Norovirus outbreak may spread among hospitals via patient sharing. Within 30 days, it spread 4.1-17.5% of the time (R0 3.74). Highlights need to cooperatively track and control outbreaks
Keywords: hospitals, intervention, norovirus, outbreak
Abstract
Background
Because hospitals in a region are connected via patient sharing, a norovirus outbreak in one hospital may spread to others.
Methods.
We utilized our Regional Healthcare Ecosystem Analyst software to generate an agent-based model of all the acute care facilities in Orange County (OC), California and simulated various norovirus outbreaks in different locations, both with and without contact precautions.
Results.
At the lower end of norovirus reproductive rate (R0) estimates (1.64), an outbreak tended to remain confined to the originating hospital (≤6.1% probability of spread). However, at the higher end of R0 (3.74), an outbreak spread 4.1%–17.5% of the time to almost all other OC hospitals within 30 days, regardless of the originating hospital. Implementing contact precautions for all symptomatic cases reduced the probability of spread to other hospitals within 30 days and the total number of cases countywide, but not the number of other hospitals seeing norovirus cases.
Conclusions.
A single norovirus outbreak can continue to percolate throughout a system of different hospitals for several months and appear as a series of unrelated outbreaks, highlighting the need for hospitals within a region to more aggressively and cooperatively track and control an initial outbreak.
BACKGROUND
Norovirus is a highly contagious source of viral gastroenteritis and is associated with extensive community and healthcare outbreaks and an estimated 21 million cases of sporadic disease annually in the United States [1, 2]. Its rapid transmissibility and short incubation period make it a serious [3, 4] and costly [3, 4] problem in healthcare settings. In the United States, reports of healthcare-associated outbreaks have been largely limited to the experience of a single reporting hospital [4–8]. However, our previous work has shown how hospitals are extensively connected to each other by patient sharing [9, 10], raising the possibility that a norovirus outbreak in one hospital could spread to others. Indeed, the existence of asymptomatic yet infectious individuals and prolonged virus shedding could allow patients contracting the virus to then carry the pathogen to other facilities. The implications of patient sharing across healthcare facilities on outbreak control measures remain unclear [11, 12]. Therefore, to determine whether and how an outbreak can spread to other facilities and the potential implications for norovirus control measures, we utilized our Regional Healthcare Ecosystem Analyst (RHEA) software to perform an agent-based simulation model (ABM) of the acute care hospitals in Orange County (OC), California.
METHODS
We used our previously described RHEA software [13, 14], and our OC ABM, a detailed virtual representation of all 29 acute care hospitals (including 5 long-term acute care [LTAC] facilities) in OC serving adult patients and the simulated virtual patients (ie, agents) admitted to and discharged from these hospitals and transferring between them (admissions and discharges to and from other hospitals) and the community on each simulated day [13–19]. This model uses parameters primarily derived from actual extensive data sources of OC, including hospital bed capacity, number of patients admitted to and discharged from each hospital, patient length of stay, and the distribution and locations of interfacility patient transfers. Our knowledge of these transfer distributions and locations included those that occurred directly between facilities as well as those that occurred with an intervening stay at home or elsewhere. Table 1 summarizes the relevant input parameters for our study.
Table 1.
Model Inputs and Hospital Characteristics
| Parameter | Median | Range | Source |
|---|---|---|---|
| Orange County Hospital Characteristics | |||
| Annual admissions | 6932 | 388–26 292 | [40] |
| Average daily census | 102 | 17–364 | [40] |
| Average length of stay (days) | 5.4 | 3.3–37.2 | [40] |
| Number of hospital readmissions within 365 days | 2214 | 187–8329 | [40] |
| Norovirus Characteristics | |||
| Probability of asymptomatic infectiona | 0.337 | 0.309–0.365 | [20, 21] |
| Low R0b | 1.64 | [24] | |
| High R0 | 3.74 | 3.2–4.3 | [29] |
| Incubation period (days) | 1–2 | [12] | |
| Duration of viral shedding (infectious period; days) | 14 | [38, 41–43] | |
| Duration of symptoms (days) | 3–6 | [12, 44] | |
| Duration of immunity (years)b | 5.1 | 4.0–6.7 | [24] |
a Among those exposed.
b For referenced Model B.
Norovirus Disease States and Transmission
The model simulates 4 possible states for each virtual patient (agent) and the daily probabilities of changing from one state to another. These states are mutually exclusive: Susceptible (S; not infected with norovirus but able to become infected), Exposed (E; exposed and infected but not yet able to transmit to others), Infectious (I; infected and able to transmit to others), Recovered (R; not infected with norovirus and immune to symptomatic disease, but susceptible to asymptomatic infection). A patient in the S state has a probability of moving to the E state, representing the chance that exposure leads to an infected patient who is incubating the virus (ie, not all patients clinically exposed to an infectious individual will move to the E state). Once in the E state, the patient would progress to the I state after the incubation period elapsed. The duration of this incubation period for a given patient results from a draw from the incubation duration distribution, as shown in Table 1. Likewise, a patient remained in the I state for the infectious period, determined by a draw from the duration distribution shown in Table 1.
An infectious patient would have a probability of being symptomatic versus asymptomatic (likeliest: 33.7% [20, 21]). Viral shedding or the duration of infectiousness (ie, how long an infected person can transmit the virus to others) was assumed to be the same for both symptomatic and asymptomatic patients [11]. However, symptomatic patients were not discharged until their symptoms resolved, whereas asymptomatic patients could be discharged or transferred to another hospital (due to unrelated reasons and occurs at the actual rate for known OC data), thus allowing for potential transmission of norovirus once he/she leaves the hospital. Once the duration of infectiousness elapsed, patients moved to the R state, where they remained immune from disease (ie, symptomatic norovirus) for the duration of immunity (Table 1). Although the duration of natural immunity to norovirus has been reported to be between 6 months and 2 years, these estimates come from highly unnatural challenge studies [22, 23], and a recent modeling study found immunity to norovirus gastroenteritis (ie, symptomatic norovirus, not asymptomatic infection) to be 4.1 to 8.7 years [24]. In addition, we assumed that the R state was protective of disease but not infection [24, 25]; therefore, patients in the R state could become asymptomatically infected, transitioning between states as described above, and transmit to others.
Norovirus transmission occurred within each hospital general ward and intensive care unit (ICU) where patients mixed homogenously within their respective wards. Regarding patient transfers between wards, only the transfer of ICU patients to general wards was modeled, and no other interaction occurred between wards. The Reed-Frost formula [26] determined a susceptible individual's infection risk as
where p is the probability of an effective contact between 2 specific individuals each day (t) and is assumed to be the same as β for a single day time step and I is the number of infectious individuals within each ward. The parameter β is a ward's transmission coefficient, calculated from the reproductive number (R0; the number of secondary cases generated by an infected individual in a completely susceptible population), the infectious period duration, and the ward size using the following formula:
Evidence has shown that asymptomatically infected individuals contribute to transmission at a lower rate than those with symptomatic infection [27]. We therefore assumed that asymptomatic patients were half (50%) as infectious as symptomatic patients.
Experiments
Although highly unlikely, the initial set of experiments simulated an unmitigated (ie, no intervention) norovirus outbreak in a single hospital, with different scenarios choosing the largest (Hospital A), median-sized (Hospital B), and smallest non-LTAC hospital (Hospital C) based on annual admissions. In the next set of experiments, we implemented contact isolation control measures (ie, mitigated) to attempt to control these outbreaks. These control measures consisted of placing symptomatic patients under contact isolation precautions (ie, the use of gloves and gowns) from the first day of symptoms until 2 simulated days after symptom resolution per the Centers for Disease and Control and Prevention guidance [11, 28]. The effectiveness of the intervention on transmission is equal to the efficacy of contact precautions multiplied by staff compliance with contact precautions. For mitigated outbreaks, an individual's infection risk at time t was computed from
where p was again assumed to be β, pCP is β*(1- contact precaution effectiveness), CP indicates those on contact precautions, and NCP indicates those not on contact precautions. The total number of infectious (It) is the sum of ICP and INCP.
Each simulation experiment (day 0) began with all patients being susceptible, except for the introduction of a single infected patient in a random ward in the target hospital experiencing the outbreak. An additional scenario began with 27% of patients immune [25] from infection and starting in the R state. For each scenario, we conducted a total of 100 000 simulation runs. Sensitivity analysis explored the impact of the R0 distribution, low R0 was a constant value of 1.64, and high R0 was a triangular distribution with likeliest value 3.74 (Table 1) [27, 29]. We also varied the effectiveness (combination of compliance and efficacy) of contact precautions from 50% to 60%. To evaluate the role of asymptomatic transmission, we utilized the same transmission rate for asymptomatic and symptomatic patients. Each experimental run continued for 5 simulated years.
RESULTS
Unmitigated Outbreak
When R0 was low (ie, 1.64) and 100% of the population was susceptible, the outbreak tended to remain within the originating hospital: the probability of spreading was only 1%–1.2%, 1.6%–4.2%, and 2.1%–6.1% for outbreaks in Hospital A, B, and C, respectively, within 14 and 30 days. Countywide, no norovirus cases remained after outbreaks in any of the hospitals after 5.5 months (168 days).
High R0 (ie, likeliest value 3.74) outbreaks spread throughout the county 0.8%–3.7% of the time within 14 days and 4.1%–17.5% of the time within 30 days (100% of population susceptible). Although the likelihood of spreading to other hospitals may not be high, it spread diffusely, leaving only a few hospitals norovirus free. Within 14 days, 22–24 other OC hospitals had norovirus. Table 2 shows how the number of cases change with time (in months) after the initial outbreak.
Table 2.
Impact of Unmitigated and Mitigated Norovirus Outbreaks With a High R0 (R0 Triangular Distribution = 3.20, 3.74, 43.0) in the Largest (A), Median-Sized (B), and Smallest Hospital (C) (Based on Annual Admissions) on Orange County Hospitals
| Outbreak Hospital | Time After Initial Outbreak (Months) |
||||
|---|---|---|---|---|---|
| 1 | 3 | 6 | 9 | 12 | |
| Average Total Number of Norovirus Cases Countywide Within Time Interval | |||||
| Unmitigated | |||||
| Hospital A | 17.3 | 47.7 | 77.7 | 100.6 | 123.6 |
| Hospital B | 15.4 | 39.5 | 64.8 | 87.5 | 112.7 |
| Hospital C | 19.8 | 75.3 | 177.2 | 294.2 | 428.8 |
| Mitigated (Contact Precaution Effectiveness = 50%) | |||||
| Hospital A | 10.0 | 18.2 | 20.7 | 21.2 | 21.3 |
| Hospital B | 8.9 | 14.7 | 16.3 | 16.8 | 16.9 |
| Hospital C | 12.0 | 29.9 | 41.1 | 45.0 | 46.4 |
| Mitigated (Contact Precaution Effectiveness = 60%) | |||||
| Hospital A | 7.7 | 11.2 | 11.8 | 11.9 | 11.9 |
| Hospital B | 7.7 | 11.3 | 11.9 | 11.9 | 12.0 |
| Hospital C | 10.4 | 22.5 | 27.3 | 28.2 | 28.4 |
Assuming that the population was 100% susceptible at the start of the simulation, an outbreak in the largest hospital (Hospital A) spread to other hospitals in 0.8% of the simulation runs with the high R0. This 0.8% probability of spread was within 14 days (duration of viral shedding) and affected 22 hospitals. Within 30 days, the probability of the initial outbreak spreading was 4.1% and 27 other OC hospitals were affected. The downstream effects of this spread and the propagation of the outbreak due to secondary transmission were substantial. Table 2 shows the countywide effects of the unmitigated outbreak with a high R0 several months after the initial outbreak. When 27% of the population started the simulation with natural immunity, the magnitude of the outbreak was smaller, but it still had a regional impact. Within 14 days the outbreak spread 0.7% of the time, reaching 22 other hospitals, and within 30 days it spread 3.8% of the time, reaching 27 other hospitals. After 1 month, there were a total of 15.6 cases countywide per initial outbreak, 41.6 cases within 3 months, 66.6 within 6 months, and 105.3 within 1 year. Setting asymptomatic transmission equal to that of symptomatic patients (i.e., equally infectious) increased the probability and speed of norovirus spread to other hospitals. The initial outbreak spread within 14 days in 0.9% of simulations and within 30 days in 7.0% of simulations (compared to 0.8% and 4.1%, respectively).
For an unmitigated outbreak in Hospital B (100% of population susceptible), the probability of spread was 2.7% within 14 days and 11.3% within 30 days, spreading to 23 and 27 other hospitals, respectively. Again, Table 2 shows how the average total number of cases countywide per outbreak changes over time. Norovirus was more likely to spread when patients were equally infectious, increasing the probability of spread to 3.8% within 14 days and 7.0% within 30 days.
An outbreak in the smallest hospital spread to other hospitals within 14 days 3.7% of the time, reaching 24 other hospitals (100% of population susceptible). Within 30 days the initial outbreak spread 17.5% of the time, reaching all 28 other OC hospitals. An outbreak in the smallest hospital had a larger impact (generated more cases) than the outbreaks in either Hospital A or B (Table 2). Although this is the smallest non-LTAC hospital in OC, it directly transfers a greater proportion of its admissions (3.4%) than the other target hospitals (0.3% and 1.4%), thereby increasing the likelihood of transferring an exposed patient and spreading norovirus beyond the originating hospital. When asymptomatic and symptomatic patients were equally infectious, the initial outbreak was more likely to spread (5.4% and 30.2% of the time within 14 and 30 days, respectively).
Figures 1 and 2 show the impact of R0 on the spread of norovirus outbreaks throughout the region (100% susceptible, asymptomatic half as infectious). Figure 1 depicts the likelihood of spread to other hospitals and the number of hospitals affected. The left axis shows the probability that an outbreak will spread from the initial hospital, and the right axis shows the number of other OC hospital with norovirus cases after it spread, for different values of R0. As expected, increasing R0 increased the likelihood of spread beyond the originating hospital, but the increase was more dramatic within 30 days than within 14 days (Figure 1). The likelihood of spread also increased between day 14 and 30, because more norovirus cases in the originating hospital have the potential to be transferred to another hospital. Although all 3 outbreaks tended to affect most of OC's hospitals, outbreaks in Hospital C (Figure 1c) tended to spread more often, having a 26.6% probability of spreading given an R0 of 5, affecting all 28 other OC hospitals. Figure 2 shows the magnitude of spread, depicting the average total number of norovirus cases countywide within 1, 3, and 6 months when an outbreak occurs. As can be seen, the amount of subsequent transmission was substantial, leading to 409 (Hospital A) to 1097 (Hospital C) cases countywide within 6 months of an outbreak with an R0 of 5. This rate more than doubled (2686–9106) within 1 year. Even lower R0 values generated a large number of cases; our high R0 value estimate (3.74) generated 71.2–162 total countywide within 6 months of the initial outbreak (Figure 2).
Figure 1.
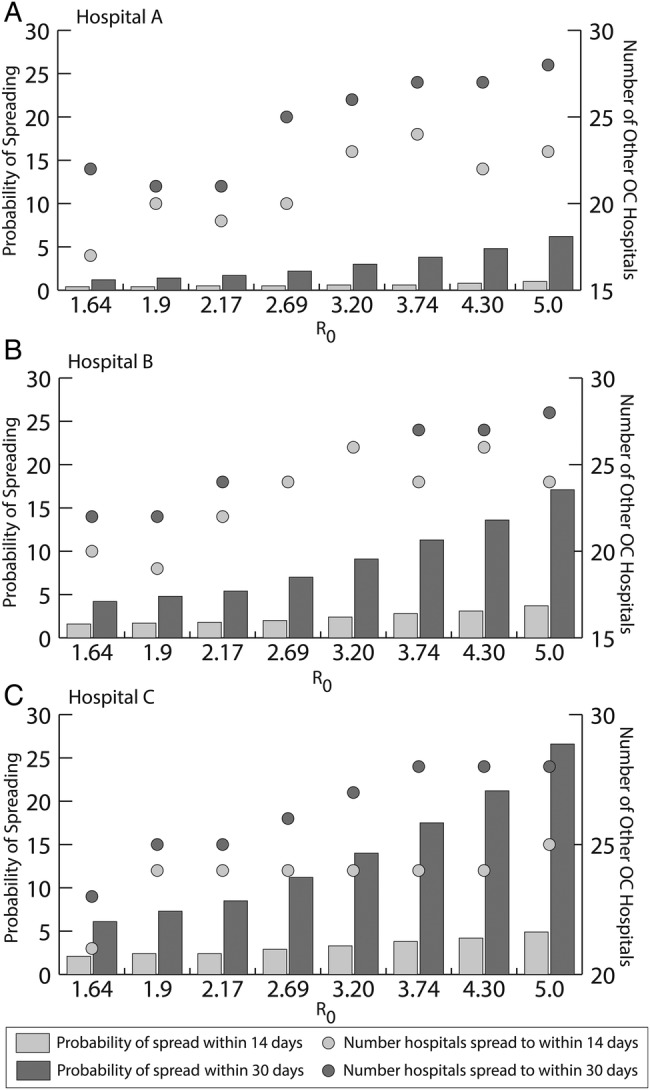
Impact of R0 on the probability of spread to other hospitals and the maximal number of other hospitals affected within 14 days and 30 days for unmitigated outbreaks in Hospital A, B, and C. (Note: The same number of hospitals are affected for an R0 of 2.69 and 3.20 within 14 and 30 days for an outbreak in Hospital B.)
Figure 2.
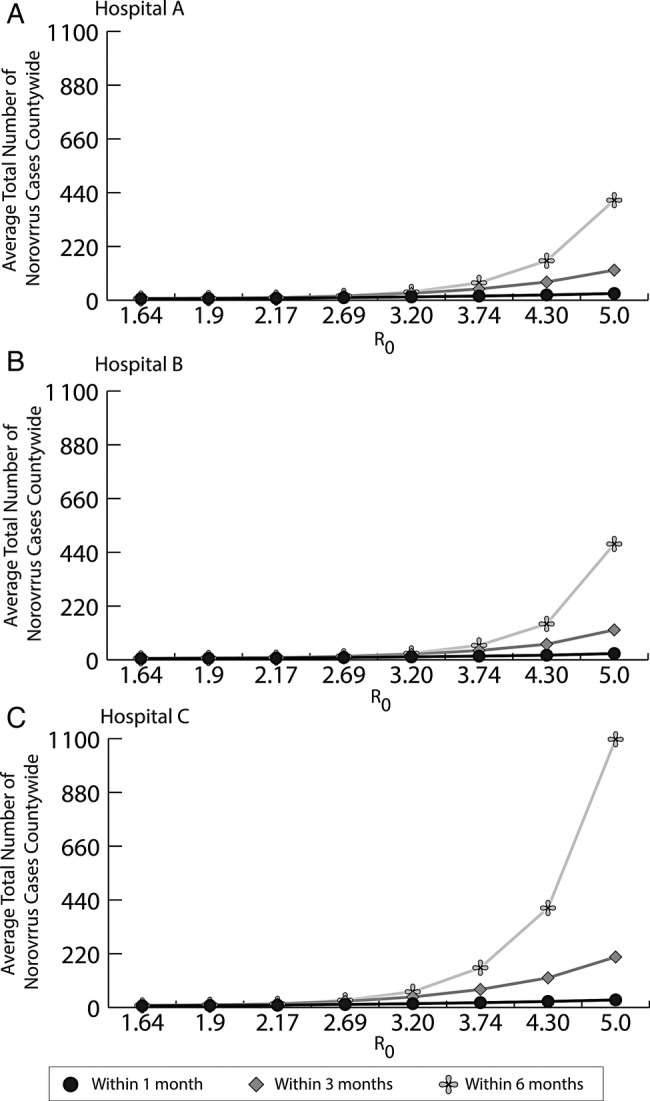
Impact of R0 on the average total number of norovirus cases countywide after unmitigated outbreaks in Hospitals A, B, and C within 1, 3, and 6 months of the initial outbreak.
Use of Contact Precautions
The use of contact precautions for all symptomatic cases limited the spread of norovirus to other OC hospitals (assuming 100% of population susceptible and asymptomatic patients are half as infectious as symptomatic patients). Although the norovirus outbreaks still spread to other hospitals, the probability of spread was reduced. In Hospital A, 0.5% of simulations spread within 14 days, regardless of contact precaution effectiveness; within 30 days, 2.6% (50% effectiveness) and 2.4% (60% effectiveness) of simulations spread. This is a 1.6- to 2-fold reduction in spread to other hospitals. However, once norovirus got out of the originating hospital, it still spread to most of the other OC hospitals. Both Hospital B and C saw a 1.2-fold decrease in spread within 14 days and a 1.4-fold decrease within 30 days when contact precautions were in place, with an effectiveness of 50% or 60%.
Table 2 shows how the average total number of cases countywide (per outbreak) decreased for the different levels of contact precaution effectiveness. The largest benefits of contact precautions were on reducing the overall transmission (eg, primary and secondary transmission) and limiting the further accumulation of cases. For some outbreaks, the number of cases levels off between 3 and 6 months, with contact precaution effectiveness of 60% (Table 2). This result occurs when the initial spread of the outbreak has reached its maximum, but there is still transmission due to secondary cases and a few lingering cases are generated.
DISCUSSION
Our study shows that unmitigated outbreaks of a highly contagious pathogen (ie, high attack rate) with a short incubation period can affect multiple hospitals across a county. Norovirus outbreaks can quickly spread beyond the initial hospital, when they do spread, with other hospitals experiencing ramifications within 14 to 30 days. This activity is especially important given the frequency of norovirus outbreaks, which account for 89% (1419 of 2259) of all person-to-person acute gastroenteritis outbreaks reported with an etiology to the National Outbreak Reporting System in the United States from 2009 to 2010 and causing 59% (32–54) of those outbreaks occurring in hospitals [30]. Surveys of US hospital infection preventionists (in 289 hospitals) reported 386 outbreaks, of which norovirus caused 18% [31]. As a result of this spread, multiple sequential outbreaks in different facilities could actually be just the ongoing manifestation of a single initial outbreak in 1 facility. This means that better identification and control measures at the origin of the outbreak could prevent considerably more cases than originally thought. A prime reason for dissemination beyond the initial location is the previously described extensive patient sharing that occurs among different facilities in a region [9, 10]. This patient sharing includes both direct transfers and patients returning to the community before being subsequently readmitted to another facility. The sharing can cross many miles and involve multiple facilities. The highest volume of sharing does not necessarily occur between hospitals that are geographically closest to each other. Although having an unmitigated norovirus outbreak is highly unlikely, modeling this situation is important because we can learn about the R0 at which norovirus will spread beyond a single facility and the magnitude and speed at which this can occur. The smallest hospital in OC (Hospital C) had the largest effect (of all the tested scenarios) on other hospitals in the region, because a larger fraction of its admitted patients (3.4%) are directly transferred to other OC hospitals compared to the Hospitals A and B (0.3% and 1.4%, respectively).
We also demonstrate that contact precautions (the most practical means of interrupting transmission [28]) can be instrumental in mitigating the outbreak when applied rapidly, even at 50% effectiveness. However, even when controlled with the use of contact precautions, norovirus could still spread and breakthrough multihospital outbreaks can still occur. Evidence shows that norovirus prevention and containment methods during outbreaks vary, with an increased use of gloves and gowns applied in only 9.3% of outbreaks [32]. Contact precautions should be put in place rapidly during outbreaks, and efforts should be made to ensure high compliance [11, 28]. In addition, temporary universal preemptive contact precautions [33] in an affected unit may be able to accelerate containment, although we did not model this scenario. Other commonly used concomitant interventions, such as environmental cleaning, have been shown to be essential to containing outbreaks but were not modeled here [32].
Although asymptomatic infectious patients can elude identification, prevention strategies can still mitigate norovirus outbreaks. Control measures, such as environmental cleaning of an infected ward, hand hygiene, and isolating those who may have contacted known infectious individuals, ensure communication of patients who require contact precautions on transfer, and ward closure may help to reduce spread beyond the initial hospital. In practice, many infection-control measures are used to manage and quell a norovirus outbreak [4, 8, 34], and patient sharing should be taken into consideration when deciding on which measures to apply. This process can be facilitated by improved communication and better awareness of where patients are from and their infection status, which can help implement appropriate control measures. In addition, hospital personnel should be aware of any ongoing outbreaks in hospitals to which they are highly connected (ie, they share a lot of patients) and the patients they receive from these hospitals.
Our study showed how R0 determines the likelihood of spread beyond an initial facility, emphasizing the importance of knowing this value better. Although we studied a conservative range for R0, the literature reports a wide range for the norovirus R0, with 1 study reporting a minimum of 1.20 [35] and another a maximum of 7.26 [36]. This wide variability may be due to either the variability in study method or true variation in the R0 of norovirus strains [37]. The R0 depends on a number of different factors, including strain, viral shedding, contact patterns, and virulence of that virus. In addition, R0 may be different between healthcare settings and the community, where the number and intimacy or proximity of contacts and other environmental exposures may vary. By better characterizing these factors and how they may influence the value of R0, we could help to identify more effective control measures.
Our model may be conservative about the spread of norovirus. The duration of viral shedding is highly variable and has been reported to last up to 8 weeks [38]. Our model assumed an infectious duration of 14 days. We assumed contact precautions to have an overall effectiveness of 50% or 60%; however, this rate is highly variable across institutions, because it depends on compliance as well as efficacy. In future studies of norovirus outbreaks, an attempt should be made to better quantify (1) the role of asymptomatic carriers, because they have high potential to spread disease, and (2) the value of R0. As our study shows, norovirus' R0 has an impact on an outbreak's spread among hospitals.
Limitations
Models, by definition, are simplifications of real life, and as such they cannot account for every possible factor [39]. Although a vast majority of patients (87%) in OC stay within the county for hospital care, some do cross county lines. Our study focused on adults and did not include pediatric hospital or patients. Although our model does not directly consider hospital staff, the transmission coefficients incorporated contact among patients as well as contact mediated by healthcare workers, staff, and objects such as surfaces. In addition, our model assumes the duration of viral shedding to be the same in all infected patients regardless of symptoms. Other more stringent interventions (eg, unit closure, restricting patient movements, or admissions) were not undertaken and would have likely further mitigated an outbreak.
CONCLUSIONS
During an unmitigated outbreak, norovirus can readily spread among healthcare facilities in a region due to patient sharing and can percolate throughout the region, appearing as unrelated outbreaks. Even outbreaks in small hospitals had a large impact. Infection control strategies such as contact precautions (even at 50% compliance) can help mitigate the effects, but these strategies alone may not completely stop the spread of norovirus. R0 and asymptomatic infectiousness (compared to symptomatic patients) determined the likelihood of norovirus spread, emphasizing the importance of knowing these values better. Temporary universal contact precautions or a higher compliance with contact precautions along with enhanced environmental cleaning could be essential to ensuring containment across a variety of hospitals.
Notes
Disclaimer. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Financial support. This study was supported by the National Institute of Allergy and Infectious Diseases (Grant 1RC4A1092327-01); the Pennsylvania Department of Health; and the National Institute of General Medical Sciences Models of Infectious Disease Agent Study (Grants 1U54GM088491-0109 and 1U01 GM076672). This research was supported in part by the University of Pittsburgh Center for Simulation and Modeling through the supercomputing resources provided.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States - major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall AJ, Rosenthal M, Gregoricus N, et al. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004–2005. Emerg Infect Dis. 2011;17:1381–8. doi: 10.3201/eid1708.101533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee BY, McGlone SM, Bailey RR, et al. Economic impact of outbreaks of norovirus infection in hospitals. Infect Control Hosp Epidemiol. 2011;32:191–3. doi: 10.1086/657910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston CP, Qiu H, Ticehurst JR, et al. Outbreak management and implications of a nosocomial norovirus outbreak. Clin Infect Dis. 2007;45:534–40. doi: 10.1086/520666. [DOI] [PubMed] [Google Scholar]
- 5.Weber DJ, Sickbert-Bennett E, Vinje J, et al. Lessons learned from a norovirus outbreak in a locked pediatric inpatient psychiatric unit. Infect Control Hosp Epidemiol. 2005;26:841–3. doi: 10.1086/502504. [DOI] [PubMed] [Google Scholar]
- 6.Doshi M, Woodwell S, Kelleher K, et al. An outbreak of norovirus infection in a bone marrow transplant unit. Am J Infect Control. 2012;41:820–3. doi: 10.1016/j.ajic.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Turcios-Ruiz RM, Axelrod P, St. John K, et al. Outbreak of necrotizing enterocolitis caused by norovirus in a neonatal intesive care unit. J Pediatr. 2008;153:339–44. doi: 10.1016/j.jpeds.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu HM, Fornek M, Schwab KJ, et al. A norovirus outbreak at a long-term-care facility: the role of environmental surface contamination. Infect Control Hosp Epidemiol. 2005;26:802–10. doi: 10.1086/502497. [DOI] [PubMed] [Google Scholar]
- 9.Lee BY, McGlone SM, Song Y, et al. Social network analysis of patient sharing among hospitals in Orange County, California. Am J Public Health. 2011;101:707–13. doi: 10.2105/AJPH.2010.202754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BY, Song Y, Bartsch SM, et al. Long-term care facilities: important participants of the acute care facility social network? PLoS One. 2011;6:e29342. doi: 10.1371/journal.pone.0029342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacCannell T, Umscheid CA, Agarwal RK, et al. Guideline for prevention and management of norovirus outbreaks in healthcare settings. Infect Control Hosp Epidemiol. 2011;32:939–69. doi: 10.1086/662025. [DOI] [PubMed] [Google Scholar]
- 12.Patel MM, Hall AJ, Vinje J, et al. Noroviruses: a comprehensive review. J Clin Virol. 2009;44:1–8. doi: 10.1016/j.jcv.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Lee BY, McGlone SM, Wong KF, et al. Modeling the spread of methicillin-resistant Staphylococcus aureus (MRSA) outbreaks throughout the hospitals in Orange County, California. Infect Control Hosp Epidemiol. 2011;32:562–72. doi: 10.1086/660014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee BY, Wong KF, Bartsch SM, et al. The Regional Healthcare Ecosystem Analyst (RHEA): simulation modeling tool to assist infectious disease control in a health system. J Am Med Inform Assoc. 2013;20:e139–46. doi: 10.1136/amiajnl-2012-001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang SS, Avery TR, Song Y, et al. Quantifying interhospital patient sharing as a mechanism for infectious disease spread. Infect Control Hosp Epidemiol. 2010;31:1160–9. doi: 10.1086/656747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BY, Bartsch SM, Wong KF, et al. Simulation shows hospitals that cooperate on infection control obtain better results than hospitals acting alone. Health Affairs. 2012;31:2295–303. doi: 10.1377/hlthaff.2011.0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee BY, Singh A, Bartsch SM, et al. The potential regional impact of contact precaution use in nursing homes to control methicillin resistant Staphylococcus Aureus (MRSA) Infect Control Hosp Epidemiol. 2013;34:151–60. doi: 10.1086/669091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BY, Bartsch SM, Wong KF, et al. The importance of nursing homes in the spread of methicillin-resistant Staphylococcus aureus (MRSA) among hospitals. Med Care. 2013;51:205–15. doi: 10.1097/MLR.0b013e3182836dc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee BY, Yilmaz SL, Wong KF, et al. Modeling the regional spread and control of vancomycin-resistant enterococci (VRE) Am J Infect Control. 2013;41:668–73. doi: 10.1016/j.ajic.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham DY, Jiang X, Tanaka T, et al. Norwalk virus infection of volunteers: new insights based on improved assays. J Infect Dis. 1994;170:34–43. doi: 10.1093/infdis/170.1.34. [DOI] [PubMed] [Google Scholar]
- 21.Gray JJ, Cunliffe C, Ball J, et al. Detection of immunoglobulin M (IgM), IgA, and IgG Norwalk virus-specific antibodies by indirect enzyme-linked immunosorbent assay with baculovirus-expressed Norwalk virus capsid antigen in adult volunteers challenged with Norwalk virus. J Clin Microbiol. 1994;32:3059–63. doi: 10.1128/jcm.32.12.3059-3063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parrino TA, Schreiber DS, Trier JS, et al. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N Engl J Med. 1977;297:86–9. doi: 10.1056/NEJM197707142970204. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PC, Mathewson JJ, DuPont HL, et al. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J Infect Dis. 1990;161:18–21. doi: 10.1093/infdis/161.1.18. [DOI] [PubMed] [Google Scholar]
- 24.Simmons K, Gambhir M, Leon J, et al. Duration of immunity to norovirus gastroenteritits. Emerg Infect Dis. 2013;19:1260–7. doi: 10.3201/eid1908.130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopman B, Simmons K, Gambhir M, et al. Epidemiologic implications of asymptomatic reinfection: a mathematical modeling study of norovirus. Am J Epidemiol. 2014;179:507–12. doi: 10.1093/aje/kwt287. [DOI] [PubMed] [Google Scholar]
- 26.Vynnycky E, White RG. An Introduction to Infectious Disease Modelling. New York: Oxford University Press; 2010. [Google Scholar]
- 27.Sukhrie FH, Teunis P, Vennema H, et al. Nosocomial transmission of norovirus is mainly caused by symptomatic cases. Clin Infect Dis. 2012;54:931–7. doi: 10.1093/cid/cir971. [DOI] [PubMed] [Google Scholar]
- 28.Hall AJ, Vinje J, Lopman B, et al. Updated norovirus outbreak management and disease prevention guidelines. MMWR Recomm Rep. 2011;60:1–18. [PubMed] [Google Scholar]
- 29.Vanderpas J, Louis J, Reynders M, et al. Mathematical model for the control of nosocomial norovirus. J Hosp Infect. 2009;71:214–22. doi: 10.1016/j.jhin.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Wikswo ME, Hall AJ. Outbreaks of acute gastroenteritis transmitted by person to person contact - United States, 2009–2010. MMWR Surveill Summ. 2012;61:1–12. [PubMed] [Google Scholar]
- 31.Rhinehart E, Walker S, Murphy D, et al. Frequency of outbreak investigations in US hospitals: results of a national survey of infection preventionists. Am J Infect Control. 2012;40:2–8. doi: 10.1016/j.ajic.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Greig JD, Lee MB. A review of nosocomial norovirus outbreaks: infection control interventions found effective. Epidemiol Infect. 2012;140:1151–60. doi: 10.1017/S0950268811002731. [DOI] [PubMed] [Google Scholar]
- 33.Hansen S, Stamm-Balderjahn S, Zuschneid I, et al. Closure of medical departments during nosocomial outbreaks: data from a systematic analysis of the literature. J Hosp Infect. 2007;65:348–53. doi: 10.1016/j.jhin.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zingg W, Colombo C, Jucker T, et al. Impact of an outbreak of norovirus infection on hospital resources. Infect Control Hosp Epidemiol. 2005;26:263–7. doi: 10.1086/502537. [DOI] [PubMed] [Google Scholar]
- 35.Heijne JC, Rondy M, Verhoef L, et al. Quantifying transmission of norovirus during an outbreak. Epidemiology. 2012;23:277–84. doi: 10.1097/EDE.0b013e3182456ee6. [DOI] [PubMed] [Google Scholar]
- 36.Heijne JC, Teunis P, Morroy G, et al. Enhanced hygiene measures and norovirus transmission during an outbreak. Emerg Infect Dis. 2009;15:24–30. doi: 10.3201/1501.080299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopman B, Vennema H, Kohli E, et al. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet. 2004;363:682–8. doi: 10.1016/S0140-6736(04)15641-9. [DOI] [PubMed] [Google Scholar]
- 38.Atmar RL, Opekun AR, Gilger MA, et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis. 2008;14:1553–7. doi: 10.3201/eid1410.080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee BY. Digital decision making: computer models and antibiotic prescribing in the twenty-first century. Clin Infect Dis. 2008;46:1139–41. doi: 10.1086/529441. [DOI] [PubMed] [Google Scholar]
- 40.Office of Statewide Health Planning and Development. MIRCal - Inpatient Data Reporting Manual. 6th Edition. 2008. Available at: http://www.oshpd.ca.gov/HID/MIRCal/IPManual.html . Accessed 4 May 2008.
- 41.Aoki Y, Suto A, Mizuta K, et al. Duration of norovirus excretion and the longitudinal course of viral load in norovirus-infected elderly patients. J Hosp Infect. 2010;75:42–6. doi: 10.1016/j.jhin.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Furuya D, Kuribayashi K, Hosono Y, et al. Age, viral copy number, and immunosuppressive therapy affect the duration of norovirus RNA excretion in inpatients diagnosed with norovirus infection. Jpn J Infect Dis. 2011;64:104–8. [PubMed] [Google Scholar]
- 43.Tu ET, Bull R, Kim MJ, et al. Norovirus excretion in an aged-care setting. J Clin Microbiol. 2008;46:2119–21. doi: 10.1128/JCM.02198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glass RI, Parashar UE, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361:1776–85. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]


