Abstract
Background
New first-line drug regimens for treatment of tuberculosis (TB) are in clinical trials: emergence of resistance is a key concern. Because population-level data on resistance cannot be collected in advance, epidemiological models are important tools for understanding the drivers and dynamics of resistance before novel drug regimens are launched.
Methods
We developed a transmission model of TB after launch of a new drug regimen, defining drug-resistant TB (DR-TB) as resistance to the new regimen. The model is characterized by (1) the probability of acquiring resistance during treatment, (2) the transmission fitness of DR-TB relative to drug-susceptible TB (DS-TB), and (3) the probability of treatment success for DR-TB versus DS-TB. We evaluate the effect of each factor on future DR-TB prevalence, defined as the proportion of incident TB that is drug-resistant.
Results
Probability of acquired resistance was the strongest predictor of the DR-TB proportion in the first 5 years after the launch of a new drug regimen. Over a longer term, however, the DR-TB proportion was driven by the resistant population's transmission fitness and treatment success rates. Regardless of uncertainty in acquisition probability and transmission fitness, high levels (>10%) of drug resistance were unlikely to emerge within 50 years if, among all cases of TB that were detected, 85% of those with DR-TB could be appropriately diagnosed as such and then successfully treated.
Conclusions
Short-term surveillance cannot predict long-term drug resistance trends after launch of novel first-line TB regimens. Ensuring high treatment success of drug-resistant TB through early diagnosis and appropriate second-line therapy can mitigate many epidemiological uncertainties and may substantially slow the emergence of drug-resistant TB.
Keywords: Mycobacterium tuberculosis, TB drug regimens, TB drug resistance, TB mathematical model
For the first time in many decades, new first-line drug regimens are being considered for treatment of tuberculosis (TB) disease [1, 2]. These include regimens that optimize existing drugs to shorten the duration of treatment (eg, using fluoroquinolones—currently second-line therapy—in shorter first-line regimens), or regimens that use novel compounds (eg, PA-824 in conjunction with moxifloxacin and pyrazinamide) [3]. There is even the hope for a regimen consisting entirely of new drugs to which all existing Mycobacterium tuberculosis strains would presumably be susceptible [4].
A key concern with the launch of new first-line drug regimens for TB is the potential for rapid emergence of resistance, thereby negating the benefits of novel regimens and in some cases even causing greater harm. For example, fluoroquinolones and (to a lesser extent) pyrazinamide are critical to the current treatment of multidrug-resistant (MDR)-TB [5]; if resistance to these 2 drugs grows after the introduction of new fluoroquinolone- or pyrazinamide-containing regimens, then treatment options for those with first-line resistance may be even more limited than before. As such, it is critical to understand the likely trajectories of emerging drug resistance after introduction of new TB drug regimens, whether short-term surveillance can be used to predict longer-term trends, and the best general strategies for preventing new drug resistance after deployment of a new regimen. Because population-level data cannot be collected on resistance to regimens that have not yet been implemented, mathematical models are important tools for advancing our understanding of these dynamics [6]. Previous models of TB drug resistance have studied the relationships between TB natural history and the reproductive fitness of drug resistance [7, 8], the role of mixed infections of susceptible and resistant strains [9, 10], amplification [11] of drug-resistant TB when heterogeneities in resistance exist, and the roles of TB control and treatment [12, 13] and noncompliance [14] in the contribution to resistance. However, it is now important to consider and refine these insights in the current context of potential new first-line regimens, including the potential public health impact of various strategies (eg, improved detection and treatment of drug-resistant TB [DR-TB]) that aim to prevent emergence of resistance to new regimens.
Therefore, the main objective of this analysis is to understand the role of factors that may drive the future prevalence of drug resistance subsequent to the launch of new first-line regimens. In particular, we explore the role of 3 mechanistically different factors: (1) the probability of acquiring drug resistance during treatment (via de novo mutations); (2) the transmission fitness of DR-TB (taken in relation to drug-susceptible TB [DS-TB]); and (3) probability of success in treating DR-TB (compared with DS-TB). To inform these key questions, we draw on prior efforts and here construct a novel model of a TB epidemic into which a new first-line treatment regimen is introduced. We then use this model to elucidate the key drivers of TB drug resistance and the implications for TB control programs in the setting of launching a new first-line regimen for TB chemotherapy.
MATERIALS AND METHODS
Model Structure
Using classic models of DR-TB as a guide [7, 8], we constructed a compartmental model of TB to simulate the introduction of a novel first-line drug regimen for TB treatment (Figure 1). In this model, we consider 2 bacterial populations of TB, one that is susceptible to the novel regimen (DS-TB) and one that is resistant (DR-TB). We do not consider resistance levels to regimens other than the novel regimen; thus, the “DS-TB” strain may include strains that are resistant to existing regimens but susceptible to the new regimen. We make this simplification for purposes of evaluating key principles and providing generalizable insight in the absence of data on the true spectrum of drug resistance after the launch of any given novel regimen. We consider that the 2 forms of TB differ in 3 important ways: (1) DS-TB may convert to DR-TB during treatment—drug resistance is acquired through spontaneous mutation, but bacterial populations with such acquired resistance only expand within hosts to the point of becoming transmissible when subjected to selective pressure during treatment with the novel regimen; (2) DS-TB is successfully transmitted at a higher per-person rate than DR-TB (differential transmission fitness); and (3) probability of success upon treatment with the novel regimen is higher for DS-TB than DR-TB (differential treatment success).
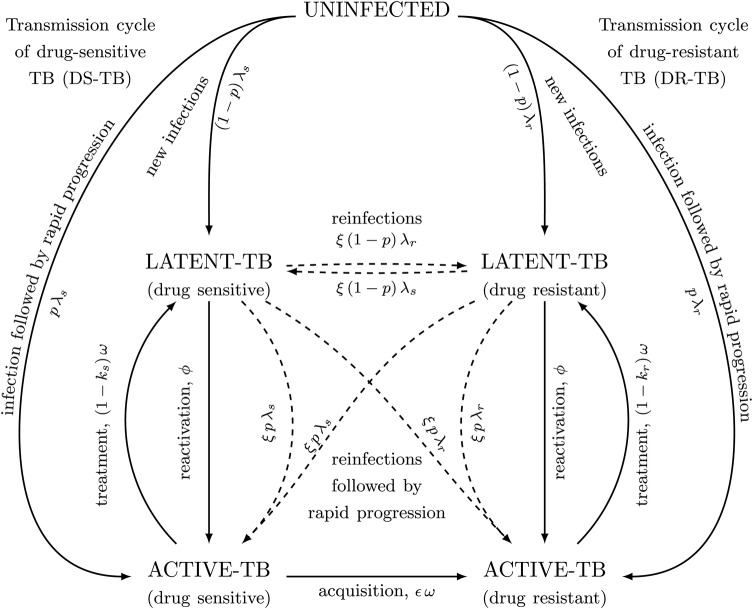
Figure 1.
An epidemiological model of drug-susceptible (DS) and drug-resistant (DR) tuberculosis (TB) to investigate the future prevalence of drug resistance following a roll-out of novel first-line regimens. Tuberculosis subpopulations are broadly classified as either susceptible to the new regimen (DS-TB) or resistant (DR-TB). After successful infection with either of these subpopulations, individuals can develop active TB disease or latent TB infection (LTBI), with probabilities p and 1−p, respectively. Individuals with LTBI can subsequently progress to active TB, at per capita rate ϕ. Individuals with active TB are diagnosed and treatment is initiated after an average composite duration of 1/ω. Successful treatment is modeled as individuals returning to latent class, the probability of which is 1-ks and 1-kr for the susceptible and resistant strain, respectively; unsuccessful treatment is modeled as remaining in the active infectious state. Resistance can be acquired through spontaneous mutation, but we assume that such resistance can expand in an individual to the point of becoming transmissible only when selective pressure is applied during treatment, at a probability of ε per treatment. Individuals with LTBI may be reinfected with either the same or the opposite strain, but previous infections impart partial immunity, where the degree of protection is given by 1−ξ. Demographic turnovers are present in the model, but they are omitted here for simplicity.
As presented schematically in Figure 1 and described in detail in the Supplementary Materials, our model includes a TB-uninfected state, latent TB infection (which includes individuals who have recovered from prior active disease either by treatment or spontaneous resolution), and active infectious disease. In this model, infection with TB may result in either primary progression to active TB or establishment of latency. Such infections may occur multiple times, but in cases of superinfection (DS-TB reinfection in an individual with latent DR-TB, or vice versa), the relative transmission fitness of DR-TB is used to assign a single “dominant” population within the host that will cause any future reactivations (which may in turn result in further transmission); for simplicity, mixed-strain (ie, DR- and DS-TB) infection is not considered further here. We assume that DR-TB can be acquired either through spontaneous mutations that are selected during treatment with the novel regimen or directly through infection or reinfection (ie, after contact with an infectious case of DR-TB).
Effective Reproductive Ratio
We used this model to analytically derive the effective reproductive ratio (REFF) of DR-TB. This is defined as the expected number of new DR-TB cases resulting from a single DR-TB case, when introduced into a population that is at equilibrium with only DS-TB. This quantity differs from the classic quantity R0 in that R0 considers introduction into an entirely susceptible population, whereas REFF considers introduction of a new strain into a stable epidemic with an existing strain, as would be the case upon launch of any new first-line drug regimen for TB. Hence, the effective reproductive ratio determines the ability of drug-resistant strains to proliferate in the population in the presence of drug-sensitive TB. A larger effective reproductive ratio results in a higher expected prevalence of DR-TB, albeit the expected prevalence may take a long time to be realized due to the slow dynamics of TB.
Model Calibration
We initialized the model at steady-state with constant population size and no migration. Because the goal of the model was to draw generalizable insight and elucidate key “first principles,” we did not seek to model any specific epidemiological setting or drug regimen. As shown in Table 1, we populated the model with data from the literature. The TB transmission rate was calibrated to reflect an incidence of 150 per 100 000/year. We benchmarked the probability of acquired resistance during treatment in the baseline scenario to a meta-analysis of acquired resistance to rifampin [16] and the probability of treatment success for DR-TB to existing data on treatment outcomes for MDR-TB [17]. Because the population-level transmission fitness of MDR-TB is unknown, the baseline value of this parameter is taken to generate a prevalence of DR-TB at 50 years that is similar to the prevalence of MDR-TB at present (ie, approximately 50 years after the introduction of rifampin [18]).
Table 1.
Model Parametersa
| Parameter Description | Parameter Symbol | Baseline Value | References |
|---|---|---|---|
| Per capita mortality rate for individuals actively infected with TB | µA | 0.187 per year | [28] |
| Fraction of successful infections that progress rapidly to active TB | p | 0.14 | [29] |
| Per capita reactivation rate | ϕ | 0.0015 per year | [30] |
| Average duration of active TB until diagnosis and initiation of treatment | 1/ω | 12 months | [15] |
| Treatment success proportion, DS-TB | 1–ks | 95% | [15] |
| Treatment success proportion, DR-TB | 1–kr | 60% | [31, 32] |
| Relative probability of successful infection in a host with LTBI | ξ | 0.33 | [33-36] |
| Per capita transmission rate | β | 7.36 per infectious person-year | [37]b |
| Probability of acquiring new drug resistance during treatment | ε | 0.008 | [16] |
| Relative transmission fitness: DR-TB vs DS-TB | f | 0.6 | c |
| Differential in treatment success between DR-TB and DS-TB (kr–ks) | Δk | 0.35 | [17] |
Abbreviations: DR-TB, drug-resistant tuberculosis; DS-TB, drug-susceptible TB; HIV, human immunodeficiency virus; LTBI, latent TB infection; MDR, multidrug-resistant.
a We consider a population of 100 000 (N), where the background mortality rate (μ and µL) is taken to be 0.02 per year. Baseline parameter values generate an equilibrium condition with a TB incidence of 150 per 100 000 per year, and in which the DR-TB proportion reaches 4%, 50 years after the introduction of a novel TB regimen (resembling MDR-TB prevalence in high-burden, low-HIV settings such as Southeast Asia [38]).
b Calibrated to provide an incidence of 150 per 100 000 per year.
c Calibrated such that Mycobacterium tuberculosis would achieve similar level of drug resistance (4%) at 50 years as is currently seen with MDR-TB after 50 years of treatment with rifampin [18].
Analysis
Our primary outcome was the DR-TB proportion, defined as the proportion of all active TB cases that was drug-resistant, at 5 years (short-term) and 50 years (long-term) after immediate introduction of a novel first-line regimen for TB therapy. We also provide results in terms of the absolute burden of DR-TB (ie, prevalence of DR-TB cases) in the supplement that, in the simulations considered here, have similar trajectories as the DR-TB proportion. We consider these projected trajectories of drug resistance as a function of the 3 aforementioned parameters (“drivers”) that differentiate the DR-TB and DS-TB populations, namely: (1) the probability of acquiring DR-TB (ε); (2) the relative transmission fitness of DR-TB (f); and (3) the absolute difference in treatment success proportion (Δk). The treatment success of DR-TB reflects the mean probability of success resulting from all treatment attempts after launching the new regimen (ie, including empiric treatment, inappropriate treatment using the new regimen despite resistance, appropriate treatment after drug susceptibility testing, etc). Thus, for example, if the new regimen were rolled out in such a way that drug-susceptibility testing (DST) could identify individuals with resistance to the new regimen and place those individuals on alternative regimens with higher probability of treatment success, the value of Δk would decrease.
Sensitivity and Uncertainty Analysis
We carried out a series of formal one-way and multiway sensitivity analyses to identify the epidemiological factors that most strongly affect the trajectories of the DR-TB proportion after launch of a novel regimen. For one-way sensitivity analyses, we calculated the changes in the DR-TB proportion 50 years after the introduction of the new first-line drug regimen, which would result from changing each of the model parameters in a way that would either halve or double the baseline incidence. The results are summarized in Supplementary Table S2. We also carried out a time-dependent analysis to address multivariate uncertainty. We conducted >100 000 simulations in which all parameter values were simultaneously varied uniformly across ranges (provided in Supplementary Figure S2) using Latin Hypercube Sampling. Using these simulations, we calculated partial correlation coefficients, both linear (Pearson) and ranked (Spearman), comparing each parameter value with the proportion of DR-TB at yearly intervals up to 50 years after introduction of the new drug regimen [19, 11].
In addition, we evaluated the roles of the level of preexisting resistance in the population before the launch of the new regimen (eg, fluoroquinolone monoresistance before the introduction of a new regimen containing a fluoroquinolone) and the proportion of active TB due to reactivation versus recent infection. In exploring the proportion of active TB due to reactivation versus recent infection, we considered 2 additional scenarios: (1) recent-infection dominant scenario, in which 5% of the active TB reflects reactivation; and (2) reactivation-dominant scenario, in which 80% of the active TB reflects reactivation. Each of these scenarios was constructed by altering the reactivation rate ϕ and the transmission rate β such that the baseline incidence of TB remained constant.
RESULTS
Relationship Between Key Drivers and the Effective Reproductive Ratio
The effective reproductive ratio (REFF) is the expected number of new DR-TB cases resulting from a single DR-TB case, when introduced into a population that is at equilibrium with only DS-TB. It describes the ability of DR-TB to proliferate in the presence of DS-TB (also interpretable as the composite fitness of DR-TB relative to DS-TB). We first derived an algebraic expression for the effective reproductive ratio, which delineates how different drivers feature in its composition:
Here, U*, L*S, and A*S, are, respectively, equilibrial levels of uninfected, latent TB, and active TB populations when only DS-TB is circulating. Note that this equilibrium occurs in the absence of DR-TB and is therefore independent of all 3 drivers associated with DR-TB. Expressions for this DS equilibrium, and the details of the derivation, are provided in the Supplementary Materials.
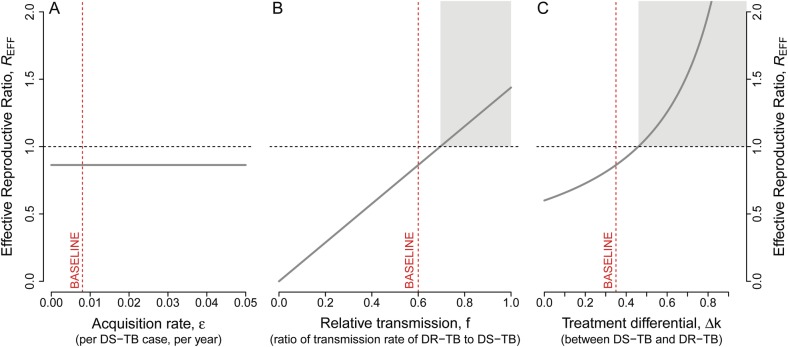
By examining how each of the 3 drivers affects the effective reproductive ratio, we can understand their individual roles in the ability of DR-TB to proliferate after launch of a new drug regimen. The 3 drivers of drug resistance had markedly different effects on REFF (Figure 2). The acquisition rate had no effect on the effective reproductive ratio, because REFF is independent of the acquisition rate (ε) (Figure 2A). In contrast, the relative transmission fitness of DR-TB (f) had a linear relationship with REFF, as seen by its presence as a single term in the numerator of the expression of REFF (and graphically in Figure 2B). Likewise, the treatment success differential had a positive relationship with REFF. Furthermore, the effect of Δk on REFF increased as Δk increased (Figure 2C). Hence, the DR-TB proportion at any time not only grows, but does so progressively faster, as the differential in treatment success increases.
Figure 2.
The effective reproductive ratio and its association with the 3 potential drivers of drug resistance. The effective reproductive ratio (REFF) compares the ability of drug-resistant tuberculosis (TB) to propagate through populations (ie, “composite fitness”) relative to drug-susceptible TB. The derived expression of the effective reproductive ratio is independent of the probability of acquiring drug resistance during treatment, ε (as can be seen from the mathematical expression provided in the text), and hence has no effect on REFF (A); REFF remains fixed for the entire range of ε. In contrast, transmission fitness (f) has a strong linear relationship (B); REFF increases in a linear fashion with increase in f; and treatment success differential Δk has an even stronger effect (C); REFF increases in supralinear fashion with increase in Δk. Dashed vertical red lines show the baseline values of each of the parameters, as provided in Table 1. The shaded gray region indicates the parameter values that lead to an effective reproductive ratio of greater than 1. Abbreviations: DR-TB, drug-resistant tuberculosis; DS-TB, drug-susceptible TB.
Short- and Long-Term Trajectories of Emerging Drug Resistance
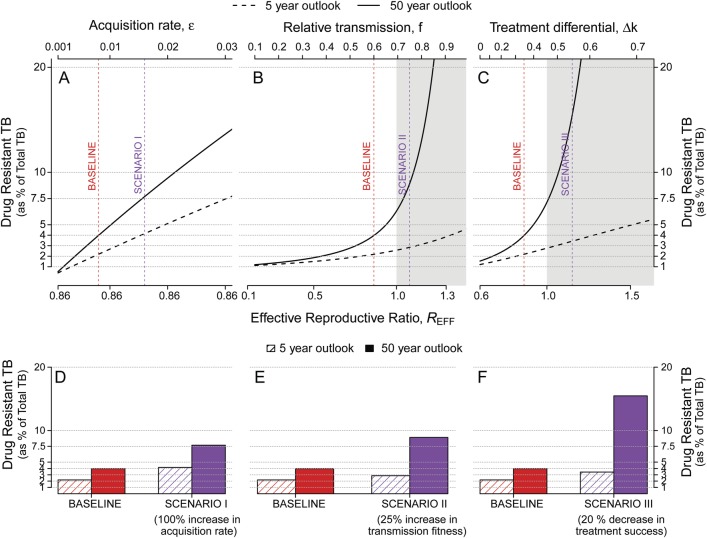
Whereas short-term dynamics of drug resistance (as a proportion of all active TB cases) after introducing a novel TB drug regimen are mostly driven by the probability at which DR-TB emerges under selective pressure from treatment, the projected trajectories at 50 years are more reflective of the contributions of the different drivers to the effective reproductive ratio, REFF (Figure 3). For example, doubling the probability of de novo acquisition of resistance essentially doubles the proportion of DR-TB at 5 years (Figure 3D, red bars)—an impact more pronounced than by increasing the transmission fitness of DR-TB by a relative 25% (Figure 3E) or decreasing treatment success for DR-TB by an absolute 20% (Figure 3F). However, the effect of these smaller changes in transmission fitness or treatment success was much more important at 50 years than that of doubling the acquisition probability (Figure 3D–F, purple bars), reflecting stronger relationships with REFF (Figure 3A–C). As a result, scenarios that produced similar 5-year projections had very different 50-year trajectories.
Figure 3.
Variation in 5- and 50-year projections of drug-resistant tuberculosis (DR-TB) proportion as a function of the acquisition rate, relative transmission, and treatment differential. Plotted are projections of the prevalence of DR-TB, defined as the percentage of all active TB cases that are DR-TB, at 5 (dashed lines) and 50 (solid lines) years after the introduction of a new first-line TB drug regimen. Changes from the baseline condition (dashed vertical red line) are achieved by sequentially varying (A) the probability of acquiring de novo resistance during treatment, ε; (B) the relative transmission fitness (f) of DR-TB (vs drug-susceptible TB [DS-TB]); and (C) the absolute difference in treatment success for DR-TB vs DS-TB, Δk. The shaded gray region indicates the parameter values that lead to an effective reproductive ratio of greater than 1. Three alternative scenarios are marked in purple: doubling the probability of de novo drug acquisition (Scenario I); increasing the transmission fitness of DR-TB by a relative 25% (Scenario II); and lowering the treatment success for DR-TB by an absolute 20% (Scenario III). As shown in D–F, Scenario I has a modest effect on both 5-year and 50-year projections, whereas Scenarios II and III (representing much smaller relative changes in corresponding parameter values) have little impact on 5-year projections but tremendous impact on the emergence of DR-TB at 50 years. The effects on projections of raw prevalence of DR-TB show similar pattern (see Supplementary Figure S3).
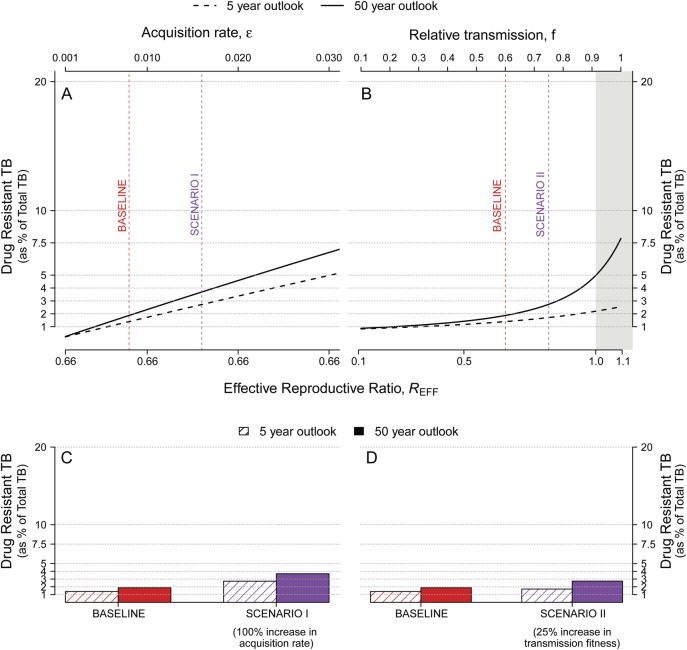
To simulate the effect of improving treatment of DR-TB, we projected long- and short-term trajectories under the assumption that detection of resistance (among diagnosed cases of TB) is complete and treatment success of DR-TB reaches 85% (ie, Δ k = 0.1). Such improvement may be achievable through universal DST and rapid initiation of appropriate second-line therapy. As shown in Figure 4, the short- and the long-term projections of DR-TB proportion were markedly reduced from baseline (Figure 3). Even under the assumption that drug resistance incurred no transmission fitness cost (ie, f = 1), the proportion of TB with drug resistance did not reach 8% by 50 years (Figure 4B). These results are qualitatively similar when measuring the absolute prevalence, rather than the proportion, of DR-TB (see Supplementary Figures S3 and S4).
Figure 4.
Five- and 50-year projections of drug-resistant tuberculosis (DR-TB) proportion with high levels of treatment success for DR-TB. Plotted are projections of the DR-TB proportion, defined as the percentage of all active TB that is DR-TB, at 5 (dashed lines) and 50 (solid lines) years after the introduction of a new first-line TB drug regimen. Here, the probability of treatment success for DR-TB is taken to be 85% (compared with baseline of 60% in Figure 3; equivalent to decreasing Δk to 0.1 from 0.35). This represents a potential scenario in which DR-TB is rapidly detected with drug-susceptibility testing, and then effectively treated with second-line drugs [18]. Changes from the baseline condition (dashed vertical red line) are achieved by sequentially varying (A) the probability of acquiring de novo resistance during treatment, ε; and (B) the relative transmission fitness (f) of DR-TB (vs drug-susceptible TB). The shaded gray region indicates the parameter values that lead to an effective reproductive ratio of greater than 1. Two alternative scenarios are marked in purple: doubling the probability of de novo drug acquisition (Scenario I, C); and increasing the transmission fitness of DR-TB by a relative 25% (Scenario II, D). In contrast to Figure 3, both scenarios (C vs Figure 3D and D vs Figure 3E) result in low DR-TB proportions even after 50 years, when high DR-TB treatment success is achieved.
Trends in the Associations Between Proportion of Drug-Resistant Tuberculosis and the Three Drivers
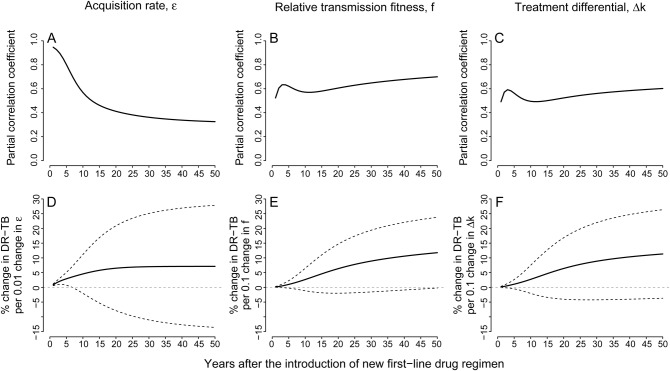
The trends observed in partial correlation coefficients (PCC) show the variation in the strength of associations through time (Figure 5A–C); higher PCC's suggest that, holding all other parameter values constant, a given parameter is more closely associated with DR-TB proportion in the population, at a given time after launch of a new regimen. Using this metric, the acquisition rate was strongly correlated with DR-TB proportion at year 5, but the correlation steadily decreased over time (Figure 5A). In contrast, both relative transmission fitness and treatment differential became more strongly associated with the proportion of drug resistance over time (Figure 5B and C). More importantly, the predicted effects of the 3 drivers on proportion of DR-TB also showed substantial variation through time (Figure 5D–F). The effect of acquisition rate (ε) only persisted for a shorter time, such that any effects of acquisition on DR-TB proportion occurred within the first 20 years (Figure 5D), whereas the effects of transmission fitness (f) and treatment success (Δk) continued to grow over time, remaining strong determinants of DR-TB proportion even at 50 years (Figure 5E and F). These trends are similar to those observed in partial ranked correlation coefficients (Supplementary Figure S2).
Figure 5.
Time-varying effects of the 3 potential drivers on drug-resistant tuberculosis (DR-TB) proportion. Plotted on the top row are the partial correlation coefficients (PCC) between (A) acquisition rate (ε); (B) relative transmission fitness (f); and (C) treatment differential (Δk), and DR-TB proportion over the first 50 years after the introduction of a new drug regimen. Plotted on the bottom row are predicted effects of (D) acquisition rate (ε); (E) relative transmission fitness (f); and (F) treatment differential (Δk), on DR-TB proportion over the first 50 years after the introduction of a new drug regimen. These predictions are directly related to the PCC: whereas the PCCs indicate the strengths of associations of with each parameter, the solid lines in this graph represent the slopes of the best-fit linear prediction between each parameter value and the projected DR-TB proportions. The dotted lines above and below the solid lines are ± standard deviations.
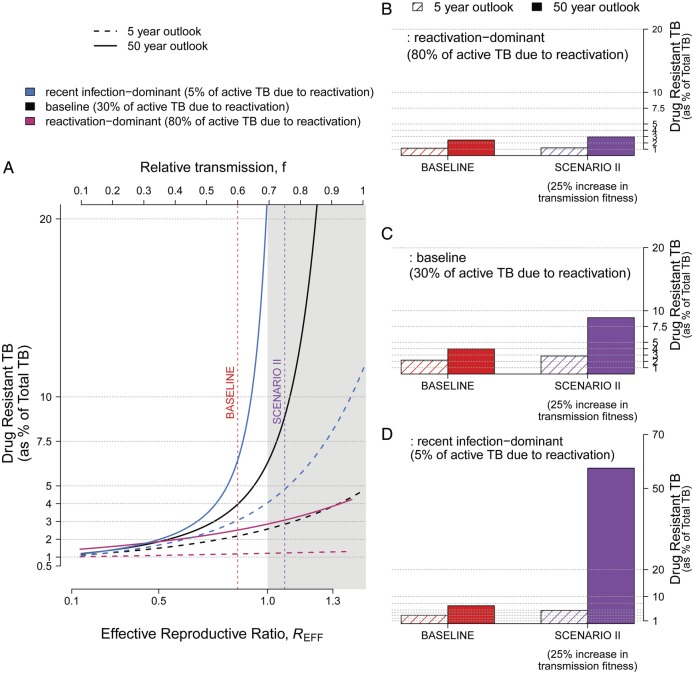
Relationship Between Emergence of Drug Resistance and the Reactivation/Recent Infection Ratio
The proportion of active TB resulting from recent infection versus reactivation of remote infection was also a major determinant of the emerging drug resistance. In a setting where the majority of active TB reflected reactivation disease (Figure 6A, pink lines, and Figure 6B), DR-TB was very slow to emerge; even in settings where REFF was 1.5, no more than 10% of TB was DR-TB by 50 years after roll-out of a novel regimen. In contrast, where the majority of active TB was due to recent infection (Figure 6A, blue and black lines, and Figure 6C and D), DR-TB could rapidly account for a sizeable proportion of all TB; at REFF of 1 in either of these scenarios, over 20% of all TB was DR-TB after 50 years. This was true whether 5% or 30% of active TB was due to reactivation (Figure 6C vs D, or A, blue vs black lines).
Figure 6.
Proportion of drug-resistant tuberculosis (DR-TB) as a function of the balance between new infection and reactivation. Active TB can develop either via recent infection of uninfected and latent individuals (followed by rapid progression to active disease) or via reactivation of latent TB infection. In our baseline scenario (shown in center [black] lines), 30% cases are due to reactivation. Keeping the incidence level at 150 cases per 100 000, we consider 2 extreme scenarios: reactivation-dominant (80% of cases result from reactivation, shown in the lower [pink] lines) and recent infection-dominant (5% of the cases result from reactivation, shown in the upper [blue] line). A shows trajectories of emerging resistance at 5 and 50 years after introduction of a new TB treatment regimen (analogous to Figure 3C). B–D compare the proportion of DR-TB at baseline to Scenario III (treatment success for DR-TB in decreased by an absolute 20%); as the TB due to reactivation decreases, the rate at which drug resistance emerges increases.
DISCUSSION
This compartmental model of TB epidemiology provides a number of key insights into the drivers and likely trajectories of drug resistance after implementation of novel first-line regimens. First, as demonstrated in other models [9, 11], the rate at which M tuberculosis acquires de novo resistance to new regimens has some effect on the short-term emergence of DR-TB but little long-term importance. Second, short-term levels of resistance do not have a predictable relationship with long-term proportion of DR-TB, suggesting the importance of understanding the causal mechanisms behind emerging resistance to new regimens (eg, transmission vs acquisition vs failed treatment) rather than simply conducting surveillance. Third, despite substantial uncertainty regarding natural history of DR-TB for novel regimens, high levels of detection and effective treatment of DR-TB may be the most effective way to prevent resistant strains from rapidly accounting for most cases of TB in a population. This effect is achieved by reducing the duration of time that people with those strains remain infectious. Of importance, this model defines treatment success as the mean success of all treatment attempts—whether empiric, inappropriate, or appropriate. Thus, wider use of high-quality DST linked to appropriate therapy could dramatically improve treatment success simply by reducing the number of people with DR-TB initiated on inappropriate regimens. Finally, DR-TB may emerge much more rapidly in settings where the majority of active TB represents recent infection rather than reactivation; by contrast, long-term projections of resistance are not very sensitive to preexisting resistance to new regimens (See Supplementary Figure S1).
Our findings suggest that the rate of DR-TB emergence (as a proportion of all TB) may be unpredictable, in that the most important drivers—ie, transmission fitness and “on the ground” treatment success differentials—are more difficult to measure in epidemiological studies than are other variables, including preexisting resistance levels. Given that high treatment success and low levels of recent transmission are both associated with lower future proportions of DR-TB, the ideal settings for early adoption of novel regimens may be those in which TB incidence is falling and in which high coverage of DST and effective therapy for DR-TB can be assured.
Our finding of treatment success as a critical determinant of future DR-TB proportion is consistent with existing knowledge about MDR-TB, where epidemics of MDR-TB have been effectively contained with improved infrastructure and high-quality second-line therapy [20, 21]. Our results also echo earlier model findings [7-9, 11], including the value of improving treatment of DR-TB and the limited role of acquisition during treatment in long-term projection of drug resistance [9, 11], while contextualizing them in the modern setting of emerging first-line regimens for TB.
As with any modeling analysis, and especially one that intends to draw insight about future events (ie, implementation of novel regimens for TB therapy), the present analysis has limitations. We intentionally adopted a highly simplified framework for purposes of easy comparison to existing models and transparent conclusions given a paucity of actual data regarding emerging resistance. As such, this model cannot describe any single specific situation, and differences across mycobacterial strains (eg, transmission fitness) and epidemiological settings (eg, treatment success differential) are likely to make the emergence of DR-TB a highly heterogeneous phenomenon. Such heterogeneity is a hallmark of current MDR-TB epidemics, the prevalence of which varies from less than 2% to greater than 30% of all TB cases worldwide [18]. Likewise, emergence of resistance has proven highly drug-specific (with resistance to isoniazid and streptomycin emerging much more rapidly than to rifampin, for example [18]), and it is difficult to predict how resistance will emerge when novel selection pressure is applied (in the form of first-line treatment with the same drug for many months), even for existing drugs. Although we started from a baseline of low resistance and did not model scenarios where the overall incidence of DS-TB was changing dramatically, it is possible that our selected outcome of DR-TB as a proportion of all TB may not reflect the true burden of DR-TB (for example, if the incidence of DS-TB declines, the proportion of DR-TB will rise simply because of a smaller denominator). However, for the scenarios studied in this work, the outcomes (in terms of proportion of DR-TB among all TB) closely reflect the effect on the raw prevalence of DR-TB (Supplementary Figures S3 and S4). Additional considerations include the role of small numbers and stochastic variability early in any DR-TB epidemic (eg, DR-TB may emerge much more rapidly as a result of a small number of “superspreaders” [22, 23]), non-homogenous mixing at the population level [24], increased fitness of resistant strains over time through compensatory mutation [25, 26], and the role of mixed-strain infections and interstrain competition [9, 10, 27], which are greatly simplified in this model and may lead this model to underestimate the rate of emerging resistance. Models that carefully and more comprehensively explore such dynamics in sequential fashion may yield important insights.
This analysis may also inform the direction of empirical data collection efforts as new regimens are rolled out. It suggests that, in addition to high-quality surveillance to detect early emergence of DR-TB, specific studies to estimate the relative transmission fitness of resistant strains (eg, intensive contact investigations with advanced molecular epidemiology, comparing DS-TB with DR-TB source cases) may be very helpful in projecting future trajectories. Well conducted observational studies of real-world treatment success (eg, close follow-up of all treated cases to assess default and relapse proportions), comparing DS-TB with different DR-TB strains (ie, different patterns of resistance to drugs in the new regimen), will also be important. Finally, studies of the probability of acquired drug resistance after first-line treatment with novel regimens will be important in projecting short-term dynamics, although their relevance for long-term resistance patterns may be less profound.
CONCLUSIONS
In summary, this simplified model of emerging drug resistance after deployment of novel first-line treatment regimens for TB suggests that relative transmission fitness and differentials in treatment success are likely to drive the proportion of TB that is drug-resistant in the long term, especially in settings of high ongoing transmission. Short-term dynamics may not predict long-term trajectories, but lowering the treatment success differential by deploying DST and effective DR-TB treatment is likely to be the most important weapon in preventing new resistance from representing a substantial proportion of overall incident TB. Future empirical studies to inform these parameter values will be essential accompaniments to the launch of any new TB drug regimens. Novel first-line treatment regimens are appropriately being heralded as key tools in the fight against TB worldwide; these modeling insights can help to ensure that such regimens are deployed in a way that preserves their efficacy and maximizes their utility in both the near future and over the longer term.
Supplementary Material
Acknowledgments
Disclaimer. The funders had no involvement in the design, collection, analysis or interpretation of the data, in writing the report, or in the decision to submit.
Financial support. This work was funded by a contract from the Bill and Melinda Gates Foundation (Work Order 10). R. G. W. is additionally funded by the Medical Research Council (UK) (MR/J005088/1), the Bill and Melinda Gates Foundation (Grants 21675/OPP1084276 and 19790.01), CDC/PEPFAR via the Aurum Institute (U2GPS0008111), and USAID/IUTLD/The Union North America (TREAT TB: Technology, Research, Education, and Technical Assistance for Tuberculosis).
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ma Z, Lienhardt C, McIlleron H, et al. Global tuberculosis drug development pipeline: the need and the reality. Lancet. 2010;375:2100–9. doi: 10.1016/S0140-6736(10)60359-9. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg AM. Tuberculosis drug development: Progress, challenges, and the road ahead. Tuberculosis. 2010;90:162–7. doi: 10.1016/j.tube.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Lienhardt C, Raviglione M, Spigelman M, et al. New drugs for the treatment of tuberculosis: Needs, challenges, promise, and prospects for the future. J Infect Dis. 2012;205:S241–9. doi: 10.1093/infdis/jis034. [DOI] [PubMed] [Google Scholar]
- 4.Dooley K, Nuermberger E, Diacon A. Pipeline of drugs for related diseases: tuberculosis. Curr Opin HIV AIDS. 2013;8:579–85. doi: 10.1097/COH.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. World Health Organization; 2009. WHO/HTM/TB/2009.420. [PubMed] [Google Scholar]
- 6.Cohen T, Dye C, Colijn C, et al. Mathematical models of the epidemiology and control of drug-resistant TB. Expert Rev Respir Med. 2009;3:67–79. doi: 10.1586/17476348.3.1.67. [DOI] [PubMed] [Google Scholar]
- 7.Blower S, Small P, Hopewell P, et al. Control strategies for tuberculosis epidemics: New models for old problems. Science. 1996;273:497. doi: 10.1126/science.273.5274.497. [DOI] [PubMed] [Google Scholar]
- 8.Blower SM, Gerberding JL. Understanding, predicting and controlling the emergence of drug-resistant tuberculosis: a theoretical framework. J Mol Med. 1998;76:624–36. doi: 10.1007/s001090050260. [DOI] [PubMed] [Google Scholar]
- 9.Cohen T, Murray M. Modeling epidemics of multidrug-resistant M. tuberculosis of heterogeneous fitness. Nat Med. 2004;10:1117–21. doi: 10.1038/nm1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colijn C, Cohen T, Murray M. Latent coinfection and the maintenance of strain diversity. Bull Math Biol. 2009;71:247–63. doi: 10.1007/s11538-008-9361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blower SM, Chou T. Modeling the emergence of the ‘hot zones’: Tuberculosis and the amplification dynamics of drug resistance. Nat Med. 2004;10:1111–6. doi: 10.1038/nm1102. [DOI] [PubMed] [Google Scholar]
- 12.Dye C, Williams BG. Criteria for the control of drug-resistant tuberculosis. Proc Natl Acad Sci. 2000;97:8180–5. doi: 10.1073/pnas.140102797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dye C, Williams BG, Espinal MA, et al. Erasing the world's slow stain: Strategies to beat multidrug-resistant tuberculosis. Science. 2002;295:2042–6. doi: 10.1126/science.1063814. [DOI] [PubMed] [Google Scholar]
- 14.Lipsitch M, Levin B. Population dynamics of tuberculosis treatment: mathematical models of the roles of non-compliance and bacterial heterogeneity in the evolution of drug resistance. Int J Tuberc Lung Dis. 1998;2:187–99. [PubMed] [Google Scholar]
- 15.World Health Organization. World Health Organization; 2013. Global tuberculosis report 2013. Available at: http://www.who.int/tb/publications/global_report/ . Accessed 21 August 2014. [Google Scholar]
- 16.Lew W, Pai M, Oxlade O, et al. Initial drug resistance and tuberculosis treatment outcomes: systematic review and meta-analysis. Ann Intern Med. 2008;149:123–34. doi: 10.7326/0003-4819-149-2-200807150-00008. [DOI] [PubMed] [Google Scholar]
- 17.Espinal M, Kim S, Suarez P, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283:2537–45. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. World Health Organization; 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Available at: http://www.who.int/tb/publications/2010/978924599191/en/ . Accessed 21 August 2014. [Google Scholar]
- 19.Sanchez MA, Blower SM. Uncertainty and sensitivity analysis of the basic reproductive rate: Tuberculosis as an example. Am J Epidemiol. 1997;145:1127–37. doi: 10.1093/oxfordjournals.aje.a009076. [DOI] [PubMed] [Google Scholar]
- 20.Frieden TR, Fujiwara PI, Washko RM, et al. Tuberculosis in new york city–turning the tide. N Engl J Med. 1995;333:229–33. doi: 10.1056/NEJM199507273330406. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly J. Estonia lauded for tuberculosis control efforts. Lancet. 2012;379:1090. doi: 10.1016/s0140-6736(12)60448-6. [DOI] [PubMed] [Google Scholar]
- 22.Gardy JL, Johnston JC, Sui SJ, et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med. 2011;364:730–9. doi: 10.1056/NEJMoa1003176. [DOI] [PubMed] [Google Scholar]
- 23.Ypma RJ, Altes HK, van Soolingen D, et al. A sign of superspreading in tuberculosis: highly skewed distribution of genotypic cluster sizes. Epidemiology. 2013;24:395–400. doi: 10.1097/EDE.0b013e3182878e19. [DOI] [PubMed] [Google Scholar]
- 24.Dowdy DW, Golub JE, Chaisson RE, et al. Heterogeneity in tuberculosis transmission and the role of geographic hotspots in propagating epidemics. Proc Natl Acad Sci. 2012;109:9557–62. doi: 10.1073/pnas.1203517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen T, Sommers B, Murray M. The effect of drug resistance on the fitness of Mycobacterium tuberculosis. Lancet Infect Dis. 2003;3:13–21. doi: 10.1016/s1473-3099(03)00483-3. [DOI] [PubMed] [Google Scholar]
- 26.Brandis G, Wrande M, Liljas L, et al. Fitness-compensatory mutations in rifampicin-resistant RNA polymerase. Mol Microbiol. 2012;85:142–51. doi: 10.1111/j.1365-2958.2012.08099.x. [DOI] [PubMed] [Google Scholar]
- 27.Cohen T, van Helden PD, Wilson D, et al. Mixed-strain Mycobacterium tuberculosis infections and the implications for tuberculosis treatment and control. Clin Microbiol Rev. 2012;25:708–19. doi: 10.1128/CMR.00021-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dye C, Garnett GP, Sleeman K, et al. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Lancet. 1998;352:1886–91. doi: 10.1016/s0140-6736(98)03199-7. [DOI] [PubMed] [Google Scholar]
- 29.Vynnycky E, Fine P. The annual risk of infection with Mycobacterium tuberculosis in England and Wales since 1901. Int J Tuberc Lung Dis. 1997;1:389–96. [PubMed] [Google Scholar]
- 30.Horsburgh CR, Jr, O'Donnell M, Chamblee S, et al. Revisiting rates of reactivation tuberculosis: A population-based approach. Am J Respir Crit Care Med. 2010;182:420. doi: 10.1164/rccm.200909-1355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–61. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 32.Falzon D, Jaramillo E, Wares F, et al. Universal access to care for multidrug-resistant tuberculosis: an analysis of surveillance data. Lancet Infect Dis. 2013;13:690–7. doi: 10.1016/S1473-3099(13)70130-0. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland I, Svandova E, Radhakrishna S. The development of clinical tuberculosis following infection with tubercle bacilli: 1. A theoretical model for the development of clinical tuberculosis following infection, linking from data on the risk of tuberculous infection and the incidence of clinical tuberculosis in the Netherlands. Tubercle. 1982;63:255–68. doi: 10.1016/s0041-3879(82)80013-5. [DOI] [PubMed] [Google Scholar]
- 34.Vynnycky E, Fine P, et al. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;119:183–201. doi: 10.1017/s0950268897007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basu S, Orenstein E, Galvani AP. The theoretical influence of immunity between strain groups on the progression of drug-resistant tuberculosis epidemics. J Infect Dis. 2008;198:1502–13. doi: 10.1086/592508. [DOI] [PubMed] [Google Scholar]
- 36.Andrews JR, Noubary F, Walensky RP, et al. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis. 2012;54:784–91. doi: 10.1093/cid/cir951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Leth F, van der Werf M, Borgdorff M. Prevalence of tuberculous infection and incidence of tuberculosis: a re-assessment of the Styblo rule. Bull World Health Organ. 2008;86:20–6. doi: 10.2471/BLT.06.037804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zignol M, van Gemert W, Falzon D, et al. Surveillance of anti-tuberculosis drug resistance in the world: an updated analysis, 2007-2010. Bull World Health Organ. 2012;90:111–9. doi: 10.2471/BLT.11.092585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Driesche P, Watmough J. Reproduction number and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci. 2002;180:29–48. doi: 10.1016/s0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.