A systematic review of studies conducted in Africa found high herpes simplex virus (HSV)-2 acquisition rates in both general and high-risk cohorts; thereby identifying an efficient setting for preventative HSV-2 vaccine trials.
Keywords: acquisition, Africa, herpes simplex virus type 2, incidence rate, vaccine
Abstract
The burden of HSV type 2 varies substantially by region, with the highest incidence and prevalence in sub-Saharan Africa. We undertook a systematic review to identify studies reporting prospective data on incidence rates in men and women in Africa. Of 18 eligible studies, 7 were conducted in high-risk populations. Among women, incidence rates appeared to be higher in those with high-risk sexual behavior, with rates ranging from 3 to 23 per 100 person-years. In contrast, incidence rates in men appeared to be lower, ranging from 1 to 12 per 100 person-years. Risk factors for HSV-2 in women included prevalent human immunodeficiency virus (HIV) infection, younger age at sexual initiation, and sexual activity. Among men, condom use and circumcision had a protective effect, whereas prevalent HIV increased the risk of HSV-2 acquisition. This review draws attention to the high HSV-2 acquisition rates reported in Africa, thereby identifying an efficient setting for preventative HSV-2 vaccine trials.
Globally, 536 million people were estimated to be infected with herpes simplex virus (HSV) type 2 in 2003, with more than 15% of individuals in the 25- to 29-year-old age group reported to be living with this sexually transmitted infection (STI) [1]. However, the HSV-2 prevalence varies considerably by region, with parts of sub-Saharan Africa reporting a prevalence as high as 80% among men and women aged 35 years and older [2]. The human immunodeficiency virus (HIV) epidemic in sub-Saharan Africa is known to be fueled by HSV-2 among other factors, consequently leading to an increased focus on the transmission of HSV-2 in this region in recent years [3–6]. In addition, genital herpes is a leading cause of genital ulcer disease worldwide [7–10].
According to World Health Organization estimates, in 2003 23.6 million new cases of HSV-2 infection occurred worldwide with more than 20% acquired in sub-Saharan Africa [1]. Notably, approximately 28% of these incident HSV-2 infections were in African women aged 15–19 years. Unlike bacterial STIs, HSV-2 is a lifelong infection and hence its prevalence and incidence continue to remain high in sub-Saharan Africa, with seroprevalence as high as 90% in persons infected with HIV [5, 11–13].
A comprehensive measurement and description of the burden of HSV-2 in Africa entails estimation of the prevalence and incidence in this population. In 2001, Smith and Robinson [2] compiled available literature on HSV-2 in Africa and reported its age-specific prevalence by country. Likewise, Looker et al [1] used HSV-2 seroprevalence data to obtain model estimates of new HSV-2 infections in the various geographic regions in 2003. They found that HSV-2 incidence was highest in the youngest age groups (15–19 years), and sub-Saharan Africa accounted for 15% of global incident infections. Despite this significant regional burden, HSV-2 incidence in Africa has not been systematically reviewed. Because incidence is a better measure of the risk of infection in a population [14], we have undertaken a systematic review of published estimates of incidence rates from individual studies conducted in Africa to measure the scale of the epidemic in this region and to identify potential populations for conducting clinical trials of candidate HSV-2 vaccines.
METHODS
Search Strategy and Selection Criteria
This review was conducted as per the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and checklist [15]. Five electronic databases, namely, Pubmed/Medline, Web of Science, CINAHL, EMBASE (Ovid), and Scopus, were searched from their original start date to July 2013. The general outline of the search was [herpes] AND [incidence] AND [Africa], using combinations of the following terms: herpes simplex, herpes, genital herpes, herpes simplex virus infection, HSV, HSV type 2, herpes simplex virus type 2 or HSV-2 AND incidence, acquisition, or risk, AND Africa. In addition, the search term “sexually transmitted diseases” (STD) was also included in place of the HSV keyword to broaden the search filter and to identify studies that referred to general STIs in their abstracts.
Eligible studies reported prospective data on the incidence of HSV-2 in Africa. In addition, studies were only eligible for the review if participants were tested for HSV-2 antibody with type-specific antibody assays as part of study procedures. Case studies, cross-sectional studies, review articles, and studies conducted outside of Africa were excluded. Studies were excluded if they only tested symptomatic participants. Based on the title and abstract, all identified references were reviewed to determine study eligibility for inclusion in the review. Two authors (S. R. and A. M.) independently conducted a full text review of references selected from the initial screening.
Data Extraction
Data were extracted onto a standardized table by 1 author (S. R.) and independently verified by a second author (A. M.). The data abstracted from each article included study characteristics (year of publication, country, study design and objective), serologic testing, population studied (eg, general, antenatal, or commercial sex worker), age, gender, sample size, HIV status at enrollment, baseline HSV-2 prevalence, and incidence, and key findings were summarized. To assess generalizability, we also reported (1) loss to follow-up either as proportion of visits attended or proportion of enrolled persons included in analysis, whichever was available, and (2) proportion of eligible subjects who enrolled, where available. In addition, incident rate ratios for HSV-2 acquisition associated with risk factors such as age, gender, and HIV infection were abstracted from each article and summarized separately. To assess the methodological quality of included studies, we considered potential sources of selection bias, at the time of study enrollment, as well as retention rates.
RESULTS
Using our search strategy, we identified 1394 abstracts after removing duplicates (Figure 1). Approximately 1366 abstracts were excluded based on criteria described above. The remaining 28 studies (2%) that met our prespecified eligibility criteria were selected for a full text review [16–43]. Subsequently, 10 additional articles were excluded, 5 of which had insufficient information on HSV-2 incidence rates [21, 30, 33, 38, 42] and [19] 1 paper that reported estimates from incident symptomatic cases alone. Authors concurred on the inclusion of the 18 studies in the systematic review [17, 18, 20, 22, 24–29, 31, 32, 34–37, 39, 43].
Figure 1.
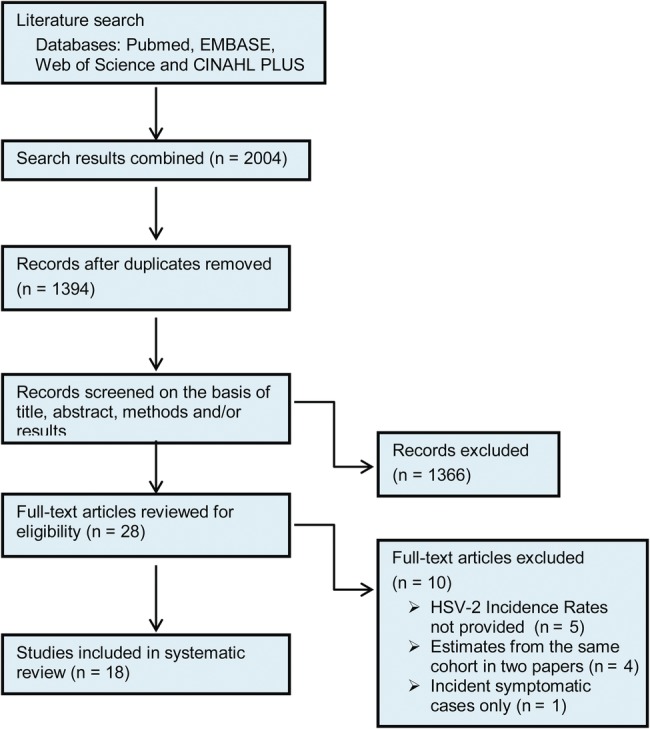
Study selection flow diagram.
Demographics
Study sites included 6 studies from Uganda, 4 from Zimbabwe, 3 each in South Africa, Kenya, and Tanzania, and 1 in Ethiopia (Tables 1 and 2). All studies were conducted between 1990 and 2012. Studies had a median of 1360 participants (range, 126–6396). Fifty-one percent of participants included in all 18 studies were women, and the age of participants ranged from 14 to 64 years of age.
Table 1.
Summary of Studies Reporting HSV-2 Incidence Among Women in Africa
| Citation and Study Setting | Median Age (Range) | % HIV Positive | Baseline HSV-2 Prevalence (Number Enrolled) | HSV-2 Incidence/100 Person-Years (Number Susceptible) | % Eligible Who Enrolled | % of Person-Years Completed (or % of Follow-Up Visits Completed | Study Design and Objective |
|---|---|---|---|---|---|---|---|
| Brown et al [17], women seeking reproductive services in UG and ZW | UG: 23 (20–26) | 0% | UG: 51.5% (2235) | UG: 9.6 (1084) | UNK | 91% | Prospective cohort study of HSV-2 infection as a risk factor for HIV acquisition |
| ZW: 24 (21–27) | ZW: 53.2% (2296) | ZW: 8.8 (1075) | |||||
| Chohan et al [18], women sex workers in Mombasa, Kenya | 23 (20–26) | 0% | 79.9% (1506) | 23.0 (297) | UNK | UNK | Prospective cohort for HSV-2 incidence |
| De Bruyn et al [20], sexually active women in Harare, ZW; Durban and Johannesburg South Africa | *21 (18–49) | 0% | 58.9% (4904) | 6.8 (2016) | UNK | 77% | Randomized open-label trial of vaginal diaphragm and lubricant gel in addition to male condoms to prevent HSV-2 acquisition |
| Jewkes et al [22], randomized villages in Mthatha, South Africa | *18 (15–26) | 11.2% | 29.2% (1416) | 6.48 (1009) | UNK | 75% | Cluster randomized trial of an educational intervention to prevent HIV and HSV-2 |
| Kamali et al [24], adult residents in 15 villages in Southwest UG | *27 (15–54) | †4.9% | 71.5% (541) | 12.3 (154) | †80% | †60% | Retrospective cohort study measuring HSV-2 prevalence and incidence |
| Kamali et al [25], 18 rural communities in Masaka, UG | *†18 (13–29) | †10% | 38% (3818) | †3.1 (2379) | †71% | †75% | Randomized trial of a behavioral intervention to prevent HIV transmission |
| Kebede et al [26], factory workers in Addis Ababa, Ethiopia | 33 (19–46) | †40.1% | 59.5% (407) | 3.7 (165) | UNK | 86% | Prospective cohort examining risk factors for HIV acquisition and disease progression |
| Munjoma et al [29], pregnant women in Harare, ZW | 23 (20–28) | 24.7% | 49.1% (340) | 13.9 (173) | 99% | 97% | Prospective cohort study examining HSV-2 prevalence and incidence in pregnant and postpartum women |
| Okuku et al [31], adult sex workers in Mombasa, Kenya | 26 (22–31) | 0% | 50.8% (469) | 22.1 (248) | UNK | 77% | Prospective cohort study examining risk factors for HSV-2 acquisition in HIV-1 seronegative sex workers |
| Riedner et al [32], female bar workers in Mbeya Region, Tanzania. | *27 (16–39) | 68% | 86.8% (600) | 17.3 (79) | UNK | UNK | Prospective cohort study examining trends in STI prevalence and HIV incidence in bar workers |
| Tassiopoulos et al [35], female bar and hotel workers in Moshia, Tanzania | *27 (14–64) | 19% | 56.3% (1045) | 14.2 (457) | UNK | 79% | Ancillary study of HSV-2 acquisition within a prospective cohort examining risk factors for HIV acquisition |
| Tobian et al [37], HIV and HSV-2 negative women in Rakai, UG | *23 (15–49) | 0% | 54.8% (1638) | 6.2 (740) | 69% | 87.6% | Ancillary study of transmission of HSV-2 to female partners within a randomized trial of male circumcision to prevent HIV acquisition |
| Vallely et al [39], women working in restaurants, bars and guesthouses in Mwanza City, Tanzania | *30 (20–35+) | 26.5% | 69% (1020) | 12.7 (316) | 63% | 68.4% | Feasibility study of enrollment, retention, STI prevalence, and incidence, for future microbicide trials |
| Wagner et al [43], adults from 2 neighboring villages in UG | *34 (15–49) | †7.8% | 74.4% (168) | 21.1 (19) | UNK | 56% | Retrospective cohort examining STIs among retained participant subset within larger prospective cohort |
Abbreviations: HIV, human immunodeficiency virus; HSV, herpes simplex virus; STI, sexually transmitted infection; UG, Uganda; UNK, unknown; ZW, Zimbabwe.
* Estimated age from range. Study citations in bold indicate high-risk populations.
† Indicates a measure assessed on men and women combined.
Table 2.
Summary of Studies Reporting HSV-2 Incidence Among Men in Africa
| Citation and Study Setting | Median Age (Range) | % HIV Positive | Baseline HSV-2 Prevalence (Number Enrolled) | HSV-2 Incidence/100 Person-Years (Number Susceptible) | % Eligible Who Enrolled | % of Person-Years Completed (or % of Follow-up Visits Completed | Study Design and Objective |
|---|---|---|---|---|---|---|---|
| Jewkes et al [22], randomized villages in Mthatha, South Africa | *19 (15–26) | 1.9% | 10.0% (1360) | 1.74 (1225) | UNK | 73% | Cluster randomized trial of an educational intervention to prevent HIV and HSV-2 |
| Kamali et al [24], adult residents in 15 villages in Southwest Uganda | *26 (15–54) | †4.9% | 36% (357) | 7.3 (225) | †80% | †60% | Retrospective cohort study measuring HSV-2 prevalence and incidence |
| Kamali et al [25], 18 rural communities in Masaka, Uganda | *†18 (13–29) | †10% | 16% (3214) | †3.1 (2706) | †71% | †75% | Community randomized trial of a behavioral intervention and STI treatment to prevent HIV transmission |
| Kebede et al [26], factory workers in Addis Ababa, Ethiopia | 35 (19–62) | †40.1% | 34.6% (1205) | 1.4 (788) | UNK | 86% | Prospective cohort examining risk factors for HIV acquisition and disease progression |
| McFarland et al [27], male factory workers in Zimbabwe. | *28 (18–46+) | 16.6% | 39.8% (2397) | 6.2 (1444) | 71% | UNK | Retrospective cohort examining prevalence and incidence of HSV-2 |
| Mehta et al [28], 18–24 year old uncircumcised men Kenya | 20 (17–28) | 0% | 26.5% (2748) | 6.0 (2021) | 61% | 77% | Randomized trial of male circumcision to prevent HSV-2, GUD and HIV |
| Okuku et al [31], adult sex workers in Mombasa, Kenya | 26 (22–33) | 0% | 22.0% (803) | 9.0 (626) | UNK | 71% | Prospective cohort study examining risk factors for HSV-2 acquisition in HIV-1 seronegative sex workers |
| Sobngwi-Tambekou et al [34], uncircumsized men in Orange Farm, South Africa | *21 (18–24) | 4.4% | 5.9% (3274) | 2.9 (3080) | UNK | 96% | Ancillary study within randomized trial of male circumcision to prevent HIV acquisition, examining additional influence of HSV-2 |
| Tobian et al [36], men in Rakai, Uganda | *25 (15–49) | 13.4% | 33.8% (6396) | 4.9 (4237) | UNK | 68.3% | Ancillary study of HSV-2 incidence within 2 randomized trials of male circumcision to prevent HIV acquisition |
| Wagner et al [43], adults from 2 neighboring villages in Uganda | *34 (15–49) | †7.8% | 57.0% (126) | 11.8 (17) | UNK | UNK | Retrospective cohort examining STIs among retained participant subset within larger prospective cohort |
Abbreviations: GUD,genital ulcer disease; HIV, human immunodeficiency virus; HSV, herpes simplex virus; STI, sexually transmitted infection; UNK, unknown.
* Estimated age from range. Study citations in bold indicate high-risk populations.
† Indicates a measure assessed on men and women combined.
Settings
Participants were recruited from high-risk groups as well as the general population (Tables 1 and 2). Seven studies were conducted in high-risk populations, with a median sample size of 1311 (range, 407–2397) [18, 26, 27, 31, 32, 35, 39]. High-risk settings included food and recreational facilities (hotels, guesthouses, bars), specialty clinics (municipal sex worker clinic, STD clinics), and male factory workers enrolled in HIV prevention projects. The remaining 11 studies, with a median sample size of 1360 (range, 126–6396), recruited participants from the general population [17, 20, 22, 24, 25, 28, 29, 34, 36, 37, 43]. Targeted populations included pregnant women, women seeking reproductive services, women's health facilities, rural communities and townships, and participants in HIV prevention circumcision trials.
Study Design
We identified 11 cohort studies [17, 18, 24, 26, 27, 29, 31, 32, 35, 39, 43] and 7 randomized clinical trials [20, 22, 25, 28, 34, 36, 37]: 10 of the 18 studies assessed HSV-2 incidence and risk factors for HSV-2 acquisition as their primary endpoint with follow-up ranging from 10 months to 4 years (Table 3) [18, 24, 26, 27, 29, 31, 32, 34–36]. There were 8 studies in women [17, 18, 20, 29, 32, 35, 37, 39], 4 studies in men [27, 28, 34, 36], and 6 studies recruiting both men and women (Tables 1 and 2) [22, 24–26, 31, 43].
Table 3.
Summary of Incident Risk Ratios for HSV-2 Acquisition Among Men and Women in Africa*
| Citation and Study Setting | HIV Status | Age at Sexual Initiation | Age | Gender | Factors Adjusted for in Multivariate Analysis |
|---|---|---|---|---|---|
| Chohan [18], women sex workers in Mombasa, Kenya | Ref: <25 years HR, 25–34: 0.72 (0.48–1.10) HR, 35+: 0.41 (0.17–1.03) |
None (age was no longer significant in multivariate analysis) | |||
| Kamali [24], adult residents in 15 villages in Southwest Uganda. |
Incident HIV 3.70 (2.10–6.60) |
Ref: Men Age-adjusted 1.70 (1.10–2.70) |
For HIV: age and sex | ||
| Kebede [26], factory workers in Addis Ababa, Ethiopia |
Prevalent HIV 2.80 (1.30–6.01) |
Ref: 20–29 years 30–39: 1.33 (0.60–2.80) 40–49: 1.67 (0.80–3.50) |
Ref: Men 2.43 (1.39–4.27) |
Sex, HSV-2 serostatus of partner, syphilis infection at baseline (age was no longer significant in multivariate analysis) | |
| McFarland [27], male factory workers in Zimbabwe |
Prevalent HIV HR: 4.70 (3.30–6.70) Incident HIV HR: 3.90 (2.60–5.80) |
Ref: ≤20 years HR, 21–25: 1.10 (0.70–1.70) HR, 26–30: 0.90 (0.50–1.50) HR, 31–35: 1.30 (0.80–2.30) HR, 36–40: 1.10 (0.6–2.0) HR, 41–45: 0.90 (0.40–2.0) HR, 46+: 0.70 (0.30–1.40) |
Widowed, history of genital ulcer, history of any STD, number of sex partners | ||
| Munjoma [29], pregnant women in Harare, Zimbabwe | Ref: >16 years ≤16 years: 3.60 (1.60–8.30) |
Ref: <20 20–24: 0.80 (0.30–12.10) 25–29: 0.20 (0–1.80) 30–34: 1.00 (0.20–4.50) >34: 0.70 (0.10–5.00) |
None | ||
| Okuku [31], adult sex workers in Mombasa, Kenya |
Among men Incident HIV 3.95 (1.25–12.43) Among women Incident HIV 8.88 (3.62–21.78) |
Among men Ref: 18–24 years 25–34: 1.97 (0.79–4.92) >34: 2.83 (0.99–8.11) Among women Ref: 18–24 years 25–34: 1.26 (0.65–2.44) >34: 1.69 (0.58–4.94) |
Among men: marital status, genital washing, new sex partners, condom use. Among women: marital status, genital washing. |
||
| Riedner [32], female bar workers in Mbeya Region, Tanzania |
Prevalent or incident HIV OR: 3.75 (1.14–12.36) |
Workplace, duration of work as bar worker, change of residence during 12 months before enrollment | |||
| Sobngwi-Tambekou [34], uncircumsized men in Orange Farm, South Africa |
Prevalent HIV 2.48 (1.28–4.81) |
Ref: <21 years 2.38 (1.58–3.60) |
Circumcision status, religion, ethnicity, alcohol use, marital status, sexual behavior, nonspousal sex partners | ||
| Tassiopoulos [35], female bar and hotel workers in Moshi, Tanzania |
Prevalent or incident HIV HR: 3.36 (1.44–7.87) |
Ref: ≥21 years HR, <21: 6.0 (1.39–25.97) Missing: HR, 16.70 (3.01–92.46) Not active: HR, 5.77 (0.85–39.16) |
Ref: ≤20 years HR, 21–30: 1.94 (0.82–4.59) HR, 31–40: 1.29 (0.37–4.43) HR, ≥41: 2.07 (0.32–13.38) |
Ethnicity, condom use, number of sex partners and alcohol intake | |
| Tobian [36], men in Rakai, Uganda | Prevalent HIV: 1.38 (0.71–2.69) |
Ref: 15–19 years 20–24: 1.21 (0.83–1.77) 25–29: 0.94 (0.59–1.50) 30–49: 0.65 (0.39–1.09) |
Education, employment, marital status, alcohol use, circumcision status, condom use, number of sex partners |
Unless otherwise specified as ‘OR’ or ‘HR’, effects are incident rate ratios (IRR).
Abbreviations:HIV, human immunodeficiency virus; HR, hazards ratio; HSV, herpes simplex virus; OR, odds ratio; Ref, reference; STD, sexually transmitted disease.
* Effects in bold represent results from multivariate analyses.
Few studies supplied information on the proportion of eligible persons who enrolled into the study. In the 7 studies that did report this criterion, the proportion enrolled from among the eligible ranged from 61% to 99% (Tables 1 and 2) [24, 25, 27–29, 37, 39]. Commonly cited reasons for nonenrollment included lack of interest and withdrawal of consent. Information on loss to follow-up or retention rates was reported by 15 studies; the proportion of participants completing the study ranged from 56%–97% with a median of 76%.
HSV-2 Detection
Herpes simplex virus-2 serological tests used by the different studies included Focus HerpeSelect 2 enzyme-linked immunosorbent assay (ELISA) (n = 8) [17, 18, 20, 22, 26, 29, 31, 35], Kalon HSV-2 ELISA (n = 6) [22, 28, 32, 34, 36, 37], monoclonal blocking enzyme-linked immunoassay (n = 1) [25], Chiron RIBA HSV Type 1/Type 2 strip immunoassay (n = 1) [27], and Western blot (n = 2) [24, 43].
HSV-2 Prevalence and Incidence in Women
Across the 6 studies conducted in high-risk cohorts, the baseline prevalence of HSV-2 ranged from 51% to 87%, with Riedner et al [32] reporting the highest prevalence in female bar workers in Mbeya, Tanzania (Table 1). In these studies, the incidence rates ranged from 4 to 23 per 100 person-years with a median of 16 per 100 person-years [18, 26, 31, 32, 35, 39]. Some of the highest incidence rates were reported by studies enrolling high-risk occupational cohorts in Kenya. Chohan et al [18] estimated an incidence rate of 23 per 100 person-years among women attending a municipal STI clinic in Mombasa, Kenya. Of note, a large proportion of this cohort was HSV-2 seropositive at enrollment (80%).
Eight studies recruited women from the general population (Table 1) [17, 20, 22, 24, 25, 29, 37, 43]. In 5 of these studies, more than half of the cohort was HSV-2 seropositive at enrollment. Among the 8 studies, the incidence ranged from 3 to 21 cases per 100 person-years, with the highest rate measured in a rural population cohort in Masaka, Uganda [43]. Notably, more than 70% of this cohort was HSV-2 seropositive at enrollment.
HSV-2 Prevalence and Incidence in Men
Overall, 10 studies reported HSV-2 prevalence and incidence among men; 7 were conducted in the general population (Table 2) [22, 24, 25, 28, 34, 36, 43]. Between 22% and 40% of high-risk cohorts were HSV-2 positive at enrollment. In high-risk populations, the incidence rates ranged from 1 to 9 per 100 person-years, with a notably low incidence reported among Ethiopian factory workers [26].
In comparison with high-risk cohorts, the baseline prevalence in general populations appeared to be lower, ranging from 6% to 57% with a median of 27%. In addition, incidence appeared to be lower among studies that recruited men from the general population compared with women. Among men, the reported rates ranged from 2 to 12 per 100 person-years with a median of 5 compared with 3 to 21 per 100 person-years with a median of 8 per 100 person-years in women (Figure 2).
Figure 2.
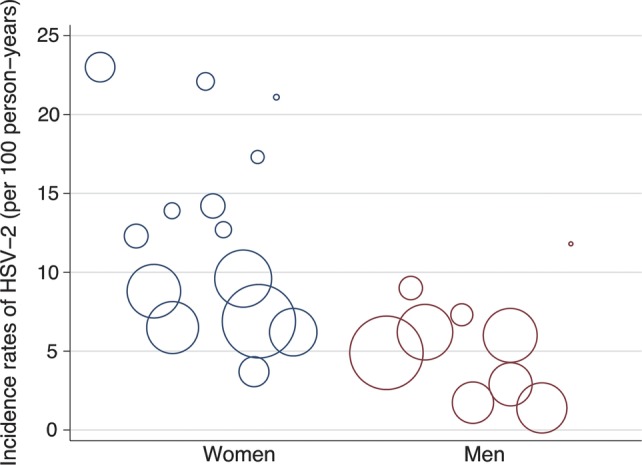
Incidence rates of herpes simplex virus (HSV)-2 reported in men and women among studies eligible for the systematic review. The area of the circle is proportional to the person-years accrued in a given study.
Risk Factors for Incident HSV-2 Infection in Women
Four studies assessed age as a risk factor for HSV-2 acquisition in women [18, 29, 31, 35], with 3 of them enrolling high-risk populations (Table 3; Figure 3A) [18, 31, 35]. None of these studies reported age as an independent risk factor in multivariate analysis. However, younger age at sexual initiation was found to be significantly associated with increased HSV-2 acquisition in the 2 studies that assessed this risk factor [29, 35]. Munjoma et al [29] estimated that women who initiated sexual activity at or below 16 years of age acquired HSV-2 at a rate 3.6 (95% confidence interval [CI], 1.6–8.3; P < .001) times higher than women who were older at their age of sexual debut. Likewise, Tassiopoulos et al [35] found that, after adjusting for age, women who were 21 years or younger when they initiated sexual activity were at an increased risk for HSV-2 acquisition (adjusted hazard ratio [aHR], 6.00; 95% CI, 1.39–25.97; P = .01).
Figure 3.
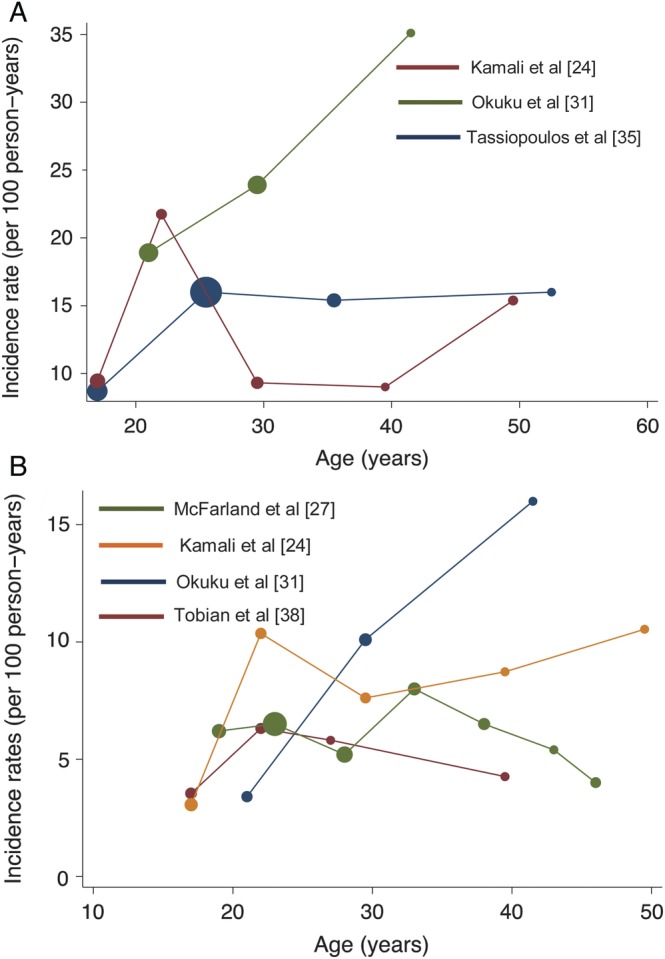
(A) Incidence rates of herpes simplex virus (HSV)-2 in women, stratified by age. Each circle corresponds to the mean in a given age group. The area of the circle is proportional to the person-years accrued in each age group. (B) Incidence rates of HSV-2 in men, stratified by age. Each circle corresponds to the mean in a given age group. The area of the circle is proportional to the person-years accrued in each age group. Please note that the y-axis differs in the two figures above.
Prevalent and incident HIV-1 infections were identified as significant risk factors for HSV-2 incidence in all 5 studies that assessed HIV status (Table 3) [24, 26, 31, 32, 35]. Among the 3 studies that examined risk factors in women [31, 32, 35], after adjustments for age, number of sex partners, and other markers of sexual exposure, the adjusted risk for HSV-2 acquisition in women with HIV-1 infection at baseline or follow-up and incident HSV-2 ranged from 3.36 (95% CI, 1.44–7.87) to 8.88 (95% CI, 3.62–21.78).
Four studies examined the association between HSV-2 acquisition and various measures of sexual activity among high-risk women [18, 26, 31, 35]. Independent risk factors identified in multivariate analysis included being in a discordant relationship with an HSV-2-positive partner (adjusted incidence rate ratio [aIRR], 15.19; 95% CI, 3.30–69.91) [26], having a male partner who had other partners (aHR, 4.91; 95% CI, 1.39–17.31) [35], and reporting 2 or more sexual partners per week compared with none (aHR, 2.70; 95% CI, 1.25–5.84) [18]. There was mixed evidence for the effect of condom use on HSV-2 acquisition. Chohan et al [18] reported a borderline protective effect among those who used condoms 25%–75% of the time compared with 25% or lower (aHR, 0.43; 95% CI, 0.19–0.99).
Risk Factors for Incident HSV-2 Infection in Men
Age was evaluated as a risk factor HSV-2 acquisition in 4 studies [27, 31, 34, 36], 2 of which were conducted in the general population (Table 3; Figure 3B). Although age was not found to be an independent risk factor in high-risk cohorts, Sobngwi-Tambekou et al [34] found that men from the general population who were 21 years or older were significantly more likely to acquire HSV-2 than their younger counterparts (aIRR, 2.38; 95% CI, 1.58–3.60; P < .001). In all 4 studies, the aIRRs for the association between older age groups (>20 years) and risk of HSV-2 acquisition ranged from 1.21 (95% CI, .83–1.77) to 2.38 (95% CI, 1.58–3.60).
Baseline and incident HIV infections were found to increase the risk of HSV-2 acquisition in 5 of the 6 studies that examined this association [24, 26, 27, 31, 34, 36] (Table 3). Men who acquired HIV infection during the course of follow-up had a 2.48 (aIRR; 95% CI, 1.28–4.81) to 4.7 (aIRR; 95% CI, 3.3–6.7) times higher acquisition rate of HSV-2. As a notable exception, Tobian et al [36] did not observe a significant difference in HSV-2 incidence rate across HIV-1 enrollment status in their male circumcision trial in Uganda (aIRR, 1.38; 95% CI, .71–2.69).
Male circumcision was found to have a protective effect on the risk of HSV-2 acquisition in 2 of the 4 studies that examined this association [28, 31, 34, 36]. In their multivariate analyses, Sobngwi-Tambekou et al [34] and Tobian et al [36] found a lower risk of HSV-2 acquisition among circumcised men, with adjusted IRRs ranging from 0.45 (95% CI, .24–.82) to 0.70 (95% CI, .55–.91), respectively. In contrast, univariate analyses from 2 other studies did not show any effect of circumcision on HSV-2 acquisition [28, 31].
Five studies assessed measures of sexual activity among men [26, 27, 31, 34, 36], with 3 of them enrolling high-risk cohorts [26, 27, 31]. Consistent condom use was found to have a protective effect in all 3 studies that assessed it [31, 34, 36]. In particular, Okuku et al [31] observed that men who had never used a condom in the past 3 months were 2.36 times (95% CI, 1.07–5.23) more likely to acquire HSV-2 compared with men reporting consistent use. Likewise, in a cohort of uncircumcised men in Orange Farm, South Africa, men who reported at least 1 sexual contact without condom use during follow-up were at an elevated risk for HSV-2 acquisition (aIRR, 1.87; 95% CI, 1.21–2.89) [34]. Among 5 studies that examined other measures of sexual activity, independent risk factors for HSV-2 acquisition included having a HSV-2-positive partner (aIRR, 15.19; 95% CI, 3.30–69.91) [26] and number of sex partners (aHR, 1.10; 95% CI, 1.00–1.30) [27].
Gender and Incidence of HSV-2
Among 6 studies that enrolled both genders [22, 24–26, 31, 43], 3 studies examined gender as a risk factor for HSV-2 acquisition [24, 26, 43]. Two studies reported that women were at a higher risk for HSV-2 acquisition (P < .001; Table 3), with incidence rate ratios ranging from 1.70 (95% CI, 1.10–2.70) to 2.43 (95% CI, 1.39–4.27) and as high as 4.21 (95% CI, .93–18.98) in a subset [24, 26]. In addition, Wagner et al [43] observed this in their prospective cohort recruited from the general population but did not report any formal comparisons.
DISCUSSION
In our systematic review of HSV-2 incidence within sub-Saharan Africa, we found that HSV-2 incidence is overall high but variable across countries and populations. Not surprisingly, incidence appeared to be higher among women who report higher risk sexual behavior, such as bar and hotel workers. Despite being a common significant predictor of HSV-2 prevalence, age was not found to be associated with HSV-2 incidence in women. Our findings confirm that prevalent and incident HIV infection is a significant risk factor for incident HSV-2 in both men and women, even after adjusting for age, sexual activity, condom use, and circumcision.
This review draws attention to the high HSV-2 acquisition rates in both general and high-risk populations in sub-Saharan Africa; as high as 22 per 100 person-years and 23 per 100 person-years in 2 high-risk Kenyan cohorts [18, 31]. Notably, both studies were conducted in HIV-seronegative populations. Such high acquisition rates likely reflect the high HSV-2 prevalence in the community, age mixing in partnerships, and sexual networks characterized by higher partner concurrency [44]. For example, Kenyon et al [45, 46] reported a significant correlation of more than 70% between country-specific partner concurrency rates and the HSV-2 prevalence. Thus, the risk of HSV-2 may be less predicated upon individual sexual behavior and more on network characteristics.
Few preventative strategies to lower sexual HSV-2 transmission have been shown to be effective. Disclosure of HSV-2 status to the uninfected partner and condom use appear protective in observational studies [47, 48]. Corey et al [49] found that once-daily suppressive therapy with valacyclovir significantly reduces the risk of transmission among immunocompetent HSV-2 discordant couples; an effect that did not extend to couples where the index partner was coinfected with HSV-2 and HIV-1 and acyclovir was used as the antiviral [50]. Tenofovir is another antiviral drug that has been shown to have anti-HSV activity in vitro [51]. However, Tan et al [52] report that standard doses of oral tenofovir did not reduce genital HSV shedding in HIV and HSV-2 coinfected adults.
Among treatment as prevention strategies, the recent CAPRISA-004 trial showed a 51% reduction in HSV-2 acquisition among women assigned to the tenofovir 1% vaginal gel, in an effect independent of reducing HIV acquisition [53]. In addition, preliminary data from the Pre-Exposure Prophylaxis (PrEP) trial showed daily tenofovir to be 21% efficacious in the prevention of HSV-2 acquisition among susceptible partners in HIV and HSV-2 serodiscordant relationships in Africa [54]. In contrast, results from the Pre-exposure Prophylaxis Initiative (iPrEx) study did not find oral tenofovir to prevent HSV-2 acquisition among men who have sex with men [55].
Prior systematic reviews and meta-analyses have focused on global patterns of HSV-2 prevalence; to our knowledge, this is the first systematic synthesis of HSV-2 incidence in sub-Saharan Africa, a region severely affected by both HIV and HSV-2. Although surveillance systems in resource-constrained countries typically rely on HIV and HSV-2 prevalence data, cross-sectional disease prevalence is not an optimal maker of risk of infection. In addition, trends in acquisition across demographic groups can help identify transmission patterns and accordingly tailor disease prevention strategies by identifying sub-populations most at-risk. A limitation to this review includes the lack of standardized HSV-2 antibody tests across studies. Although all included studies relied on a type-specific antibody assay, the sensitivity and specificity of a given serological test varies between the different commercially available tests and may be more problematic with sera from sub-Saharan Africa, potentially resulting in misclassification of HSV-2 status [56, 57].
CONCLUSIONS
In general, HSV-2 incidence has been derived from cohort studies that enroll a variety of populations that correspond to the aim of the study and measure HSV-2 incidence as a secondary outcome or a potential confounder. Yet estimates of incidence are critical for planning prevention trials, because expected number of incident cases is a key determinant of number of participants required to enroll. All preventative trials of HSV-2 vaccines to date have been conducted in the United States; thus far, none of the candidate vaccines have shown efficacy for prevention of HSV-2 acquisition. In the most recent Herpevac trial, >8000 HSV-seronegative women were enrolled and observed for up to 20 months [58]. In the control arm of the study, the incidence of HSV-2 was 1.1 per 100 person-years [59]. This striking contrast in HSV-2 incidence rates between Africa and the United States has significant implications for HSV vaccine research. An efficacy trial of a candidate prophylactic vaccine may not be feasible in the United States because of the sample size required. For example, at such low incidence, 10 240 persons would need to be observed for 1 year to demonstrate a 50% reduction in HSV-2 incidence with 80% power. In contrast, the higher acquisition rate of HSV-2 among men and women in sub-Saharan Africa confirms an advantage for rapid evaluation of candidate vaccines [60]. Candidate HSV-2 vaccines will need to prevent HSV-2 in persons seropositive for HSV-1, because almost all persons in Africa acquire HSV-1 in childhood. In addition, the availability of the product in Africa will need to be addressed, if the trials will enroll local populations.
In conclusion, our data suggest that in sub-Saharan Africa the rates of HSV-2 acquisition are exceptionally high, especially among women, presenting an opportunity to efficiently conduct clinical trials of candidate vaccines.
Acknowledgments
Author contributions. A. W. conceived of and designed the study. S. R. conducted the literature search and identified studies for full text review. A. M. and S. R. independently assessed all full-text articles for inclusion, extracted data, and interpreted findings. S. R. drafted the manuscript. N. M. and A. W. critically reviewed the manuscript drafts, and all authors have contributed to the drafts of the manuscript and approve the final report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgment. The authors thank Drs. Helen Weiss and Frances Cowan for their careful review of our manuscript.
Disclaimer. The funding source had no role in the conception, design, analysis, or drafting of this report.
Financial support. This work was supported by the National Institutes of Health (grant numbers P01 AI030731 [to A. W., N. M., and A. M.] and K24 AI071113 [to A. W.]).
Potential conflicts of interest. A. M. received personal fees from Immune Design Corporation and AiCuris. A. W. received grants from National Institutes of Health; personal fees from Aicuris, Amgen, and Eisai; and monies for clinical trials to the University of Washington from Genentech, Gilead, Vical, Agenus, and Genocea.
References
- 1.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008;86:805. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186(Suppl 1):S3–28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 3.O'Farrell N. Increasing prevalence of genital herpes in developing countries: implications for heterosexual HIV transmission and STI control programmes. Sex Transm Infect. 1999;75:377–84. doi: 10.1136/sti.75.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wald A. Synergistic interactions between herpes simplex virus type-2 and human immunodeficiency virus epidemics. Herpes. 2004;11:70–6. [PubMed] [Google Scholar]
- 5.Abu-Raddad LJ, Magaret AS, Celum C, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One. 2008;3:e2230. doi: 10.1371/journal.pone.0002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biraro S, Kamali A, White R, et al. Effect of HSV-2 on population-level trends in HIV incidence in Uganda between 1990 and 2007. Trop Med Int Health. 2013;18:1257–66. doi: 10.1111/tmi.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24A–35A. [PubMed] [Google Scholar]
- 8.World Health Organization. Department of HIV/AIDS, UNAIDS, London School of Hygiene and Tropical Medicine. Herpes simplex virus type 2: programmatic and research priorities in developing countries: report of a WHO/UNAIDS/LSHTM workshop; 14–16 February 2001; London. Geneva: World Health Organization; 2001. [Google Scholar]
- 9.Halioua B, Malkin JE. Epidemiology of genital herpes - recent advances. Eur J Dermatol. 1999;9:177–84. [PubMed] [Google Scholar]
- 10.Weiss HA, Buvé A, Robinson NJ, et al. The epidemiology of HSV-2 infection and its association with HIV infection in four urban African populations. AIDS. 2001;15(Suppl 4):S97–108. doi: 10.1097/00002030-200108004-00011. [DOI] [PubMed] [Google Scholar]
- 11.Korenromp EL, Bakker R, de Vlas SJ, et al. HIV dynamics and behaviour change as determinants of the impact of sexually transmitted disease treatment on HIV transmission in the context of the Rakai trial. AIDS. 2002;16:2209–18. doi: 10.1097/00002030-200211080-00014. [DOI] [PubMed] [Google Scholar]
- 12.Korenromp EL, Bakker R, De Vlas SJ, et al. Can behavior change explain increases in the proportion of genital ulcers attributable to herpes in sub-Saharan Africa? A simulation modeling study. Sex Transm Dis. 2002;29:228–38. doi: 10.1097/00007435-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Kaul R, Kimani J, Nagelkerke NJ, et al. Monthly antibiotic chemoprophylaxis and incidence of sexually transmitted infections and HIV-1 infection in Kenyan sex workers: a randomized controlled trial. JAMA. 2004;291:2555–62. doi: 10.1001/jama.291.21.2555. [DOI] [PubMed] [Google Scholar]
- 14.Hallett TB, Zaba B, Todd J, et al. Estimating incidence from prevalence in generalised HIV epidemics: methods and validation. PLoS Med. 2008;5:e80. doi: 10.1371/journal.pmed.0050080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baeten JM, Benki S, Chohan V, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007;21:1771–7. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 17.Brown JM, Wald A, Hubbard A, et al. Incident and prevalent herpes simplex virus type 2 infection increases risk of HIV acquisition among women in Uganda and Zimbabwe. AIDS. 2007;21:1515–23. doi: 10.1097/QAD.0b013e3282004929. [DOI] [PubMed] [Google Scholar]
- 18.Chohan V, Baeten JM, Benki S, et al. A prospective study of risk factors for herpes simplex virus type 2 acquisition among high-risk HIV-1 seronegative women in Kenya. Sex Transm Infect. 2009;85:489–92. doi: 10.1136/sti.2009.036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couppié P, Sarazin F, Clyti E, et al. Increased incidence of genital herpes after HAART initiation: a frequent presentation of immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients. AIDS Patient Care STDS. 2006;20:143–5. doi: 10.1089/apc.2006.20.143. [DOI] [PubMed] [Google Scholar]
- 20.de Bruyn G, Shiboski S, van der Straten A, et al. The effect of the vaginal diaphragm and lubricant gel on acquisition of HSV-2. Sex Transm Infect. 2011;87:301–5. doi: 10.1136/sti.2010.047142. [DOI] [PubMed] [Google Scholar]
- 21.Heffron R, Chao A, Mwinga A, et al. High prevalent and incident HIV-1 and herpes simplex virus 2 infection among male migrant and non-migrant sugar farm workers in Zambia. Sex Transm Infect. 2011;87:283–8. doi: 10.1136/sti.2010.045617. [DOI] [PubMed] [Google Scholar]
- 22.Jewkes R, Nduna M, Levin J, et al. Impact of stepping stones on incidence of HIV and HSV-2 and sexual behaviour in rural South Africa: cluster randomised controlled trial. BMJ. 2008;337:a506. doi: 10.1136/bmj.a506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jewkes RK, Dunkle K, Nduna M, et al. Associations between childhood adversity and depression, substance abuse and HIV and HSV2 incident infections in rural South African youth. Child Abuse Negl. 2010;34:833–41. doi: 10.1016/j.chiabu.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamali A, Nunn AJ, Mulder DW, et al. Seroprevalence and incidence of genital ulcer infections in a rural Ugandan population. Sex Transm Infect. 1999;75:98–102. doi: 10.1136/sti.75.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamali A, Quigley M, Nakiyingi J, et al. Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: a community randomised trial. Lancet. 2003;361:645–52. doi: 10.1016/s0140-6736(03)12598-6. [DOI] [PubMed] [Google Scholar]
- 26.Kebede Y, Dorigo-Zetsma W, Mengistu Y, et al. Transmission of herpes simplex virus Type 2 among factory workers in Ethiopia. J Infect Dis. 2004;190:365–72. doi: 10.1086/422038. [DOI] [PubMed] [Google Scholar]
- 27.McFarland W, Gwanzura L, Bassett MT, et al. Prevalence and incidence of herpes simplex virus type 2 infection among male Zimbabwean factory workers. J Infect Dis. 1999;180:1459–65. doi: 10.1086/315076. [DOI] [PubMed] [Google Scholar]
- 28.Mehta SD, Moses S, Parker CB, et al. Circumcision status and incident herpes simplex virus type 2 infection, genital ulcer disease, and HIV infection. AIDS. 2012;26:1141–9. doi: 10.1097/QAD.0b013e328352d116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munjoma MW, Kurewa EN, Mapingure MP, et al. The prevalence, incidence and risk factors of herpes simplex virus type 2 infection among pregnant Zimbabwean women followed up nine months after childbirth. BMC Womens Health. 2010;10:2. doi: 10.1186/1472-6874-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyiro JU, Sanders EJ, Ngetsa C, et al. Seroprevalence, predictors and estimated incidence of maternal and neonatal herpes simplex virus type 2 infection in semi-urban women in Kilifi, Kenya. BMC Infect Dis. 2011;11:155. doi: 10.1186/1471-2334-11-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okuku HS, Sanders EJ, Nyiro J, et al. Factors associated with herpes simplex virus type 2 incidence in a cohort of human immunodeficiency virus type 1-seronegative Kenyan men and women reporting high-risk sexual behavior. Sex Transm Dis. 2011;38:837–44. doi: 10.1097/OLQ.0b013e31821a6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riedner G, Hoffmann O, Rusizoka M, et al. Decline in sexually transmitted infection prevalence and HIV incidence in female barworkers attending prevention and care services in Mbeya Region, Tanzania. AIDS. 2006;20:609–15. doi: 10.1097/01.aids.0000210616.90954.47. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez M, Obasi A, Mosha F, et al. Herpes simplex virus type 2 infection increases HIV incidence: a prospective study in rural Tanzania. AIDS. 2002;16:451–62. doi: 10.1097/00002030-200202150-00018. [DOI] [PubMed] [Google Scholar]
- 34.Sobngwi-Tambekou J, Taljaard D, Lissouba P, et al. Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. J Infect Dis. 2009;199:958–64. doi: 10.1086/597208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tassiopoulos KK, Seage G, Sam N, et al. Predictors of herpes simplex virus type 2 prevalence and incidence among bar and hotel workers in Moshi, Tanzania. J Infect Dis. 2007;195:493–501. doi: 10.1086/510537. [DOI] [PubMed] [Google Scholar]
- 36.Tobian AA, Charvat B, Ssempijja V, et al. Factors associated with the prevalence and incidence of herpes simplex virus type 2 infection among men in Rakai, Uganda. J Infect Dis. 2009;199:945–9. doi: 10.1086/597074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tobian AA, Kigozi G, Redd AD, et al. Male circumcision and herpes simplex virus type 2 infection in female partners: a randomized trial in Rakai, Uganda. J Infect Dis. 2012;205:486–90. doi: 10.1093/infdis/jir767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobian AA, Kigozi G, Wawer MJ, et al. Herpes simplex virus type-2 assay specificity and male circumcision to reduce herpes simplex virus type-2 acquisition. AIDS. 2013;27:147–9. doi: 10.1097/QAD.0b013e32835aa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallely A, Hambleton IR, Kasindi S, et al. Are women who work in bars, guesthouses and similar facilities a suitable study population for vaginal microbicide trials in Africa? PLoS One. 2010;5:e10661. doi: 10.1371/journal.pone.0010661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van de Wijgert JH, Morrison CS, Brown J, et al. Disentangling contributions of reproductive tract infections to HIV acquisition in African Women. Sex Transm Dis. 2009;36:357–64. doi: 10.1097/OLQ.0b013e3181a4f695. [DOI] [PubMed] [Google Scholar]
- 41.Venkatesh KK, Mayer KH, Blanchard K, et al. African women recently infected with HIV-1 and HSV-2 have increased risk of acquiring Neisseria gonorrhoeae and Chlamydia trachomatis in the Methods for Improving Reproductive Health in Africa trial. Sex Transm Dis. 2011;38:562–70. doi: 10.1097/OLQ.0b013e31820a8c2c. [DOI] [PubMed] [Google Scholar]
- 42.Venkatesh KK, Cheng H, Montgomery ET, et al. The relative contribution of viral and bacterial sexually transmitted infections on HIV acquisition in Southern African women in the Methods for Improving Reproductive Health in Africa study. Int J STD AIDS. 2011;22:218–24. doi: 10.1258/ijsa.2010.010385. [DOI] [PubMed] [Google Scholar]
- 43.Wagner HU, Van Dyck E, Roggen E, et al. Seroprevalence and incidence of sexually transmitted diseases in a rural Ugandan population. Int J STD AIDS. 1994;5:332–7. doi: 10.1177/095646249400500509. [DOI] [PubMed] [Google Scholar]
- 44.Morris M, Epstein H, Wawer M. Timing is everything: international variations in historical sexual partnership concurrency and HIV prevalence. PLoS One. 2010;5:e14092. doi: 10.1371/journal.pone.0014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenyon C, Colebunders R, Hens N. Determinants of generalized herpes simplex virus-2 epidemics: the role of sexual partner concurrency. Int J STD AIDS. 2013;24:375–82. doi: 10.1177/0956462412472816. [DOI] [PubMed] [Google Scholar]
- 46.Kenyon C, Colebunders R, Buve A, et al. Partner-concurrency associated with herpes simplex virus 2 infection in young South Africans. Int J STD AIDS. 2013;24:804–12. doi: 10.1177/0956462413482810. [DOI] [PubMed] [Google Scholar]
- 47.Wald A, Krantz E, Selke S, et al. Knowledge of partners' genital herpes protects against herpes simplex virus type 2 acquisition. J Infect Dis. 2006;194:42–52. doi: 10.1086/504717. [DOI] [PubMed] [Google Scholar]
- 48.Wald A, Langenberg AG, Link K, et al. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. JAMA. 2001;285:3100–6. doi: 10.1001/jama.285.24.3100. [DOI] [PubMed] [Google Scholar]
- 49.Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 50.Mujugira A, Magaret AS, Celum C, et al. Daily acyclovir to decrease herpes simplex virus type 2 (HSV-2) transmission from HSV-2/HIV-1 coinfected persons: a randomized controlled trial. J Infect Dis. 2013;208:1366–74. doi: 10.1093/infdis/jit333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrei G, Lisco A, Vanpouille C, et al. Topical tenofovir, a microbicide effective against HIV, inhibits herpes simplex virus-2 replication. Cell Host Microbe. 2011;10:379–89. doi: 10.1016/j.chom.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan DH, Kaul R, Raboud JM, et al. No impact of oral tenofovir disoproxil fumarate on herpes simplex virus shedding in HIV-infected adults. AIDS. 2011;25:207–10. doi: 10.1097/QAD.0b013e328341ddf7. [DOI] [PubMed] [Google Scholar]
- 53.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.HIV Prevention at CROI. 20th Conference on Retroviruses and Opportunistic Infections 2013. Available at: http://www.natap.org/2013/CROI/croi_75.htm . Accessed 14 February 2014.
- 55.Marcus JL, Glidden DV, McMahan V, et al. Daily oral emtricitabine/tenofovir preexposure prophylaxis and herpes simplex virus type 2 among men who have sex with men. PLoS One. 2014;9:e91513. doi: 10.1371/journal.pone.0091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Dyck E, Buvé A, Weiss HA, et al. Performance of commercially available enzyme immunoassays for detection of antibodies against herpes simplex virus type 2 in African populations. J Clin Microbiol. 2004;42:2961–5. doi: 10.1128/JCM.42.7.2961-2965.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magder LS, Hughes JP. Logistic regression when the outcome is measured with uncertainty. Am J Epidemiol. 1997;146:195–203. doi: 10.1093/oxfordjournals.aje.a009251. [DOI] [PubMed] [Google Scholar]
- 58.Belshe RB, Leone PA, Bernstein DI, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bernstein DI, Bellamy AR, Hooklll EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56:344–51. doi: 10.1093/cid/cis891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freeman EE, White RG, Bakker R, et al. Population-level effect of potential HSV2 prophylactic vaccines on HIV incidence in sub-Saharan Africa. Vaccine. 2009;27:940–6. doi: 10.1016/j.vaccine.2008.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]


