Nucleic acid amplification test, age, race and sex are associated with increased community-associated Clostridium difficile infection (CDI) incidence, while age and number of inpatient-days are associated with increased healthcare-associated CDI incidence. Comparison of CDI incidence across regions should account for these factors.
Abstract
Background
Clostridium difficile infection (CDI) is no longer restricted to hospital settings, and population-based incidence measures are needed. Understanding the determinants of CDI incidence will allow for more meaningful comparisons of rates and accurate national estimates.
Methods
Data from active population- and laboratory-based CDI surveillance in 7 US states were used to identify CDI cases (ie, residents with positive C difficile stool specimen without a positive test in the prior 8 weeks). Cases were classified as community-associated (CA) if stool was collected as outpatients or ≤3 days of admission and no overnight healthcare facility stay in the past 12 weeks; otherwise, cases were classified as healthcare-associated (HA). Two regression models, one for CA-CDI and another for HA-CDI, were built to evaluate predictors of high CDI incidence. Site-specific incidence was adjusted based on the regression models.
Results
Of 10 062 cases identified, 32% were CA. Crude incidence varied by geographic area; CA-CDI ranged from 28.2 to 79.1/100 000 and HA-CDI ranged from 45.7 to 155.9/100 000. Independent predictors of higher CA-CDI incidence were older age, white race, female gender, and nucleic acid amplification test (NAAT) use. For HA-CDI, older age and a greater number of inpatient-days were predictors. After adjusting for relevant predictors, the range of incidence narrowed greatly; CA-CDI rates ranged from 30.7 to 41.3/100 000 and HA-CDI rates ranged from 58.5 to 94.8/100 000.
Conclusions
Differences in CDI incidence across geographic areas can be partially explained by differences in NAAT use, age, race, sex, and inpatient-days. Variation in antimicrobial use may contribute to the remaining differences in incidence.
BACKGROUND
Clostridium difficile infection (CDI) is a global public health problem with increases in incidence and severity of disease being reported from several countries [1–4]. In the United States, an estimated 14 000 deaths associated with CDI occurred in 2007 based on death certificate data, with an estimated excess in medical costs related to CDI of up to $4.8 billion in US acute care facilities [5, 6]. Clostridium difficile infection is now recognized to occur outside acute care hospitals, and reports of CDI among individuals in the community or in nursing homes have become increasingly common in the last few years [7–10]. Some of these patients do not require hospitalization for CDI treatment and are treated in outpatient or in nonacute care settings where the diagnosis of CDI was made [10, 11]. Therefore, population-level data are important for understanding the total burden of CDI at the national level.
Accurate estimates of CDI nationally will require accounting for factors that influence CDI rates. Several factors such as age, sex, or type of C difficile diagnostic assay may influence incidence measures. Incidence of CDI is known to be higher among the elderly population, especially those over 65 years of age [1, 12, 13]. In addition, females seem to be more affected than males in some studies focusing on community-associated (CA) CDI [14, 15]. Finally, at least 8 nucleic acid amplification tests (NAATs) have been approved by the US Food and Drug Administration since 2009 [16]. These assays have a higher sensitivity than the traditional C difficile toxin assays, and some studies have shown that use of NAAT can increase the rate of diagnosed CDI at least 2-fold [17–19].
Understanding the population- or diagnostic-specific factors that influence CDI incidence measures is important for meaningful comparisons of CDI rates across facilities, states, and regions and for accurate national burden estimates.
METHODS
Surveillance Population and Definitions
The Emerging Infections Program (EIP) conducts active laboratory- and population-based surveillance for C difficile. The EIP CDI surveillance was approved by the institutional review boards at the Centers for Disease Control and Prevention and participating surveillance sites. The requirement to obtain informed consent to review medical records was waived.
In 2010, surveillance was conducted in selected counties across 7 US states for the full calendar year: California (San Francisco County: 805 235 persons), Colorado (Adams, Arapahoe, Denver, Douglas, and Jefferson Counties: 2 433 772 persons), Connecticut (18 towns in New Haven County: 594 811 persons), Georgia (Clayton, Cobb, DeKalb, Douglas, Fulton, Gwinnett, Newton, and Rockdale Counties: 3 682 873 persons), Minnesota (Benton, Morrison, Stearns ,and Todd Counties: 247 186 persons), New York (Monroe County: 744 344 persons), and Oregon (Klamath County: 66 380 persons). The surveillance methods have been described elsewhere [20]. In brief, surveillance staff at each site identified all positive C difficile stool specimens by either toxin or molecular assay from inpatient and outpatient laboratories serving surveillance area residents. Clostridium difficile testing was based on provider discretion. A CDI case was defined as a positive C difficile toxin or molecular assay on a stool specimen from a resident of the surveillance catchment area ≥1 year of age who did not have a positive assay in the previous 8 weeks. Clostridium difficile infection cases were then classified as CA if a positive C difficile specimen was collected as an outpatient or within 3 days after hospital admission and the patient had no documented overnight stays in a healthcare facility in the prior 12 weeks; otherwise, they were classified as healthcare-associated (HA) and subdivided into 3 mutually exclusive categories: (1) community-onset healthcare facility-associated (CO-HCFA) if the positive specimen was collected as an outpatient or within 3 days after hospital admission from a private residence and the patient had a documented overnight stay in a healthcare-facility in the prior 12 weeks; (2) hospital-onset (HO) if the positive specimen was collected more than 3 calendar days after hospital admission or in a long-term acute care hospital; or (3) nursing HO (NHO) if the positive specimen was collected in a nursing home or within 3 days after hospital admission from a nursing home.
In 5 of the 7 EIP surveillance sites, medical record review was performed for all CDI cases identified to collect demographic and healthcare exposure data. Two EIP sites with the largest surveillance populations, Georgia and Colorado, performed full medical record review on a random sample of the CDI cases identified. Sampling was stratified by 4 age groups (ie, 1–17, 18–44, 45–64, ≥65 years) and gender category for a total of 8 strata (4 age groups × 2 gender categories). All male and female pediatric CDI cases (ie, 1–17 years) were selected for full medical record review due to the low numbers of cases, whereas a 33% sampling was performed for the other 6 age group and gender strata. The sampled cases were then used to estimate the epidemiologic class and race of all CDI cases identified across these 2 sites.
Additional Data Sources
We used data from the 2009–2010 Area Resource File [21] to obtain county-level information on (1) healthcare utilization: average number of outpatient visits per hospital, average number of inpatient-days per hospital, and percentage of the population residing in nursing homes; and (2) population characteristics: percentage of population in urban centers and percentage of persons between 18 and 64 years of age without health insurance. County-level data were aggregated at the EIP surveillance site level.
Data on NAAT use for C difficile diagnosis were obtained through a survey of all laboratories (N = 72) serving the surveillance catchment population. The laboratory survey is conducted annually as part of the EIP CDI surveillance and assesses the type of diagnostic test used for C difficile, as well as any changes in testing algorithms during the calendar year and the date those changes occurred. Nucleic acid amplification test use was defined by the proportion of 2010 CDI cases identified by NAAT used as either a first- or second-line test. Finally, the 2010 population figures were obtained from the US Census for the incidence denominator. Persons <1 year of age were excluded from the denominator because the surveillance case definition only included persons 1 year of age or older.
Statistical Analysis
Clostridium difficile infection cases with missing race (18%), including sampled cases from Georgia and Colorado, had race imputed based on the distribution of known race by age, sex, and surveillance site. A multiple imputation method was used to account for the uncertainty associated with imputing unknown values. After race imputation was performed, a domain (subpopulation) analysis was used to estimate CDI cases by epidemiological class and race in Georgia and Colorado.
Two regression models were built, one for CA-CDI and another for HA-CDI. A generalized linear mixed model with negative binomial distribution was used to evaluate the association of demographic, diagnostic, socioeconomic, and healthcare utilization factors with increased CA- or HA-CDI incidence. Because CDI incidence varied across surveillance sites, a random intercept was specified to account for surveillance site variations. For the CA-CDI model, the candidate variables for the model included age, sex, race, percentage of urban population, NAAT usage, percentage of population between 18 and 64 years of age without health insurance, and average of outpatient visits per hospital in each surveillance site; for the HA-CDI model, candidate variables included age, sex, race, NAAT usage, percentage of population in nursing homes, and average inpatient-days per hospital in each surveillance site. Final models were obtained using backward selection with a stay criterion of P ≤ .05. The parameters in the final models from multiple imputed data were summarized and used to calculate standardized CA- and HA-CDI rates. Analyses were performed using SAS software version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
From January 1 through December 31, 2010, a total of 10 062 CDI cases among 9450 unique patients were identified; 8890 (94.1%) patients had a single incident episode, 512 (5.4%) patients had 2 incident episodes, 44 (0.5%) patients had 3 incident episodes, and 4 patients had 4 incident episodes during the calendar year. Of the 10 062 CDI cases identified, 2777 (27.6%) were estimated to be diagnosed by NAAT based on laboratory survey. Minnesota was the site with the highest percentage of cases (88.1%) detected by NAAT, followed by New York (41.9%), Colorado (29.5%), California (26.7%), Georgia (9.8%), and Connecticut (4.5%). Oregon was the only site with no cases identified by NAAT.
Of the 10 062 CDI cases, 32% were CA, 26% were NHO, 23% were HO, and 19% were CO-HCFA (Table 1). The proportion of CDI cases in each epidemiologic class varied greatly across sites. Minnesota and Oregon, the only 2 sites with rural counties, had the highest proportion of CA-CDI cases, 51% and 53%, respectively (Table 1).
Table 1.
Clostridium difficile Infection (CDI) Cases by Surveillance Site and Epidemiologic Category, 2010
| Surveillance Site | N of Cases | Community-Associated |
Healthcare-Associated |
||
|---|---|---|---|---|---|
| Community-Onset Healthcare Facility-Associated |
Hospital-Onset |
Nursing Home-Onset |
|||
| N (%) | N (%) | N (%) | N (%) | ||
| San Francisco County, CA | 836 | 225 (27) | 147 (18) | 276 (33) | 188 (22) |
| Adams, Arapahoe, Denver, Douglas, Jefferson Counties, COa | 3298 | 1033 (31) | 611 (19) | 645 (19) | 1009 (31) |
| [95% CI, 931 (28)– 1136 (34)] | [95% CI, 530 (16)– 691 (21)] | [95% CI, 562 (17)– 728 (22)] | [95% CI, 904 (27)– 1114 (34)] | ||
| New Haven and Waterbury area, CT | 862 | 174 (21) | 147 (17) | 239 (28) | 302 (35) |
| Clayton, Cobb, Douglas, DeKalb, Fulton, Gwinnett, Newton, Rockdale Counties, GAa | 3066 | 1133 (37) | 573 (19) | 739 (24) | 621 (20) |
| [95% CI, 1027 (34)– 1239 (40)] | [95% CI, 494 (16)– 652 (21)] | [95% CI, 650 (21)– 829 (27)] | [95% CI, 538 (18)– 703 (23)] | ||
| Stearns, Benton, Morrison, Todd Counties, MN | 377 | 193 (51) | 87 (23) | 51 (14) | 46 (12) |
| Monroe county, NY | 1566 | 419 (27) | 316 (20) | 390 (25) | 441 (28) |
| Klamath County, OR | 57 | 30 (53) | 10 (18) | 5 (9) | 12 (21) |
| Total | 10 062 | 3207 (32) | 1891 (19) | 2345 (23) | 2619 (26) |
| [95% CI, 3063 (30)– 3351 (33)] | [95% CI, 1775 (18)– 2007 (20)] | [95% CI, 2220 (22)– 2470 (25)] | [95% CI, 2484 (25)– 2754 (27)] | ||
Abbreviation: CI, confidence interval.
a Site did stratified sampling of cases based on age and sex; point estimate and 95% CIs are presented for each specific epidemiologic category.
Clostridium difficile Infection Incidence
Aggregated CDI incidence across the 7 surveillance sites was higher among persons 65 years of age or older, females, and persons of white race for both CA and HA infections (Table 2). Among pediatric patients (1–17 years) and young adult patients (18–44 years), incidence of CA-CDI was higher than HA-CDI, 13.6 vs 6.3 and 23.3 vs 16.3 per 100 000 population, respectively. With advancing age, the incidence of HA-CDI was higher than CA-CDI (Table 2).
Table 2.
Age-, Gender-, Race-Specific Clostridium difficile Infection (CDI) incidence by Epidemiologic Category Aggregated Across 7 Emerging Infections Program Sites, 2010
| Demographic | Community-Associated CDI |
Healthcare-Associated CDI |
Total |
|||
|---|---|---|---|---|---|---|
| Rate per 100 000 Population | 95% CI | Rate per 100 000 Population | 95% CI | Rate per 100 000 Population | 95% CI | |
| Sex | ||||||
| Male | 29.8 | 27.5–32.2 | 69.2 | 65.9–72.4 | 99.0 | 93.4–104.6 |
| Female | 45.4 | 42.6–48.2 | 91.9 | 88.4–95.6 | 137.4 | 131.0–143.8 |
| Age, years | ||||||
| 1–17 | 13.6 | 12.0–15.2 | 6.3 | 5.2–7.4 | 19.9 | 17.2–22.6 |
| 18–44 | 23.3 | 21.0–25.7 | 16.3 | 14.4–18.3 | 39.6 | 35.4–44.0 |
| 45–64 | 50.4 | 46.0–54.8 | 69.2 | 64.3–74.1 | 119.6 | 110.3–128.9 |
| ≥65 | 114.4 | 104.1–124.7 | 517.4 | 499.6–535.1 | 631.8 | 613.7–659.8 |
| Race | ||||||
| White | 45.2 | 42.8–47.5 | 94.9 | 92.0–97.8 | 140.1 | 134.8–145.3 |
| Black | 28.2 | 24.7–31.8 | 64.2 | 58.8–69.5 | 92.4 | 83.5–101.3 |
| Other | 13.0 | 9.9–16.2 | 30.8 | 26.3–35.2 | 43.8 | 36.2–51.4 |
Abbreviations: CI, confidence interval.
Predictors of High Clostridium difficile Infection Incidence
In bivariate analysis for CA-CDI, older age (P < .0001), white race (P = .007), NAAT usage (P = .009), female sex (P = .02), and a lower percentage of urban population (P = .05) were associated with increased incidence. In the multivariable model, independent predictors of high CA-CDI were age, sex, race, and NAAT use. Table 3 provides the incidence rate ratio for each predictor, controlling for the other factors that remained in the final CA-CDI model. For each 10% increase in the number of cases detected by NAAT, CA-CDI rates were expected to increase 11% (95% confidence interval [CI], 6%–16%) after controlling for age, sex and race. Incidence of CA-CDI was 41% (95% CI, 23%–61%) higher among females compared with males, 45% (95% CI, 21%–75%) higher among whites compared with non-whites, and approximately 7-fold (95% CI, 5-fold to 9-fold) higher among those 65 and older compared with persons 1–17 years of age.
Table 3.
Multivariable Modeling Analysis for Predictors of High Community- and Healthcare-Associated Clostridium difficile Infection (CDI) Incidence, 2010
| Community-Associated CDI Multivariable Modela | ||||
|---|---|---|---|---|
| Predictor | Category | Incidence Rate Ratiob | 95% CI | P |
| Age | 1–17 | 1.00 (reference) | ||
| 18–44 | 1.72 | 1.38–2.14 | <.0001 | |
| 45–64 | 3.42 | 2.77–4.24 | <.0001 | |
| ≥65 | 7.17 | 5.76–8.94 | <.0001 | |
| Sex | Male | 1.00 (reference) | ||
| Female | 1.41 | 1.23–1.61 | <.0001 | |
| Race | Non-white | 1.00 (reference) | ||
| White | 1.45 | 1.21–1.75 | <.0001 | |
| Test | NAAT use by 10% increase | 1.11 | 1.06–1.16 | <.0001 |
| Healthcare-Associated CDI Multivariable Modelc | ||||
| Predictor | Category | Incidence Rate Ratiod | 95% CI | P |
| Age | 1–17 | 1.00 (reference) | ||
| 18–44 | 2.67 | 2.01–3.54 | <.0001 | |
| 45–64 | 11.23 | 8.54–14.76 | <.0001 | |
| ≥65 | 68.79 | 52.56–90.03 | <.0001 | |
| Inpatient-days | Increase in 10 000 inpatient-days per hospital | 1.10 | 1.01–1.20 | .02 |
| Test | NAAT use by 10% increase | 1.06 | .99–1.14 | .13 |
Abbreviations: CI, confidence interval; NAAT, nucleic acid amplification test.
a Candidate variables included in the model: age, sex, race, NAAT usage, percentage of urban population, percentage of 18–64 years without health insurance, and average number of outpatient visits per hospital in each surveillance site.
b Adjusted for the factors that remained in the final model (age, sex, race, test).
c Candidate variables included in the model: age, sex, race, NAAT usage, average number of inpatient-days per hospital, and average number of nursing home patients in each surveillance site.
d Adjusted for the factors that remained in the final model (age, inpatient-days, test).
In bivariate analysis for HA-CDI, only age (P < .0001) and the average number of inpatient-days per hospital in the EIP surveillance site (P = .04) were significantly associated with increased incidence. These predictors remained associated with high HA-CDI incidence in the multivariable model (Table 3). Even though NAAT use was not significant in the final HA-CDI model, this factor was forced into the model due to reports from healthcare facilities of increased CDI rates after NAAT adoption [17, 18, 22]. Incidence of HA-CDI was approximately 68-fold (95% CI, 52-fold to 90-fold) higher among persons ≥65 years compared with persons aged 1–17 years after controlling for molecular diagnostic test use and healthcare utilization (ie, average number of inpatient-days per hospital in the region). In addition, a 10% (95% CI, 1%–20%) increase in HA-CDI incidence was found for every increase of 10 000 inpatient-days.
Variability of Clostridium difficile Infection Incidence Across Geographic Locations
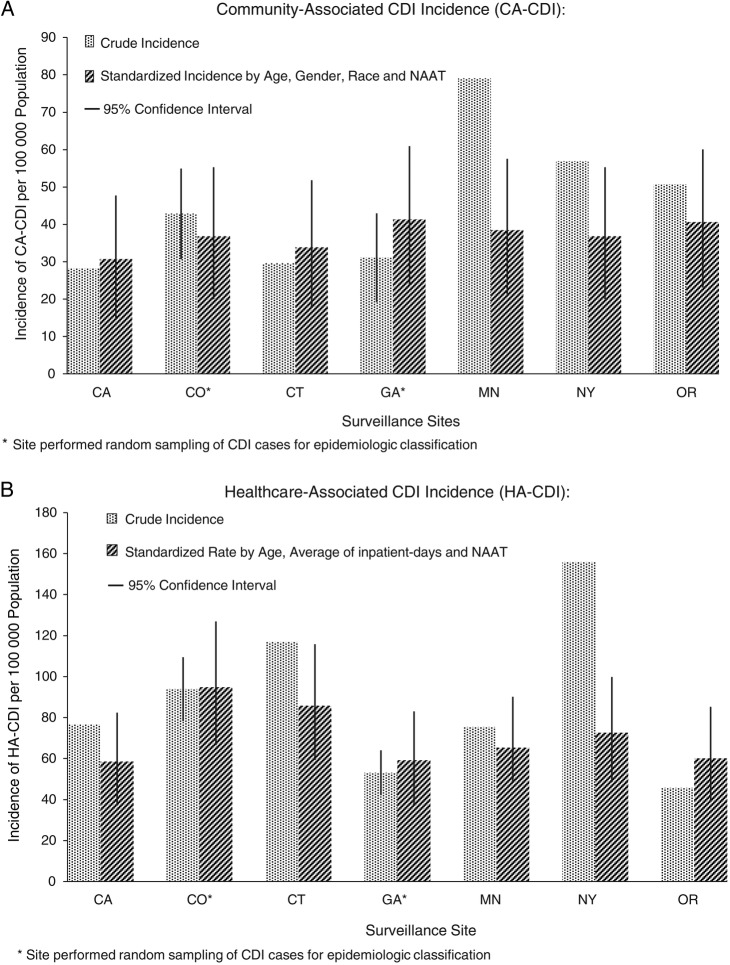
Crude CA-CDI incidence was lowest in California (28.2/100 000 population) and highest in Minnesota (79.1/100 000 population). The differences in CA-CDI incidence across sites decreased substantially after rates were standardized by age, sex, race, and NAAT use; after adjustment, the lowest incidence was 30.7 (95% CI, 15.1–47.7)/100 000 population in California and the highest was 41.3 (95% CI, 23.6–60.9)/100 000 population in Georgia (Figure 1A).
Figure 1.
Crude and standardized community-associated (CA) and healthcare-associated (HA) Clostridium difficile infection (CDI) incidence per surveillance site, 2010.
The differences in HA-CDI incidence also decreased across sites after rates were adjusted based on the results of multivariable model. Crude incidence varied from as low as 45.7/100 000 population in Oregon to as high as 155.9/100 000 population in New York. After adjusting by age, inpatient-days, and NAAT use, the differences in HA-CDI incidence decreased, ranging from 58.5 (95% CI, 37.2–82.4)/100 000 population in California to 94.8 (95% CI, 66.5–126.9)/100 000 population in Colorado (Figure 1B).
DISCUSSION
This analysis including data from 7 US states, encompassing a population of ∼10 million persons, showed that elderly patients, females, and those of white race seem to be at higher risk of CDI. Incidence of CDI has been reported to be higher among persons 65 years of age or older in several studies [1, 12, 13]. This result may be related to increased exposures to healthcare settings, increased antibiotic use, and impaired immune response to infectious pathogens [23–25]. Some studies have reported higher CDI incidence in females compared with males for CA infections; however, we observed that for both CA- and HA-CDI, females appear to be at higher risk [14, 15]. Although C difficile has been increasingly reported among peripartum women [26, 27], and females seem to be at increased risk of recurrent CDI [28], the reasons for the higher risk of CDI in females compared with males are still not clear. However, some possible explanations have been raised but not proven. Females may have greater exposure to infants who are known to have a high C difficile colonization rate, and infant exposure has been described as a risk factor for CA-CDI [8]. In addition, females are more likely to seek medical care, resulting in increased exposure to antibiotics [29]. A study in England and Wales showed that antibiotic prescribing rates in females were 40% higher compared with males [30]. Finally, host factors related to immune system may explain some of the differences in CDI rates between females and males [31].
Another interesting finding in our study was the ∼50% higher incidence among whites compared with blacks and 3-fold higher incidence of whites compared with other nonblack races. The relationship between white race and CDI has not been previously described. Many CDI published studies have either not collected race information or have not found a significant correlation between race and CDI. Although the reasons for white individuals to be at increased risk of CDI compared with other races are not known: one can hypothesize that greater access to healthcare and, therefore, the potential for more antibiotic exposure or increased diagnostic testing may play a role in the differences in race-specific CDI incidence we observed [32, 33].
According to our results, differences in CDI incidence across geographic locations can be partially explained by the type of diagnostic tests used for C difficile; demographic characteristics of the population such as age, sex, and race; and increased healthcare utilization. The remaining differences in adjusted CDI rates across states, especially for HA-CDI, suggest that other factors not accounted for in our analyses, such as differences in antibiotic prescribing practices, infection control practices, or the occurrence of C difficile outbreaks during the surveillance period, may also play a role in the variability of CDI incidence across states. Although some studies have shown an association of NAAT with increased CDI incidence [17–19], we did not find NAAT use to be significantly associated with population level differences in HA-CDI incidence across states. It is possible that in contrast to CA-CDI rates, HA-CDI rates may be driven more by patient age and other healthcare facility characteristics, such as volume of patients admitted and severity of illness of patients receiving care at the facilities, rather than the type of diagnostic test used. Nevertheless, our findings are critical to informing comparisons of CDI population-based rates across states and regions and to developing more robust national burden estimates of CDI that account for major determinants when projecting CDI rates to the national level. We plan to use these factors when we analyze EIP CDI surveillance data in the future to inform national rates.
Finally, the 32% of CA-CDI cases we found is higher than the rates of 20%–27% reported in Canada and in one region of the United States using a similar definition [14, 34]. This higher proportion of CA-CDI cases may be explained by the inclusion of large commercial and outpatient laboratories in the surveillance. Similar to a study done in the United Kingdom [8], we also found that the 2 EIP sites, Minnesota and Oregon, with a more rural population under surveillance had a higher prevalence of CA-CDI cases compared with other sites with a more urban population, which may be related to either healthcare utilization factors or different sources of C difficile exposures. In addition, the crude incidence of CA-CDI we observed across sites of 28–79/100 000 population is higher than the crude incidence of 20–40/100 000 population that has been reported in other population-based studies before the introduction of molecular diagnostic tests for C difficile [8, 35]. Despite this large proportion of CA-CDI cases, the majority of CDI cases (68%) are still HA with disease onset either in nursing homes, hospitals, or within 12 weeks after discharge from a healthcare facility. Moreover, as previously reported, the majority of CA-CDI cases are related to the receipt of outpatient care [11].
This study has several limitations. First, we were not able to capture antibiotic prescribing data in our surveillance sites. Antibiotics are known to be a major risk factor for CDI [36, 37], and variability in prescribing practices across regions has been documented [24]. Second, the data on NAAT utilization were captured through a survey of participating laboratories, and it is possible that some CDI cases may have been misclassified as being identified by NAAT, especially in laboratories using NAAT as a second-line test. Third, classification of CA-CDI cases was based on medical record documentation and, therefore, some cases may have been misclassified if no documentation of prior hospitalization was available. However, a previous study using the same surveillance population [11] showed that misclassification only occurred in ∼6% of CA-CDI cases. Finally, the incidence calculation relied on positive C difficile test, and it is possible that some patients may have been misclassified as cases if C difficile stool testing was not limited to patients with ≥3 unformed stools per 24 hour period as recommended by laboratory practice guidelines [38].
CONCLUSION
In conclusion, CA-CDI rates vary by age, sex, race, and type of diagnostic test, whereas HA-CDI rates vary by age and average number of inpatient-days per hospitals in a region. These factors must be taken into account for meaningful comparison of CDI incidence across different geographic locations and for more reliable projections of CDI burden in the United States.
Notes
Acknowledgments. We acknowledge the following individuals for their contributions with implementation of surveillance and collection of data: Joelle Nadle, MPH, Erin Garcia, MPH, Erin Parker, MPH (California Emerging Infections Program); Wendy Bamberg, MD, Helen Johnston, MPH (Colorado Emerging Infections Program); Carol Lyons, MPH (Connecticut Emerging Infections Program); Leigh Ann Clark, MPH, Andrew Revis, MPH (Georgia Emerging Infections Program); Ruth Lynfield, MD (Minnesota Emerging Infections Program); Rebecca Tsay, MPH, Deborah Nelson, RN (New York Emerging Infections Program); Valerie Ocampo, RN (Oregon Emerging Infections Program).
Drs. Fernanda Lessa and Yi Mu had full access to data and take responsibility for the integrity of the data and accuracy of the data analysis.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. F. C. L. and Y. M. have full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial support. This work was funded by the Emerging Infections Program Cooperative Agreement between the 7 EIP sites and the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis. 2006;12:409–15. doi: 10.3201/eid1203.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerg Infect Dis. 2007;13:1417–9. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burckhardt F, Friedrich A, Beier D, Eckmanns T. Clostridium difficile surveillance trends, Saxony, Germany. Emerg Infect Dis. 2008;14:691–2. doi: 10.3201/eid1404.071023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gravel D, Miller M, Simor A, et al. Canadian Nosocomial Infection Surveillance Program. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis. 2009;48:568–76. doi: 10.1086/596703. [DOI] [PubMed] [Google Scholar]
- 5.Hall AJ, Curns AT, McDonald LC, et al. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis. 2012;55:216–23. doi: 10.1093/cid/cis386. [DOI] [PubMed] [Google Scholar]
- 6.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(Suppl 2):S88–92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Severe Clostridium difficile-associated disease in populations previously at low risk—four states, 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1201–5. [PubMed] [Google Scholar]
- 8.Wilcox MH, Mooney L, Bendall R, et al. A case-control study of community-associated Clostridium difficile infection. J Antimicrob Chemother. 2008;62:388–96. doi: 10.1093/jac/dkn163. [DOI] [PubMed] [Google Scholar]
- 9.Campbell RJ, Giljahn L, Machesky K, et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30:526–33. doi: 10.1086/597507. [DOI] [PubMed] [Google Scholar]
- 10.Pawar D, Tsay R, Nelson DS, et al. Burden of Clostridium difficile infection in long-term care facilities in Monroe County, New York. Infect Control Hosp Epidemiol. 2012;33:1107–12. doi: 10.1086/668031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chitnis AS, Holzbauer SM, Belflower M, et al. Epidemiology of community-associated Clostridium difficile infection, 2009–2011. JAMA Intern Med. 2013;173:1359–67. doi: 10.1001/jamainternmed.2013.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucado J, Gould C, Elixhauser A. Clostridium difficile Infections (CDI) in Hospital Stays, 2009: Statistical Brief #124. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Health Care Policy and Research (US); 2006–2012. Available at: http://www.ncbi.nlm.nih.gov/books/NBK92613/pdf/sb124.pdf . Accessed 14 July 2014. [PubMed] [Google Scholar]
- 13.Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693–703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 14.Kutty PK, Woods CW, Sena AC, et al. Risk factors for and estimated incidence of community-associated Clostridium difficile infection, North Carolina, USA. Emerg Infect Dis. 2010;16:197–204. doi: 10.3201/eid1602.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Surveillance for community-associated Clostridium difficile—Connecticut, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:340–3. [PubMed] [Google Scholar]
- 16.Brecher SM, Novak-Weekley SM, Nagy E. Laboratory Diagnosis of Clostridium difficile infections: there is light at the end of the colon. Clin Infect Dis. 2013;57:1175–81. doi: 10.1093/cid/cit424. [DOI] [PubMed] [Google Scholar]
- 17.Fong KS, Fatica C, Hall G, et al. Impact of PCR testing for Clostridium difficile on incident rates and potential on public reporting: is the playing field level? Infect Control Hosp Epidemiol. 2011;32:932–3. doi: 10.1086/661789. [DOI] [PubMed] [Google Scholar]
- 18.Moehring RW, Lofgren ET, Anderson DJ. Impact of change to molecular testing for Clostridium difficile infection on healthcare facility-associated incidence rates. Infect Control Hosp Epidemiol. 2013;34:1055–61. doi: 10.1086/673144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould CV, Edwards JR, Cohen J, et al. Effect of nucleic acid amplification testing on population-based incidence rates of Clostridium difficile infection. Clin Infect Dis. 2013;57:1304–7. doi: 10.1093/cid/cit492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Measuring the scope of Clostridium difficile infection in the United States. Available at: http://www.cdc.gov/hai/eip/clostridium-difficile.html . Accessed 17 July 2013.
- 21.Health Resources and Service Administration, U.S. Department of Health and Human Services. Area Resource File. Available at: http://arf.hrsa.gov/ . Accessed 26 June 2013.
- 22.Centers for Disease Control and Prevention. Risk adjustment for Healthcare Facility-Onset C. difficile and MRSA Bacteremia Laboratory-identified Event Reporting in NHSN. Available at: http://www.cdc.gov/nhsn/PDFs/mrsa-cdi/RiskAdjustment-MRSA-CDI.pdf . Accessed 21 October 2013.
- 23.Simor AE. Diagnosis, management, and prevention of Clostridium difficile infection in long-term care facilities: a review. J Am Geriatr Soc. 2010;58:1556–64. doi: 10.1111/j.1532-5415.2010.02958.x. [DOI] [PubMed] [Google Scholar]
- 24.Hicks LA, Taylor TH, Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461–2. doi: 10.1056/NEJMc1212055. [DOI] [PubMed] [Google Scholar]
- 25.Ginaldi L, De Martinis M, D'Ostilio A, et al. The immune system in the elderly: II. Specific cellular immunity. Immunol Res. 1999;20:109–15. doi: 10.1007/BF02786467. [DOI] [PubMed] [Google Scholar]
- 26.Unger JA, Whimbey E, Gravett MG, et al. The emergence of Clostridium difficile infection among peripartum women: a case-control study of a C. difficile outbreak on an obstetrical service. Infect Dis Obstet Gynecol. 2011;2011:267249. doi: 10.1155/2011/267249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuntz JL, Yang M, Cavanaugh J, et al. Trends in Clostridium difficile infection among peripartum women. Infect Control Hosp Epidemiol. 2010;31:532–4. doi: 10.1086/652454. [DOI] [PubMed] [Google Scholar]
- 28.Fekety R, McFarland LV, Surawicz CM, et al. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;24:324–33. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 29.Bertakis KD, Azari R, Helms LJ, et al. Gender differences in the utilization of health care services. J Fam Pract. 2000;49:147–52. [PubMed] [Google Scholar]
- 30.Majeed A, Moser K. Age- and sex-specific antibiotic prescribing patterns in general practice in England and Wales in 1996. Br J Gen Pract. 1999;49:735–6. [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson DL, Gange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune disease in the United States. Clin Immunol Immunopathol. 1997;84:223–43. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 32.Schappert SM, Burt CW. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001–02. Vital Health Stat 13. 2006;(159):1–66. [PubMed] [Google Scholar]
- 33.Kullgren JT, Galbraith AA, Hinrichsen VL, et al. Health care use and decision making among lower-income families in high-deductible health plans. Arch Intern Med. 2010;170:1918–25. doi: 10.1001/archinternmed.2010.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambert PJ, Dyck M, Thompson LH, et al. Population-based surveillance of Clostridium difficile infection in Manitoba, Canada, by using interim surveillance definitions. Infect Control Hosp Epidemiol. 2009;30:945–51. doi: 10.1086/605719. [DOI] [PubMed] [Google Scholar]
- 35.Norén T, Akerlund T, Bäck E, et al. Molecular epidemiology of hospital-associated and community-acquired Clostridium difficile infection in a Swedish county. J Clin Microbiol. 2004;42:3635–43. doi: 10.1128/JCM.42.8.3635-3643.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens V, Dumyati G, Fine LS, et al. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis. 2011;53:42–8. doi: 10.1093/cid/cir301. [DOI] [PubMed] [Google Scholar]
- 37.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 38.Sharp S, Gilligan P. A practical guidance document for the laboratory detection of toxigenic Clostridium difficile. Washington, DC: American Society for Microbiology; 2010. Available at: http://www.asm.org/images//Clinical/clostridiumdifficile9-21.pdf . Accessed 7 March 2014. [Google Scholar]