Novel, post-vancomycin fidaxomicin chaser or fidaxomicin taper regimens may decrease subsequent CDI episodes and improve outcomes compared to standard 10 day treatment of mrCDI episodes with fidaxomicin, but randomized prospective comparative data are not yet available.
Keywords: Clostridium difficile infection, fidaxomicin, recurrence
Abstract
Reports of fidaxomicin treatment for patients with multiple recurrent Clostridium difficile infections ([mrCDI] ie, more than 2 CDI episodes) indicate symptomatic response to this agent, but 50% have subsequent mrCDI episodes. In an effort to improve outcomes in patients with mrCDI we used novel regimens of fidaxomicin based on strategies used with vancomycin. Of 8 patients who received a 10-day chaser of fidaxomicin given twice daily after a course of vancomycin, 3 (38%) experienced a subsequent recurrence. Two (18%) of 11 patients who completed a 14- to 33-day course of fidaxomicin in a tapering dose experienced a recurrence, both of whom received additional antibiotics before that recurrence. The median symptom-free interval (SFI) after fidaxomicin taper was greater than the median SFI after the most effective prior regimen for those patients (257 days [interquartile range, 280] vs 25 days [interquartile range, 30], respectively; P = .003). A fidaxomicin chaser or taper regimen may be effective in patients with mrCDI, but the number of patients treated is small, and randomized comparative data are not available.
Over the last several decades, the management of primary and recurrent Clostridium difficile infections (CDI) has involved a standard antimicrobial course with either metronidazole or vancomycin. In this setting, both therapies lead to high initial cure rates; however, recurrences after initial cure are problematic, and each recurrence increases the risk of subsequent recurrences [1]. First recurrences after therapy have been reported in up to 25% of patients; of this population, retreatment can leave over half of these patients with a second recurrence [1–3]. For these treatment refractory patients, standard therapies are typically ineffective at breaking the cycle of recurrent CDI (rCDI), leaving clinicians to choose nontraditional therapies.
A variety of empiric strategies for managing patients with multiple rCDI (mrCDI) episodes have been reported (ie, more than 2 CDI episodes). The most widely used regimen for patients with mrCDI refractory to standard treatment has included a vancomycin taper [4]. The effectiveness of this approach is supported by a retrospective study by McFarland et al [2], who evaluated a subgroup of placebo patients enrolled in 2 clinical trials for the probiotic Saccharomyces boulardii. This uncontrolled study found significantly lower subsequent recurrence rates in patients treated with a vancomycin taper (31%, 9 of 29) or a pulse regimen given once every 2–3 days (14.3%, 1 of 7), compared with the recurrence rates observed in patients receiving a regimen of vancomycin (250 mg) given 4 times daily (71.4%, 10 of 14).
A rifaximin chaser given immediately after the completion of oral vancomycin therapy has also been reported to be effective in a case-series of patients with mrCDI, and a randomized, placebo-controlled pilot study showed promise for this approach [5–7]. Other potentially effective mrCDI therapies include treatment with nitazoxanide and immunoglobulin therapy administered concurrently with active CDI therapy [8, 9]. Fecal microbiota transplantation (FMT) has increasingly been used as an alternative for managing patients with mrCDI based on the positive findings from a randomized controlled study after years of anecdotal experience [10]. Although this approach has been effective, screening of donors and other logistics involved in the preparation and delivery of specimens is costly, and there are concerns about unanticipated consequences after introduction of a new microbiota. In addition, determination of which patients would benefit most from FMT is still unclear [4].
Fidaxomicin has been studied in randomized, controlled trials for the treatment of patients with primary CDI and with first CDI recurrences. Although phase 3 studies indicated non-inferiority for the primary outcome of cure compared with vancomycin, fidaxomicin showed an overall significant reduction in recurrence rates [11, 12]. To date, there are 3 reports of fidaxomicin used to treat patients with mrCDI episodes (ie, more than 2 episodes) and 1 description of fidaxomicin use in a post-vancomycin, chaser strategy [13–16]. Three case series have been published reporting on the use of a 10- to 14-day course of fidaxomicin 200 mg given twice daily in adults and a pediatric patient with recurrent CDI [13, 14]. Five of 8 adult patients in 1 report of fidaxomicin treatment had mrCDI (2–5 prior CDI episodes), 1 of whom experienced a recurrence (without exposure to non-CDI antimicrobials) 75 days after completing fidaxomicin therapy [13]. This patient received another course of fidaxomicin without subsequent recurrence over a follow-up period of 17 months. The other 4 patients with mrCDI (followed for 60 days to 21 months) did not have another CDI episode. A 10-year-old patient received fidaxomicin for his 6th CDI episode after successfully completing a 6-week vancomycin taper 2 weeks prior [14]. After completing therapy, the patient was without symptoms at his 1-month follow-up visit, but he experienced a subsequent recurrence after he was prescribed clarithromycin for pneumonia several months later. Contrary to the success described in these reports, an additional report of 2 patients with mrCDI suggested that this 10-day course was ineffective in patients with a history of multiple recurrences [15]. Both of these patients had multiple prior CDI recurrences. Both patients responded to fidaxomicin but had subsequent mrCDI episodes. In summary, 8 patients with mrCDI responded to fidaxomicin treatment, but 4 (50%) had subsequent mrCDI episodes.
We reported our initial experience using fidaxomicin as a postvancomycin, chaser regimen in 3 patients with mrCDI [16]. Fidaxomicin was not used to treat symptomatic episodes in these patients, but it was administered after a prolonged, suppressive low dose of vancomycin that was given until fidaxomicin became available. Our subsequent uncontrolled experience using this and an alternate approach is described below.
CLINIC BACKGROUND AND METHODS
Over the last decade, patients with mrCDI within Chicago and surrounding areas have been referred to our clinic for management. Most of these patients have been referred after treatment for several prior CDI episodes and have considered FMT as a last treatment resort. After prescribing CDI therapy, all patients were closely monitored via telephone and subsequent clinic visits for CDI recurrence, defined as the reappearance of diarrheal stools with or without laboratory diagnostic testing. All patients had at least 1 of the episodes confirmed by stool assay for C difficile, and confirmation was attempted at the time of symptomatic recurrence. However, symptomatic recurrence that was clinically consistent with CDI recurrence was sometimes treated without obtaining a diagnostic specimen.
A prolonged vancomycin taper has been typically effective for a majority of these patients and is often still effective if patients fail a first vancomycin taper. Because some patients were refractory to vancomycin taper, we next used a regimen of rifaximin immediately after a treatment course of vancomycin, which we termed a “post-vancomycin rifaximin chaser” strategy [5, 6]. In theory, a chaser approach using a CDI-active, “microbiota-sparing” antimicrobial given immediately after vancomycin therapy (used to reduce C difficile counts to minimize rifaximin resistance development) would allow the microbiota to recover and re-establish colonization resistance while C difficile is actively controlled. Limited options after rifaximin chaser failures and the risk for selection of rifaximin-resistant isolates have prompted us to seek alternative approaches for managing mrCDI.
In this study, we describe our recent observational experience with fidaxomicin using novel treatment regimens for patients with mrCDI. Patients were also evaluated for the duration of time that they have been symptom-free (symptom-free interval [SFI]) after the completion of the fidaxomicin regimens. Follow up stopped and the SFI data were censored on January 9, 2014. The SFIs after the fidaxomicin regimens were compared to the SFIs of the most effective prior regimens by Mann–Whitney U test and expressed as medians and interquartile ranges (IQRs). Other demographic data (age and number of prior CDI treatment regimens) were analyzed by the Student t-test. The Institutional Review Board for Human Studies at Loyola University Medical Center (Maywood, IL) approved the patient chart review for the study.
RESULTS
Fidaxomicin Chaser Regimen
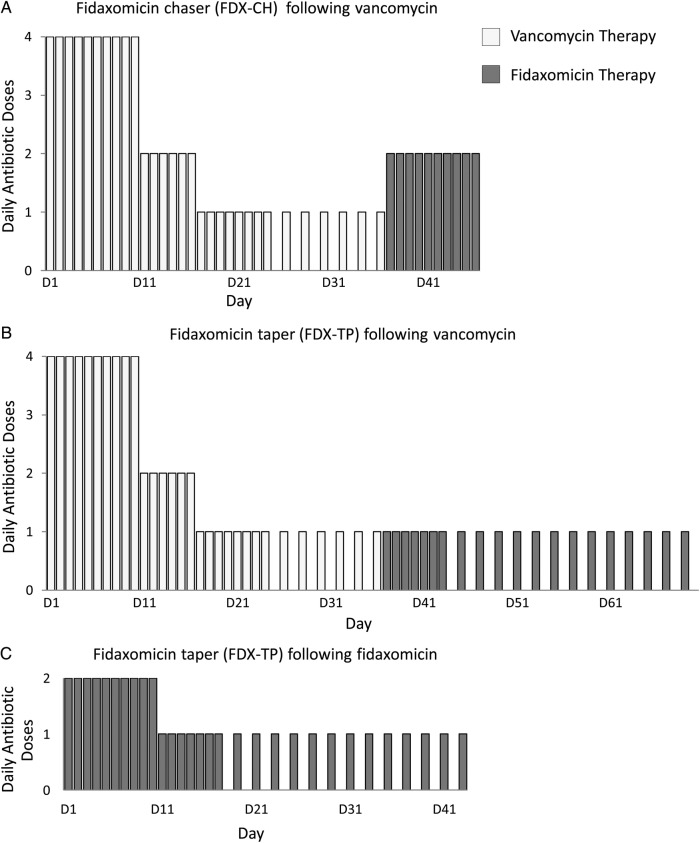
Based on review of the literature, 50% of mrCDI patients who received a standard fidaxomicin treatment course experienced a subsequent recurrence. Therefore, we tried an alternate approach using fidaxomicin as a post-vancomycin “chaser.” Ten days of fidaxomicin (200 mg given twice daily) was administered immediately after the completion of a vancomycin regimen (Figure 1). In 2012, we reported our experience using this approach in 3 patients (patients 1–3 in Table 1) [16]. Since this publication, 5 additional patients have received treatment with fidaxomicin chaser (Table 1).
Figure 1.
Recurrent Clostridium difficile management strategies using novel fidaxomicin regimens. A fidaxomicin chaser (A) and a fidaxomicin taper (B) were given immediately after a vancomycin taper for 8 and 9 patients, respectively. For the fidaxomicin chaser regimen, fidaxomicin 200 mg was administered BID for 10 days and for the fidaxomicin taper, fidaxomicin 200 mg was administered QD for 7 days, then QOD for 7–26 days. The duration of the vancomycin taper varied from weeks to months. A fidaxomicin taper (C) was also administered after a 10-day course of fidaxomicin treatment (200 mg BID) for 3 patients.
Table 1.
Demographic Characteristics and Outcomes of Patients With mrCDI Who Received a FDX-CH Regimen*
| Age, Years/Sex | No. of CDI Episodes | Prior Regimens | Additional Antimicrobial Exposure After the Last Prior Regimen | Longest SFI for Non-FDX-CH Course, Days (Antibiotic) | SFI Post FDX-CH, Days | Recurrence After FDX-CH | Additional Antimicrobial Exposure After FDX-CH Regimens | |
|---|---|---|---|---|---|---|---|---|
| Patient 1† | 67/M | 4 | M, M, VT, VT, VT, VT | None | 18 (VT) | 857 | No | None |
| Patient 2† | 83/F | 5 | V,V,V,V,VT,VT,VT | None | 20 (VT) | 92 | Yes | Yes |
| Patient 3† | 32/F | 8 | M,M,VT,VT,V + RC,V + RC,VT + IVIG,VT | None | 42 (RC) | 839 | No | None |
| Patient 4 | 68/F | 4 | M,M,VT | None | 64 (VT) | 19 | Yes | None |
| Patient 5 | 46/F | 10 | M, V, V, VT, V + FMT, V, RC, VT, VT, VT | None | 102 (V) | 34 | Yes | None |
| Patient 6 | 90/F | 3 | V,VT,VT | None | 82 (V) | 689 | No | None |
| Patient 7 | 79/M | 6 | U,U,U,VT,VT,VT | None | 90 (VT) | 419 | No | None |
| Patient 8 | 70/F | 4 | M + V,V,VT,VT | None | 49 (VT) | 137 | No | None |
| Mean ± SD | 66.9 ± 19 | 5.5 ± 2 | Median (IQR) | 56.5 (48) | 278 (649)‡ |
Abbreviations: CDI, Clostridium difficile infection; FDX, standard fidaxomicin treatment regimen; FDX-CH, fidaxomicin chaser; FDX-TP, fidaxomicin taper; FMT, fecal microbiota transplantation; IQR, interquartile range; IVIG, intravenous immunoglobulin; M, metronidazole; mrCDI, multiple recurrent CDI; RC, rifaximin chaser; SD, standard deviation; SFI, symptom-free interval; U, unknown; V, standard vancomycin treatment course; VT, vancomycin taper.
* The SFI after the most effective prior treatment is compared to the SFI after FDX-CH.
† Patients 1–3 were previously reported [16].
‡ P = .066 compared with non-FDX-CH SFI, Mann–Whitney U test.
The average age of the patients who received fidaxomicin chaser was 66.9 ± 19 years (mean ± SD) and 75% were female (Table 1). Patients treated with fidaxomicin chaser had a mean of 5 (range 3–10) previous CDI episodes. The median SFI postfidaxomicin chaser was 278 days (IQR, 649), and the median SFI for the most effective prior CDI treatment regimen was 56.5 days (IQR, 48) (P = .066).
None of the patients received additional antimicrobials after their last prior regimen and before the recurrent CDI episode for which fidaxomicin chaser was prescribed (Table 1). Of the 8 patients who received fidaxomicin chaser, 38% (3 of 8) experienced a subsequent recurrence. One of these 3 patients (Patient 2) had their recurrence after receiving systemic antimicrobial therapy and has been described previously [16].
Fidaxomicin Taper Regimen
Although patients receiving fidaxomicin chaser experienced fewer subsequent recurrences compared with those who received a standard fidaxomicin treatment course, a tapered strategy of fidaxomicin was pursued with the goal of minimizing recurrence rates further. Immediately after either vancomycin taper or a standard fidaxomicin treatment course, fidaxomicin 200 mg was administered once daily for 7 days and then once every other day for 7–26 days, for a total taper duration of 14–33 days (Figure 1). The empiric dosing regimen selected was adopted from a previously reported vancomycin tapering strategy [2]. Initially, patients were given a 14- to 21-day fidaxomicin taper after a 10-day treatment course of fidaxomicin administered twice daily (patients 4, 5, 9; Table 2). Subsequently, most patients received a 33-day fidaxomicin taper after a vancomycin course that was tapered to vancomycin 125 mg once daily before the fidaxomicin taper. This 33-day taper included fidaxomicin given once daily for 7 days and once every other day for 26 days. The regimen was chosen to accommodate a typical fidaxomicin prescription of 20 tablets.
Table 2.
Demographic Characteristics and Outcomes of Patients With mrCDI Who Received a FDX-TP Regimen: The SFI After the Most Effective Prior Treatment Is Compared With the SFI After FDX-TP
| Age, Years/Sex | No. of CDI Episodes | Prior Regimens | Additional Antimicrobial Exposure After the Last Prior Regimen | Longest SFI for Non-FDX-TP Course, Days (Antibiotic) | SFI Post FDX-TP, Days | Recurrence After FDX-TP | Additional Antimicrobial Exposure After FDX-TP Regimens | |
|---|---|---|---|---|---|---|---|---|
| Patient 4 | 68/F | 5 | M, M, VT, V + FDX-CH, FDX | None | 64 (VT) | 232 | No | None |
| Patient 5 | 46/F | 11 | M, V, V, VT, V + FMT, V, RC, VT, VT, VT + FDX-CH, FDX | None | 102 (V) | 462 | No | None |
| Patient 9 | 46/F | 3 | VT, FDX, FDX | None | 29 (FDX) | 571 | No | None |
| Patient 10 | 65/M | 4 | U, M + V, VT, VT | None | 21 (V) | 429 | No | None |
| Patient 11 | 37/F | 4 | M, M, VT, V + FDX | None | 15 (M) | 375 | No | None |
| Patient 12 | 54/F | 4 | M, V, VT | None | 35 (V) | 298 | No | None |
| Patient 13 | 68/F | 3 | M, VT, VT | None | 8 (M) | 121 | No | None |
| Patient 14 | 70/F | 4 | VT, VT, VT, VT | None | 81 (VT) | 79 | No | None |
| Patient 15* | 86/M | 8,9 | M,M,V,V,VT,V,VT,VT + FDX-TP,VT | None, Amoxicillin | 41 (V) | 282 | Yes, No | Yes |
| Patient 16 | 67/M | 5 | M,M,VT,V,VT | None | 17 (V) | 118 | Yes | Yes |
| Patient 17 | 66/M | 6 | U,M,M,V,VT, VT | None | 20 (V) | 0 | N/A** | None |
| Patient 18 | 91/M | 4 | M,M + VT,VT, VT | None | 9 (VT) | 23 | No | None |
| Mean ± SD: | 63.6 ± 16 | 5.1 ± 2 | Median (IQR) | 25 (30) | 257 (280)*** |
Abbreviations: CDI, Clostridium difficile infection; FDX, standard fidaxomicin treatment regimen; FDX-CH, fidaxomicin chaser; FDX-TP, fidaxomicin taper; FMT, fecal microbiota transplantation; IQR, interquartile range; IVIG, intravenous immunoglobulin; M, metronidazole; mrCDI, multiple recurrent CDI; N/A, not applicable; RC, rifaximin chaser; SD, standard deviation; SFI, symptom-free interval; U, unknown; V, standard vancomycin treatment course; VT, vancomycin taper.
* Patient was given 2 FDX-TP courses.
** Patient did not complete FDX-TP regimen.
*** P = .003 compared with non-FDX-TP SFI, Mann–Whitney U test.
The average age of the patients who received fidaxomicin taper was 63.7 ± 16 years (mean ± SD) and 58% were female (Table 2). Fidaxomicin taper was prescribed after a mean of 5 CDI episodes (range 3–11). The median SFI after fidaxomicin taper was 257 days (IQR, 280) and was greater than the median SFI after the most effective prior regimen (25 days; IQR, 30) (P = .003).
Twelve patients received 13 courses of fidaxomicin taper, and 92% (11 of 12) of patients experienced resolution of their diarrheal symptoms while receiving fidaxomicin taper. One patient (patient 15) received amoxicillin after fidaxomicin taper and promptly developed a subsequent recurrent CDI episode, but he responded to vancomycin, which was followed by a second fidaxomicin taper and he had no further recurrence (Table 2). Twelve fidaxomicin taper courses prescribed to 11 patients were evaluable for recurrence; patient 17 did not complete his fidaxomicin taper course due to worsening of his symptoms while on therapy and therefore could not be evaluated for recurrence. Of those fidaxomicin taper courses that were completed, 83% (10 of 12) were not associated with a subsequent CDI episode. Both of the CDI recurrences post fidaxomicin taper occurred after additional antimicrobial exposure; patient 15 experienced a recurrence after amoxicillin treatment for a sore throat taken 8 days after completing fidaxomicin taper, and patient 16 received cephalexin for a urinary tract infection. Before receiving cephalexin, patient 16 had been symptom free for 118 days after fidaxomicin taper. This length of time contrasts with his former longest SFI of 17 days after vancomycin taper. Patient 15 was retreated with fidaxomicin taper after his recurrence and has been symptom free for 282 days. When recurrences associated with additional antimicrobial exposure are excluded, all patients who completed a fidaxomicin taper course (10 of 10) have been symptom free to date.
Unlike the other patients receiving fidaxomicin taper, patient 17 experienced a worsening of diarrheal symptoms before completing fidaxomicin taper for his 6th CDI episode (5th recurrence). He was a 66-year-old man with a history of pancreatic necrosectomy for necrotizing pancreatitis and subsequent hepaticoduodenostomy and gastrojejunostomy. For his 4th recurrence, he had received 85 days of a prolonged vancomycin taper and experienced an increase in diarrheal stools (his 5th recurrence) as he attempted to wean the vancomycin daily dose to every other day. He was restarted on a chronic suppressive dose of vancomycin 125 mg once daily until fidaxomicin was approved by his insurance company. At this time, vancomycin was discontinued and fidaxomicin taper was initiated. Within 5 days of starting the fidaxomicin taper regimen at a dose 200 mg daily, he experienced an increase in diarrheal stools and restarted vancomycin 125 mg 4 times daily. He later reported inadequate control of his diarrheal symptoms while on the suppressive dose of vancomycin that preceded fidaxomicin taper, indicating that the fidaxomicin taper may have been initiated prematurely.
DISCUSSION
Disruption of the normal colonic microbiota after antimicrobial therapy is a mechanism proposed for the pathogenesis of recurrent CDI as well as primary CDI [17, 18]. Normal intestinal microbiota help promote “colonization resistance” of C difficile. The exact mechanism of protection is unknown; however, it has been suggested that the gastrointestinal microbiota can directly inhibit C difficile proliferation by competing for nutrients or releasing antimicrobial products active against the bacteria [19]. Indigenous bacteria can also facilitate the transformation of bile acid products that can negatively affect C difficile spore germination and vegetative cells. Dysbiosis due to both CDI and non-CDI antimicrobial therapy can help explain the observed high first and second recurrence rates ranging between 25% and 50% associated with vancomycin and metronidazole.
A vancomycin tapering strategy is typically effective in managing CDI recurrences. Conceptually, the beginning of the taper would provide higher exposure of vancomycin within the colon to control the initially high C difficile bacterial load. Because vancomycin has activity against commensal anaerobes, exposure at high doses consequently alters the normal colonic microbiota [20]. Tapering the vancomycin dose (eg, from 4 times daily to twice daily to once daily and pulsing every 2–3 days) may minimize further disruption of the fecal microbiota while keeping residual C difficile in check. Applying a taper approach using fidaxomicin has effectively controlled subsequent recurrences among our patients who have failed a prolonged vancomycin taper. It has been shown that fidaxomicin spares Bacteroidetes and Firmicutes; its narrower spectrum of activity compared with vancomycin may better promote recolonization of the normal microbiota [20, 21].
Non-CDI antimicrobial exposure after CDI resolution is a known risk factor for recurrent CDI, and, despite the lower recurrence rates seen after fidaxomicin treatment, concomitant antibiotics compromise the response rate and durability of response seen with fidaxomicin [1, 22]. Both of our patients who experienced a recurrence after completing fidaxomicin taper received additional antimicrobial treatment. Patient 16 has a history of chronic urinary tract infections and received a course of cephalexin, whereas patient 15 received a course of amoxicillin. It has been shown that patients recovering from CDI are at the highest risk for recurrences within the first 2–3 weeks after disease resolution [18]. Patient 15 was exposed to antimicrobials within 11 days of completing therapy, and his recurrence was in line with this risk period. In contrast, patient 16 received treatment for a urinary tract infection almost 4 months after resolution of mrCDI and completion of fidaxomicin taper. In addition, patient 2 was symptom free post fidaxomicin chaser for 92 days until she experienced her 8th CDI episode 2 weeks after receiving levofloxacin for a urinary tract infection. Although reinfection with a new strain or recurrence due to the same strain are possible mechanisms for recurrence, the delayed onset of mrCDI months after completing CDI therapy and so soon after starting a new antibiotic suggests that some patients may carry low levels of C difficile for prolonged periods without symptomatic recurrence after CDI treatment including fidaxomicin [23].
In summary, our experience suggests that a fidaxomicin taper strategy for patients with mrCDI refractory to commonly used salvage therapies may be effective in breaking the cycle of mrCDI. At this time, the number of patients treated is small, but results suggest that this approach should be investigated further in more rigorous, systematically designed prospective randomized trials.
Acknowledgments
We thank Mary M. O′Driscroll for help in supporting the longitudinal care of our clinic patients.
Financial support. S. J. and D. N. G. are supported by the US Department of Veterans Affairs Merit Review Program. D. N. G. has also received research grants from GOJO and the Centers for Disease Control and Prevention.
Potential conflicts of interest. L. H. D. is on the speaker's board for Cubist and Astellas. S. J. has served as a consultant for Bio-K+ and Summit PLC. D. N. G. holds patents for the treatment and prevention of CDI licensed to ViroPharma/Shire and is a consultant for Sanofi Pasteur, Merck, Rebiotix, ViroPharma, Cubist, Summit, and Actelion.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fekety R, McFarland LV, Surawicz CM, et al. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997;24:324–33. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 2.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769–75. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 3.Barbut F, Richard A, Hamadi K, et al. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000;38:2386–8. doi: 10.1093/gao/9781884446054.article.t031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakken JS, Polgreen PM, Beekmann SE, et al. Treatment approaches including fecal microbiota transplantation for recurrent Clostridium difficile infection (rCDI) among infectious disease physicians. Anaerobe. 2013;24:20–4. doi: 10.1016/j.anaerobe.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Johnson S, Schriever C, Galang M, et al. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44:846–8. doi: 10.1086/511870. [DOI] [PubMed] [Google Scholar]
- 6.Johnson S, Schriever C, Patel U, et al. Rifaximin redux: treatment of recurrent Clostridium difficile infections with rifaximin immediately post-vancomycin treatment. Anaerobe. 2009;15:290–1. doi: 10.1016/j.anaerobe.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Garey KW, Ghantoji SS, Shah DN, et al. A randomized, double-blind, placebo-controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhoea in patients with Clostridium difficile infection. J Antimicrob Chemother. 2011;66:2850–5. doi: 10.1093/jac/dkr377. [DOI] [PubMed] [Google Scholar]
- 8.Musher DM, Logan N, Mehendiratta V, et al. Clostridium difficile colitis that fails conventional metronidazole therapy: response to nitazoxanide. J Antimicrob Chemother. 2007;59:705–10. doi: 10.1093/jac/dkl553. [DOI] [PubMed] [Google Scholar]
- 9.Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 10.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 11.Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(Suppl 2):S154–61. doi: 10.1093/cid/cis462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 13.Bono BR, Culshaw DL, Mitropoulos IF, et al. Patients with recalcitrant Clostridium difficile-associated diarrhea treated successfully with fidaxomicin: a case series. Infect Dis Clin Pract. 2014 doi:10.1097/IPC.0b013e31829ff59b. [Google Scholar]
- 14.Smeltzer S, Hassoun A. Successful use of fidaxomicin in recurrent Clostridium difficile infection in a child. J Antimicrob Chemother. 2013;68:1688–9. doi: 10.1093/jac/dkt079. [DOI] [PubMed] [Google Scholar]
- 15.Orenstein R. Fidaxomicin failures in recurrent Clostridium difficile infection: a problem of timing. Clin Infect Dis. 2012;55:613–4. doi: 10.1093/cid/cis495. [DOI] [PubMed] [Google Scholar]
- 16.Johnson S, Gerding DN. Fidaxomicin “chaser” regimen following vancomycin for patients with multiple Clostridium difficile recurrences. Clin Infect Dis. 2013;56:309–10. doi: 10.1093/cid/cis833. [DOI] [PubMed] [Google Scholar]
- 17.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435–8. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 18.Abujamel T, Cadnum JL, Jury LA, et al. Defining the vulnerable period for re-establishment of Clostridium difficile colonization after treatment of C. difficile infection with oral vancomycin or metronidazole. PLoS One. 2013;8:e76269. doi: 10.1371/journal.pone.0076269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Britton RA, Young VB. Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends Microbiol. 2012;20:313–9. doi: 10.1016/j.tim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louie TJ, Cannon K, Byrne B, et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Infect Dis. 2012;55(Suppl 2):S132–42. doi: 10.1093/cid/cis338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tannock GW, Munro K, Taylor C, et al. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology. 2010;156(Pt 11):3354–9. doi: 10.1099/mic.0.042010-0. [DOI] [PubMed] [Google Scholar]
- 22.Mullane KM, Miller MA, Weiss K, et al. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin Infect Dis. 2011;53:440–7. doi: 10.1093/cid/cir404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson S, Adelmann A, Clabots CR, et al. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J Infect Dis. 1989;159:340–3. doi: 10.1093/infdis/159.2.340. [DOI] [PubMed] [Google Scholar]