In multivariate analysis, a leukocyte score that adds 1 point each for neutropenia, lymphopenia and monocytopenia was associated with 30-day mortality in 192 patients with bacteremic pneumococcal pneumonia. By reflecting immunoparalysis, this score could improve clinical outcome predictions in BPP.
Keywords: bacteremia, leukocyte, mortality, pneumonia, Streptococcus pneumonia
Abstract
Background
Bacteremic pneumococcal pneumonia (BPP) is associated with high and early mortality. A simple procedure to predict mortality is crucial.
Methods
All adult patients with BPP admitted from 2005 through 2013 to the University Hospital of Dijon, France, were enrolled to study 30-day mortality and associated factors, particularly leukocyte counts. A simple leukocyte score was created by adding 1 point each for neutropenia (<1500 cells/mm3), lymphopenia (<400), and monocytopenia (<200).
Results
One hundred and ninety-two adult patients (mean age, 69 years; standard deviation [SD], 19 years) who had developed and were hospitalized for BPP (58% community-acquired) were included. The 30-day crude mortality rate was 21%. The mean Pneumonia Severity Index score was high at 127.3 (SD = 41.3). Among the 182 patients who had a white blood cell count, 34 (19%) had a high leukocyte score (≥2). Multivariate analysis revealed that mortality was significantly associated with a high leukocyte score (odds ratio, 6.28; 95% confidence interval, 2.35–16.78), a high respiratory rate, a low serum bicarbonate level, and an altered mental status (all P < .05). The leukocyte score was not significantly dependent on the previous state of immunosuppression, alcoholism, or viral coinfection, but it did correlate with an acute respiratory distress syndrome and a low serum bicarbonate level.
Conclusions
This new leukocyte score, in combination with the well known predictive factors, seems of interest in predicting the risk of death in BPP. A high score correlated with organ dysfunction and probably reflects the level of immunoparalysis. Its predictive value has to be confirmed in other cohorts.
Community-acquired pneumonia (CAP) is the major cause of infection-related death in developed countries and Streptococcus pneumoniae is the principal causative agent [1]. In healthcare-associated pneumonia (HCAP), S pneumoniae is one of the most frequent causative pathogens, and patients infected with this bacterium have a greater risk of in-hospital death [2].
Even though less than 20% of patients with pneumococcal pneumonia also present a bloodstream infection [3, 4], bacteremic pneumococcal pneumonia (BPP) is a particularly severe form with a first month mortality rate ranging from 15% to 29% [4–6]. The microbial characteristics, such as serotypes and antimicrobial resistance, have also been shown to be different from those in non-BPP [4]. Several prognostic factors, including age, male gender, comorbidities, severity of sepsis, low blood pressure, high respiratory rate, altered mental status, multilobar involvement, late antibiotic treatment, or discordant therapy have been identified in BPP [7, 8].
Leukopenia is a marker of systemic inflammatory response syndrome and is also a risk factor of death following CAP, especially Staphylococcus aureus-necrotizing CAP [9, 10]. Leukopenia, occurring in approximately 7% to 17% of cases [5, 8, 11], was previously described as a poor prognostic factor in BPP [5]. However, many studies considered the mean leukocyte counts, which mainly reflect the neutrophil count and give limited information about monocytes and lymphocytes [12]. It is important to study markers of the innate immune response, especially in light of the increasing evidence of sepsis-induced immunosuppression and the related mortality [13, 14].
Thus, we conducted a study to assess the association between leukocytes and 30-day mortality in a cohort of patients with proven BPP.
PATIENTS AND METHODS
Study Design
A cohort of adult patients hospitalized for BPP (either community acquired or healthcare -related) at the Hospital of Dijon was prospectively observed from January 1, 2005 to March 31, 2013 (French National Hospital Clinical Research Program [PHRC] 2004/37). Data were collected in standardized report forms. This cohort was retrospectively increased by patients hospitalized with BPP during the same period, in the same hospital and who had not been included in the prospective cohort study for logistical reasons. The study was carried out in accordance with the Declaration of Helsinki and National standards. The collection of nominative data was approved by the national authority for the protection of privacy and personal data and by the local ethics committee (Comité de protection des personnes Est I).
Inclusion and Noninclusion Criteria
For both retrospective and prospective inclusions, the patients needed to meet the following 3 criteria: (1) age over 18 years; (2) blood cultures obtained at the time of hospitalization and positive for S pneumoniae; and (3) diagnosis of pneumonia, defined as an acute illness (<10 days of symptoms) with the presence of a new pulmonary infiltrate on chest radiograph at the time of hospitalization, plus either a new or increased cough with or without sputum production, or an abnormal temperature (<35.6°C or >37.8°C), or an abnormal serum leukocyte count: ie, leukocytosis (leukocyte count ≥ 10.106/L), left shift, or leukopenia (leukocyte count <4.106/L).
Hospital-acquired pneumonia (HAP), HCAP, and CAP were defined according to the guidelines of the American Thoracic Society/Infectious Diseases Society of America [15, 16]. Healthcare-associated pneumonia corresponded to any of the following: hospitalization for ≥2 days in the preceding 90 days; residence in a nursing home; home infusion therapy; long-term dialysis within 30 days; and home wound care. Hospital-acquired pneumonia was defined as pneumonia that occurred 48 hours or more after admission, which was not incubating at the time of admission.
Noninclusion criteria were concomitant meningitis, endocarditis, spondylitis, or arthritis at diagnosis.
Study Variables
Demographic data, medical history, initial clinical presentation, and biological findings (first 24 hours), antibiotic treatment, microbiological culture results, and outcome were all recorded. Comorbidity was assessed using the Charlson Comorbidity Index [17]. To evaluate disease severity, we collected the first 24-hour clinical and biological data for the Pneumonia Severity Index (PSI) score as defined by Fine et al. [18], the Simplified Acute Physiology Score II (SAPS II) [19], and the CURB65 score (confusion, urea >7 mM, respiratory rate ≥30 breaths min−1, blood pressure <90 mm Hg systolic or ≤60 mm Hg diastolic, aged ≥65 years old) [20]. The following therapeutic data were recorded: vasoactive drugs, renal replacement therapy, mechanical ventilation, corticosteroid treatment.
Immunosuppression included human immunodeficiency virus seropositivity; daily administration of corticosteroids (at least 5 mg per day of prednisone or an equivalent drug); immunosuppressive therapy; chemotherapy for an underlying malignancy during the 6 months before hospital admission; and primary or secondary hypogammaglobulinemia, hypocomplementemia, and splenectomy [2].
Septic shock was defined as the requirement for vasopressor for more than 4 hours or hypotension (systolic blood pressure <90 mm Hg) for more than 1 hour, despite adequate fluid challenge, plus either a change in mental status, oliguria, organ dysfunction, or lactate >2 mmol/L [21]. Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition [22].
The susceptibility of bacterial strains to antibiotics was assessed according to the recommendations of the Antibiogram Committee of the French Society for Microbiology [23] for antimicrobial susceptibility testing and breakpoints. The following definitions were used: penicillin minimal inhibitory concentration (MIC) ≤ 0.06 mg/L; intermediate, 0.06 mg/L < MIC ≤ 1 mg/L; resistant, MIC > 1 mg/L.
The antimicrobial treatment was considered inappropriate when the isolated strain of S pneumoniae was not susceptible in vitro to the first line of antibiotics administered. Appropriate antimicrobial treatment had to be prescribed within 24 hours of hospital admission.
Leukocyte Score
A simple leukocyte score was created by adding up the points related to the white blood cell count: neutropenia (neutrophil count <1.5.106/L), 1 point; severe lymphopenia (lower than the 30th percentile), 1 point; and monocytopenia (lower than the 30th percentile), 1 point; with a minimum of 0 and a maximum of 3.
Outcome/Judgment Criterion
Thirty-day mortality was reported as the proportion of patients who died within 30 days after hospitalization. The time to follow-up was recorded as the number of days from the date the blood sample was received at the Microbiology Department to death or to the 30-day censoring point. Early death was defined as death occurring during the first 48 hours.
Statistical Analysis
Continuous variables were expressed as means and standard deviations (SDs), and categorical variables were expressed as frequencies (percentages). Continuous data were compared using the Mann–Whitney U–test, and categorical data were compared using the χ2 test (and Fisher's exact test when appropriate). Survival was studied in different classes of the leukocyte score by the log-rank test and presented by Kaplan-Meier curves. Forward stepwise logistic regression was used for the multivariate analysis, which included all of the variables with P ≤ .2 in the univariate analysis. Although included in the univariate analysis, the usual severity scores (SAPS-II, PSI, CURB-65, Charlson) were not individually included in the multivariate model, because they partially overlap and comprise other variables that were analyzed, per se, in particular age, chronic diseases, and clinical and biological findings. Odds ratios (OR) and 95% confidence intervals (CIs) were calculated for each variable. In a second step, sensitivity analysis was performed by excluding immunosuppressed patients. Finally, receiver operating characteristic (ROC) curves for the leukocyte score, the CURB-65 score and the PSI, for 48-hour and 30-day mortality were drawn, and areas under the curves (AUC) were compared. Statistical significance was defined as P < .05. Statistical analyses were computed using SPSS version 19 software.
RESULTS
Among the 238 adult patients with pneumococcal bacteremia from January 2005 to March 2013, 200 patients were hospitalized at Dijon University Hospital with a diagnosis of pneumonia and without early complications. Of these, 8 were excluded from the analysis because of missing data in the follow-up. Of the remaining 192 patients (112 CAP [58%], 60 HCAP [31%], and 20 HAP [10%]), 82 were prospectively included in the PHRC cohort and 110 were retrospectively analyzed (56 patients before 2009 and 54 from 2009 onwards). No patient was lost during the follow-up period. The total 30-day mortality rate was 21% (16% for CAP, 28% for HCAP), and 21 of the 41 deaths (51%) occurred during the first 48 hours.
White Blood Cells Analysis: Elaboration of the Leukocyte Score
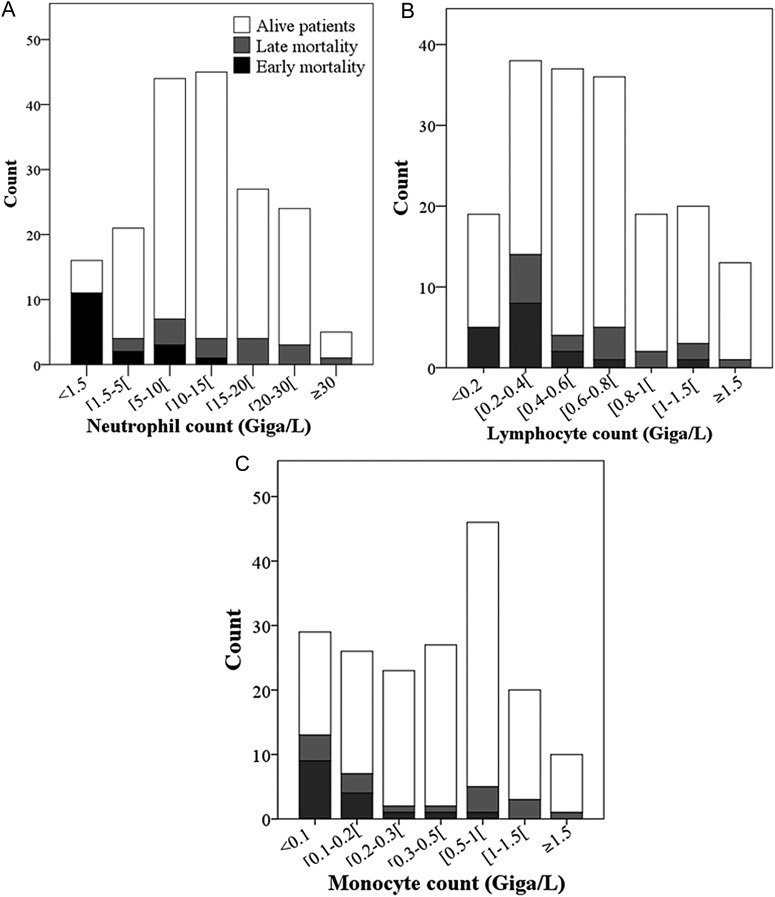
The white blood cell count was available in 182 (95%) patients, and leukopenia <4000 cells/mm3 was present in 24 (13%) of them. The distribution of neutrophils, lymphocytes, and monocytes is shown in Figure 1. Note that patients who died were mainly in the lower values of each subclass of leukocytes. We created a simple leukocyte score by adding the points related to the white blood cells counts: neutropenia (neutrophil count <1.5.106/L), 1 point; severe lymphopenia (<400 cells/mm3, the 30th percentile), 1 point; and monocytopenia (<200 cells/mm3, the 30th percentile), 1 point; with a minimum of 0 and a maximum of 3. Univariate analysis revealed that 30-day mortality was significantly associated with neutropenia (OR, 13.68; 95% CI, 4.35–42.99), severe lymphopenia (OR, 3.67; 95% CI, 1.70–7.93), and monocytopenia (OR, 4.61; 95% CI, 2.11–10.07). Two groups were defined: low leukocyte score (<2) and high leukocyte score (≥2), according to the split between the 2 curves (leukocyte score = 1 and leukocyte score = 2) in the Kaplan-Meier plot of 30-day survival (Figure 2).
Figure 1.
The histogram shows the distribution of leukocytes in different strata for the 182 patients with bacteremic pneumococcal pneumonia and an available leukocyte count: (A) neutrophil count distribution; (B) lymphocyte count distribution; (C) monocyte count distribution. The clear bar represents survivors and the black bars represent nonsurvivors at 30 days.
Figure 2.
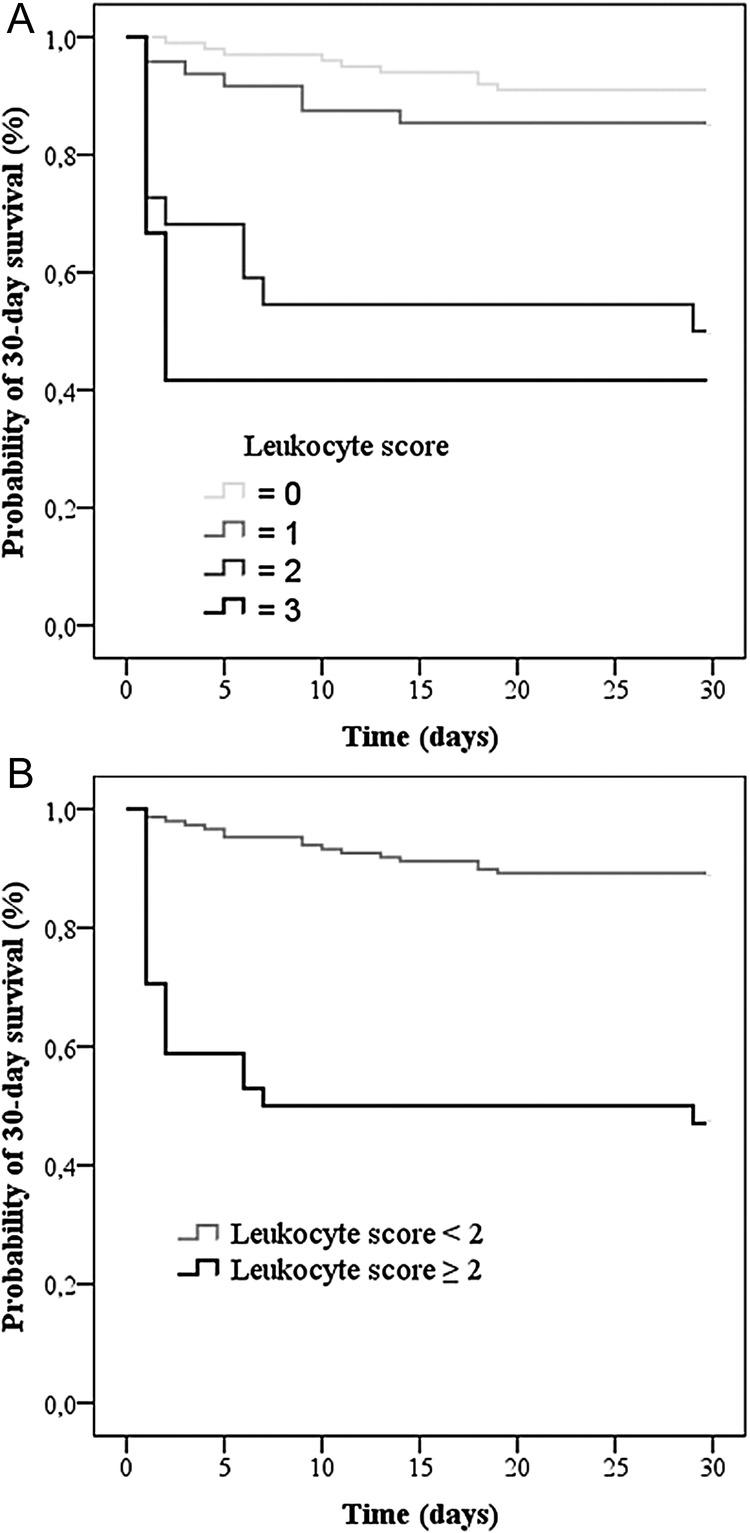
Kaplan-Meier survival analysis comparing 182 patients with bacteremic pneumococcal pneumonia according to their leukocyte score. (A) Patients with different classes of the leukocyte score (0–3) were compared (log rank: P < .0001). (B) Patients with a low (<2) leukocyte score (gray curve) were compared with patients with a high (≥2) leukocyte score (black curve) (log rank: P < .0001).
Thirty-four of the 182 patients (19%) had a high leukocyte score, including 13 of 39 immunocompromised patients (33%) and 21 of 143 non-immunocompromised patients (15%).
Patients' Characteristics
The main characteristics of the clinical outcomes are presented in Table 1. The mean age of patients at admission was 68.9 (SD = 18.8) years; 111 patients (58%) were male, and the mean duration of hospitalization was 18.4 (24.0) days. More than 75% of the deaths occurred in patients aged >65 years. One hundred and forty-two patients (74%) had at least 1 chronic comorbidity.
Table 1.
Baseline Demographic and Clinical Characteristics of 192 Patients With Bacteremic Pneumococcal Pneumoniaa
| Survivor | Nonsurvivor | Univariate Analysis, OR [95% CI] | P Value | Multivariate Analysis, OR [95% CI] | |
|---|---|---|---|---|---|
| n = 151 | n = 41 | ||||
| Demographic data | |||||
| Age, median (IQR) | 70 (54–84) | 77 (65–85) | .06 | ||
| Age ≥65 years | 91 (60) | 32 (78) | 2.34 [1.05–5.26] | .04 | |
| Comorbid conditions | |||||
| HAP | 12 (8) | 8 (20) | 2.81 [1.06–7.42] | .04 | |
| Chronic heart disease | 39 (26) | 16 (39) | 1.84 [0.89–3.80] | .10 | |
| Diabetes mellitus | 30 (20) | 4 (10) | 0.44 [0.14–1.32] | .13 | |
| Recent or progressive cancer | 25 (17) | 13 (32) | 2.34 [1.07–5.13] | .03 | |
| Immunosuppression | 35 (23) | 11 (27) | 1.27 [0.58–2.80] | .56 | |
| Clinical features on first day | |||||
| Heart rate ≥110/min | 69 (46) | 25 (61) | 1.86 [0.92–3.76] | .08 | |
| Systolic blood pressure < 90 mm Hg | 28 (19) | 21 (51) | 4.61 [2.21–9.64] | <.0001 | |
| Respiratory rate ≥30/min | 59 (39) | 32 (78) | 5.54 [2.47–12.45] | <.0001 | 4.83 [1.71–13.61] |
| Altered mental status | 21 (14) | 18 (44) | 4.85 [2.24–10.46] | <.0001 | 4.17 [1.52–11.46] |
| Septic shock | 11 (7) | 13 (32) | 5.91 [2.40–14.53] | <.0001 | |
| ARDS | 9 (6) | 15 (37) | 9.10 [3.61–22.98] | <.0001 | |
| Cardiac failure | 26 (17) | 13 (32) | 2.21 [1.01–4.84] | .04 | |
| Biological feature on first day | |||||
| Bicarbonates <21 mmol/L (150/41)b | 8 (5) | 14 (34) | 9.14 [3.50–23.90] | <.0001 | 3.80 [1.11–13.00] |
| Blood urea ≥10 mmol/L (150/41)b | 77 (51) | 33 (81) | 3.91 [1.70–9.02] | .001 | |
| High leukocyte score (148/34)b | 16 (11) | 18 (53) | 9.28 [3.97–21.72] | <.0001 | 6.28 [2.35–16.78] |
| Radiological findings | |||||
| Multilobar infiltrates | 45 (30) | 32 (78) | 8.38 [3.70–18.97] | <.0001 | |
| Treatments, first 24 h | |||||
| Amoxicillin | 88 (58) | 12 (29) | 0.30 [0.14–0.63] | <.0001 | |
| Third-generation cephalosporin | 50 (33) | 23 (56) | 2.58 [1.28–5.22] | .007 | |
| ≥2 antimicrobial treatments | 55 (36) | 27 (66) | 3.67 [1.63–6.96] | .001 | |
| Other treatments | |||||
| ICU admission | 37 (25) | 19 (46) | 2.64 [1.29–5.40] | .007 | |
| Mechanical ventilation | 17 (11) | 19 (46) | 6.81 [3.08–15.07] | <.0001 | |
| Dialysis | 8 (5) | 8 (20) | 4.33 [1.52–12.39] | .01 | |
| Steroids | 37 (25) | 15 (37) | 1.78 [0.85–3.71] | .12 | |
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; HAP, hospital-acquired pneumonia; ICU, intensive care unit; IQR, interquartile range; OR, odds ratio.
a Data are expressed as number (%), excepted age: median (IQR).
b (n1/n2): data available.
The mean Charlson, SAPS-II, PSI, and CURB-65 scores were 1.7 (SD = 1.8), 35.6 (SD = 16.9), 127.3 (SD = 41.3), and 2.7 (SD = 1.2), respectively, thereby reflecting severe critical illness. Sixty-three (33%) patients had severe BPP that required admittance to an intensive care unit or caused early death. Only 5 patients had a viral coinfection (3 influenza virus infections, 1 respiratory syncytial virus infection, and 1 parainfluenza 3 virus infection).
Thirty-Day Mortality
Table 1 summarizes variables with P ≤ .2 in univariate analysis. Gender, HCAP, active tobacco use, active alcohol abuse, chronic obstructive pulmonary disease, chronic renal insufficiency, immunosuppression, pleural effusion, penicillin-nonsusceptible pneumococci, first anti-biotherapy including a macrolide, and inappropriate antimicrobial treatment were not associated with 30-day mortality.
The following severity scores had lower values in survivors than in nonsurvivors: SAPS-II (3.7 vs 54.6, P < .0001), PSI (117.2 vs 167.9, P < .0001), and CURB-65 (2.4 vs 3.7, P < .0001). The mean Charlson comorbidity score tended to be lower in survivors (1.6 vs 2.3, P = .06).
In multivariate analysis, a respiratory rate ≥30 breaths/min (OR, 4.83; 95% CI, 1.71–13.61), a low serum bicarbonate (HCO3) level < 21 mmol/L (OR, 3.80; 95% CI, 1.11–13.00), a high leukocyte score (OR, 6.28; 95% CI, 2.35–16.78), and an altered mental status (OR, 4.17; 95% CI, 1.52–11.46) were associated with 30-day mortality.
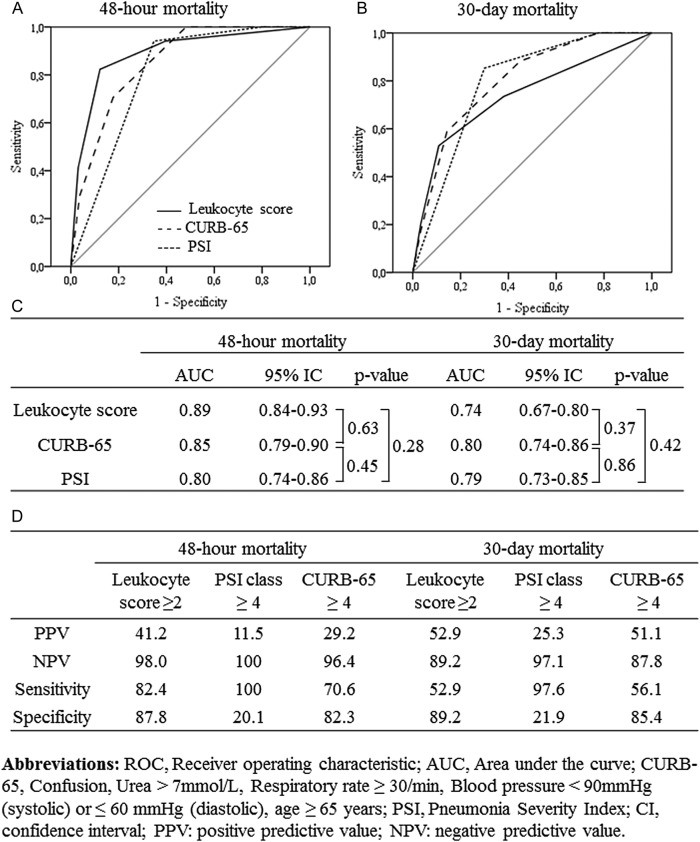
A high leukocyte score correlated remarkably well with early death (<48 h). To predict 30-day mortality, a high leukocyte score had a positive predictive value (PPV) of 53% and a negative predictive value (NPV) of 89%. In addition, the 3 combined factors (high respiratory rate, low serum bicarbonates and altered mental status) had a PPV = 65% and NPV = 88%, but when these were associated with a high leukocyte score the PPV was higher (78%) with the same NPV.
In a sensitivity analysis (without considering the 43 immunosuppressed patients), a respiratory rate ≥30 breaths/min (OR, 14.27; 95% CI, 3.08–66.12) and a high leukocyte score (OR, 8.33; 95% CI, 2.64–26.32) remained significantly associated with death (Table 2).
Table 2.
Baseline Demographics and Clinical Characteristics of 143 Immunocompetent Patients With Bacteremic Pneumococcal Pneumoniaa
| Survivor | Nonsurvivor | Univariate Analysis, P Value | Multivariate Analysis, OR [95% CI] | |
|---|---|---|---|---|
| n = 117 | n = 26 | |||
| Demographic data | ||||
| Age ≥65 years | 71 (61) | 20 (77) | .12 | |
| Comorbid conditions | ||||
| HAP | 5 (4) | 3 (12) | .16 | |
| COPD | 20 (17) | 8 (31) | .11 | |
| Chronic heart disease | 33 (28) | 13 (50) | .03 | |
| Diabetes mellitus | 26 (22) | 2 (8) | .09 | |
| Recent or evolutive cancer | 3 (3) | 3 (12) | .07 | |
| Clinical features on first day | ||||
| Heart rate ≥110/min | 54 (46) | 16 (62) | .16 | |
| Respiratory rate ≥30/min | 49 (42) | 24 (92) | <.0001 | 14.27 [3.08–66.12] |
| Altered mental status | 19 (16) | 10 (39) | .01 | |
| Septic shock | 9 (8) | 7 (27) | .01 | |
| ARDS | 9 (8) | 10 (39) | <.0001 | |
| Cardiac failure | 22 (19) | 10 (39) | .03 | |
| Biological features on first day | ||||
| Bicarbonates <21 mmol/L | 6 (5) | 9 (35) | <.0001 | |
| Blood urea ≥10 mmol/L | 59 (50) | 21 (81) | .005 | |
| High leukocyte score | 9 (8) | 12 (46) | <.0001 | 8.33 [2.64–26.32] |
| Radiological findings | ||||
| Multilobar infiltrates | 36 (31) | 21 (81) | <.0001 | |
| Treatments, first 24 h | ||||
| Amoxicillin | 78 (67) | 7 (27) | <.0001 | |
| Third-generation cephalosporin | 30 (26) | 16 (64) | <.0001 | |
| ≥2 antimicrobial treatments | 36 (31) | 18 (69) | <.0001 | |
| Inappropriate antimicrobial treatment | 4 (3) | 3 (12) | .11 | |
| Other treatments | ||||
| ICU admission | 30 (26) | 14 (54) | .005 | |
| Mechanical ventilation | 14 (12) | 13 (50) | <.0001 | |
| Dialysis | 5 (4) | 6 (8) | .001 | |
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HAP, hospitalized-acquired pneumonia; ICU, intensive care unit; OR, odds ratio.
a Data are expressed as number (%).
Comparison of the Leukocyte Score With the CURB-65 Score and Pneumonia Severity Index
Receiver operating characteristic curves and performance of the leukocyte score, the CURB-65 score, and the PSI for 48-hour and 30-day mortality are depicted in Figure 3. The leukocyte score (AUC, 0.74; 95% CI, .67–.80) was as efficient as the 2 other scores in predicting 30-day mortality. Note that performances were higher in predicting 48-hour mortality (AUC, .89; 95% CI, .84–.93). The performances for CAP and HCAP/HAP were comparable (Supplementary Figure 1).
Figure 3.
Receiver operating characteristic (ROC) curves for the leukocyte score, the CURB-65 score, and the Pneumonia Severity Index (PSI), for (A) 48-hour mortality and (B) 30-day mortality; (C) area under the ROC curves; (D) measures of performance in predicting 48-hour and 30-day mortality by the 3 scores.
Factors Associated With a High Leukocyte Score
The characteristics of the 143 immunocompetent patients according to the leukocyte score are presented in Table 3. Active alcohol abuse was not significantly associated with a high leukocyte score (P = 1.0). No significant association was observed between a high leukocyte score and seasonal flu epidemics (P = .53). In multivariate analysis, ARDS (OR, 8.85; 95% CI, 2.82–27.80) and a low serum HCO3 level < 21 mmol/L (OR, 4.54; 95% CI, 1.23–16.78) were significantly associated with a high leukocyte score. To ascertain the potential interaction between a high leukocyte score and a low bicarbonate count in the logistic regression (Table 1), an interaction variable defined by the multiplication of the values of these 2 variables was calculated. This variable was not significantly associated with 30-day mortality (P = .73), and the coefficient of correlation was −.09 (inferior to .9).
Table 3.
Characteristics According to the Leukocyte Score of the 143 Immunocompetent Patients With Bacteremic Pneumococcal Pneumoniaa
| Low Leukocyte Score | High Leukocyte Sore | Univariate Analysis, P Value | Multivariate Analysis, OR [95% CI] | |
|---|---|---|---|---|
| n = 122 | n = 21 | |||
| Demographic | ||||
| Male gender | 68 (56) | 8 (38) | .14 | |
| Clinical features on first day | ||||
| Heart rate ≥110/min | 55 (45) | 15 (71) | .03 | |
| Systolic blood pressure < 90 mm Hg | 25 (21) | 8 (38) | .09 | |
| Respiratory rate ≥30/min | 57 (47) | 16 (76) | .01 | |
| Altered mental status | 22 (18) | 7 (33) | .14 | |
| Septic shock | 9 (7) | 7 (33) | .003 | |
| ARDS | 9 (7) | 10 (48) | <.0001 | 8.85 [2.82–27.80] |
| Biological features on first day | ||||
| Bicarbonates < 21 mmol/L | 8 (7) | 7 (33) | .002 | 4.54 [1.23–16.78] |
| Blood urea ≥10 mmol/L | 64 (53) | 16 (76) | .04 | |
| Microbiological findings | ||||
| Penicillin G (MIC ≥ 0.06 µg/mL) | 38 (31) | 2 (10) | .04 | |
| Third-generation cephalosporin (MIC ≥ 0.5 µg/mL) | 21 (17) | 1 (5) | .20 | |
| Documented viral coinfection | 0 | 4 (8%) | .01 | |
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; MIC, minimal inhibitory concentration; OR, odds ratio.
a Data are expressed as number (%).
DISCUSSION
The first result of this study is that BPP is associated with a high mortality rate: 21% of patients died during the following month, which is in keeping with previous studies (15%–29%) [4, 8, 11]. As also reported previously, 10% of the infections were HAP [24], and bacteremic pneumococcal HCAP and HAP were associated with higher mortality: 23% and 40%, respectively [25], whereas 30-day mortality for CAP was 17%.
Second, the main prognostic factors found are similar to those observed in previous studies. Of the conditions known to increase the incidence of invasive pneumococcal disease [26], chronic heart disease was the most frequent in our study. The Charlson comorbidity score, which reflects the vulnerability of the patients, was high. The PSI and CURB-65 scores, which have been validated as predictors of the need for intensive care and death in BPP [27], were significantly higher in the nonsurvivor group. Therefore, severe clinical signs were associated with a poorer outcome, as shown in multivariate analysis by the correlation between 30-day mortality, a higher respiratory rate, and altered mental status. In addition, a low serum HCO3 level, which reveals severe lactic acidosis [28], was an independent factor of mortality. This test can be done routinely, but it is almost never studied in BPP. As previously reported, we found no significant association between the appropriateness of initial antimicrobial therapy and 30-day mortality [29]. In addition, using third-generation cephalosporin was associated with a higher death rate, and using amoxicillin was associated with a higher survival rate in the univariate but not in the multivariate analysis. These findings reflect possible bias because third-generation cephalosporin is recommended in severe CAP or HCAP [30].
Third, we observed that neutropenia, lymphopenia, and monocytopenia were significantly associated with 30-day mortality. The incidence of leukopenia (13%) was similar to that in other studies [5, 8, 11]. When white blood cell abnormalities were combined in a leukocyte score, we found that a score of 2 or more was associated with 30-day mortality in the whole population as well as in immunocompetent patients only. The PPV of the leukocyte score alone (53%) was lower than the PPV of the other 3 factors together (65%). However, combining all 4 factors significantly improved the PPV (78%), whereas the NPV did not change (approximately 88%). More specifically, a high leukocyte score was associated with early mortality: the higher the score, the higher the mortality. Note that the leukocyte score, similar to the CURB-65 score and the PSI, seems to perform better in predicting early mortality for CAP and for HCAP/HAP.
Unlike previous studies, in our study leukopenia in pneumococcal pneumonia did not appear to be linked to the suppressive impact of chronic alcoholism on the granulopoietic response to pulmonary S pneumoniae infection [31, 32]. We also found no significant association between mortality and viral coinfections. Indeed, in influenza infections, leukopenia is commonly associated with ARDS and a poor prognosis [33] and may be related to alterations in the lympho-hematopoietic system with destruction of lymphocytes by virus-induced apoptosis, hemophagocytosis [33], and the depletion of alveolar macrophages [34]. However, viral coinfections were rarely observed in our study, and the under-diagnosis of such infections cannot be ruled out.
Nevertheless, leukopenia may be a direct consequence of the pneumococcal infection itself. The pneumococcal capsule is probably involved, leading to a failure of phagocytosis [35]. It is likely that other virulence factors, such as pneumolysin, are also responsible for leukopenia, as shown in S aureus with Panton-Valentine leukocidin (PVL) in necrotizing CAP, where severe leukopenia (≤3000 cells/mm3) is an important risk factor of mortality [10]. In such cases, PVL is known to induce both apoptosis and necrosis in human leukocytes [36]. In addition, in the study by Khanafer et al [10], there were significantly more ARDS with severe leukopenia (77% vs 18%, P < .0001). These results were confirmed in our cohort of BPP, with a significant association between ARDS and a high leukocyte score, probably reflecting the incapacity of the innate immune response to control bacterial growth.
After pneumococcal invasion, neutrophils and lymphocytes are rapidly recruited in great numbers at sites of infection [12, 37] and lead to inflammation-related lung damage. Thus, lymphopenia can be explained by lymphocyte sequestration in the peripheral tissues or apoptosis [38]. Monocytopenia might be a result of pneumolysin. First, monocytes are recruited and sequestered in the lung [39]. Then, pneumolysin depresses the monocyte respiratory burst and bactericidal activity [40]. Finally, murine models of local lung inflammation have revealed the sequestration of monocytes released from bone marrow [39].
These findings reinforce the controversial paradigm of sepsis-induced immunosuppression [14], which may partly explain the failure of many clinical trials that investigate an immunosuppressive approach in sepsis. Efforts are currently focused on new therapies to modulate the immune host response and new biomarkers that could identify patients with immunoparalysis [14]. From a clinical point of view, the leukocyte score could be a simple, readily available, and inexpensive biomarker that provides information on 3 major immune cells. However, we cannot exclude the possibility that we missed some patients with genuine BPP, either because blood sampling was not done or because effective antibiotic treatment was started before blood samples were taken. Furthermore, missing leukocyte data for 10 patients (3 survivors and 7 nonsurvivors) represent a potential bias in the analysis of the prognostic factors. The fact that we combined CAP and HCAP could also have modified the results. The single-centre nature of the study and the retrospectively collected data are also limitations. In addition, we assumed that a high leukocyte score could be related to a high initial bacterial inoculum and/or a different expression of virulence factors, without being able to demonstrate that this was indeed the case. Finally, given the great number of variables analyzed, the logistic regression model is possibly subject to overfitting. However, the 3 main variables (high leukocyte score, high respiratory rate and altered mental status) remained significantly associated with 30-day mortality after a bootstrapping procedure with 1000 replications and a CI of 95%.
CONCLUSIONS
In conclusion, a high leukocyte score was associated with mortality in this homogenous cohort of BPP in which the demographic, clinical, and biological data and prognostic factors were comparable to those in other BPP cohorts. A low white blood cell count is probably an early marker of failure of the immune system in its response to bacterial proliferation in the lung and may reflect an impaired innate immune response in some patients, possibly linked to the capsule, pneumolysin, and to other virulence factors. The predictive value of the leukocyte score in BPP has to be confirmed in other studies and should be investigated in pneumonia or sepsis, in which immunoparalysis has been found to be associated with a poor prognosis.
Supplementary material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank all the patients who participated to the study, Sandrine Gohier for help in data management, Professor Laurence Duvillard for support, Drs. Philippe d′Athis and Serge Aho for help in statistical analysis, and Philip Bastable for help in reviewing the manuscript.
Financial support. This study was supported by the French Clinical Research Program [PHRC 2004/37].
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Johansson N, Kalin M, Tiveljung-Lindell A, et al. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202–9. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polverino E, Torres A, Menendez R, et al. Microbial aetiology of healthcare associated pneumonia in Spain: a prospective, multicentre, case-control study. Thorax. 2013;68:1007–14. doi: 10.1136/thoraxjnl-2013-203828. [DOI] [PubMed] [Google Scholar]
- 3.Pereira JM, Teixeira-Pinto A, Basílio C, et al. Can we predict pneumococcal bacteremia in patients with severe community-acquired pneumonia? J Crit Care. 2013;28:970–4. doi: 10.1016/j.jcrc.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Kang CI, Song JH, Kim SH, et al. Risk factors and pathogenic significance of bacteremic pneumonia in adult patients with community-acquired pneumococcal pneumonia. J Infect. 2013;66:34–40. doi: 10.1016/j.jinf.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60:759–76. doi: 10.7326/0003-4819-60-5-759. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Vidal C, Fernández-Sabé N, Carratalà J, et al. Early mortality in patients with community-acquired pneumonia: causes and risk factors. Eur Respir J. 2008;32:733–9. doi: 10.1183/09031936.00128107. [DOI] [PubMed] [Google Scholar]
- 7.Berjohn CM, Fishman NO, Joffe MM, et al. Treatment and outcomes for patients with bacteremic pneumococcal pneumonia. Medicine (Baltimore) 2008;87:160–6. doi: 10.1097/MD.0b013e318178923a. [DOI] [PubMed] [Google Scholar]
- 8.Lujan M, Gallego M, Fontanals D, et al. Prospective observational study of bacteremic pneumococcal pneumonia: effect of discordant therapy on mortality. Crit Care Med. 2004;32:625–31. doi: 10.1097/01.ccm.0000114817.58194.bf. [DOI] [PubMed] [Google Scholar]
- 9.Menéndez R, Torres A, Zalacaín R, et al. Risk factors of treatment failure in community acquired pneumonia: implications for disease outcome. Thorax. 2004;59:960–5. doi: 10.1136/thx.2003.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanafer N, Sicot N, Vanhems P, et al. Severe leukopenia in Staphylococcus aureus-necrotizing, community-acquired pneumonia: risk factors and impact on survival. BMC Infect Dis. 2013;13:359. doi: 10.1186/1471-2334-13-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore) 2000;79:210–21. doi: 10.1097/00005792-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Kadioglu A, Gingles NA, Grattan K, et al. Host cellular immune response to pneumococcal lung infection in mice. Infect Immun. 2000;68:492–501. doi: 10.1128/iai.68.2.492-501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–74. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 16.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 19.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 20.Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–82. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. 1992. Chest. 2009;136(5 Suppl):e28. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 22.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 23.Chardon H. Pneumococcal susceptibility testing. Revue francophone des laboratoires. 2008;407:45–59. [Google Scholar]
- 24.Bouza E, Pintado V, Rivera S, et al. Nosocomial bloodstream infections caused by Streptococcus pneumoniae. Clin Microbiol Infect. 2005;11:919–924. doi: 10.1111/j.1469-0691.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- 25.Rello J, Luján M, Gallego M, et al. Why mortality is increased in health-care-associated pneumonia: lessons from pneumococcal bacteremic pneumonia. Chest. 2010;137:1138–44. doi: 10.1378/chest.09-2175. [DOI] [PubMed] [Google Scholar]
- 26.Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681–9. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- 27.Spindler C, Ortqvist A. Prognostic score systems and community-acquired bacteraemic pneumococcal pneumonia. Eur Respir J. 2006;28:816–23. doi: 10.1183/09031936.06.00144605. [DOI] [PubMed] [Google Scholar]
- 28.Surbatovic M, Radakovic S, Jevtic M, et al. Predictive value of serum bicarbonate, arterial base deficit/excess and SAPS III score in critically ill patients. Gen Physiol Biophys. 2009;28(Spec No):271–6. [PubMed] [Google Scholar]
- 29.Yu VL, Chiou CC, Feldman C, et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin Infect Dis. 2003;37:230–7. doi: 10.1086/377534. [DOI] [PubMed] [Google Scholar]
- 30.Chidiac C Société de pathologie infectieuse de langue française Agence française de sécurité sanitaire des produits de santé. Systemic antibiotherapy for the treatment of lower respiratory tract infections. Community acquired pneumonia, acute exacerbation of obstructive chronic bronchitis. Med Mal Infect. 2011;41:221–8. doi: 10.1016/j.medmal.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Perlino CA, Rimland D. Alcoholism, leukopenia, and pneumococcal sepsis. Am Rev Respir Dis. 1985;132:757–60. doi: 10.1164/arrd.1985.132.4.757. [DOI] [PubMed] [Google Scholar]
- 32.Siggins RW, Melvan JN, Welsh DA, et al. Alcohol suppresses the granulopoietic response to pulmonary Streptococcus pneumoniae infection with enhancement of STAT3 signaling. J Immunol Baltim Md 1950. 2011;186:4306–13. doi: 10.4049/jimmunol.1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.To KK, Hung IF, Li IW, et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis. 2010;50:850–9. doi: 10.1086/650581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghoneim HE, Thomas PG, McCullers JA. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol Baltim Md 1950. 2013;191:1250–9. doi: 10.4049/jimmunol.1300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadioglu A, Weiser JN, Paton JC, et al. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 36.Genestier AL, Michallet MC, Prévost G, et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J Clin Invest. 2005;115:3117–27. doi: 10.1172/JCI22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirst RA, Kadioglu A, O'callaghan C, et al. The role of pneumolysin in pneumococcal pneumonia and meningitis. Clin Exp Immunol. 2004;138:195–201. doi: 10.1111/j.1365-2249.2004.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kemp K, Bruunsgaard H, Skinhøj P, et al. Pneumococcal infections in humans are associated with increased apoptosis and trafficking of type 1 cytokine-producing T cells. Infect Immun. 2002;70:5019–25. doi: 10.1128/IAI.70.9.5019-5025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goto Y, Hogg JC, Whalen B, et al. Monocyte recruitment into the lungs in pneumococcal pneumonia. Am J Respir Cell Mol Biol. 2004;30:620–6. doi: 10.1165/rcmb.2003-0312OC. [DOI] [PubMed] [Google Scholar]
- 40.Nandoskar M, Ferrante A, Bates EJ, et al. Inhibition of human monocyte respiratory burst, degranulation, phospholipid methylation and bactericidal activity by pneumolysin. Immunology. 1986;59:515–20. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.