Using only self-reported information likely overestimates lack of awareness of HIV status for black MSM. Estimates that also incorporate laboratory and case surveillance measures do not show significant racial disparity in lack of awareness of HIV status.
Keywords: HIV, MSM, survey, testing
Abstract
Background
Lack of human immunodeficiency virus (HIV) infection awareness may be a driver of racial disparities in HIV infection among men who have sex with men (MSM). Lack of awareness is typically measured by comparing HIV test result to self-reported HIV status. This measure may be subject to reporting bias and alternatives are needed.
Methods
The InvolveMENt study examined HIV disparities between black and white MSM from Atlanta. Among HIV-positive participants who did not report knowing they were positive, we examined other measures of awareness: HIV viral load (VL) <1000 copies/mL (low VL), antiretroviral (ARV) drugs in blood, and previous HIV case surveillance report.
Results
Using self-report only, 32% (62 of 192) of black and 16% (7 of 45) of white MSM were not aware of their HIV infection (P = .03). Using self-report and low VL, 25% (48 of 192) black and 16% (7 of 45) white MSM lacked awareness (P = .18). Using self-report and ARVs, 26% (50 of 192) black and 16% (7 of 45) white MSM lacked awareness (P = .14). Using self-report and surveillance report, 15% (28 of 192) black and 13% (6 of 45) white MSM lacked awareness (P = .83).
Conclusions
Self-report only may overestimate true lack of awareness of HIV status for black MSM. If, as our data suggest, black MSM are not less likely to be aware of their HIV infection than are white MSM, then this factor is not a substantial driver of HIV disparity. Future HIV research that depends on accurate measurement of HIV status awareness should consider including additional laboratory and case surveillance data.
Over the past decade, men who have sex with men (MSM) have been the only group in the United States in which human immunodeficiency virus (HIV) incidence has increased [1]. More recently, increases in incidence have been concentrated among young MSM of color [2]. Black MSM have over twice the prevalence of HIV than white men, [3, 4] and data from the HIV Prevention Trials Network study number 061 suggest that black MSM experience an HIV incidence rate over 5 times that of white MSM [5]. The reasons for these racial disparities in HIV infection among MSM are unclear, but differences in individual-level risk behaviors likely do not account for the observed disparities [4, 6]. This same pattern of disparity exists among black MSM in Atlanta, the city with the 8th highest rate of new HIV diagnoses and 4th highest number of new HIV diagnoses among MSM in the country in 2011 [7]. Men who have sex with men comprise the largest group living with HIV in Atlanta, and black MSM are disproportionately affected, constituting approximately 60% of HIV-infected MSM, whereas black persons represent only approximately 30% of the overall Atlanta population [8].
Differences in awareness of HIV infection between black and white MSM are hypothesized to contribute to this disparity [6]. Overall, approximately 20% of persons infected with HIV in the United States are thought to be unaware of their infection; however, they account for an estimated 49% of transmission events [9]. Awareness of HIV infection results in a reduction in high-risk sexual behavior [10], and it is the first critical step in the continuum of HIV care, which ideally results in receipt of antiretroviral (ARV) therapy, achievement of an undetectable HIV viral load (VL), and reduction in HIV transmissions [11–13]. National surveillance data show that black MSM have high levels (59%) of lack of awareness of HIV infection compared with white MSM (26%) [14].
HIV surveillance projects and research studies use a similar set of testing history questions to define self-reported lack of awareness of infection that is detected through study-delivered testing. However, there are new reports that the validity of this self-reported measure may be questionable for some groups of MSM [5, 15, 16]. It remains unknown whether the validity of these measures may differ by participant race and whether viable solutions to improve the measure may be available to HIV researchers. For this study, we hypothesized that the combination of traditional survey, laboratory, and public health surveillance data could improve the measurement of awareness of HIV infection among black and white MSM participants of a research study in Atlanta, Georgia.
METHODS
InvolveMENt Study
The InvolveMENt study was a prospective cohort study designed to examine factors that may contribute to disparities in HIV and sexually transmitted infection between black and white MSM in Atlanta. MSM aged 18–39 years were recruited, regardless of HIV status, primarily using time-space venue sampling, with a sampling frame built upon that used for the Atlanta site for the second MSM cycle of the National HIV Behavioral Surveillance System (NHBS-MSM) [17, 18]. Facebook was also included as a virtual venue. The InvolveMENt study methods have been previously reported but are briefly described here [19]. Eligible participants were self-identified black and white MSM who reported sex with another man in the previous 3 months, who were not in a mutually monogamous relationship, could complete survey instruments in English, lived in the Atlanta metropolitan area, were not enrolled in another HIV prevention study, who did not identify as Hispanic/Latino, and had no plans to relocate in the subsequent 2 years. During the baseline visit consent process, participants were informed that they would be screened for HIV infection, but the criteria for which participants would be offered enrollment in the prospective component of the study were not specifically discussed.
All InvolveMENt study participants were tested for HIV using a rapid test with confirmation by enzyme-linked immunosorbent assay and Western blot analysis. Before results of HIV testing were returned, participants completed a detailed computer-assisted self-interview. All HIV-positive men had HIV VL testing (COBAS AmpliPrep/COBAS TaqMan HIV-1 test kit version 2.0; Roche Molecular Systems, Inc.), and these results were returned to participants. Participants who had a negative HIV rapid test at baseline were offered enrollment in the follow-up study. If a participant subsequently tested HIV positive at the 3-month visit, we conducted VL testing on the stored specimen from the baseline visit. Those who had detectable VL (but who were nonreactive on the HIV rapid test) were considered to have acute HIV infection at the time of their baseline visit. The InvolveMENt study staff (eg, counselors, phlebotomists, interviewers) were diverse in regards to race, ethnicity, age, and gender. The InvolveMENt study was reviewed and approved by the institutional review board of Emory University.
Measures
We used several approaches to classify HIV infection awareness. The first method (“self-reported”) defined awareness of infection using a set of questions about their experiences ever getting tested, the most recent testing experiences, and their most recent test result. In addition, during the posttest result discussion, any participant who disclosed prior knowledge of their HIV status to the study counselor was classified as “self-reported aware,” even if they did not report having a previous HIV-positive test in their survey.
Among those who were classified as not aware of their HIV infection by self-report, we also explored 2 laboratory measures and a public health surveillance measure of awareness. One laboratory measure was low VL (<1000 copies/mL or undetectable) on the baseline blood specimen. The other laboratory measure was detectable ARV drugs using either of 2 nonmutually exclusive algorithms. First, for those MSM classified as not aware of their HIV infection by self-report and who also had a low VL, we conducted a limited quantification ARV panel that included commonly prescribed nucleoside/nucleotide reverse-transcriptase inhibitors ([NRTIs] abacavir, emtricitabine, lamivudine, tenofovir, and zidovudine) [20]. This limited quantification ARV panel was the first we explored and was not done on all specimens because of the potential expense involved. Second, for all MSM classified as not aware by self-report, we used mass spectrometry to test an expanded ARV panel that included NRTIs (emtricitabine, lamivudine, tenofovir, and zidovudine), nonnucleotide reverse-transcriptase inhibitors (efavirenz and nevirapine), and protease inhibitors (atazanavir, darunavir, fosamprenavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, and tipranavir) [16]. The results of the ARV testing were not returned to participants.
For the public health surveillance measure of awareness, we used evidence of a preexisting HIV case surveillance report at the Georgia Department of Public Health. We submitted to the state health department a line-listing of black and white participants considered not aware of their status by self-report. The health department reported back on the total number of persons from the list who were already in the HIV case surveillance system and whose first HIV diagnosis in the system had occurred at least 21 days before our diagnosis. This timeframe was selected to ensure that persons who were previously diagnosed more recently would have had at least some opportunity to receive their previous test result. To protect confidentiality of case surveillance data, the health department did not return a list of persons with previous diagnoses to the study's researchers, but it only provided us with aggregate numbers. The health department also reported the mean number of days between the first HIV diagnosis in the system and our diagnosis.
Participants who enrolled through February 2012 and were not aware of their HIV infection by self-report were also invited to participate in an in-depth interview. A staff interviewer conducted one-on-one discussions with participants at a follow-up visit to the study office (ie, the qualitative interviews happened after the visit in which they tested HIV-positive). The interviewer used a semistructured qualitative interview method with a set of open-ended question domains with follow-up probes. The purpose of the interviews was to generate more detailed information about previous HIV testing experiences and diagnoses, general themes regarding disclosure of HIV status, and rephrasing activities to better understand comprehension of the HIV testing questions. Participants were not specifically asked about possible discrepancies between self-reported awareness and laboratory testing.
Analyses
We compared lack of awareness of HIV infection between black and white MSM using χ2 tests for the different measures: self-report alone, self-report plus low VL, self-report plus detectable ARV, and self-report plus previous HIV case surveillance report. Findings were considered significant if P < .05. Statistical analyses were performed using OpenEpi (www.OpenEpi.com, version 2013/04/06).
RESULTS
Enrollment occurred from July 2010 through December 2012, resulting in a total of 454 black MSM and 349 white MSM being enrolled and contributing to this analysis. The prevalence of HIV infection at the baseline visit among black MSM was 43% (197 of 454), compared with 13% (46 of 349) among white MSM (prevalence ratio = 3.3; 95% confidence interval = 2.5, 4.4) [19]. There were 5 black MSM and 1 white MSM who were considered to have acute HIV infection at the time of their baseline visit and were excluded from further analysis.
Among black MSM with HIV infection at baseline, 69 were classified as not aware on survey responses alone, and 7 disclosed knowledge of their status during posttest counseling. Among white MSM with HIV infection at baseline, 8 were classified as not aware by survey responses alone, and 1 disclosed knowledge of his status during posttest counseling. The measure of self-reported lack of awareness of HIV status was 32% (62 of 192) among black MSM and 16% (7 of 45) among white MSM. Most participants (65%, 45 of 69) who disclosed not being aware of their infection had been previously HIV tested and reported that their most recent test result was negative (Table 1).
Table 1.
Characteristics of MSM Classified as Lacking Awareness of Their HIV Infection, InvolveMENt Study, 2010–2012
| Black MSM (N = 62) |
White MSM (N = 7) |
|||
|---|---|---|---|---|
| No. | (%) | No. | (%) | |
| HIV Testing History Questions | ||||
| Never Tested | 14 | (23) | 1 | (14) |
| Ever Tested, Last Result | ||||
| Negative | 39 | (63) | 6 | (86) |
| Indeterminate | 3 | (5) | 0 | (0) |
| Didn't Get It | 6 | (10) | 0 | (0) |
| HIV Viral Load <1000 copies/mL | 14 | (23) | 0 | (0) |
| Any Antiretroviral Drug Detected | 12 | (19) | 0 | (0) |
| Mass Spectrometry | 12 | (19) | 0 | (0) |
| NRTI Quantificationa | 7 | (11) | 0 | (0) |
| Previously Reported to HIV Case Surveillanceb | 34 | (55) | 1 | (14) |
Abbreviations: HIV, human immunodeficiency virus; MSM, men who have sex with men; NRTI, nucleoside/tide reverse-transcriptase inhibitor.
a NRTI blood levels only performed among those with HIV viral load <1000 copies/mL.
b Average time between previous report and InvolveMENt report = 1590 days.
Fourteen participants (14 black MSM and 0 white MSM) who were classified as not aware by self-report had a low VL. The limited panel of ARV testing was completed for 13 of them; 7 of whom had at least 1 NRTI detected. The expanded ARV panel was completed for 60 of 69 MSM (53 of 62 black MSM and 7 of 7 white MSM); 12 of whom (all black MSM) had ARV detected. Thirteen participants had both types of ARV testing; 7 of whom had ARV detected on both tests, 4 had no ARV detected on either test, and 2 had ARV on mass spectrometry that was not detected on the quantitative NRTI test (Table 2 shows detected ARV results). Two participants with high VL (14 755 and 16 802 copies/mL) had detectable ARV, and both had only zidovudine detected.
Table 2.
Antiretroviral Drugs Detected Among Men Who Have Sex with Men Classified as Lacking Awareness of Their HIV Infection Based on Self-disclosure, InvolveMENt Study, 2010–2012
| Participant ID | HIV Viral Load (Copies/mL) | NRTI Quantification ARV Detected | Mass Spectrometry ARV Detected | ARV detected |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABC | FPV | ATV | DRV | FTC | EFV | IDV | 3TC | LPV | NFV | NVP | RTV | SQV | TDF | TPV | ZDV | ||||
| 021 | Undetectable | Yes | Yes | x | x | x | |||||||||||||
| 031 | 162 | Yes | Yes | x | x | ||||||||||||||
| 051 | 16802 | Not Performed | Yes | x | |||||||||||||||
| 081 | 14755 | Not Performed | Yes | x | |||||||||||||||
| 151 | 28 | Yes | Yes | x | x | ||||||||||||||
| 171 | Undetectable | Yes | Yes | x | x | x | |||||||||||||
| 241 | 70 | Yes | Yes | x | x | x | |||||||||||||
| 271 | 743 | No | Yes | x | x | x | x | ||||||||||||
| 451 | Undetectable | Yes | Yes | x | x | ||||||||||||||
| 521 | 181 | Not Performed | Yes | x | |||||||||||||||
| 591 | Undetectable | No | Yes | x | |||||||||||||||
| 621 | 62 | Yes | Yes | x | x | x | |||||||||||||
Abbreviations: ABC, abacavir; ARV, antiretroviral; ATV, atazanavir; DRV, darunavir; EFV, efavirenz; FPV, amprenavir; FTC, emtricitabine; IDV, indinavir; LPV, lopinavir; NFV, nelfinavir; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; NVP, nevirapine; RTV, ritonavir; SQV, saquinavir; TDF, tenofovir; TPV, tipranavir; ZDV, zidovudine; 3TC, lamivudine.
There were 35 participants (34 black MSM and 1 white MSM) who were classified as not aware on self-report and had a previous HIV diagnosis reported to the health department. The mean time between the first HIV diagnosis reported to the health department and the InvolveMENt study baseline visit was ∼4.4 years.
Based on self-report only, black MSM were significantly less likely to be aware of their HIV infection than were white MSM (Figure 1). Based on self-report combined with any of the other criteria, the proportions of black MSM not aware of their infection were not significantly higher than the proportions of white MSM not aware. Based on self-report and previous report to the health department, the proportions of black MSM and white MSM not aware of their infection was essentially the same (15% and 13%, respectively).
Figure 1.
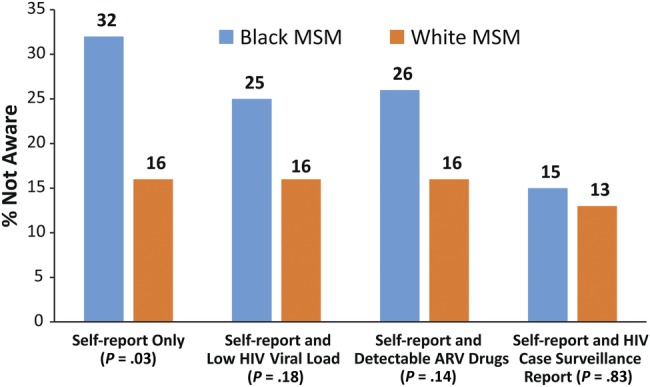
Lack of awareness of human immunodeficiency virus (HIV) serostatus among black and white men who have sex with men (MSM) in the InvolveMENt study, 2010–2012, using 3 approaches to defining lack of awareness. χ2 P values provided.
Of the 55 participants who did not self-report awareness and were invited to the in-depth interviews, 15 took part (13 black MSM and 2 white MSM). Twelve participants confirmed accuracy of their survey responses in regards to not being previously aware of their HIV infection, 2 of whom had detectable ARV. Three participants identified inaccuracies in their original survey responses: 1 reported that his most recent test was HIV-negative, but his survey response was “indeterminate”; 1 reported that his most recent test was HIV-positive, but his survey response was “didn't get result of most recent test”; and 1 was aware of his previous HIV diagnosis but had been retested and had not gotten the result of that most recent test. There were no issues noted in the comprehension of the HIV testing history questions.
DISCUSSION
Up to one half of our study participants who were not considered to be aware of their positive HIV status based on discordance between self-report and testing results may have actually been aware of their HIV infection. During qualitative interviewing with a subset of participants, we found some inconsistencies between survey responses and detailed questions about HIV testing history, but there was no evidence of systematic misinterpretation of testing questions or response options. When we used any of the alternative methods of defining awareness, the difference in awareness between black and white MSM was no longer significant.
Our study's sample size did not allow us to calculate sensitivity and specificity of the laboratory measures of awareness, but this framework may be useful to consider here. The specificity of ARV testing is likely very high as a measure of awareness of HIV status because there are probably few situations in which someone not aware of their status would have detectable ARV; one such possibility is use of HIV pre-exposure prophylaxis (PrEP) [21]. The Centers for Disease Control and Prevention guidance on PrEP use among MSM was released in the middle of our study, and although we added questions about current PrEP use in follow-up surveys, most participants were not asked this question in their baseline survey. The sensitivity of ARV detection as a measure of awareness is more questionable and likely dependent on many factors, especially being engaged in care and being adherent to an ARV regimen. The specificity of low VL as a measure of awareness is also likely high because, although it is possible that a person may have a low VL but not be aware of his HIV status (eg, an “HIV controller”), this situation is probably uncommon. Although there are no population-level studies of VL in treatment-naive persons, there was an 8% prevalence of VL <1000 copies/mL in reportedly treatment-naive participants in a large combined study of multiple prospective research cohorts of patients infected with HIV [22]. The prevalence of HIV controllers (>10 years of infection duration and 90% of VL <500 copies/mL) was estimated to be 0.22% in 1 large cohort study of persons infected with HIV in France [23]. Our findings suggest that the sensitivity of a low VL as a measure of awareness may be high because we found that all but 2 participants with detectable ARV had low VL. Future studies with larger samples of HIV-positive persons should explore further the sensitivity and specificity of these 2 measures separately and in combination.
Matching findings from research studies to HIV case surveillance reports may also be a viable means of estimating lack of awareness of HIV infection. Verification of whether a previous positive HIV test result was returned to a patient is not part of the surveillance case report. Men may have had a previous positive HIV test but not actually received the results of the test and therefore may not have been aware of their status, although there are factors that argue against this. Another study found that 90% of MSM get the results of their HIV test and did not find any significant racial difference in getting results [24]. Previous behavioral surveillance data have also reported that almost two-thirds of black MSM have had an HIV test in the past year [25], but the average time between first case surveillance report and our study's diagnosis was more than 4 years. Even if our participants did not get the result of that first reported diagnosis, the typical frequency of HIV testing among MSM makes it unlikely that they would have gone this length of time without another HIV test for which they got their test result.
It should also be noted that several participants confirmed their survey responses during the in-depth interviews, although results of ARV testing indicated that they were taking ARVs and therefore likely aware of their HIV status at the time of enrollment. These participants were also aware that researchers already knew of their status through the study-delivered testing. We did not have the results of all of the ARV testing at the time of these interviews, and participants were not specifically asked about possible discrepancies between the different measures of awareness. Regardless, these interviews still underscore that some part of the research process is creating an environment in which black MSM do not feel inclined to disclose knowledge of their HIV status to researchers. This may be due to HIV-related stigma or distrust in HIV research, both of which have been reported among black MSM [26–28].
The significant racial differences in self-reported awareness of HIV infection in our study is similar to that reported by NHBS-MSM [29]. The HIV testing history questions used in our study were the same as those used in the first cycle of NHBS-MSM, but more recent versions of the NHBS-MSM survey include an additional question about ever having had an HIV diagnosis. There was no significant change in self-reported awareness of HIV status between the first and second versions of the NHBS-MSM survey [30]; therefore, it is also unlikely that the addition of the more sensitive “ever positive” question would have substantially altered our findings. In addition, only 1 participant of the in-depth interviews reported awareness of his status based on ever having a positive HIV test result.
Human immunodeficiency virus case surveillance data estimates that 19.4% of MSM living with HIV infection in the United States have not yet been diagnosed [31]. Although these surveillance estimates should not be interpreted to be equivalent to lack of awareness of HIV status, the wide discrepancy between the most recent NHBS-MSM estimate (34%) and the surveillance-based estimate are problematic for public health [32]. Our study gives a potential explanation for this discrepancy: that self-report alone may overestimate lack of awareness among black MSM because of misclassification.
There is evidence from other research that underreporting of awareness to researchers is occurring. A recently published study from enrollment of black MSM in a community-randomized HIV prevention trial in 5 cities (including Atlanta) determined that among 155 HIV-positive black MSM who said that they were not aware of their HIV status, 54% had a VL of <1000 copies/mL and 78% of those had detectable ARVs [5, 16]. These results are substantially higher than those we observed in our Atlanta cohort of black and white MSM. That other study specifically recruited and enrolled black MSM who believed themselves to be HIV-negative, which may have produced the discrepancy between our findings. Underreporting of awareness by HIV-positive black MSM may also not be isolated to just the research environment. Other studies have reported that from one third to one half of HIV-positive black MSM do not disclose their serostatus to sexual partners [32, 33].
The current paradigm for racial disparity in HIV infection dictates that the higher rates of HIV acquisition among black MSM are due in part to (1) an increased likelihood for black MSM to have black male sex partners; (2) a higher rate of HIV prevalence among black MSM; and (3) a lower awareness of HIV status among HIV-positive black MSM [4, 6]. This third pillar of the paradigm is based on a premise that black MSM are less likely to be aware of their HIV status than are white MSM and are therefore less able act on this knowledge to protect their susceptible sexual partners from exposure to HIV. At a minimum, the magnitude of disparity in lack of awareness of HIV status for black MSM may be overstated with the use of only self-reported data. If, as our data suggest, black MSM are equally aware of their HIV status compared with white MSM, then the field would need to reconsider this paradigm and the HIV prevention programs upon which it is built.
Several limitations should be noted for this analysis. Our study involved incentivized research and had relatively small sample sizes for some groups. This not only limited our ability to conduct more detailed statistical analysis, but it would also limit generalizability, especially to other HIV testing programs or surveillance activities with MSM. Although the insights gained from the in-depth interviews were valuable and allowed for added verification of the quantitative findings, we also had a limited number of these interviews, and it is likely that those who agreed to an interview were different from those who refused, resulting in selection bias. A previous surveillance case report does not equal a person knowing their HIV status. The surveillance case match results were only provided on an aggregate level; therefore, we could not determine how laboratory and surveillance case-match measures aligned for individual participants. Finally, it should be acknowledged that another jurisdiction's public health regulations and policies may not allow for replication of the case-match approach.
CONCLUSIONS
Gaining knowledge of one's own HIV status will remain a cornerstone of HIV prevention, but additional research is clearly warranted to understand the context in which black MSM do or do not report awareness of their HIV status to researchers or to testing program personnel. This research should involve larger studies that apply multiple measures of determining awareness of status and that include qualitative interviews to specifically explore situations in which there is a discrepancy in these measures. Until a potential gold standard for awareness of HIV status can be determined from these larger studies, future HIV research that relies on the accuracy of this indicator should consider using multiple ways to measure it.
Acknowledgments
Author contributions. T. H. S. and N. L. conducted the quantitative analyses. R. C. collected the qualitative data, and T. H. S. and R. C. conducted the qualitative analyses. R. L. conducted the surveillance case match. S. T. and W. C. conducted the ARV testing, and C. F. K. conducted analysis and wrote these sections of the paper. E. R. and B. O. created the data structure and verified analyses. P. F., L. F. S., C. d. R., and P. S. S. designed the study. P. S. S. led the study. All authors contributed to the drafting of the manuscript and read and approved the final version.
Financial support. This work supported by the National Institute of Mental Health (grant R01MH085600); Minority Health and Health Disparities (grant RC1MD004370); Eunice Kennedy Shriver National Institute for Child Health and Human Development (grant R01HD067111); National Institutes of Health (grant P30AI050409)-the Emory Center for AIDS Research; and the National Center for Advancing Translational Sciences (grant UL1TR000454).
References
- 1.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–9. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006-2009. PloS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purcell DW, Johnson CH, Lansky A, et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. Open AIDS J. 2012;6:98–107. doi: 10.2174/1874613601206010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maulsby C, Millett G, Lindsey K, et al. HIV among Black men who have sex with men (MSM) in the United States: a review of the literature. AIDS Behav. 2014;18:10–25. doi: 10.1007/s10461-013-0476-2. [DOI] [PubMed] [Google Scholar]
- 5.Koblin BA, Mayer KH, Eshleman SH, et al. Correlates of HIV acquisition in a cohort of Black men who have sex with men in the United States: HIV prevention trials network (HPTN) 061. PloS One. 2013;8:e70413. doi: 10.1371/journal.pone.0070413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millett GA, Flores SA, Peterson JL, et al. Explaining disparities in HIV infection among black and white men who have sex with men: a meta-analysis of HIV risk behaviors. AIDS. 2007;21:2083–91. doi: 10.1097/QAD.0b013e3282e9a64b. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Diagnoses of HIV Infection in the United States and Dependent Areas. 2011. http://www.cdc.gov/hiv/library/reports/surveillance/2011/surveillance_Report_vol_23.html . Accessed 17 February 2014.
- 8.Georgia Department of Public Health. National HIV Behavioral Surveillance Secondary Data Report: Men Who Have Sex With Men, Cycle 3. http://dph.georgia.gov/sites/dph.georgia.gov/files/MSM3_Secondary%20Report.pdf . Accessed 17 February 2014.
- 9.Hall HI, Holtgrave DR, Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS. 2012;26:893–6. doi: 10.1097/QAD.0b013e328351f73f. [DOI] [PubMed] [Google Scholar]
- 10.Marks G, Crepaz N, Senterfitt JW, et al. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–53. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall HI, Frazier EL, Rhodes P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013;173:1337–44. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 13.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balaji AB, Bowles KE, Le BC, et al. High HIV incidence and prevalence and associated factors among young MSM, 2008. AIDS. 2013;27:269–8. doi: 10.1097/QAD.0b013e32835ad489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogel JM, Wang L, Parsons TL, et al. Undisclosed antiretroviral drug use in a multinational clinical trial (HIV Prevention Trials Network 052) J Infect Dis. 2013;208:1624–8. doi: 10.1093/infdis/jit390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzinke MA, Clarke W, Wang L, et al. Nondisclosure of HIV status in a clinical trial setting: antiretroviral drug screening can help distinguish between newly diagnosed and previously diagnosed HIV infection. Clin Infect Dis. 2014;58:117–20. doi: 10.1093/cid/cit672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacKellar DA, Gallagher KM, Finlayson T, et al. Surveillance of HIV risk and prevention behaviors of men who have sex with men--a national application of venue-based, time-space sampling. Public Health Rep. 2007;122(Suppl 1):39–47. doi: 10.1177/00333549071220S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. HIV infection and HIV-associated behaviors among injecting drug users - 20 cities, United States, 2009. MMWR Morb Mortal Wkly Rep. 2012;61:133–8. [PubMed] [Google Scholar]
- 19.Sullivan PS, Peterson J, Rosenberg ES, et al. Understanding racial HIV/STI disparities in black and white men who have sex with men: a multilevel approach. PloS One. 2014;9:e90514. doi: 10.1371/journal.pone.0090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung BH, Rezk NL, Bridges AS, et al. Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2007;21:1095–104. doi: 10.1002/bmc.865. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65–8. [PubMed] [Google Scholar]
- 22.Egger M, May M, Chêne G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 23.Grabar S, Selinger-Leneman H, Abgrall S, et al. Prevalence and comparative characteristics of long-term nonprogressors and HIV controller patients in the French Hospital Database on HIV. AIDS. 2009;23:1163–9. doi: 10.1097/QAD.0b013e32832b44c8. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan PS, Lansky A, Drake A. Failure to return for HIV test results among persons at high risk for HIV infection: results from a multistate interview project. J Acquir Immune Defic Syndr. 2004;35:511–8. doi: 10.1097/00126334-200404150-00009. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. HIV testing among men who have sex with men--21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep. 2011;60:694–9. [PubMed] [Google Scholar]
- 26.Radcliffe J, Doty N, Hawkins LA, et al. Stigma and sexual health risk in HIV-positive African American young men who have sex with men. AIDS Patient Care and STDs. 2010;24:493–9. doi: 10.1089/apc.2010.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smit PJ, Brady M, Carter M, et al. HIV-related stigma within communities of gay men: a literature review. AIDS Care. 2012;24:405–12. doi: 10.1080/09540121.2011.613910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchinson AB, Begley EB, Sullivan P, et al. Conspiracy beliefs and trust in information about HIV/AIDS among minority men who have sex with men. J Acquir Immune Defic Syndr. 2007;45:603–5. doi: 10.1097/QAI.0b013e3181151262. [DOI] [PubMed] [Google Scholar]
- 29.Wejnert C, Le B, Rose CE, et al. HIV infection and awareness among men who have sex with men-20 cities, United States, 2008 and 2011. PloS One. 2013;8:e76878. doi: 10.1371/journal.pone.0076878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Prevalence and awareness of HIV infection among men who have sex with men --- 21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59:1201–7. [PubMed] [Google Scholar]
- 31.Chen M, Rhodes PH, Hall IH, et al. Prevalence of undiagnosed HIV infection among persons aged >/=13 years--National HIV Surveillance System, United States, 2005-2008. MMWR Morb Mortal Wkly Rep. 2012;61(suppl):57–64. [PubMed] [Google Scholar]
- 32.Tieu HV, Xu G, Bonner S, et al. Sexual partner characteristics, serodiscordant/serostatus unknown unprotected anal intercourse and disclosure among human immunodeficiency virus-infected and uninfected black men who have sex with men in New York City. Sex Transm Dis. 2011;38:548–54. doi: 10.1097/OLQ.0b013e318203e2d7. [DOI] [PubMed] [Google Scholar]
- 33.Winter AK, Sullivan PS, Khosropour CM, Rosenberg ES. Discussion of HIV status by serostatus and partnership sexual risk among internet-using MSM in the United States. J Acquir Immune Defic Syndr. 2012;60:525–9. doi: 10.1097/QAI.0b013e318257d0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]


