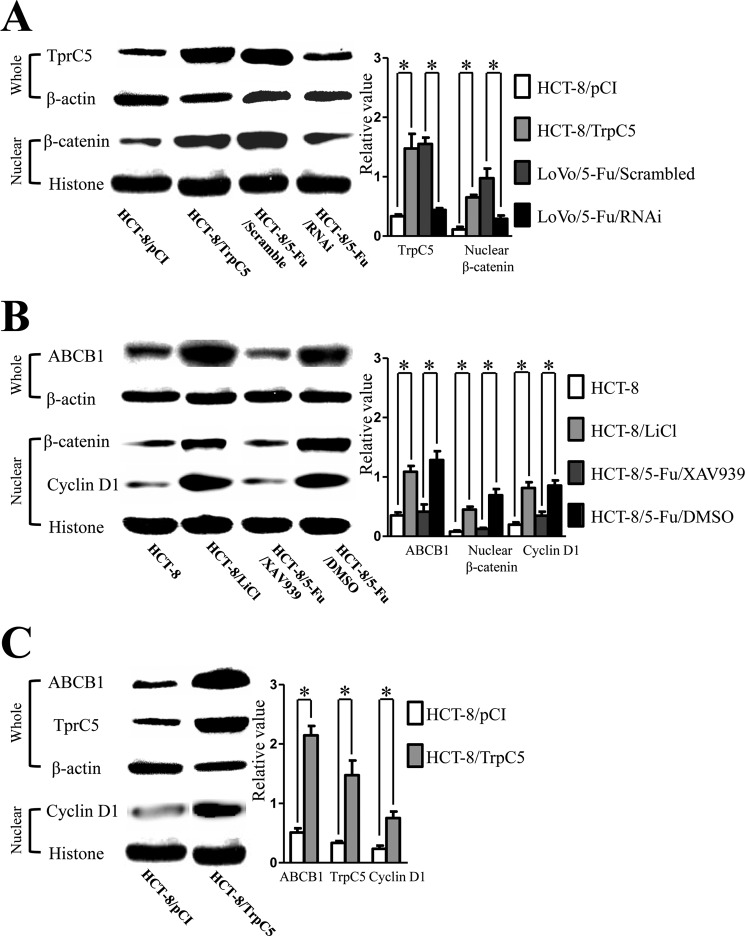
TrpC5 is considered to increase [Ca2+]i, and this has been reported to facilitate β-catenin translocation into the nucleus (13). In HCT-8/5-Fu and LoVo/5-Fu cells, together with higher membrane expression of TrpC5, β-catenin showed more stable accumulation in the nucleus than in HCT-8 and LoVo cells (Fig. 1, B and C). Suppressing TrpC5 expression with TrpC5-specific siRNA in HCT-8/5-Fu cells resulted in decreased nuclear accumulation of β-catenin, and on the contrary, enforcing TrpC5 expression in HCT-8 (HCT-8/TrpC5) cells increased the nuclear accumulation of β-catenin (Fig. 4A).
FIGURE 4.
A, Western blot showed that nuclear accumulation of β-catenin was down-regulated in HCT-8/5-Fu/RNAi cells compared with scrambled siRNA in HCT-8/5-Fu cells, whereas enforcing TrpC5 expression in HCT-8 (HCT-8/TrpC5) cells increased the nuclear accumulation of β-catenin. *, p < 0.05 (Student's t test). B, Western blot showed more nuclear expression of cyclin D1 protein in HCT-8/5-Fu/DMSO than HCT-8 cells. Administration of XAV939 (an inhibitor of the canonical Wnt/β-catenin signal pathway) decreased the nuclear accumulation of β-catenin in HCT-8/5-Fu cells, as well as ABCB1 expression and the nuclear expression of cyclin D1. On the contrary, exposure to LiCl (an activator of the canonical Wnt/β-catenin signal pathway) resulted in more nuclear accumulation of β-catenin, as well as greater nuclear expression of cyclin D1 and ABCB1 membrane expression in HCT-8 cells. *, p < 0.05 (Student's t test). C, Western blot showed that up-regulation of TrpC5 expression in HCT-8 cells led to translocation of β-catenin to the nucleus and up-regulated expression of cyclin D1 and ABCB1. *, p < 0.05 (Student's t test). Whole, whole cell protein; Nuclear, nuclear protein.
We next explored the possible mechanism by which β-catenins involved in the regulation of ABCB1 induction by TrpC5. Cyclin D1 and ABCB1 are considered to be the target genes of β-catenin in the activated canonical Wnt/β-catenin signal pathway (15, 20–22). Western blot showed more cyclin D1 protein expression in the nucleus of HCT-8/5-Fu/DMSO than HCT-8 cells (Fig. 4B). Administration of XAV939 decreased the nuclear accumulation of β-catenin in HCT-8/5-Fu cells, decreased ABCB1 expression, and decreased the nuclear expression of cyclin D1. Also, the 5-Fu IC50 decreased to 35.34 ng/ml (95% CI: 31.73–39.36 mg/liter). On the contrary, exposure to LiCl increased the nuclear accumulation of β-catenin, as well as the nuclear expression of cyclin D1 and ABCB1 expression in HCT-8 cells (Fig. 4B). Furthermore, up-regulation of TrpC5 expression in HCT-8 cells led to translocation of β-catenin to the nucleus and up-regulated expression of cyclin D1 and ABCB1 (Fig. 4C).
DISCUSSION
Resistance to chemotherapy occurs universally during malignant tumor treatment and contributes to most cancer-related deaths. The most common and standard strategy to overcome resistance is to use other cytotoxic agents, but this does not work in most cases because of multidrug resistance (MDR).
MDR tumor cells can become resistant to the drugs originally used to treat them and cross-resistant to other drugs with different mechanisms of action. MDR is believed to cause treatment failure in >90% of patients with metastatic cancer (23). A typical example is the outcome of the randomized GERCOR study (24), in which previously untreated advanced CRC patients received FOLFIRI (Irinotecan, 5-Fu, and leucovorin) followed by FOLFOX (oxaliplatin, 5-Fu, and leucovorin) or the reverse sequence. Although both regimes achieved response rates of >50% in first line therapy, considerably fewer patients (4 and 15%) responded to second line therapy with both regimes. A reasonable explanation for this is MDR.
ABCB1, also known as P-glycoprotein, coded by the ABCB1/MDR1 gene, is an ATP-dependent membrane transport protein, and its high expression in cancer cells is considered to be the major cause of MDR and the failure of chemotherapy. ABCB1 has been a research focus for overcoming resistance, but many ABCB1 antagonists have failed, because of either toxicity or intolerable pharmacokinetic interactions (2).
[Ca2+]i is involved in the transcriptional regulation of ABCB1 and is a promising target for modulating ABCB1 production (19, 25, 26). Ca2+ signaling is indispensable for maintaining physiological functions in all living cells. Studies linking Ca2+ signaling to nonmalignant tumors have emerged (as well as cardiovascular (27) and neurological diseases (28)) over many years, whereas only recently, increasing numbers of studies have been concerned with Ca2+ signaling in malignant tumors and have demonstrated its involvement in many key aspects of cancer development and progression. Among these, Trp channels have received the most attention. Trp channels are expressed in a wide variety of tissues and have many functions including distinguishing sensations such as pain, temperature, taste, and pressure (29). Recently, abnormal expression of Trp channels has been found in many tumors and is considered to participate in the biological behavior of cancer cells, including invasion (30, 31), proliferation (6), differentiation (32), and tumor vascularization (33).
As Ca2+ entry channels, Trp channels have recently been associated with ABCB1 induction. TrpC5 is essential for ABCB1 overproduction in adriamycin-resistant breast cancer cells (MCF-7/ADM) via the TrpC5-Ca2+-NFATc3-ABCB1 signaling cascade (11), and the transfer of TrpC5-containing extracellular vesicles from MCF-7/ADM cells to the parental line MCF-7/WT cells allows recipient cells to acquire TrpC5, consequently stimulating ABCB1 production and thus conferring chemoresistance on nonresistant cells (16).
In this study, ABCB1 expression was higher in HCT-8/5-Fu and LoVo/5-Fu cells than in the parental HCT-8 and LoVo cells, consistent with previous studies (34, 35). Also, TrpC5 was overexpressed in HCT-8/5-Fu and LoVo/5-Fu cells compared with HCT-8 and LoVo cells. Application of La3+ elicited a rise in [Ca2+]i in HCT-8/5-Fu and LoVo/5-Fu but not in HCT-8 and LoVo cells, and this was inhibited by T5E3, indicating that TrpC5 in HCT-8/5-Fu and LoVo/5-Fu cells has constitutive activity that is required for the increase of [Ca2+]i. We next knocked down TrpC5 expression in HCT-8/5-Fu and LoVo/5-Fu cells using TrpC5-siRNA. As a result, the mRNA and protein levels of ABCB1 decreased significantly. Rhodamine 123, a fluorescent cationic dye, is an established substrate for ABCB1. It has been used as a molecular probe in studies pertaining to MDR phenotypes (36, 37). We assessed the RFI of rhodamine 123 in cells by confocal microscopy and found that the RFI in HCT-8 cells was markedly higher than in HCT-8/5-Fu cells, and the RFI in HCT-8/5-Fu cells increased after TrpC5 was down-regulated by siRNA. MTT assays showed that inhibition of TrpC5 using TrpC5-siRNA reversed the 5-Fu resistance in HCT-8/5-Fu and LoVo/5-Fu cells. These results showed that overexpression of ABCB1 contributes to the resistance of HCT-8/5-Fu and LoVo/5-Fu cells to 5-Fu and is modulated by Ca2+ entry through TrpC5. In this process, TrpC5 is the key modulator that regulates Ca2+ entry and ABCB1 expression. This is in accord with our prior findings, in which we identified the role of TrpC5 and ABCB1 in breast cancer cell resistance to adriamycin (11).
Wnt-related signaling includes the canonical Wnt/β-catenin and the noncanonical Wnt/Ca2+ pathways. In the activated canonical Wnt/β-catenin pathway, phosphorylation of glycogen synthase kinase 3β results in the down-regulation of glycogen synthase kinase 3β activity, decreased β-catenin degradation in the cytoplasm, and subsequently the accumulation of β-catenin in the nucleus (38), where it binds to lymphoid enhancer factor/T-cell factor and activates Wnt target genes including cyclin D1 and ABCB1 (15, 20–22). Activation of canonical Wnt/β-catenin signaling has been demonstrated to induce chemoradioresistance in cancer cells (39–41). Recently, it was shown to be associated with up-regulation of ABCB1 and to induce MDR in 5-Fu-resistant human cholangiocarcinoma cells (22). In the present study, we found the same phenomenon in the 5-Fu-resistant human CRC cells HCT-8/5-Fu.
The two Wnt-related signaling pathways were thought to operate independently (42), but recent studies indicated that they act in a coordinated manner (13). Activation of the Wnt/Ca2+ pathway results in an increase of [Ca2+]i and depolarization of both the cell and nuclear membranes, subsequently promoting β-catenin translocation into the nucleus. This indicates a novel pathway through which TrpC5 regulates the induction of ABCB1. As a nonselective Ca2+-permeant cation channel, TrpC5 could modulate Ca2+ entry into the cytoplasm. Thus, overexpression of TrpC5 might increase [Ca2+]i, cause the nuclear translocation of β-catenin, and induce ABCB1 gene production. This hypothesis was validated by our studies. TrpC5 overexpression and greater accumulation of stabilized β-catenin in the nucleus were simultaneously found in 5-Fu-chemoresistant cells (HCT-8/5-Fu and LoVo/5-Fu cells). Furthermore, knockdown of TrpC5 by TrpC5-siRNA caused a remarkable decrease in the nuclear accumulation of β-catenin in HCT-8/5-Fu cells, whereas enforcing TrpC5 expression increased the nuclear accumulation of β-catenin in HCT-8 cells. We carried out additional experiments and demonstrated that the Wnt target genes cyclin D1 and ABCB1 were regulated by the nuclear accumulation of β-catenin in HCT-8 and HCT-8/5-Fu cells. Importantly, we found that enforcing TrpC5 expression resulted in the increased nuclear accumulation of β-catenin and subsequent up-regulated expression of the Wnt target genes cyclin D1 and ABCB1. Thus, we hypothesize the existence of a TrpC5-Ca2+-β-catenin-ABCB1 signal pathway (Fig. 5).
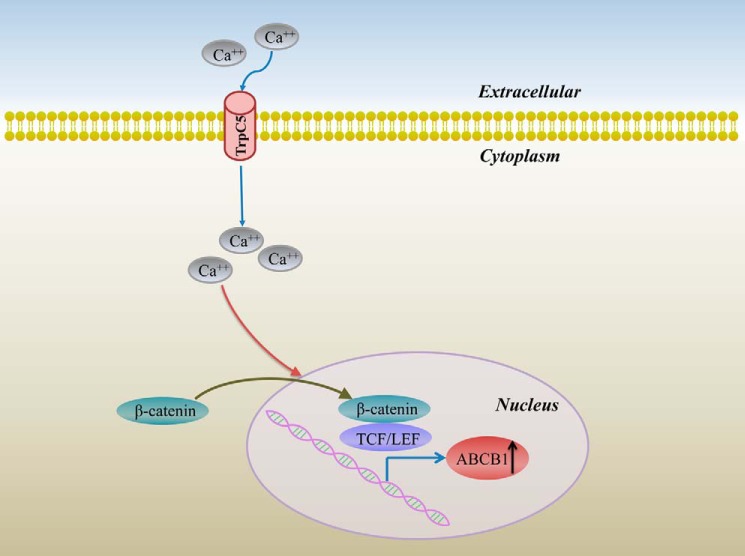
FIGURE 5.
Schematic of the role of TrpC5 in ABCB1 production in HCT-8/5-Fu cells. Overexpression of TrpC5 leads to increased [Ca2+]i, facilitates the translocation of β-catenin into the nucleus, and induces ABCB1 gene production. Inhibiting TrpC5 inhibits nuclear β-catenin accumulation, reduces the induction of ABCB1, and causes a remarkable reversal of 5-Fu resistance in HCT-8/5-Fu cells.
In our previously study (11), the TrpC5-Ca2+-NFATc3-ABCB1 signal pathway was identified as the mechanism by which TrpC5 regulates ABCB1 production. It has been reported that inhibition of glycogen synthase kinase 3β promotes the accumulation of both NFATc3 and β-catenin in the nucleus (43, 44), but to date no link between NFATc3 and β-catenin has been demonstrated. Thus, the TrpC5-Ca2+-β-catenin-ABCB1 signal pathway may be a novel means by which TrpC5 regulates ABCB1 production.
It is generally acknowledged that ABCB1 overexpression contributes to most of the chemotherapy resistance. In addition to our previous findings in breast cancer, TrpC5 was demonstrated to be essential in the induction of ABCB1-mediated resistance to 5-Fu in CRC cells. The underlying mechanism involves the translocation of β-catenin into the nucleus facilitated by an increase of [Ca2+]i triggered by TrpC5. These findings help to understand the complicated underlying mechanism of MDR. Moreover, our study puts forward the idea that TrpC5 may be a target for the reversal of clinical CRC chemoresistance.
This work was supported by the Natural Science Foundation for Distinguished Young Scholars of Jiangsu Province BK20140004 (to X. M.); the National High Technology Research and Development Program of China SQ2015AA0201349 (863 Program; to X. M.); the Program for New Century Excellent Talents in University of The Ministry of Education of China NCET-12-0880 (to X. M.); National Natural Science Foundation of China Grants 81100185, 91439131 (to X. M.) and 81273437 (to J. J.); Fundamental Research Funds for the Central Universities Grant JUSRP51311A (to X. M. and J. J.), Jiangsu Province National Natural Science Foundation Grant BK2010161 (to D. H.), Jiangsu Province Clinical Medical Science and Technology Specialized Research Fund Grant BL2014019 (to D. H.), scientific and technological development funds from Wuxi science and technology bureau CSE31N1419 (to T. W.), and Strategic Priority Research Program Grant XDA01040000 from the Chinese Academy of Sciences (to J. J.).
- CRC
- colorectal cancer
- 5-Fu
- 5-fluorouracil
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- RFI
- relative fluorescence intensity
- CI
- confidence interval
- MDR
- multidrug resistance.
REFERENCES
- 1. Haggar F. A., Boushey R. P. (2009) Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 22, 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goda K., Bacsó Z., Szabó G. (2009) Multidrug resistance through the spectacle of P-glycoprotein. Curr. Cancer Drug Targets 9, 281–297 [DOI] [PubMed] [Google Scholar]
- 3. Lehen'kyi V., Prevarskaya N. (2011) Oncogenic TRP channels. Adv. Exp. Med. Biol. 704, 929–945 [DOI] [PubMed] [Google Scholar]
- 4. Ding X., He Z., Zhou K., Cheng J., Yao H., Lu D., Cai R., Jin Y., Dong B., Xu Y., Wang Y. (2010) Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. J. Natl. Cancer Inst. 102, 1052–1068 [DOI] [PubMed] [Google Scholar]
- 5. El Hiani Y., Ahidouch A., Lehen'kyi V., Hague F., Gouilleux F., Mentaverri R., Kamel S., Lassoued K., Brûlé G., Ouadid-Ahidouch H. (2009) Extracellular signal-regulated kinases 1 and 2 and TRPC1 channels are required for calcium-sensing receptor-stimulated MCF-7 breast cancer cell proliferation. Cell Physiol. Biochem. 23, 335–346 [DOI] [PubMed] [Google Scholar]
- 6. Yang S. L., Cao Q., Zhou K. C., Feng Y. J., Wang Y. Z. (2009) Transient receptor potential channel C3 contributes to the progression of human ovarian cancer. Oncogene 28, 1320–1328 [DOI] [PubMed] [Google Scholar]
- 7. Aydar E., Yeo S., Djamgoz M., Palmer C. (2009) Abnormal expression, localization and interaction of canonical transient receptor potential ion channels in human breast cancer cell lines and tissues: a potential target for breast cancer diagnosis and therapy. Cancer Cell Int. 9, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bomben V. C., Sontheimer H. W. (2008) Inhibition of transient receptor potential canonical channels impairs cytokinesis in human malignant gliomas. Cell Prolif 41, 98–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El Boustany C., Bidaux G., Enfissi A., Delcourt P., Prevarskaya N., Capiod T. (2008) Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology 47, 2068–2077 [DOI] [PubMed] [Google Scholar]
- 10. Thebault S., Flourakis M., Vanoverberghe K., Vandermoere F., Roudbaraki M., Lehen'kyi V., Slomianny C., Beck B., Mariot P., Bonnal J. L., Mauroy B., Shuba Y., Capiod T., Skryma R., Prevarskaya N. (2006) Differential role of transient receptor potential channels in Ca2+ entry and proliferation of prostate cancer epithelial cells. Cancer Res. 66, 2038–2047 [DOI] [PubMed] [Google Scholar]
- 11. Ma X., Cai Y., He D., Zou C., Zhang P., Lo C. Y., Xu Z., Chan F. L., Yu S., Chen Y., Zhu R., Lei J., Jin J., Yao X. (2012) Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proc. Natl. Acad. Sci. U.S.A. 109, 16282–16287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slusarski D. C., Yang-Snyder J., Busa W. B., Moon R. T. (1997) Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev. Biol. 182, 114–120 [DOI] [PubMed] [Google Scholar]
- 13. Thrasivoulou C., Millar M., Ahmed A. (2013) Activation of intracellular calcium by multiple Wnt ligands and translocation of β-catenin into the nucleus: a convergent model of Wnt/Ca2+ and Wnt/β-catenin pathways. J. Biol. Chem. 288, 35651–35659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arce L., Yokoyama N. N., Waterman M. L. (2006) Diversity of LEF/TCF action in development and disease. Oncogene 25, 7492–7504 [DOI] [PubMed] [Google Scholar]
- 15. Yamada T., Takaoka A. S., Naishiro Y., Hayashi R., Maruyama K., Maesawa C., Ochiai A., Hirohashi S. (2000) Transactivation of the multidrug resistance 1 gene by T-cell factor 4/β-catenin complex in early colorectal carcinogenesis. Cancer Res. 60, 4761–4766 [PubMed] [Google Scholar]
- 16. Ma X., Chen Z., Hua D., He D., Wang L., Zhang P., Wang J., Cai Y., Gao C., Zhang X., Zhang F., Wang T., Hong T., Jin L., Qi X., Chen S., Gu X., Yang D., Pan Q., Zhu Y., Chen Y., Chen D., Jiang L., Han X., Zhang Y., Jin J., Yao X. (2014) Essential role for TrpC5-containing extracellular vesicles in breast cancer with chemotherapeutic resistance. Proc. Natl. Acad. Sci. U.S.A. 111, 6389–6394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 18. Xu S. Z., Sukumar P., Zeng F., Li J., Jairaman A., English A., Naylor J., Ciurtin C., Majeed Y., Milligan C. J., Bahnasi Y. M., Al-Shawaf E., Porter K. E., Jiang L. H., Emery P., Sivaprasadarao A., Beech D. J. (2008) TRPC channel activation by extracellular thioredoxin. Nature 451, 69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Riganti C., Campia I., Polimeni M., Pescarmona G., Ghigo D., Bosia A. (2009) Digoxin and ouabain induce P-glycoprotein by activating calmodulin kinase II and hypoxia-inducible factor-1α in human colon cancer cells. Toxicol. Appl. Pharmacol. 240, 385–392 [DOI] [PubMed] [Google Scholar]
- 20. Hiendlmeyer E., Regus S., Wassermann S., Hlubek F., Haynl A., Dimmler A., Koch C., Knoll C., van Beest M., Reuning U., Brabletz T., Kirchner T., Jung A. (2004) β-Catenin up-regulates the expression of the urokinase plasminogen activator in human colorectal tumors. Cancer Res. 64, 1209–1214 [DOI] [PubMed] [Google Scholar]
- 21. Brabletz T., Jung A., Reu S., Porzner M., Hlubek F., Kunz-Schughart L. A., Knuechel R., Kirchner T. (2001) Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. U.S.A. 98, 10356–10361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shen D. Y., Zhang W., Zeng X., Liu C. Q. (2013) Inhibition of Wnt/β-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. Cancer Sci. 104, 1303–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Longley D. B., Johnston P. G. (2005) Molecular mechanisms of drug resistance. J. Pathol. 205, 275–292 [DOI] [PubMed] [Google Scholar]
- 24. Tournigand C., André T., Achille E., Lledo G., Flesh M., Mery-Mignard D., Quinaux E., Couteau C., Buyse M., Ganem G., Landi B., Colin P., Louvet C., de Gramont A. (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J. Clin. Oncol. 22, 229–237 [DOI] [PubMed] [Google Scholar]
- 25. Shtil A. A., Azare J. (2005) Redundancy of biological regulation as the basis of emergence of multidrug resistance. Int. Rev. Cytol. 246, 1–29 [DOI] [PubMed] [Google Scholar]
- 26. Komoto C., Nakamura T., Yamamori M., Ohmoto N., Kobayashi H., Kuwahara A., Nishiguchi K., Takara K., Tanigawara Y., Okamura N., Okumura K., Sakaeda T. (2007) Reversal effects of Ca2+ antagonists on multidrug resistance via down-regulation of MDR1 mRNA. Kobe J. Med. Sci. 53, 355–363 [PubMed] [Google Scholar]
- 27. Bush E. W., Hood D. B., Papst P. J., Chapo J. A., Minobe W., Bristow M. R., Olson E. N., McKinsey T. A. (2006) Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J. Biol. Chem. 281, 33487–33496 [DOI] [PubMed] [Google Scholar]
- 28. Vennekens R., Menigoz A., Nilius B. (2012) TRPs in the brain. Rev. Physiol. Biochem. Pharmacol. 163, 27–64 [DOI] [PubMed] [Google Scholar]
- 29. Song M. Y., Yuan J. X. (2010) Introduction to TRP channels: structure, function, and regulation. Adv. Exp. Med. Biol. 661, 99–108 [DOI] [PubMed] [Google Scholar]
- 30. Fixemer T., Wissenbach U., Flockerzi V., Bonkhoff H. (2003) Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene 22, 7858–7861 [DOI] [PubMed] [Google Scholar]
- 31. Lehen'kyi V., Flourakis M., Skryma R., Prevarskaya N. (2007) TRPV6 channel controls prostate cancer cell proliferation via Ca2+/NFAT-dependent pathways. Oncogene 26, 7380–7385 [DOI] [PubMed] [Google Scholar]
- 32. Jiang H. N., Zeng B., Zhang Y., Daskoulidou N., Fan H., Qu J. M., Xu S. Z. (2013) Involvement of TRPC channels in lung cancer cell differentiation and the correlation analysis in human non-small cell lung cancer. PLoS One 8, e67637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fiorio Pla A., Avanzato D., Munaron L., Ambudkar I. S. (2012) Ion channels and transporters in cancer. 6. Vascularizing the tumor: TRP channels as molecular targets. Am. J. Physiol. Cell Physiol. 302, C9–C15 [DOI] [PubMed] [Google Scholar]
- 34. Han Y., Bu L. M., Ji X., Liu C. Y., Wang Z. H. (2005) Modulation of multidrug resistance by andrographolid in a HCT-8/5-FU multidrug-resistant colorectal cancer cell line. Chin. J. Dig. Dis. 6, 82–86 [DOI] [PubMed] [Google Scholar]
- 35. Yu Z. W., Zhao P., Liu M., Dong X. S., Tao J., Yao X. Q., Yin X. H., Li Y., Fu S. B. (2004) Reversal of 5-fluoroucial resistance by adenovirus-mediated transfer of wild-type p53 gene in multidrug-resistant human colon carcinoma LoVo/5-FU cells. World J. Gastroenterol. 10, 1979–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shapiro A. B., Ling V. (1998) The mechanism of ATP-dependent multidrug transport by P-glycoprotein. Acta Physiol. Scand. Suppl. 643, 227–234 [PubMed] [Google Scholar]
- 37. Molnár J., Engi H., Hohmann J., Molnár P., Deli J., Wesolowska O., Michalak K., Wang Q. (2010) Reversal of multidrug resitance by natural substances from plants. Curr. Top. Med. Chem. 10, 1757–1768 [DOI] [PubMed] [Google Scholar]
- 38. Frame S., Cohen P. (2001) GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 359, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chikazawa N., Tanaka H., Tasaka T., Nakamura M., Tanaka M., Onishi H., Katano M. (2010) Inhibition of Wnt signaling pathway decreases chemotherapy-resistant side-population colon cancer cells. Anticancer Res. 30, 2041–2048 [PubMed] [Google Scholar]
- 40. Janikova M., Skarda J. (2012) Differentiation pathways in carcinogenesis and in chemo- and radioresistance. Neoplasma 59, 6–17 [DOI] [PubMed] [Google Scholar]
- 41. Zhang M., Atkinson R. L., Rosen J. M. (2010) Selective targeting of radiation-resistant tumor-initiating cells. Proc. Natl. Acad. Sci. U.S.A. 107, 3522–3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McEwen D. G., Peifer M. (2000) Wnt signaling: moving in a new direction. Curr. Biol. 10, R562–R564 [DOI] [PubMed] [Google Scholar]
- 43. Gómez-Sintes R., Lucas J. J. (2010) NFAT/Fas signaling mediates the neuronal apoptosis and motor side effects of GSK-3 inhibition in a mouse model of lithium therapy. J. Clin. Invest. 120, 2432–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pansters N. A., van der Velden J. L., Kelders M. C., Laeremans H., Schols A. M., Langen R. C. (2011) Segregation of myoblast fusion and muscle-specific gene expression by distinct ligand-dependent inactivation of GSK-3β. Cell Mol. Life Sci. 68, 523–535 [DOI] [PMC free article] [PubMed] [Google Scholar]