Abstract
Primary mediastinal B-cell lymphoma (PMBL) is a subtype of diffuse large B-cell lymphoma (DLBCL) that is putatively derived from a thymic B cell. Accounting for up to 10% of cases of DLBCL, this subtype predominantly affects women in the third and fourth decades of life. Its clinical and molecular characteristics are distinct from other subtypes of DLBCL and, in fact, closely resemble those of nodular sclerosing Hodgkin lymphoma (NSHL). Recently, mediastinal lymphomas with features intermediate between PMBL and NSHL, called mediastinal gray-zone lymphomas, have been described. The optimal management of PMBL is controversial, and most standard approaches include a combination of immunochemotherapy and mediastinal radiation. Recently, the recognition that mediastinal radiation is associated with significant long-term toxicities has led to the development of novel approaches for PMBL that have shown excellent efficacy and challenge the need for routine mediastinal radiation.
Introduction
Primary mediastinal B-cell lymphoma (PMBL), originally described in the 1980s, accounts for up to 10% of diffuse large B-cell lymphomas (DLBCL). It is epidemiologically, clinically, and biologically distinct from the other subtypes of DLBCL (germinal center B-cell-like [GCB] DLBCL and activated B-cell-like DLBCL). Similar to nodular sclerosing Hodgkin lymphoma (NSHL) arising in the mediastinum, it is likely derived from a thymic B cell and typically presents in adolescents and young adults with an anterior mediastinal mass, which may invade local structures. Studies of gene expression profiling demonstrate a significant overlap between PMBL and NSHL and, interestingly, mediastinal lymphomas, with pathologic features that are intermediate and transitional between PMBL and NSHL (mediastinal gray-zone lymphomas; MGZLs) have been described.
The optimal therapeutic approach to PMBL is controversial, with a paucity of prospective studies. Although there are many retrospective studies, one of the challenges in interpreting them is that older studies likely included cases that would not meet the clinicopathologic definition of PMBL today. For the most part, it has been treated in the same way as the other subtypes of DLBCL, with R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone). However, the high efficacy of increased dose intensity regimens in this disease suggests it requires a unique therapeutic approach. The major controversies in PMBL therapeutics are the need for consolidation radiation, the role of fluorodeoxyglucose-positron emission tomography (FDG-PET) scanning, and whether or not there are superior chemotherapy platforms to CHOP.
Clinical features
PMBL usually affects adolescents and young adults, with a female propensity, and typically presents in the third and fourth decades of life, which is much earlier than the other subtypes of DLBCL.1 Symptoms at diagnosis are typically caused by an anterior mediastinal mass, and complications such as superior vena cava syndrome are common at presentation. PMBL tends to stay confined to the mediastinum and sometimes may invade local structures such as the anterior chest wall and lungs. Disseminated disease may occur at diagnosis when extranodal sites such as the kidney, liver, and adrenal gland may be involved. NSHL, arising in the mediastinum, shares many clinical characteristics with PMBL and also typically presents in young women. Recently, MGZLs with clinical and pathologic features intermediate between PMBL and classical Hodgkin lymphoma have been recognized. MGZLs predominantly affect men and appear to have an inferior outcome compared with PMBL.1,2
Pathology
PMBL is putatively derived from a medullary thymic B cell. Morphologically, these are medium to large cells having round or lobulated nuclei and abundant cytoplasm. In most cases, compartmentalizing sclerosis is observed, and sometimes tumor cells can resemble Hodgkin/Reed-Sternberg cells. The nodal architecture is typically diffuse, with occasional cases showing focal nodularity, and necrosis is sometimes seen.3 PMBL has a B-cell phenotype and expresses CD20 and pan B-cell markers such as CD79a, but tumor cells do not express surface immunoglobulin; therefore, monoclonality cannot be established by κ and λ staining, in contrast to most B-cell neoplasms (Figure 1).4,5 B-cell transcription factors including PAX5, OCT2, and BOB1 are typically strongly expressed. CD30 is typically expressed but is dim in comparison with classical Hodgkin lymphoma (CHL), whereas CD15 is usually negative.3-5 The germinal center markers CD10, BCL6, and CD23 are expressed in most cases of PMBL, in keeping with its thymic B-cell origin.6,7 Distinguishing PMBL from NSHL can sometimes be challenging for the pathologist: NSHL has a nodular pattern of growth, as well as the presence of lacunar variants of Hodgkin/Reed-Sternberg cells with a characteristic immunophenotype. In contrast to PMBL, cells are typically CD15-positive and are strongly positive for CD30. The expression of B-cell markers such as CD20, CD79a, and PAX5 is often weak or negative.8,9
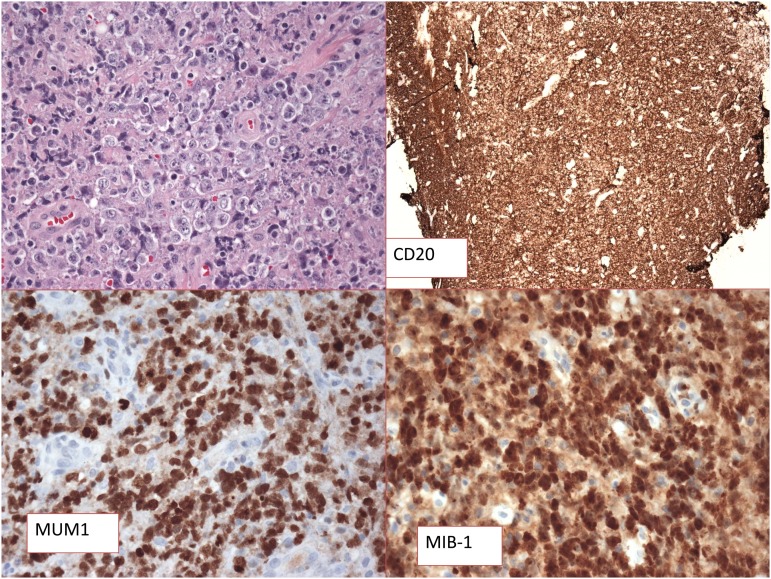
Figure 1.
Primary mediastinal B-cell lymphoma. Hematoxylin and eosin is shown and CD20 and MUM1 staining are positive. MIB-1 scoring is high. (Figure courtesy of Stefania Pittaluga.)
The morphological and immunohistochemical features of MGZLs are intermediate and transitional between PMBL and NSHL.10-12 As in the case of both PMBL and NSHL, surface immunoglobulin is not expressed. B-cell markers such as CD20 and CD79a are typically expressed, CD30 is usually positive, and there is variable expression of CD15. PAX5, OCT2, and BOB1 are also typically expressed. In cases of MGZL, an asynchrony between morphology and immunophenotype can be seen: cases can have a PMBL-like morphology but with immunophenotypical features of NSHL, or vice versa. The factors that transform a thymic B cell into one or another of these diseases are not well understood, but it is likely there is plasticity in these events, given that it is not uncommon to see them recur as 1 of the other entities (eg, PMBL recurs as NSHL or NSHL as MGZLs, etc; Figure 2A-B).11
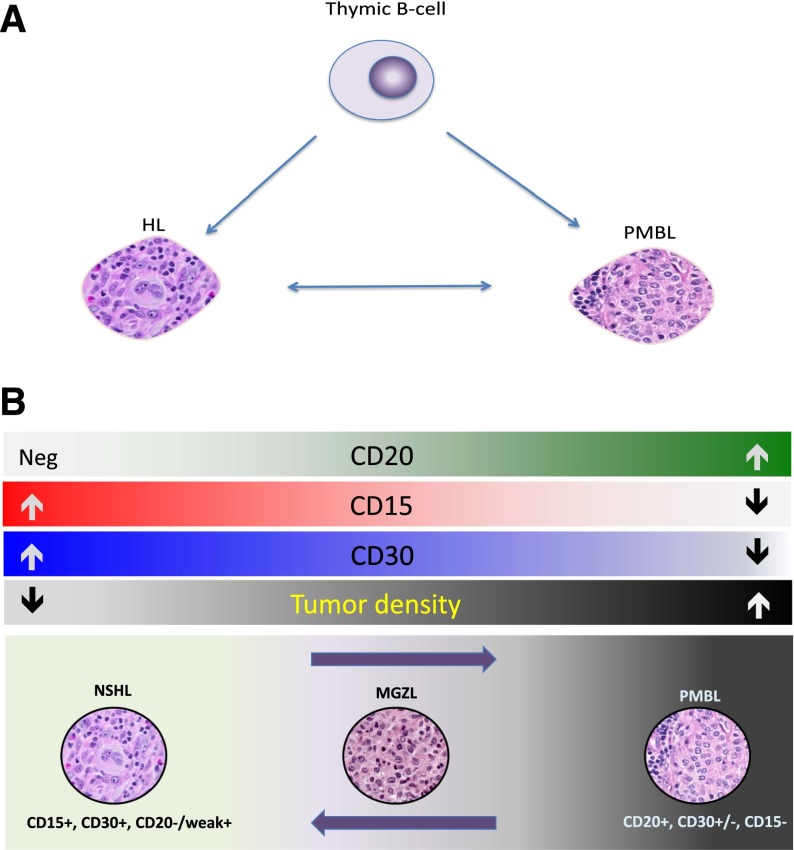
Figure 2.
Spectrum of mediastinal B-cell lymphomas. (A) Both PMBL and Hodgkin lymphoma (HL) are putatively derived from a thymic B cell. The events that transform a thymic B cell into PMBL or HL are poorly understood, but there appears to be plasticity in these events, as HL can recur as PMBL and vice versa. (B) Although NSHL is CD15- and CD30-positive and PMBL is CD20-positive, there are mediastinal lymphomas between these 2 entities with histologic and immunohistochemical features intermediate and transitional between NSHL and PMBL. These diseases are MGZLs.
Genetic and molecular characteristics of PMBL
Gene expression profiling studies have demonstrated that the genotype of PMBL has much more in common with that of NSHL than with the other subtypes of DLBCL (ie, GCB DLBCL and activated B-cell-like DLBCL)13,14 (Figure 3). In fact, PMBL shares a third of its genes with NSHL.13 Among the most common genetic alterations in PMBL are abnormalities on chromosome 9p (up to 75%) and 2p (approximately 50%). Although these abnormalities have been described in NSHL, they are typically not found in the other DLBCL subtypes.1 The 9p region encodes Janus kinase 2 (JAK2), which then activates the transcription factor signal transducer and activator of transcription (STAT) 6 through phosphorylation.13,15 Recent work has demonstrated that phosphorylated STAT 6 can transcriptionally repress BCL6 in PMBL.16 Suppressor of cytokine signaling 1 suppresses JAK signaling and is mutated in a high proportion of PMBL and CHL cases.17 Also in the 9p region, programmed death ligands (PDLs) 1 and 2 are rearranged at a frequency of 20%, whereas gains or amplifications of c-REL may be seen at 2p.13,18 Approximately one-third of PMBL cases may have gains in chromosome X. Recently, whole-genome and whole-transcriptome sequencing have identified recurrent somatic coding-sequence mutations in the PTPN1 gene; these are also commonly found in Hodgkin lymphoma cases.19 In PMBL, in contrast to other subtypes of DLBCL, rearrangements of BCL2, BCL6, and MYC are typically absent.1 PMBL and CHL both have constitutively activated nuclear factor κ-B, and PMBL cell line survival is dependent on nuclear factor κ-B target genes.
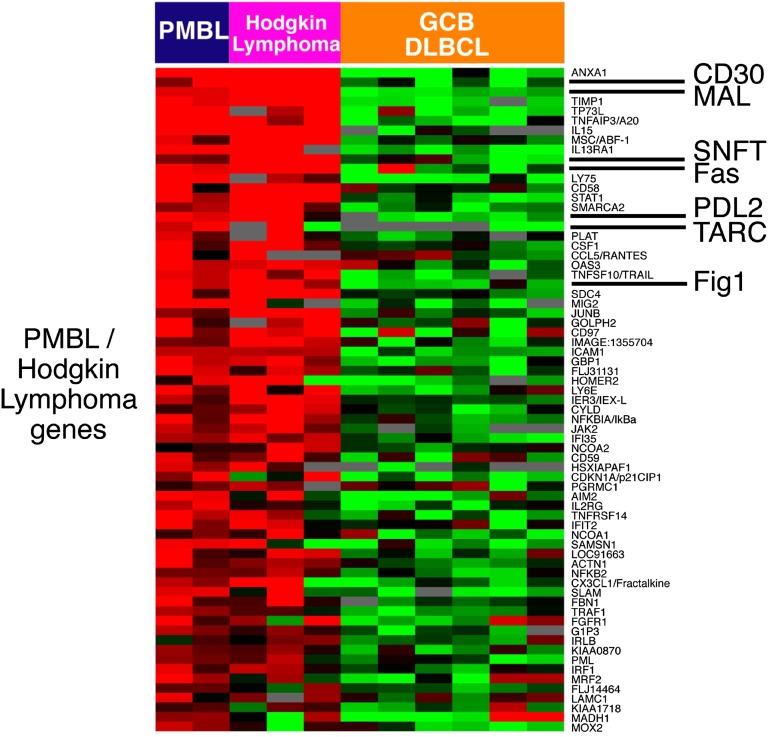
Figure 3.
Relationship of PMBL to Hodgkin lymphoma. Relative gene expression is shown in primary PMBLs (average of all biopsy samples), the PMBL cell line K1106, 3 Hodgkin lymphoma cell lines, and 6 GCB DLBCL cell lines. Red represents high gene expression and green low expression. PMBL signature genes that are also expressed at high levels in Hodgkin lymphoma cell lines compared with GCB DLBCL cell lines. (Figure courtesy of Louis Staudt.)
Because of its rarity, the molecular characteristics of GZLs have not been well studied. However, a study that looked at chromosomal aberrations in GZLs showed gains including amplifications in 2p16.1 (REL/BCL11A locus) in 33% of all cases, alterations of the JAK2/PDL2 locus in 9p24.1 in 55%, rearrangement of the CIITA locus at 16p13.13 in 27%, and gains of 8q24 (MYC) in 27%.10 A recent large-scale methylation analysis that included PMBL, CHL, and MGZL cases showed that these entities shared many epigenetic characteristics and that MGZL had a distinct epigenetic signature.20
Diagnosis of PMBL and prognostic factors
The diagnostic work up for PMBL should include the same routine tests performed for any other patient with DLBCL. Most important, the tissue biopsy should be evaluated by a pathologist who is an expert in the diagnosis of lymphoma. For the aforementioned reasons, it can sometimes be challenging to distinguish PMBL from NSHL. A thorough history and physical examination; a complete evaluation of hematological and biochemical parameters; computerized tomography of the chest, abdomen, and pelvis; and a bone marrow aspirate and biopsy should be performed. Although central nervous system involvement is very rare at initial diagnosis, the cerebrospinal fluid should be checked by cytology and flow cytometry in the presence of clinical characteristics that are associated with a higher risk for central nervous system spread.21 It is common for pleural and pericardial effusions to occur at presentation, so it may be useful to perform an echocardiogram. Although the international prognostic index (IPI) is useful in DLBCL, its use in PMBL specifically is limited by the young age distribution of the disease and its typical confinement to the mediastinum.22 Various studies have looked at the role of IPI, but it is not clear that it is helpful in predicting outcome.23 Some retrospective studies have suggested that factors such as lactate dehydrogenase level, male sex, performance status, and advanced-stage disease may be useful predictors of survival, but this is controversial and has not been validated in prospective studies.24,25
Primary treatment and outcome
As PMBL is relatively rare and has been only recently described, there is a paucity of prospective treatment data and a lack of randomized studies (Table 1). Therefore, controversies abound about what the optimal therapeutic approach should be and which regimen is best. The cure rate for progressive or recurrent disease after primary therapy is low, so it is critical to optimize up-front outcomes. Most effective approaches to date have incorporated consolidation radiation; although high cure rates are achieved with combined modality therapy, it is increasingly apparent, especially from follow-up data on long-term survivors of Hodgkin lymphoma, that mediastinal radiation is associated with significant late sequelae, and particularly a high risk for breast tumors in women.26,27 Although some studies suggest that lower doses of radiation and more focused treatment fields may reduce these complications, this has not been clearly demonstrated, and some studies contest this.28
Table 1.
Selected published studies of chemotherapy/immunochemotherapy regimens with and without radiation treatment in PMBL
| Authors | Treatment | Study type | Patient characteristic | Outcome | |
|---|---|---|---|---|---|
| Chemotherapy | RT+/− | ||||
| Zinzani et al29 | MACOP-B | Yes | Prospective study, n = 50 | All IPI groups | At 39 months, 93% who achieved complete response were relapse-free; at 8 years, OS was 82% |
| Todeschini et al30 | MACOP-B, VACOP-B or CHOP | Yes | Retrospective study, n = 138 | All IPI groups | At 66 months, EFS was 39% for CHOP and 76% for MACOP-B/VACOP-B |
| Zinzani et al25 | MACOP-B, VACOP-B, ProMACE-CytaBOM, CHOP, or HDS/ABMT | Yes | Retrospective study, n = 426 | All IPI groups | At 10 years, PFS was 35% for CHOP/CHOP-like, 67% for MACOP-B/VACOP-B, and 78% for HDS/ABMT |
| Hamlin et al23 | CHOP/CHOP like, NHL-15, ASCT | No | Retrospective study, n = 141 | All IPI groups | At 11 years, EFS was 34% for CHOP/CHOP-like, 60% for NHL-15, and 60% for ASCT |
| Savage et al24 | CHOP/R-CHOP/MACOP-B/VACOP-B | Variable | Retrospective study, n = 153 | All IPI groups | At 5 years, overall PFS was 69%; only MACOP-B/VACOP-B vs CHOP-like regimens were significantly different |
| Rieger et al33 | CHOP/R-CHOP | Variable: RT intended in 87% | Retrospective analysis, n = 87 | Confined to AA-IPI of 0-1 | At 3 years, EFS was 78% for R-CHOP and 52% for CHOP |
| Soumerai et al37 | R-CHOP | Yes: 77% of responding patients | Retrospective study, n = 63 | All IPI groups | At 5 years, PFS was 68% |
| Dunleavy et al34 | DA-EPOCH-R | No | Prospective study, n = 51 | All IPI groups | At 5 years, EFS was 93% |
| Woessmann et al41 | DA-EPOCH-R | No | Ongoing case series, n = 15 | All IPI groups, age younger than 18 years | At 19 months, EFS was 92% |
| Martelli et al36 | R-MACOP-B, R-VACOP-B, R-CHOP | Yes | Prospective study, n = 125 | All IPI | Estimated 5-year PFS is 86% |
AA, age-adjusted; ASCT, autologous stem cell transplantation; HDS/ABMT, high-dose sequential chemotherapy and autologous bone marrow transplantation; NHL, non-Hodgkin lymphoma.
It is therefore important in PMBL to develop platforms that obviate the need for routine radiation, and thus eliminate these complications.
Radiation and dose intensity
Early studies in PMBL suggested that consolidation radiation was a critical component of curative therapy. One of these studies that led to this widely held acceptance was a study of MACOP-B (methotrexate, leucovorin, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin) followed by mediastinal radiation therapy in 50 untreated patients with PMBL.29 Although 66% of patients were gallium scan-positive (this study was done in the pre-FDG-PET era), at the end of chemotherapy, only 19% were gallium-positive after radiation, which supported a combined modality approach that is taken by most clinicians today. Early (albeit retrospective) studies also suggested a benefit to increasing dose intensity, which has been shown to be important in Hodgkin lymphoma, a closely related disease clinically and biologically. One such study retrospectively compared MACOP-B and VACOP-B (etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin) with CHOP in 138 patients. Those patients who received CHOP had a worse outcome, suggesting a role for dose-intensity.30 The largest study to look at the dose-intensity question was conducted by the International Extranodal Lymphoma Study Group and evaluated 426 newly diagnosed patients with PMBL who received MACOP-B, VACOP-B, ProMACE-CytaBOM (cyclophosphamide, doxorubicin, etoposide cytozar, bleomycin, vincristine, methotrexate and prednisone), or CHOP.25 Although response rates were similar among the groups, projected long-term progression-free survival (PFS) and overall survival (OS) rates were higher in patients who received third-generation regimens. Additional retrospective series from the British Columbia Cancer Agency and Memorial Sloan Kettering Cancer Center groups also suggested that increased dose-intensity regimens might be superior to CHOP-like approaches in this disease.23,24 Nonetheless, prospective comparisons of increased dose-intensity vs CHOP-like regimens have not been performed in PMBL. Although the Southwest Oncology Group prospectively compared second- and third-generation regimens with CHOP in DLBCL, the outcome of PMBL was not assessed, as it was not a recognized disease entity at this time.31
The role of rituximab
Although the addition of rituximab to CHOP chemotherapy in DLBCL has been shown to improve survival in several different studies, this has not been well studied or established in PMBL because of the rarity of the disease.32 A subgroup analysis of the prospective, randomized, phase 3 MabThera International Trial evaluated the role of rituximab in combination with CHOP-like regimens in patients with PMBL with an age-adjusted IPI of 1 or less.33 The rituximab group was clearly superior in terms of 3-year event-free survival (EFS; 78% vs 52% in the chemotherapy group alone), but no statistically significant difference in OS was detected because of the small numbers of patients. When the outcome of patients who received dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin (DA-EPOCH) alone in the prerituximab era was compared with those who received DA-EPOCH-R, although it was a nonrandomized comparison, there was significantly better EFS and OS in the group that received rituximab.34 Although an earlier retrospective study from the British Columbia Cancer Agency in the pre- and postrituximab periods demonstrated no survival advantage in patients when rituximab was added to CHOP, a more recent report from the group showed an improved time to progression and longer OS in those receiving rituximab.25,35 The International Extranodal Lymphoma Study Group recently reported a projected 5-year PFS of 86% in patients receiving rituximab in combination with MACOP-B or VACOP-B predominantly, followed by involved field radiation therapy.36
Can mediastinal radiation be omitted?
Despite a lack of prospective studies, there does appear to be a benefit to adding rituximab to chemotherapy in PMBL. Therefore, can rituximab abrogate any advantage of dose-intensive platforms over CHOP and obviate the need for routine radiation? Although the subset analysis in the MabThera International Trial study demonstrated improved responses and EFS in patients receiving rituximab, preplanned radiotherapy (RT) was still administered to 73% of patients in the immunochemotherapy group, and adding radiation improved remission rates. In addition, and importantly, the study was confined to patients with a low IPI score (≤1) who represent a favorable subset of patients. A recent retrospective analysis of R-CHOP (followed by mediastinal radiation in 77% of responders) in 58 patients with PMBL that included all IPI groups showed a high rate of primary induction failures (21%) and an overall PFS of 68% at 5 years.37 Another retrospective analysis from the Memorial Sloan Kettering Cancer Center group evaluated R-CHOP followed by ifosfamide, cyclophosphamide, and etoposide without radiation and reported a PFS of 78% at 3 years.38 A British Columbia study that looked at the outcome of PMBL in the rituximab era reported on a subset of patients in whom an FDG-PET-guided RT approach (ie, FDG-PET-negative cases were not radiated) was used. Despite this, a sizeable proportion of PET-negative cases subsequently relapsed.36 Because of the observation that dose intensity has been important in PMBL, a National Cancer Institute study evaluated dose-adjusted EPOCH-R without radiation in PMBL and included all clinical risk groups.39,40 In 51 patients, at a median follow-up of 5 years, EFS and OS were 93% and 97%, respectively, and just 2 patients required consolidation radiation.34 In an additional 16 patients with PMBL who received the regimen at Stanford University Medical Center, both EFS and OS were 100% without radiation. This approach is now being tested in multicenter studies, and an early report from a pediatric/adolescent study suggested high efficacy without the need for radiation in this population.41
FDG-PET evaluation after therapy
At the completion of treatment of PMBL, a residual mediastinal mass is commonly present, particularly in cases in which there had been a large mass at initial diagnosis or a large fibrotic component to the mass. It is not uncommon for these masses to persist for several months after the completion of therapy, which is an important consideration in the interpretation of follow-up imaging. Hence, computed tomography alone is limited in its scope to assess the presence of residual disease. Gallium scanning was used as an adjunct imaging test in the past for this purpose, but it is now regarded as a cumbersome test and is infrequently used today. Although studies looking at the role of end-of-treatment FDG-PET, and its ability to guide the use/need for consolidation radiation, are limited, the technique has been found to have a very high negative predictive value in this disease.34 However, the positive predictive value of FDG-PET in PMBL is poor in contrast to the high clinical accuracy of FDG-PET in other aggressive lymphomas34,41 (Figure 4). After DA-EPOCH-R, 18 of 36 patients who underwent an FDG-PET scan had a maximum standardized value above the mediastinal blood pool, but only 3 of these patients were found to have residual lymphoma. One retrospective study that looked at interim PET in patients with PMBL receiving R-VACOP-B also reported a low positive predictive value.43 This is likely a result of ongoing inflammatory activity in residual mediastinal masses that may be FDG-PET-avid. Therefore, it is not a very accurate technique for determining the presence of residual disease at the end of treatment, and alternative, more specific imaging modalities should be investigated.
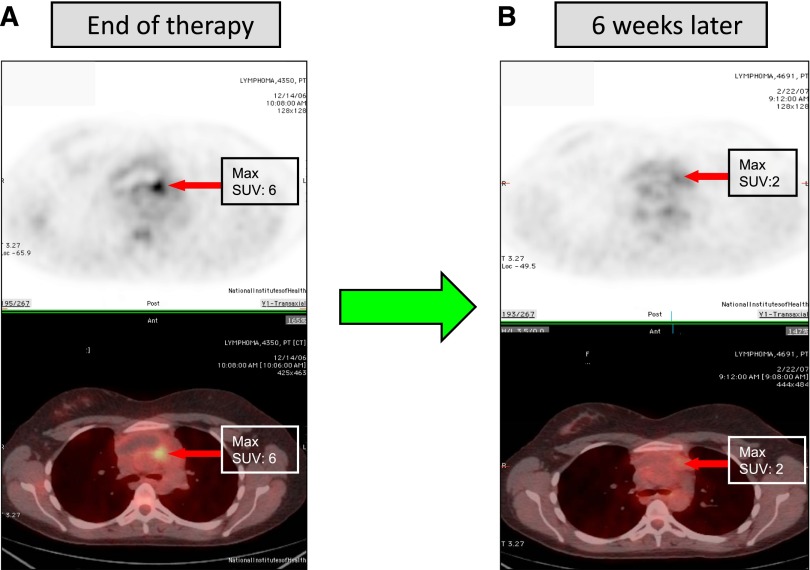
Figure 4.
FDG-PET imaging after completion of therapy in PMBL. These are sequential FDG-PET scans of a patient with PMBL who received 6 cycles of DA-EPOCH-R. (A) Three weeks after the completion of therapy, there was an FDG-PET avid lesion in the anterior mediastinum with an standardized value of 6. (B) The patient was followed, and approximately 6 weeks later had another FDG-PET (B). The lesion now had a standardized value of 2. A subsequent FDG-PET scan 6 weeks later was entirely normal, and the patient remains in complete remission several years later.
Therapeutic decision making
In making decisions about the initial treatment of PMBL, one must consider the long-term complications of mediastinal radiation in this population of patients who are predominantly young women.27 Although R-CHOP followed by radiation has been effective in low-risk patients, it appears to be insufficient therapy for patients with high-risk disease and is associated with a high rate of primary refractory disease.37 On the basis of its promising efficacy in a National Cancer Institute study, we recommend DA-EPOCH-R without radiation while confirmatory studies are in progress. After this regimen without radiation, end-of-therapy FDG-PET has an excellent negative predictive value but low positive predictive value, so end-of-therapy-positive FDG-PETs need to be interpreted cautiously with regard to decisions about consolidation radiation. A prospective study of FDG-PET-directed therapy by the International Extranodal Lymphoma Study Group is currently underway.
Treatment of mediastinal gray-zone lymphomas
These very rare tumors have histological and immunophenotypic features that are transitional between PMBL and NSHL. Because of their rarity and recent identification, they have been poorly studied. In the past, these tumors were likely called “anaplastic large-cell lymphoma Hodgkin-like,” which was reported to have a poor prognosis with short median survivals.43 Their indeterminate pathobiology has led to uncertainty about what the optimal therapeutic strategy should be. One retrospective study reported that the 5-year EFS for this entity was worse than that for classical Hodgkin lymphoma (International Database on Hodgkin’s Disease), suggesting adverse biology and a high rate of treatment resistance.43 Recently, a prospective study looked at the clinical characteristics and outcome of MGZL after treatment with DA-EPOCH-R and reported a worse outcome compared with that of PMBL, despite a patient population with similar clinical characteristics (EFS and OS of 62% and 74%, respectively, versus 93% and 97% for PMBL).44,45 Studies investigating the molecular characteristics and biological basis for this inferior outcome are ongoing.
Treatment of relapsed or refractory disease
In PMBL, relapses tend to occur relatively early after the completion of treatment, and most are observed in the first year or 18 months after therapy. Relapsed disease may stay confined to the mediastinum or spread to extranodal sites such as the liver, kidneys, or central nervous system. Optimal therapy for relapsed disease has not been well defined and should be decided on the basis of the pattern of relapse and prior treatments received. For relapses localized to the mediastinum, chemotherapy with radiation treatment (with or without autologous stem cell transplantation) may be a curative option, especially in patients who did not receive mediastinal radiation initially. For nonlocalized relapses, salvage chemotherapy followed by high-dose therapy may be considered.46 Allogeneic transplantation is another experimental option that can be considered. The outcome for patients with MGZL is inferior to that of PMBL, and relapses should be approached similarly to PMBL.
Novel agents
The antibody drug conjugate directed against CD30, brentuximab vedotin, has shown activity in patients with relapsed Hodgkin lymphoma and is currently being studied in PMBL, where CD30 is variably expressed.47 Novel strategies in PMBL and other mediastinal lymphomas should focus on combining targeted agents with effective immunochemotherapy platforms. As the nuclear factor κ-B signaling pathway is one of the most important deregulated pathways in PMBL, inhibitors of this pathway are rational strategies in PMBL. A region on chromosome 9p24 is amplified in 70% of cases of PMBL that constitute critical targets, including JAK2 and the PD1 ligands PDL 1 (PD-L1) and PD-L2.48-50 Selective JAK2 inhibition has been shown to specifically decrease mediastinal large B-cell lymphoma growth in vitro and in vivo.51 Agents such as JAK-STAT pathway inhibitors or neutralizing antibodies to PD1, such as pidilizumab, are worthwhile investigating in PMBL.52
Conclusions
Controversies abound as to what the optimal regimen is for PMBL, but evidence from several studies suggests a benefit to regimens with increased dose-intensity. Recent data demonstrate that DA-EPOCH-R can obviate the need for routine mediastinal radiation. These results are now being validated in multicenter settings in distinct patient groups. Although FDG-PET is routinely used for end-of-treatment assessment in PMBL, it has a low positive predictive value that limits its usefulness in the assessment of residual masses.
Acknowledgment
The authors receive funding from the Intramural Program of the National Cancer Institute.
Authorship
Contribution: K.D. and W.H.W. contributed to the writing of the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wyndham H. Wilson, Metabolism Branch, National Cancer Institute, Building 10, Room 4N-115, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: wilsonw@mail.nih.gov.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. 4th ed. Lyon, France: IARC; 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 2.Dunleavy K, Grant C, Eberle FC, Pittaluga S, Jaffe ES, Wilson WH. Gray zone lymphoma: better treated like hodgkin lymphoma or mediastinal large B-cell lymphoma? Curr Hematol Malig Rep. 2012;7(3):241–247. doi: 10.1007/s11899-012-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pileri SA, Zinzani PL, Gaidano G, et al. International Extranodal Lymphoma Study Group. Pathobiology of primary mediastinal B-cell lymphoma. Leuk Lymphoma. 2003;44(Suppl 3):S21–S26. doi: 10.1080/10428190310001623810. [DOI] [PubMed] [Google Scholar]
- 4.Möller P, Moldenhauer G, Momburg F, et al. Mediastinal lymphoma of clear cell type is a tumor corresponding to terminal steps of B cell differentiation. Blood. 1987;69(4):1087–1095. [PubMed] [Google Scholar]
- 5.Pileri SA, Gaidano G, Zinzani PL, et al. Primary mediastinal B-cell lymphoma: high frequency of BCL-6 mutations and consistent expression of the transcription factors OCT-2, BOB.1, and PU.1 in the absence of immunoglobulins. Am J Pathol. 2003;162(1):243–253. doi: 10.1016/s0002-9440(10)63815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calaminici M, Piper K, Lee AM, Norton AJ. CD23 expression in mediastinal large B-cell lymphomas. Histopathology. 2004;45(6):619–624. doi: 10.1111/j.1365-2559.2004.01969.x. [DOI] [PubMed] [Google Scholar]
- 7.Salama ME, Rajan Mariappan M, Inamdar K, Tripp SR, Perkins SL. The value of CD23 expression as an additional marker in distinguishing mediastinal (thymic) large B-cell lymphoma from Hodgkin lymphoma. Int J Surg Pathol. 2010;18(2):121–128. doi: 10.1177/1066896909331994. [DOI] [PubMed] [Google Scholar]
- 8.Schmid C, Pan L, Diss T, Isaacson PG. Expression of B-cell antigens by Hodgkin’s and Reed-Sternberg cells. Am J Pathol. 1991;139(4):701–707. [PMC free article] [PubMed] [Google Scholar]
- 9.Zukerberg LR, Collins AB, Ferry JA, Harris NL. Coexpression of CD15 and CD20 by Reed-Sternberg cells in Hodgkin’s disease. Am J Pathol. 1991;139(3):475–483. [PMC free article] [PubMed] [Google Scholar]
- 10.Eberle FC, Salaverria I, Steidl C, et al. Gray zone lymphoma: chromosomal aberrations with immunophenotypic and clinical correlations. Mod Pathol. 2011;24(12):1586–1597. doi: 10.1038/modpathol.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traverse-Glehen A, Pittaluga S, Gaulard P, et al. Mediastinal gray zone lymphoma: the missing link between classic Hodgkin’s lymphoma and mediastinal large B-cell lymphoma. Am J Surg Pathol. 2005;29(11):1411–1421. doi: 10.1097/01.pas.0000180856.74572.73. [DOI] [PubMed] [Google Scholar]
- 12.García JF, Mollejo M, Fraga M, et al. Large B-cell lymphoma with Hodgkin’s features. Histopathology. 2005;47(1):101–110. doi: 10.1111/j.1365-2559.2005.02175.x. [DOI] [PubMed] [Google Scholar]
- 13.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198(6):851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102(12):3871–3879. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- 15.Guiter C, Dusanter-Fourt I, Copie-Bergman C, et al. Constitutive STAT6 activation in primary mediastinal large B-cell lymphoma. Blood. 2004;104(2):543–549. doi: 10.1182/blood-2003-10-3545. [DOI] [PubMed] [Google Scholar]
- 16.Ritz O, Rommel K, Dorsch K, et al. STAT6-mediated BCL6 repression in primary mediastinal B-cell lymphoma (PMBL). Oncotarget. 2013;4(7):1093–1102. doi: 10.18632/oncotarget.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melzner I, Bucur AJ, Brüderlein S, et al. Biallelic mutation of SOCS-1 impairs JAK2 degradation and sustains phospho-JAK2 action in the MedB-1 mediastinal lymphoma line. Blood. 2005;105(6):2535–2542. doi: 10.1182/blood-2004-09-3701. [DOI] [PubMed] [Google Scholar]
- 18.Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood. 2014;123(13):2062–2065. doi: 10.1182/blood-2013-10-535443. [DOI] [PubMed] [Google Scholar]
- 19.Gunawardana J, Chan FC, Telenius A, et al. Recurrent somatic mutations of PTPN1 in primary mediastinal B cell lymphoma and Hodgkin lymphoma. Nat Genet. 2014;46(4):329–335. doi: 10.1038/ng.2900. [DOI] [PubMed] [Google Scholar]
- 20.Eberle FC, Rodriguez-Canales J, Wei L, et al. Methylation profiling of mediastinal gray zone lymphoma reveals a distinctive signature with elements shared by classical Hodgkin’s lymphoma and primary mediastinal large B-cell lymphoma. Haematologica. 2011;96(4):558–566. doi: 10.3324/haematol.2010.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Besien K, Ha CS, Murphy S, et al. Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood. 1998;91(4):1178–1184. [PubMed] [Google Scholar]
- 22.A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 23.Hamlin PA, Portlock CS, Straus DJ, et al. Primary mediastinal large B-cell lymphoma: optimal therapy and prognostic factor analysis in 141 consecutive patients treated at Memorial Sloan Kettering from 1980 to 1999. Br J Haematol. 2005;130(5):691–699. doi: 10.1111/j.1365-2141.2005.05661.x. [DOI] [PubMed] [Google Scholar]
- 24.Savage KJ, Al-Rajhi N, Voss N, et al. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol. 2006;17(1):123–130. doi: 10.1093/annonc/mdj030. [DOI] [PubMed] [Google Scholar]
- 25.Zinzani PL, Martelli M, Bertini M, et al. International Extranodal Lymphoma Study Group (IELSG) Induction chemotherapy strategies for primary mediastinal large B-cell lymphoma with sclerosis: a retrospective multinational study on 426 previously untreated patients. Haematologica. 2002;87(12):1258–1264. [PubMed] [Google Scholar]
- 26.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117(6):1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunleavy K, Bollard CM. Sobering realities of surviving Hodgkin lymphoma. Blood. 2011;117(6):1772–1773. doi: 10.1182/blood-2010-11-317131. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien MM, Donaldson SS, Balise RR, Whittemore AS, Link MP. Second malignant neoplasms in survivors of pediatric Hodgkin’s lymphoma treated with low-dose radiation and chemotherapy. J Clin Oncol. 2010;28(7):1232–1239. doi: 10.1200/JCO.2009.24.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zinzani PL, Martelli M, Magagnoli M, et al. Treatment and clinical management of primary mediastinal large B-cell lymphoma with sclerosis: MACOP-B regimen and mediastinal radiotherapy monitored by (67)Gallium scan in 50 patients. Blood. 1999;94(10):3289–3293. [PubMed] [Google Scholar]
- 30.Todeschini G, Secchi S, Morra E, et al. Primary mediastinal large B-cell lymphoma (PMLBCL): long-term results from a retrospective multicentre Italian experience in 138 patients treated with CHOP or MACOP-B/VACOP-B. Br J Cancer. 2004;90(2):372–376. doi: 10.1038/sj.bjc.6601460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328(14):1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 32.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 33.Rieger M, Osterborg A, Pettengell R, et al. MabThera International Trial (MInT) Group. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol. 2011;22(3):664–670. doi: 10.1093/annonc/mdq418. [DOI] [PubMed] [Google Scholar]
- 34.Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368(15):1408–1416. doi: 10.1056/NEJMoa1214561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savage KJ, Yenson PR, Shenkier T, et al. The outcome of primary mediastinal large B-cell lymphoma (PMBCL) in the R-CHOP treatment era. Oral presentation at the 54th ASH Annual Meeting and Exposition. December 10, 2012. Atlanta, GA. [Google Scholar]
- 36.Martelli M, Ceriani L, Zucca E, et al. [18F] Fluorodeoxyglucose positron emission tomography predicts survival after chemoimmunotherapy for primary mediastinal large B-cell lymphoma: results of the International Extranodal Lymphoma Study Group IELSG-26 Study. J Clin Oncol. 2014. 10;32(17):1769-1775. [DOI] [PubMed] [Google Scholar]
- 37.Soumerai JD, Hellmann MD, Feng Y, et al. Treatment of primary mediastinal B-cell lymphoma with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone is associated with a high rate of primary refractory disease. Leuk Lymphoma. 2014;55(3):538–543. doi: 10.3109/10428194.2013.810738. [DOI] [PubMed] [Google Scholar]
- 38.Moskowitz C, Hamlin PA, Maraguilia J, Meikle J, Zelenetz AD. Sequential dose-Dende R-CHOP followed by ICE consolidation (MSKCC Protocol 01-142) without radiotherapy for patients with primary mediastinal large B-cell lymphoma. Paper presented at the 52nd Annual Meeting of the American Society of Hematology. December 4–7, 2010. Orlando, FL. [Google Scholar]
- 39.Wilson WH, Bryant G, Bates S, et al. EPOCH chemotherapy: toxicity and efficacy in relapsed and refractory non-Hodgkin’s lymphoma. J Clin Oncol. 1993;11(8):1573–1582. doi: 10.1200/JCO.1993.11.8.1573. [DOI] [PubMed] [Google Scholar]
- 40.Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26(16):2717–2724. doi: 10.1200/JCO.2007.13.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woessmann W, Lisfeld J, Burkhardt B NHL-BFM Study Group. Therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;369(3):282. doi: 10.1056/NEJMc1305983. [DOI] [PubMed] [Google Scholar]
- 42.Avigdor A, Sirotkin T, Kedmi M, et al. The impact of R-VACOP-B and interim FDG-PET/CT on outcome in primary mediastinal large B cell lymphoma. Ann Hematol. 2014;93(8):1297–1304. doi: 10.1007/s00277-014-2043-y. [DOI] [PubMed] [Google Scholar]
- 43.Cazals-Hatem D, André M, Mounier N, et al. Pathologic and clinical features of 77 Hodgkin’s lymphoma patients treated in a lymphoma protocol (LNH87): a GELA study. Am J Surg Pathol. 2001;25(3):297–306. doi: 10.1097/00000478-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Dunleavy K, Pittaluga S, Shovlin M, et al. Untreated primary mediastinal B-cell (PMBL) and mediastinal grey zone (MGZL) lymphomas: comparison of biological features and clinical outcome following DA-EPOCH-R without radiation. Ann Oncol. 2011;22(4):Abstract 149. [Google Scholar]
- 45.Wilson WH, Pittaluga S, Nicolae A, et al. A prospective study of mediastinal gray-zone lymphoma. Blood. 2014;124(10):1563–1569. doi: 10.1182/blood-2014-03-564906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popat U, Przepiork D, Champlin R, et al. High-dose chemotherapy for relapsed and refractory diffuse large B-cell lymphoma: mediastinal localization predicts for a favorable outcome. J Clin Oncol. 1998;16(1):63–69. doi: 10.1200/JCO.1998.16.1.63. [DOI] [PubMed] [Google Scholar]
- 47.de Claro RA, McGinn K, Kwitkowski V, et al. U.S. Food and Drug Administration approval summary: brentuximab vedotin for the treatment of relapsed Hodgkin lymphoma or relapsed systemic anaplastic large-cell lymphoma. Clin Cancer Res. 2012;18(21):5845–5849. doi: 10.1158/1078-0432.CCR-12-1803. [DOI] [PubMed] [Google Scholar]
- 48.Joos S, Otaño-Joos MI, Ziegler S, et al. Primary mediastinal (thymic) B-cell lymphoma is characterized by gains of chromosomal material including 9p and amplification of the REL gene. Blood. 1996;87(4):1571–1578. [PubMed] [Google Scholar]
- 49.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rui L, Emre NC, Kruhlak MJ, et al. Cooperative epigenetic modulation by cancer amplicon genes. Cancer Cell. 2010;18(6):590–605. doi: 10.1016/j.ccr.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hao Y, Chapuy B, Monti S, Sun HH, Rodig SJ, Shipp MA. Selective JAK2 inhibition specifically decreases Hodgkin lymphoma and mediastinal large B-cell lymphoma growth in vitro and in vivo. Clin Cancer Res. 2014;20(10):2674–2683. doi: 10.1158/1078-0432.CCR-13-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]