Abstract
The neurobiology of stress and the neurobiology of social behavior are deeply intertwined. The social environment interacts with stress on almost every front: social interactions can be potent stressors; they can buffer the response to an external stressor; and social behavior often changes in response to stressful life experience. This review explores mechanistic and behavioral links between stress, anxiety, resilience, and social behavior in rodents, with particular attention to different social contexts. We consider variation between several different rodent species and make connections to research on humans and non-human primates.
Keywords: Stress, Anxiety, Social behavior, Sociality, Social stress, Social buffering
1. Introduction
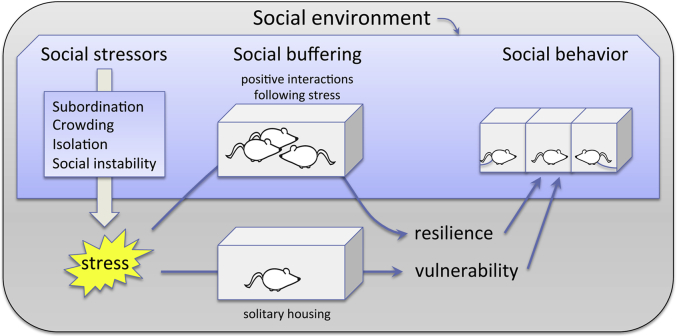
The social worlds of animals are filled with many different types of interactions, and social experience interacts with organismal stress on many levels. Social stressors have proven to be potent across a wide range of species, and their study in rodents has led to greater understanding of the role of stressor type, timing, and other factors impacting physiology and behavior. While negative social interactions can be acutely damaging, social interaction can alsomoderate stressful experiences, buffering potentially adverse impacts and contributing to resilience. In this review we explore the many interactions of stress and social behavior in research on rodents. We consider three main classes of effects: the social environment as a stressor; the effects of stress on subsequent social behavior; and social buffering of stressful experience (Fig. 1). We explore mechanisms that mediate links between stress and social behavior, and consider sex differences in these mechanisms and behavioral outcomes. Finally, we discuss data from a wide variety of rodent species wherever possible, in order to explore the universality and specificity of findings in single species.
Fig. 1.
Schematic representation of the levels at which the social environment impacts and reflects the individual. To the left and in Section 2 of this review, we consider aversive social environments as potent stressors. This stress has far-reaching impacts on individual physiology as well as on social behavior (Section 3), but these impacts are potentially moderated by social buffering (Section 4).
1.1. Measuring stress and social behavior
Responses to stress span a spectrum from detrimental immediate and long-term effects to resilience and protection against future stressors. The effects of stress exposure and consequent trajectory depend on the nature of the stressor, the severity, duration (acute vs. chronic), sex/gender, genetics, timing of exposure (early life, adolescence, adulthood or aging) as well as the perception of the stressor by the individual–for example, stressor controllability dramatically affects resilience versus vulnerability as an outcome (Maier and Watkins, 2005, Amat et al., 2010, Lucas et al., 2014). Recently it was shown that even the gender of researchers can affect rodent stress levels and influence results of behavioral tests (Sorge et al., 2014).
Stress can be assessed by both behavioral and physiological indicators. One of the most commonly measured immediate physiological responses to stress is activation of the hypothalamic–pituitary–adrenal (HPA) axis. During stressful events, corticotropin releasing factor (CRF, also called CRH) is released from the hypothalamus, and is the primary trigger of adrenocorticotropic hormone (ACTH) secretion from the anterior pituitary. ACTH then triggers systemic release of glucocorticoids (CORT) from the adrenal gland (Bale and Vale, 2004). We describe outcomes related to HPA-axis responsivity, as well as several additional neurochemical players including BDNF, serotonin, and multiple neuropeptides in the text below.
Social behavior is complex and varies with the behavioral test chosen, and whether focal individuals are tested with familiar or novel conspecifics, with same- or opposite-sex individuals, or with familiar or unfamiliar strains. The laboratory setting is a sparse environment compared to the complexity of nature, both physically and socially. Some research aims to quantify social behavior in complex housing areas such as enriched caging with social groups (e.g., artificial, visible burrow systems (Blanchard et al., 2001, Seney et al., 2006), and large, semi-natural enclosures (e.g. King, 1956, Dewsbury, 1984, Ophir et al., 2012, Margerum, 2013). Other research relies on constrained social interactions in tests designed to measure a few particular aspects of social behavior (Crawley, 2007). For example social interaction tests typically measure the amount of time spent in social contact or investigation with a conspecific. Social choice tests take place in multi-chambered apparatuses that allow investigation of either a conspecific or a non-living stimulus such as a novel object or empty restrainer (Moy et al., 2007). Variations on this test involve a choice of a familiar versus unfamiliar individual, such as in the partner preference test (Williams et al., 1992). Social habituation/dishabituation tests are often used to assess social recognition and memory for familiar individuals (Ferguson et al., 2002; Choleris et al., 2003). Social motivation may be assessed by measures of effort expended to access another individual (Lee et al., 1999), or by conditioned place preference for a social environment (Panksepp and Lahvis, 2007). Other tests measure specific aspects of social competency, such as memory and social inferences involved in hierarchy (Cordero and Sandi, 2007, Grosenick et al., 2007). Recent studies of pro-social behavior in rats have focused on latency to free a restrained rat under different scenarios (Ben-Ami Bartal et al., 2011, Ben-Ami Bartal et al., 2014).
There is no peripheral hormonal indicator of sociability, but two neuropeptides have been highly implicated in many aspects of mammalian social behavior: oxytocin (OT) and arginine vasopressin (VP). Oxytocin is produced in the hypothalamus and facilitates a wide variety of processes related to social behavior, including maternal behavior, trust, anxiolysis, and sexual pair-bond formation (reviewed in Ross and Young, 2009, Young et al., 2008, Neumann, 2008, Zucker et al., 1968, Carter et al., 2008, Donaldson and Young, 2008, Anacker and Beery, 2013). Vasopressin activity has been associated with aggression, anxiety, and social behavior (reviewed in Kelly and Goodson, 2014), as well partner preference formation in male prairie voles (Cho et al., 1999, Young and Wang, 2004). The locations and densities of oxytocin receptors (OTR) and vasopressin type 1a receptors (V1aR) have been associated with species variations, as well as with individual variations in social behavior from affiliation to aggression (e.g. Everts et al., 1997, Young, 1999, Beery et al., 2008a, Campbell et al., 2009, Beery and Zucker, 2010, Ophir et al., 2012, Calcagnoli et al., 2014). Many studies have also investigated the role of the mesolimbic dopamine system and opioid regulation of rewarding social behaviors such as pair-bonds between mates (Aragona, 2009, Resendez et al., 2012); we describe these and additional research avenues throughout.
1.2. Species diversity and the comparative perspective
In addition to considering how social behavior is assessed, we must consider the significance of the behavior to the species in which it is assessed. Social behavior encompasses skills from social recognition to social memory, as well as many distinct types of interaction, including with peers, potential reproductive partners, competitors, and offspring. Some of these interactions are better studied in some species than others; for example biparental care is only present in a few rodent species that have been studied in laboratories, namely prairie voles (Microtus ochrogaster), California mice (Peromyscus californicus), and Djungarian hamsters (Phodopus campbelli). Monogamous pairing with mates is similarly rare among rodents, and is most studied in prairie voles and California mice. Mechanisms supporting group living have been in explored in colonial rodents including naked mole-rats (Heterocephalus glaber), tuco-tucos (Ctenomys sociabilis), seasonally social meadow voles (Microtus pennsylvanicus), and others (Anacker and Beery, 2013). The idea that some problems are best studied in particular species is far from new; this principle was promoted in 1929 by the late physiologist and Nobel laureate August Krogh (Krebs, 1975). In contrast to Krogh's assertion that species should be selected for their suitability for studying particular problems, modern biological research is strongly biased towards rats and mice; in 2009 rats and mice made up approximately 90% of mammalian research subjects in physiology, up from 18% at the time Krogh's principle was articulated (Beery and Zucker, 2011 supplementary material). Lab strains of mice and rats are highly inbred and in many ways quite different from their wild peers.
Use of multiple species allows researchers to compare and contrast mechanisms across the phylogenetic tree. While the depth of mechanistic information available for non-model organisms is much less than for rats and mice, the comparative perspective is essential for understanding to what extent mechanisms underlying social behavior are unique to particular species, common across broader groups, or are variations on a theme (Phelps et al., 2010, Katz and Lillvis, 2014; Hofmann et al., 2014). In this review we focus on rats and mice for which data on stress and social behavior are most abundant, but incorporate findings from other rodent species whenever possible. And although laboratory research in rodents is heavily male-biased (Beery and Zucker, 2011), we review a substantial body of findings on the interrelationship of stress and social behavior in females.
2. The social environment as a stressor
All mammals interact with other individuals. In the wild, rodents may encounter competition for resources such as territory, food, and access to mates, and even solitary species interact with conspecifics and their chemical cues, if only to avoid them in the future. Both aversive and positive interactions are relevant features of the social environment. Widely used models of social stress in rodents include social subordination, crowding, isolation, and social instability (Fig. 1, left side). While most studies have been conducted in mice and rats, prairie voles and other social rodent species provide an opportunity to study the role of identity of the social partner, and how separation from a mate differs from isolation from a same-sex peer.
2.1. Social defeat/subordination
In humans, social rejection is used as a potent experimental stressor (Kirschbaum et al., 1993), and decades of work in humans and non-human primates have demonstrated that an individual's position in the social hierarchy has profound implications for health and well-being (Adler et al., 1994, Sapolsky, 2005). In rodents, the most prominent model of stressful social interaction is social defeat. Social defeat is typically induced by a version of the resident-intruder test in which a test subject is paired with a dominant resident in its home cage. Dominance may be assured by size, prior history of winning, strain of the resident, and/or prior housing differences (Martinez et al., 1998). Defeat may be acute or repeated, with many possible variations on the method. Social defeat is typically used as a stressor in male rodents, for whom dominance is easier to quantify and aggressive interactions related to home territory are presumed more salient. A few studies report effects of social defeat on females, particularly in Syrian hamsters in which females are highly aggressive and dominant to males (Payne and Swanson, 1970). In rats and mice, females do not always show a significant response to this task and the effect in males is far greater (Palanza, 2001, Huhman et al., 2003). Thus, other stress paradigms such as social instability are more widely used with females (Haller et al., 1999).
Social defeat can have a more substantial impact on male rodent physiology and behavior than widely used stressors such as restraint, electric shock, and chronic variable mild stress (Koolhaas et al., 1996, Blanchard et al., 1998, Sgoifo et al., 2014). In the short-term, social defeat produces changes in heart rate, hormone secretion, and body temperature, with longer-term impacts on a wide variety of additional outcomes including activity, social behavior, drug preference, disease susceptibility and others (Martinez et al., 1998, Sgoifo et al., 1999, Peters et al., 2011). Unlike physical stressors such as restraint, social defeat does not appear to be susceptible to habituation or sensitization (Tornatzky and Miczek, 1993, Sgoifo et al., 2002), and can be used in groups housed with a single dominant individual (Nyuyki et al., 2012).
Social defeat stress has profound effects on hippocampal morphology and function (reviewed in McEwen and Magarinos, 2001, Buwalda et al., 2005, Mirescu and Gould, 2006, McEwen, 2012). These effects include reduction in hippocampal volume (Czéh et al., 2001) related to dendritic remodeling and reduced neurogenesis (Magariños et al., 1996, Gould et al., 1998), Social defeat also alters the ratio of mineralocorticoid to glucocorticoid receptors in the hippocampus (Buwalda et al., 2001, Veenema et al., 2003). As with most of neurobiological research, attention has centered on neurons as the brain mediators of the biological embedding of the social world. However, following recent reports on the effects of stress (in general, and particularly social stress) on astrocytes, oligodendrocytes and microglial cells, it has become clear that glial cells are likely to play a role in this process, and deserve more attention in future studies (Braun et al., 2009, Wohleb et al., 2011, Araya-Callís et al., 2012, Chetty et al., 2014).
Social hierarchy has also been explored in settings where dominance is established through unstaged social interactions that occur on an ongoing basis (e.g. Blanchard et al., 1995, Blanchard et al., 2001). A low position in the social (and economic/resource) hierarchy appears to be stressful across a wide range of species. Negative health effects of low social status have been particularly well documented in non-human primates (e.g. Sapolsky, 1989, Sapolsky, 2005, Virgin and Sapolsky, 1997, Wu et al., 2014; Shively review, 2015). In humans, lower socioeconomic status (SES) predicts decreased mental and physical health in a graded fashion, and subjective perception of socioeconomic status may be an even more potent mediator than objective SES (Adler et al., 1994, Kawachi and Kennedy, 1999, Siegrist and Marmot, 2004, Singh-Manoux et al., 2005).
While low social status appears stressful across all instances discussed thus far, several studies have demonstrated that low status is not always stressful, in part dependent on species-particular life-history traits. For example, subordinate status is most stressful in species with despotic hierarchies, and may not be a stressor in “egalitarian” hierarchies with greater resource sharing. In the same vein, high status is more stressful in societies in which dominance must be continuously defended than in stable social hierarchies (Sapolsky, 2005). In a meta-analysis of cortisol levels in primates, Abbott et al. (2003) found that subordinates had higher basal CORT levels only when exposed to higher rates of stressors due to subordinate status, and when subordinate status afforded them few opportunities for social contact. In naked mole rats, a highly social rodent species that lives in large underground colonies, all but a few animals in each colony are reproductively suppressed subordinates (Sherman et al., 1991). In this instance, subordinates are related to breeders and are non-aggressive except in the event of loss of the breeding queen or her mates (Clarke and Faulkes, 1997). Reproductively suppressed subordinates do not have higher CORT levels than breeders and may have lower levels (Clarke and Faulkes, 1997, Clarke and Faulkes, 2001). While it is not yet clear how stress relates to status in this species, social subordination must be considered in the context of how it affects the individuals involved. Notably, social defeat may be more universally stressful than low status.
2.2. Crowding
Housing density affects rodent behavior, and both crowded and isolated social environments have been used as stressors in rodents. Crowding is a naturalistic stressor especially for social or gregarious species that relates to high population density and resource competition in the field. In house mice, several studies have shown that crowding can impair reproductive function and may be part of population size regulation (Christian and Lemunyan, 1958, Christian, 1971). In the highly social, group-living rodent species the degu (Octadon degus), increased group size is associated with greater dispersal consistent with a “social competition” hypothesis (Quirici et al., 2011).
In the laboratory, crowding typically consists of large numbers of mice or rats (e.g. >6 rats/cage (Brown and Grunberg, 1995, Reiss et al., 2007)) with ad libitum access to resources such as food and water. Crowding must be somewhat extreme to induce stressful outcomes, as group-housing (e.g. 4–6 rats or 12 mice in a sufficiently large area) is often used as a key component of environmental enrichment (Sztainberg and Chen, 2010, Simpson and Kelly, 2011).
Social crowding has been shown to impact many different physiological outcomes in male mice, rats, and prairie voles. These include changes in organ weights, hormone secretion, HPA reactivity, pain sensitivity, telomere length, and cardiac outcomes (Gamallo et al., 1986, Gadek-Michalska and Bugajski, 2003, Kotrschal et al., 2007, Grippo et al., 2010, Tramullas et al., 2012, Puzserova et al., 2013). Crowding of pregnant dams also produces changes in the offspring birth weight, pubertal timing, and reproductive behavior (e.g. Harvey and Chevins, 1987, Ward et al., 1994) and may lead to lasting changes through a subsequent generation (Christian and Lemunyan, 1958). There appear to be important sex differences in the consequences of crowding, with one study in rats finding that crowding is a stressor for males but has the capacity to calm females (Brown and Grunberg, 1995).
2.3. Isolation
At the opposite extreme, solitary housing can be a potent stressor for social species. Social isolation is employed as a stressor in previously group-housed mice and rats (Heinrichs and Koob, 2006); in both species, extended (2–13 week) solitary housing produces an “isolation syndrome” particularly in females, consisting of hyperadrenocorticism, reduced body weight, altered blood composition, and enhanced pain responsiveness among other outcomes (Hatch et al., 1965, Valzelli, 1973). These changes coincide with alterations in behavior including aggression, mating behavior, learning, and pain sensitivity (Valzelli, 1973). More recent studies have added a host of additional physiological outcomes related to stress and depressive behavior, including changes in dopamine signaling in different brain regions (Heidbreder et al., 2000), altered heart rate and cardiac function (Späni et al., 2003, Carnevali et al., 2012), and neurogenesis (Stranahan et al., 2006, Lieberwirth and Wang, 2012). Which outcomes are affected by isolation depend in part on the age at which isolation occurs (reviewed in Hall, 1998), and there are sex differences in the effects of social isolation. These suggest that isolation may be stressful for females but not necessarily to the same extent for males (Hatch et al., 1965, Palanza, 2001, Palanza et al., 2001). Assessing the impacts of both isolation and crowding share the problem of what to consider as the control comparison, as anxiety and other behavioral outcomes vary along a continuum of group sizes (Botelho et al., 2007).
In recent decades, prairie voles have become a popular model for studying social behaviors because of their unusual capacity to form socially monogamous pair-bonds with opposite sex mates (Getz et al., 1981). An additional advantage of this species is that the effects of social manipulations can be contextualized in terms of findings from field populations and semi-natural settings (e.g. Ophir et al., 2008, Mabry et al., 2011). In wild prairie voles, cohabitation with a mate or a mate and undispersed offspring is common (Getz and Hofmann, 1986), and reproductively naïve prairie voles are affiliative towards their same-sex cage mates. In the lab, separation of adult prairie voles from a sibling cage-mate for 1–2 months reduced sucrose consumption (a measure of anhedonia), and was associated with increased plasma levels of oxytocin, CORT, and ACTH, as well as increased activity of oxytocin neurons in the hypothalamus following a resident intruder test. These effects were more profound in females (Grippo et al., 2007). Further work has shown that social isolation from a sibling also leads to changes in cardiac function associated with cardiovascular disease (Grippo et al., 2011, Peuler et al., 2012), and immobility in the forced swim test (Grippo et al., 2008) – considered a measure of depressive behavior. Some physiological and behavioral sequelae were prevented or ameliorated by exposure to environmental enrichment, or by peripheral administration of oxytocin (Grippo et al., 2009, Grippo et al., 2014), as has been demonstrated in rats (Hellemans et al., 2004). Social isolation of prairie voles from weaning has been associated with higher circulating CORT, and greater CRF immunoreactivity in the paraventricular nucleus (PVN) of the hypothalamus (Ruscio et al., 2007). While the majority of current studies have focused on social isolation from a non-reproductive partner, recent investigation into disruption of opposite-sex pairs takes advantage of this unusual feature of prairie vole behavior, and suggests that mate-pair disruption has substantial autonomic and behavioral consequences for both male and female prairie voles (Bosch et al., 2009; McNeal et al., 2014).
As the work in prairie voles illustrates, it is important to consider the natural history of species when social manipulations are performed. For example, male Syrian hamsters housed in isolation are more aggressive than those housed in groups (Brain, 1972), but that is not to suggest that isolation was distressing, or produced an unusual behavioral phenotype, as this species is naturally solitary (Gattermann et al., 2001). Conversely, crowding might be a particularly potent but unnatural stressor for this species, and it has been associated with increased mortality (Germann et al., 1990, Marchleswska-Koj, 1997). Social species provide good subjects for studying the influence of social interactions on health and related outcomes, and this has been demonstrated both in the laboratory and in the field. In a species of South American burrowing rodent – the colonial tuco–tuco (C. sociabilis) – females may live alone or share a burrow with several other adults members and their young (Lacey et al., 1997). Yearling C. sociabilis that live alone (whether via dispersal in the field or investigator manipulations in the lab), have significantly higher baseline fecal glucocorticoid metabolite levels than do group-living individuals in the same environments (Woodruff et al., 2013). In a putatively monogamous species of wild guinea pig (Galea monasteriensis), social separation induces increases in cortisol secretion that are only rectified by return of the social partner (Adrian et al., 2008). The study of species in the context of their natural behavior allows us to better understand stress-related outcomes in a variety of rodent species.
2.4. Social instability
Some studies employ both crowding and isolation in alternation (for example, 24 h of each for 2 weeks), as a model for chronic social instability (e.g. Haller et al., 1999, Herzog et al., 2009). Social instability has particularly been used as a social stressor for female rats, for whom crowding and social defeat are not always effective stressors (Palanza, 2001). In the crowding phase, different social groups consisting of different numbers of males and females are formed. Females exposed to this variable social environment show increased adrenal weight, increased corticosterone secretion, decreased thymus weight, and reduced weight gain relative to females housed in stable male–female pairs (Haller et al., 1999). A second study replicated these findings and demonstrated that social instability also induced dysregulation of the hypothalmic–pituitary–gonadal (HPG) axis (elevated luteinizing hormone, prolactin, and disrupted estrus cycles), and reduced sucrose preference and food intake (Herzog et al., 2009). This stressed phenotype persisted for several weeks without habituation and led to a depressive-like phenotype. Prior history of social instability in the form of early-life separation from the mother also exacerbates vulnerability to later life chronic subordination stress (Veenema et al., 2008).
3. Social behavior responds to stress (in species, sex, and context specific ways)
In humans, stressful situations can promote affiliative behavior (Zucker et al., 1968, Teichman, 1974, Taylor, 2006) and anticipation of stressful events can promote group cohesion and liking for group members (Latané et al., 1966, Morris et al., 1976). All stress is not the same, however, and in some cases, social behavior is reduced after a stressor – in fact social withdrawal is one of the diagnostic criteria for post-traumatic stress disorder (DSM V, American Psychiatric Association, 2013). While effects of stress on social behavior are evident in humans, most of our understanding of these impacts, and of the underlying molecular and cellular mechanisms, come from rodent studies. In rodents, several stressors and manipulations of the hypothalamic–pituitary–adrenal (HPA) hormonal axis have been shown to impact a variety of subsequent social behaviors. In this case, much of what we know comes from research on prairie voles for which there appear to be important differences between the sexes, with some outcomes dependent on whether the partners are same-sex siblings or opposite-sex mates.
3.1. Stress, HPA axis regulation, and opposite-sex social behavior
As previously mentioned, prairie voles provide an opportunity to study pair-bond formation between males and females, as this species forms reproductive pair bonds both in the laboratory and in the field. Prairie voles also exhibit unusually high levels of circulating CORT relative to other rodents including montane voles, rats, and mice (DeVries et al., 1995) moderated by reduced tissue sensitivity to glucocorticoids (Taymans et al., 1997, Klein et al., 1996). Stress has opposite effects on the formation of mate preferences in male and female prairie voles. In males, stressful experiences mildly enhances the ability to form partner preferences for females. Males do not typically form a partner preference for a female after 6 h of cohabitation, however they form significant preferences within this time interval when paired after a brief swim stress (DeVries et al., 1996). Preference formation is also facilitated by CORT administration in male prairie voles, and impaired by adrenalectomy (DeVries et al., 1996). Some doses of central CRF administration also facilitate partner preference formation in males (DeVries et al., 2002). Interestingly, CORT decreases after pairing with a female, but partner preferences are not established during the early cohousing interval, and CORT levels have returned to baseline by the time male preferences have been formed (DeVries et al., 1997).
In female prairie voles, stress impairs partner preference formation, but this effect is prevented in adrenalectomized voles (DeVries et al., 1996). This phenomenon appears to be mediated by CORT, as exposure to CORT during (but not after) cohabitation with a novel male prevents partner preference formation, and adrenalectomized females form partner preferences after shorter cohabitation periods than are typically necessary (DeVries et al., 1995). CORT levels are naturally low immediately following cohousing with a male, and partner preferences are formed before they return to baseline (DeVries et al., 1995).
In rats, stress also impacts opposite-sex social behavior. In particular, stress has been shown to inhibit mating behavior in males and in naturally cycling females, via elevation of the inhibitory hypothalamic hormone RF-amide related peptide 1 (Kirby et al., 2009, Geraghty et al., 2013).
3.2. Stress, HPA axis regulation, and same-sex social behavior
Same-sex interactions have not been as well explored in prairie voles as opposite-sex affiliative interactions have been, although some data suggest same-sex affiliative behavior in prairie voles may be enhanced following a stressor (DeVries and Carter, unpublished data referenced in Carter, 1998). Same-sex affiliative behavior can be studied more broadly in rodent species that live in groups, so additional rodent species may be informative for this question.
Meadow voles are conditionally social rodents, with photoperiod-mediated seasonal variation in social huddling. While females are aggressive and territorial in summer months, they live in social groups and huddle with conspecifics in winter months or short day lengths in the laboratory (Madison et al., 1984, Madison and McShea, 1987, Beery et al., 2008b, Beery et al., 2009). Seasonal variations in huddling and partner preference formation allow for the study of the endocrine and neurobiological mechanisms underlying changes in social tolerance and peer affiliation outside the context of mate-pairing. In meadow voles, CORT varies seasonally (Boonstra and Boag, 1992, Galea and McEwen, 1999, Pyter et al., 2005) and may relate to changes in social tolerance. CRF/urocortin pathways may also link stress-reactivity and social behavior in this species, as CRF1 and CRF2 receptor densities change with day length and are associated with huddling behavior (Beery et al., 2014). Stress exposure prior to pairing impairs preference formation for a same-sex individual in female of this species (Anacker et al., 2014). Ongoing studies are examining the role of CORT and stressor timing. In addition, familiarity of the conspecific prior to the stressor may influence whether social behavior is increased or decreased.
Wild rats live in gregarious colonies, where social interactions may be beneficial for predator avoidance and under other stressful conditions (Macdonald et al., 1999). In male rats, social defeat stress leads to social avoidance – less time spent in social contact with an unfamiliar non-aggressive rat (Meerlo et al., 1996) and avoidance of the dominant rat (Lukas et al., 2011). Non-social stressors may have the opposite effect, for example, in groups of familiar male rats, rats spend more time huddling in large groups during an immediate stressor (cat fur or bright light). This effect has been termed defensive aggregation, and is facilitated by oxytocin (Bowen et al., 2012, Bowen and McGregor, 2014).
3.3. Individual variability in the social behavior response to stress – resilience vs. vulnerability
Exposure to chronic social defeat stress leads to social avoidance, altered fear acquisition and elimination, anhedonia, changes in neural circuitry and transmission, neurogenesis and metabolism in groups of exposed versus unexposed subjects (Chou et al., 2014, Donahue et al., 2014). However, looking at individual outcomes reveals a much more complex picture, even in inbred mice. For example, measuring social motivation after exposure to social defeat stress reveals a bimodal segregation of the group into affected and unaffected individuals. Affected individuals spend less time interacting with conspecific peers in the social zone, while unaffected (unsusceptible) individuals spend time in the social zone similar to unstressed individuals. Susceptibility to social aversion following social defeat is associated with a suite of other signs of stress including decreased sucrose preference, decreased body weight, and increased sensitivity to cocaine-induced conditioned place preference (Krishnan et al., 2007).
What is the difference between responders and non-responders, or a resilient vs. vulnerable trajectory? Interestingly, this resilience phenotype did not correlate with social motivation pre-stress, nor with levels of circulating glucocorticoids (Krishnan et al., 2007). However, stress-susceptibility has been correlated with stress-induced increase in levels of brain derived neurotrophic factor (BDNF), a key regulator of dopamine release in the nucleus accumbens (NAc). Following 10 days of repeated social defeat, BDNF protein levels were persistently elevated in the NAc of mice. Reduction of BDNF levels in the ventral tegmental area (VTA) via local BDNF knockdown provided an antidepressant-like effect relative to untreated, defeated mice and prevented social aversion (Berton et al., 2006). Investigation of the individual differences between susceptible and unsusceptible mice revealed that susceptibility was characterized by increased NAc BDNF, but reinforced the importance of BDNF release from the VTA, as knockdown in the VTA but not NAc promoted resilience. Susceptibility to defeat was further shown to be mediated by enhanced firing of VTA dopamine neurons, with resilience characterized by a lack of activity-dependent BDNF release (Krishnan et al., 2007).
Interestingly, unsusceptible individuals were not lacking a neural response, but in fact showed greater change in gene expression patterns in the VTA than susceptible individuals – suggesting that behavioral non-responsiveness is an active process and not merely a lack of the pathological process. Analysis of differential gene expression revealed significant down-regulation of several members of the WNT (Wingless)-dishevelled signaling cascade, including phospho-GSK3β (glycogen synthase kinase-3β), in the NAc of susceptible, but not resilient, mice (Wilkinson et al., 2011). Regulation of HPA axis activity, and specifically reduced expression of CRF (regulated by stress-induced demethylation of regulatory areas of the gene CRF1) was shown in the subset of vulnerable mice that displayed social avoidance (Elliott et al., 2010) and in mice that displayed short latency to defeat in the resident/intruder paradigm (Wood et al., 2010). Supporting this finding, knockdown of CRF levels diminished stress-induced social avoidance (Elliott et al., 2010). In a separate model of chronic subordinate colony housing, mice selectively bred for low anxiety were behaviorally resilient to subordination stress, and showed distinct HPA axis responses (Füchsl et al., 2013).
Several neurotransmission systems are implicated in social-stress resilience vs. vulnerability: in addition to BDNF-control of dopamine mentioned above, differences in the NAc dopaminergic system resulting from differential maternal behavior are correlated with increased preference for social interactions in a group of highly groomed rat offspring (Peña et al., 2014). Glutamatergic, serotonergic, and GABAergic systems appear to be involved as well. Vulnerable and resilient animals differ significantly in the expression of AMPA receptors in the dorsal hippocampus, and activation of AMPA receptor during the stress exposure prevented the physiological, neuroendocrine, and behavioral effects of chronic social stress exposure (Schmidt et al., 2010). Knockout of serotonin transporter increases the vulnerability to social avoidance following social defeat (Bartolomucci et al., 2010). Finally, supression of the GABAergic system is seen in the pre-frontal cortex of mice showing depressive symptoms following social defeat (Veeraiah et al., 2014), and in amygdala of mice exposed to peripubertal stress (Tzanoulinou et al., 2014). Similar suppression is found in the cortex of human patients with PTSD (Meyerhoff et al., 2014).
4. Resilience and social buffering: social interaction can moderate effects of a stressor
Stress exposure not only alters social interaction, but that social interaction can in turn play a role in buffering or moderating the effects of that stressor, providing adaptive value of social networks for coping with stress exposure. We can think about stress-resilience in multiple layers: life-long programming of stress-resilient individuals originating from the early life environment and in particular through maternal interactions (Parker et al., 2012; Lyons et al., 2010, Szyf et al., 2007); short-term resilience after an acute moderate stressor promoting better functioning after a secondary stressor (Kirby et al., 2013); or resilience that comes from mitigating (buffering) the effects of stress by positive, supportive social environment, or even by aggressive social interactions. For example, lower ranking baboons that show displacement of aggression on peers have lower CORT levels (Virgin and Sapolsky, 1997).
The effects of social buffering are far reaching, and in humans there is evidence that social relationships aid immune function, cardiovascular health, and other health-related outcomes (reviewed in Berkman and Kawachi, 2000). Stable natural social relationships have even been associated with increased longevity in humans and other species (humans: Holt-Lunstad et al., 2010; baboons: Silk et al., 2010; rats: Yee et al., 2008; dolphins: Stanton and Mann, 2012). The endocrine consequences of social buffering were first described in primates (Coe et al., 1978, Mendoza et al., 1978) and primate studies continue to be important particularly for our understanding of natural social buffering in the context of stress. For example in female Chacma baboons, loss of a partner results in elevated CORT and also in enhanced social behaviors such as allogrooming which may help mediate the decline to baseline levels (Engh et al., 2006). Studies of social manipulations in rodents have also played a pivotal role in our understanding of social support on a variety of behavioral, endocrine, and neurobiological outcomes (reviewed in DeVries et al., 2003, Kikusui et al., 2006).
In rodents, most studies of social buffering have focused on the presence or absence of a conspecific such as the cage-mate after a stressor. As one might imagine, many different variables may affect whether social buffering occurs, including the familiarity of the conspecific, the relative hierarchy, presence or absence during stress exposure, whether the cage-mate was also stressed, sex of the individual and partner, sensory modalities of exposure to that individual, timing of the availability of social support and so forth. While these parameters have by no means been explored in all combinations, we summarize what is known for each variable across a variety of rodent species.
4.1. Buffering effects on behavior
Social contact seeking is altered following stress exposure in male rats. Rats temporarily housed in an open field spend more time together than expected by chance (Latané, 1969), and stressed males are more likely to interact socially than non-stressed males (Taylor, 1981). Investigator-manipulated housing conditions (solitary-, pair-, or group-housing) also affect reactions to stress. Conditioned avoidance of noxious stimuli is reduced in pair-housed animals (Hall, 1955, Baum, 1969). Pair-housed rats also show reduced impacts of stress exposure relative to rats housed alone in their response to white noise (Taylor, 1981) and foot shock (Davitz and Mason, 1955, Kiyokawa et al., 2004). Group-housed rats exposed to social defeat exhibit greater growth and less anxiety behavior in repeated open field exposure relative to solitary-housed rats (Ruis et al., 1999). Solitary housing increases anxiety-like behaviors on its own (see above section); thus distinguishing between effects of isolation and effects of a stressor (and their potential interactions) requires that all housing conditions be paired with both the stressor and lack thereof. In studies where this has occurred, pair-housed animals do not show stress-induced anxiety behavior changes relative to control pair-housed animals, unlike solitary-housed individuals (Nakayasu and Ishii, 2008). More recent studies have examined novel behavioral outcomes, including social buffering effects on pain tolerance (reviewed in Martin et al., 2014) and changes in alcohol consumption (Anacker et al., 2011; Hostetler and Ryabinin, 2014).
4.2. Buffering effects on the HPA axis
Social housing impacts HPA axis responsiveness to a stressor or to hormonal stimulation via CRF. Following CRF administration, male group-housed rats have reduced CORT and ACTH relative to isolated males (Ruis et al., 1999). In young male guinea pigs, presence of the mother or an unfamiliar adult female attenuates increases in plasma ACTH, cortisol and vocalizations in response to a novel environment (Hennessy et al., 2000), with additional, subtly varying effects across the lifespan (Hennessy et al., 2006).
Studies in prairie voles allow for distinction between buffering by social peers and reproductive partners. In prairie voles, exposure to a novel individual of the opposite sex leads to a decline in serum CORT over the following 15–60 min in both males and females, while same-sex novel pairings did not influence serum CORT (DeVries et al., 1997, DeVries et al., 1995). This decline in CORT may be important for the ability of the female to form a partner preference, while it must pass in order for males to form (CORT-dependent) partner preferences (DeVries, 2002).
The nature of social buffering may be quite different within established social relationships: in prairie voles, female sibling pairs experienced elevated CORT following separation and this effect was attenuated following reunion (unpublished data referenced in Carter et al., 1995). In males, loss of a female partner also resulted in increased circulating CORT as well as increased adrenal weight (Bosch et al., 2009). The presence of a partner may provide social buffering from a stressor; female prairie voles that recovered alone from immobilization stress exhibited high levels of CORT and increased anxiety behavior, while females recovering with their male partner showed no such elevation (Smith and Wang, 2014).
While CORT is an easily measured signal that often relates to stress level, it is worth noting that measurement of glucocorticoids is not always a clear indicator of either stress exposure or stressed affect, and stress may result in both enhanced and dampened CORT profiles depending on timing and chronicity (e.g. Sapolsky et al., 2000, Beery et al., 2012).
4.3. Buffering effects on other neurochemical outcomes
Social companionship has been associated with outcomes beyond the HPA axis, although many of these changes may ultimately be related to common pathways. For example, in prairie voles, females recovering from immobilization stress with a male partner showed no CORT elevation, coupled with evidence of increased oxytocin (OT) release in the paraventricular nucleus (PVN) of the hypothalamus. Direct administration of OT to the PVN reduced CORT responses to a stressor, while oxytocin receptor antagonist (OTA) injection prevented the ameliorative effects of housing with the partner (Smith and Wang, 2014). This parallels research in humans in which OT and social buffering interact to reduce CORT responses to a social stressor (Heinrichs et al., 2003). Other neuroendocrine changes have also been documented in response to social support. For example, the presence of a conspecific in an open-field test reduces peripheral prolactin in male rats (Wilson, 2000).
4.4. Buffering effects on immune and organ systems
Relative to isolated individuals, socially housed female Siberian hamsters experience improved wound healing; an effect which is mediated by oxytocin (Detillion et al., 2004). While little is known about the natural social organization of this hamster species (Wynne-Edwards and Lisk, 1989), wound healing has also been studied in three species of Peromyscus mice for which social organization is well characterized. In the two species of monogamous or facultatively monogamous Peromyscus mice, wound healing was facilitated by social contact. This was not the case in the promiscuous species, and this species did not experience reduced CORT with pair-housing (Glasper and DeVries, 2005). This suggests that social housing was beneficial only to the species that normally resides with a partner. Some recent findings in humans suggest that higher blood oxytocin and vasopressin levels may also be associated with faster wound healing in our species (Gouin et al., 2010).
Social environment during stress has been shown to impact gastric ulcer formation in male rats following a stressor, however, only the social environment at the time of testing and not prior housing affected ulcer frequency (Conger et al., 1958). Westenbroek et al. (2005) found that group-housed chronically stressed female rats had less adrenal hypertrophy than solitary-housed, stressed females.
Social housing and support have also been shown to impact the function of the cardiovascular system. In humans, social support reduces heart rate and alters the ratio of systolic to diastolic blood pressure after performing stressful tasks (Lepore et al., 1993, Thorsteinsson et al., 1998). In mice and prairie voles, social housing has been associated with lower heart rate (Späni et al., 2003, Grippo et al., 2007), as well as other measures of cardiovascular health (Grippo et al., 2011).
4.5. Partner identity and social buffering
Not all social interactions are equal, and the effects of social companionship may differ by partner familiarity, sex, age, species, and affective state. Most studies of social buffering have explored one or two of these contexts at a time, but some evidence suggests that each of these can, but does not necessarily, impact the social buffering provided. In guinea pigs, the presence of both familiar and unfamiliar adults reduces HPA activation in response to a novel environment; however for young (pre-weaning) guinea pigs, this effect is greater with the mother (Graves and Hennessy, 2000), and the salience of different individuals changes over the life course and varies with sex (Kaiser et al., 2003). In a pair of studies in male rats, Armario et al. found the surprising result that CORT levels in an open field were higher when paired with a familiar versus an unfamiliar individual (Armario et al., 1983a, Armario et al., 1983b). In prairie voles, brief separation from a mate, but not from a same-sex sibling, increased depressive-like behavior (Bosch et al., 2009).
Partner identity/familiarity was also found to be critical in a recently developed paradigm in which helping behavior is measured in rats. In this study, rats were motivated to rescue a trapped rat from restraint only if it was matched to their own strain, or a strain they had exposure to from birth; they were uninterested in freeing rats of an unfamiliar strain (Ben-Ami Bartal et al., 2014).
The partner's affective state also influences social buffering. In rats, exposure to naïve, unshocked individuals can lessen stress responses relative to exposure to shocked individuals (Kiyokawa et al., 2004), similar to earlier findings in fear-conditioned rats (Davitz and Mason, 1955). Future research on social buffering in rodents will hopefully make progress into questions of how and when social support is helpful, and what the optimal timing and type of that support is.
5. Anxiety and depression are associated with reduced social behavior
Stress occurs as a response to an external stimulus that can be fleeting. In contrast, anxiety is a lasting state that is not an immediate response to the external environment. While stressful events can have impacts on social behavior, individual differences in anxiety also relate to variation in social behavior. For example, in humans, extraverted personality is associated with lower trait anxiety (Jylhä and Isometsä, 2006, Naragon-Gainey et al., 2014).
In rodents, the social interaction test – in which social interaction with a familiar or an unfamiliar individual are measured in an open arena – was initially developed to be an ethologically relevant measure of anxiety behavior (File and Hyde, 1978). Social interaction times of individual male and female rats are positively correlated with exploratory behavior in classic tests of anxiety-like behaviors. For example, individuals that spend more time in social interaction are more likely to spend more time in the center region of an open field or the light portion of a light-dark box (Starr-Phillips and Beery, 2014).
Maternal care, particularly maternal grooming behavior, has lasting effects on offspring anxiety behavior. High levels of maternal grooming are associated with reduced anxiety behavior in two paradigms: pup reunion after brief separation and/or handling, and natural, individual variation in maternal care (reviewed in Gonzalez et al., 2001, Meaney, 2001, Beery and Francis, 2011). Natural variations in the amount of time dams spend licking and grooming their new pups in the first week of life impacts their offspring in many ways that persist into adulthood. Reduction in stress-reactivity in rats reared by high-licking dams appears to be mediated by increased glucocorticoid receptor expression in the hippocampus (Liu et al., 1997, Weaver et al., 2004) which enhances negative feedback on the HPA axis (Sapolsky et al., 1985, Liu et al., 1997).
Recent studies have shown that natural variation in maternal care affects a wide range of outcomes beyond anxiety behavior, including social behaviors. High levels of early maternal grooming are associated with increased play behavior in juvenile male rats (Parent and Meaney, 2008, Van Hasselt et al., 2012), increased social interaction in adult offspring of both sexes (Starr-Phillips and Beery, 2014), and altered play dominance rank in adult female rats (Parent et al., 2013). Effects of maternal contact have also been described in other species; for example in prairie voles, maternal care and family structure have been associated with social investigation in adolescence, and changes in parental and mate-directed behaviors in adulthood (Ahern and Young, 2009, Perkeybile et al., 2013). Early experience of maternal care is sometimes associated with changes in oxytocin and vasopressin system regulation (reviewed in Veenema, 2012), although it is not yet clear whether such changes underlie the known differences in social behavior.
6. Sex differences in reactions to stress and implications
In a synthesis of findings across rodents, primates, and human studies, Shelly Taylor proposed that in addition to flight-or-flight responses to stress, females show pronounced “tend and befriend” responses to a stressor (Taylor et al., 2000). Taylor related “tending” to parental nurturing behaviors, based on evidence that rat dams lick their pups (tending) following separation, that oxytocin appears to be more elevated in females following a stressor, and that oxytocin can act both as an anxiolytic and to promote affiliative behavior. “Befriending” was related to the adaptive value of social support under stressful conditions, and its particular value for females that might be more vulnerable than males.
Whether or not shared history of maternal care-giving and defensive social behaviors best explains distinct female responses to stress, the existence of such sex differences in stress/social behavior interactions has been demonstrated repeatedly. We have discussed several examples in this review; first, we described sex differences in the potency of particular stressors, for example crowding is particularly stressful for males, but is either calming to females or does not have major effects on physiological endpoints (Brown and Grunberg, 1995, Kotrschal et al., 2007). Even when the same event is stressful to both males and females, the sequelae of stress exposure may differ, for example stress impairs classical conditioning in females, which is the opposite of the effect found in males (Wood and Shors, 1998).
Sex differences are also present in social behavior responses to stress: conditions of stress, high CORT, and high CRF facilitate pair-bonding in male prairie voles, while the same conditions impair pair-bonding in females voles (DeVries et al., 1996). Even where both sexes appear to be supported by their same-sex peers, male and female rats exhibit anxiety responses and adrenal reactions under different combinations of conditions (Westenbroek et al., 2005).
Some of these differences may relate to neurochemical variation in the brains of males and females. Both oxytocin and vasopressin are important for social behavior, and there are sex differences in the production and release of these neuropeptides, the location and density of their receptors, and their roles in social behavior (Bales and Carter, 2003, Carter, 2007). There are many sex differences in human psychiatric disorders, most notably anxiety and depression, which some argue are based on sex differences in responses to stress (Bangasser and Valentino, 2014).
One consequence of these findings is that we must study the interactions of stress and social behavior in both sexes in order to make meaningful conclusions about each sex. This idea is gaining greater appreciation within the scientific and funding communities (Mogil and Chanda, 2005, Cahill, 2006, Zucker and Beery, 2010, Couzin-Frankel, 2014, Clayton and Collins, 2014, Woodruff et al., 2014).
7. Conclusions
The social environment can cause stress or ameliorate the impacts of stress, and social behavior responds to stress. These effects may happen all together or at different times, and vary with individual genetic background, experience, sex, species, and other factors. While it is not feasible to study all such factors in a single study, almost a century of research has helped to show which stressors are most impactful in males and females, and how such stress is reflected in neurochemistry. Interaction time is a longstanding measure of social behavior, but recent studies have begun to employ more nuanced approaches – for instance measuring helping behavior and distinguishing preferences for familiar versus unfamiliar individuals.
While adverse social conditions (from subordination to isolation) are potent stressors, the interactions between stress and social behavior also offer multiple entry points into the study of stress resilience. Stress resilience varies with early life social environment—in particular with experience of maternal behavior and life history of exposure to mildly stressful experiences. Resilience can also arise from the mitigating or buffering effects of positive (or negative) social interactions. There is a vast body of literature linking stress and social behavior and their roles in resilience. We may learn the most from these studies when we consider the social life of the organism, and look beyond group averages to individual variability.
Acknowledgments
We are grateful to Dr. Julio Ozores for engaging discussions on this topic, and to Drs. Allison Anacker and Noopur Amin and anonymous reviewers for feedback on this manuscript. This work was supported by National Science Foundation Award #1257162 to AB, and NIH/NIMH BRAINS Innovation award #MH087495 to DK.
References
- Abbott D., Keverne E., Bercovitch F., Shively C., Mendoza S., Saltzman W., Snowdon C., Ziegler T., Banjevic M., Garlandm T., Sapolsky R. Are subordinates always stressed? a comparative analysis of rank differences in cortisol levels among primates. Horm. Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Adler N.E., Boyce T., Chesney M.A., Cohen S., Folkman S., Kahn R.L., Leonard S. Socioeconomic status and health: the challenge of the gradient. Am. Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Adrian O., Kaiser S., Sachser N., Jandewerth P., Löttker P., Epplen J.T., Hennessy M.B. Female influences on pair formation, reproduction and male stress responses in a monogamous cavy (Galea monasteriensis) Horm. Behav. 2008;53:403–412. doi: 10.1016/j.yhbeh.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Ahern T.H., Young L.J. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster) Front. Behav. Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J., Aleksejev R.M., Paul E., Watkins L.R., Maier S.F. Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience. 2010;165:1031–1038. doi: 10.1016/j.neuroscience.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Publishing; Washington, D.C: 2013. Diagnostic and Statistical Manual of Mental Disorders. DSM-5. [Google Scholar]
- Anacker A.M.J., Beery A.K. Life in groups: the roles of oxytocin in mammalian sociality. Front. Behav. Neurosci. 2013;7:185. doi: 10.3389/fnbeh.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker A.M.J., Loftis J.M., Kaur S., Ryabinin A.E. Prairie voles as a novel model of socially facilitated excessive drinking. Addict. Biol. 2011;16:92–107. doi: 10.1111/j.1369-1600.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker, A.M.J., Reitz, K.M., Gunzel, E.R., Beery, A.K., 2014. Stress inhibits partner preference in same-sex pairs of female meadow voles. Program No 64112PP14 2014 Neurosci Meet Plan Wash DC Soc Neurosci 2014 Online.
- Aragona B.J. Dopamine regulation of social choice in a monogamous rodent species. Front. Behav. Neurosci. 2009;3 doi: 10.3389/neuro.08.015.2009. http://www.frontiersin.org/Journal/10.3389/neuro.08.015.2009/full Available at: (accessed 28.02.14.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya-Callís C., Hiemke C., Abumaria N., Flugge G. Chronic psychosocial stress and citalopram modulate the expression of the glial proteins GFAP and NDRG2 in the hippocampus. Psychopharmacology (Berl) 2012;224:209–222. doi: 10.1007/s00213-012-2741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A., Luna G., Balasch J. The effect of conspecifics on corticoadrenal response of rats to a novel environment. Behav. Neural Biol. 1983;37:332–337. doi: 10.1016/s0163-1047(83)91425-5. [DOI] [PubMed] [Google Scholar]
- Armario A., Ortiz R., Balasch J. Corticoadrenal and behavioral response to open field in pairs of male rats either familiar or non-familiar to each other. Experientia. 1983;39:1316–1317. doi: 10.1007/BF01990391. [DOI] [PubMed] [Google Scholar]
- Bale T.L., Vale W.W. CRF and CRF Receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bales K.L., Carter C.S. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm. Behav. 2003;44:178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A., Carola V., Pascucci T., Puglisi-Allegra S., Cabib S., Lesch K.-P., Parmigiani S., Palanza P., Gross C. Increased vulnerability to psychosocial stress in heterozygous serotonin transporter knockout mice. Dis. Model. Mech. 2010;3:459–470. doi: 10.1242/dmm.004614. [DOI] [PubMed] [Google Scholar]
- Baum M. Extinction of an avoidance response motivated by intense fear: social facilitation of the action of response prevention (flooding) in rats. Behav. Res. Ther. 1969;7:57–62. doi: 10.1016/0005-7967(69)90049-7. [DOI] [PubMed] [Google Scholar]
- Beery A.K., Francis D.D. Adaptive significance of natural variations in maternal care in rats: a translational perspective. Neurosci. Biobehav. Rev. 2011;35:1552–1561. doi: 10.1016/j.neubiorev.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery A.K., Lacey E.A., Francis D.D. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis) J. Comp. Neurol. 2008;507:1847–1859. doi: 10.1002/cne.21638. [DOI] [PubMed] [Google Scholar]
- Beery A.K., Lin J., Biddle J.S., Francis D.D., Blackburn E.H., Epel E.S. Chronic stress elevates telomerase activity in rats. Biol. Lett. 2012;8:1063–1066. doi: 10.1098/rsbl.2012.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery A.K., Loo T.J., Zucker I. Day length and estradiol affect same-sex affiliative behavior in the female meadow vole. Horm. Behav. 2008;54:153–159. doi: 10.1016/j.yhbeh.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery A.K., Routman D.M., Zucker I. Same-sex social behavior in meadow voles: multiple and rapid formation of attachments. Physiol. Behav. 2009;97:52–57. doi: 10.1016/j.physbeh.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery A.K., Vahaba D.M., Grunberg D.M. Corticotropin-releasing factor receptor densities vary with photoperiod and sociality. Horm. Behav. 2014 doi: 10.1016/j.yhbeh.2014.08.014. http://dx.doi.org/10.1016/j.yhbeh.2014.08.014 http://www.sciencedirect.com/science/article/pii/S0018506X14002001 Available at: (accessed 26.10.14) [DOI] [PubMed] [Google Scholar]
- Beery A.K., Zucker I. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience. 2010;169:665–673. doi: 10.1016/j.neuroscience.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Beery A.K., Zucker I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami Bartal I., Decety J., Mason P. Empathy and pro-social behavior in rats. Science. 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami Bartal I., Rodgers D.A., Bernardez Sarria M.S., Decety J., Mason P. Pro-social behavior in rats is modulated by social experience. eLife. 2014;3 doi: 10.7554/eLife.01385. http://elife.elifesciences.org/content/3/e01385.abstract Available at: (accessed 28.07.14.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman L.F., Kawachi I. Oxford University Press; 2000. Social Epidemiology. [Google Scholar]
- Berton O., McClung C.A., DiLeone R.J., Krishnan V., Renthal W., Russo S.J., Graham D., Tsankova N.M., Bolanos C.A., Rios M., Monteggia L.M., Self D.W., Nestler E.J. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Blanchard D.C., Spencer R.L., Weiss S.M., Blanchard R.J., McEwen B., Sakai R.R. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Blanchard R.J., Hebert M., Sakai R.R., McKittrick C., Henrie A., Yudko E., McEwen B.S., Blanchard D.C. Chronic social stress: changes in behavioral and physiological indices of emotion. Aggress. Behav. 1998;24:307–321. [Google Scholar]
- Blanchard R.J., McKittrick C.R., Blanchard D.C. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol. Behav. 2001;73:261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Boonstra R., Boag P.T. Spring declines in Microtus pennsylvanicus and the role of steroid hormones. J. Anim. Ecol. 1992;61:339–352. [Google Scholar]
- Bosch O.J., Nair H.P., Ahern T.H., Neumann I.D., Young L.J. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho S., Estanislau C., Morato S. Effects of under- and overcrowding on exploratory behavior in the elevated plus-maze. Behav. Process. 2007;74:357–362. doi: 10.1016/j.beproc.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Bowen M.T., Keats K., Kendig M.D., Cakic V., Callaghan P.D., McGregor I.S. Aggregation in quads but not pairs of rats exposed to cat odor or bright light. Behav. Process. 2012;90:331–336. doi: 10.1016/j.beproc.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Bowen M.T., McGregor I.S. Oxytocin and vasopressin modulate the social response to threat: a preclinical study. Int. J. Neuropsychopharmacol. 2014:1–13. doi: 10.1017/S1461145714000388. [DOI] [PubMed] [Google Scholar]
- Brain P.F. Effects of isolation/grouping on endocrine function and fighting behavior in male and female golden hamsters. (Mesocricetus auratus Waterhouse. Behav. Biol. 1972;7:349–357. doi: 10.1016/s0091-6773(72)80106-8. [DOI] [PubMed] [Google Scholar]
- Braun K., Antemano R., Helmeke C., Büchner M., Poeggel G. Juvenile separation stress induces rapid region- and layer-specific changes in S100ss- and glial fibrillary acidic protein-immunoreactivity in astrocytes of the rodent medial prefrontal cortex. Neuroscience. 2009;160:629–638. doi: 10.1016/j.neuroscience.2009.02.074. [DOI] [PubMed] [Google Scholar]
- Brown K.J., Grunberg N.E. Effects of housing on male and female rats: crowding stresses males but calms females. Physiol. Behav. 1995;58:1085–1089. doi: 10.1016/0031-9384(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Buwalda B., Felszeghy K., Horváth K.M., Nyakas C., de Boer S.F., Bohus B., Koolhaas J.M. Temporal and spatial dynamics of corticosteroid receptor down-regulation in rat brain following social defeat. Physiol. Behav. 2001;72:349–354. doi: 10.1016/s0031-9384(00)00414-5. [DOI] [PubMed] [Google Scholar]
- Buwalda B., Kole M.H.P., Veenema A.H., Huininga M., de Boer S.F., Korte S.M., Koolhaas J.M. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci. Biobehav. Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat. Rev. Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Calcagnoli F., de Boer S.F., Beiderbeck D.I., Althaus M., Koolhaas J.M., Neumann I.D. Local oxytocin expression and oxytocin receptor binding in the male rat brain is associated with aggressiveness. Behav. Brain Res. 2014;261:315–322. doi: 10.1016/j.bbr.2013.12.050. [DOI] [PubMed] [Google Scholar]
- Campbell P., Ophir A.G., Phelps S.M. Central vasopressin and oxytocin receptor distributions in two species of singing mice. J. Comp. Neurol. 2009;516:321–333. doi: 10.1002/cne.22116. [DOI] [PubMed] [Google Scholar]
- Carnevali L., Mastorci F., Graiani G., Razzoli M., Trombini M., Pico-Alfonso M.A., Arban R., Grippo A.J., Quaini F., Sgoifo A. Social defeat and isolation induce clear signs of a depression-like state, but modest cardiac alterations in wild-type rats. Physiol. Behav. 2012;106:142–150. doi: 10.1016/j.physbeh.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Carter C.S. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter C.S. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behav. Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Carter C.S., DeVries A.C., Taymans S.E., Roberts R.L., Williams J.R., Chrousos G.P. Adrenocorticoid hormones and the development and expression of mammalian monogamy. Ann. N.Y. Acad. Sci. 1995;771:82–91. doi: 10.1111/j.1749-6632.1995.tb44672.x. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Grippo A.J., Pournajafi-Nazarloo H., Ruscio M.G., Porges S.W. Oxytocin, vasopressin and sociality. Prog. Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Chetty S., Friedman A.R., Taravosh-Lahn K., Kirby E.D., Mirescu C., Guo F., Krupik D., Nicholas A., Geraghty A.C., Krishnamurthy A., Tsai M.-K., Covarrubias D., Wong A.T., Francis D.D., Sapolsky R.M., Palmer T.D., Pleasure D., Kaufer D. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol. Psychiatry. 2014 doi: 10.1038/mp.2013.190. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M.M., DeVries A.C., Williams J.R., Carter C.S. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav. Neurosci. 1999;113:1071. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Choleris E., Gustafsson J.-A.A., Korach K.S., Muglia L.J., Pfaff D.W., Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou D., Huang C.-C., Hsu K.-S. Brain-derived neurotrophic factor in the amygdala mediates susceptibility to fear conditioning. Exp. Neurol. 2014;255:19–29. doi: 10.1016/j.expneurol.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Christian J.J. Population density and reproductive efficiency. Biol. Reprod. 1971;4:248–294. doi: 10.1093/biolreprod/4.3.248. [DOI] [PubMed] [Google Scholar]
- Christian J.J., Lemunyan C.D. Adverse effects of crowding on lacation and reproduction of mice and two generations of their progeny. Endocrinology. 1958;63:517–529. doi: 10.1210/endo-63-5-517. [DOI] [PubMed] [Google Scholar]
- Coe C.L., Mendoza S.P., Smotherman W.P., Levine S. Mother-infant attachment in the squirrel monkey: adrenal response to separation. Behav. Biol. 1978;22:256–263. doi: 10.1016/s0091-6773(78)92305-2. [DOI] [PubMed] [Google Scholar]
- Conger J.J., Sawrey W.L., Turrell E.S. The role of social experience in the production of gastric ulcers in hooded rats placed in a conflict situation. J. Abnorm. Soc. Psychol. 1958;57:214–220. doi: 10.1037/h0041533. [DOI] [PubMed] [Google Scholar]
- Cordero M.I., Sandi C. Stress amplifies memory for social hierarchy. Front. Neurosci. 2007;1:175–184. doi: 10.3389/neuro.01.1.1.013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin-Frankel J. Needed: more females in animal and cell studies. Science. 2014;344:679. doi: 10.1126/science.344.6185.679. [DOI] [PubMed] [Google Scholar]
- Clarke F.M., Faulkes C.G. Dominance and queen succession in captive colonies of the eusocial naked mole–rat, Heterocephalus glaber. Proc. R. Soc. Lond. B. Biol. Sci. 1997;264:993–1000. doi: 10.1098/rspb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke F.M., Faulkes C.G. Intracolony aggression in the eusocial naked mole-rat, Heterocephalus glaber. Anim. Behav. 2001;61:311–324. [Google Scholar]
- Clayton J.A., Collins F.S. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J.N. Social behavior tests for mice. 2007. http://ccsystems.us/v2mag/L018.pdf Available at: (accessed 05.08.14.)
- Czéh B., Michaelis T., Watanabe T., Frahm J., de Biurrun G., van Kampen M., Bartolomucci A., Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davitz J.R., Mason D.J. Socially facilitated reduction of a fear response in rats. J. Comp. Physiol. Psychol. 1955;48:149–151. doi: 10.1037/h0046411. [DOI] [PubMed] [Google Scholar]
- Detillion C.E., Craft T.K.S., Glasper E.R., Prendergast B.J., DeVries A.C. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29:1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- DeVries A.C. Interaction among social environment, the hypothalamic–pituitary–adrenal axis, and behavior. Horm. Behav. 2002;41:405–413. doi: 10.1006/hbeh.2002.1780. [DOI] [PubMed] [Google Scholar]
- DeVries A.C., DeVries M.B., Taymans S., Carter C.S. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries A.C., DeVries M.B., Taymans S.E., Carter C.S. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries A.C., Glasper E.R., Detillion C.E. Social modulation of stress responses. Physiol. Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- DeVries A.C., Guptaa T., Cardillo S., Cho M., Carter C.S. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology. 2002;27:705–714. doi: 10.1016/s0306-4530(01)00073-7. [DOI] [PubMed] [Google Scholar]
- DeVries A.C., Taymans S.E., Carter C.S. Social modulation of corticosteroid responses in male prairie voles. Ann. N.Y. Acad. Sci. 1997;807:494–497. doi: 10.1111/j.1749-6632.1997.tb51949.x. [DOI] [PubMed] [Google Scholar]
- Dewsbury D.A. Aggression, copulation, and differential reproduction of deer mice (Peromyscus maniculatus) in a semi-natural enclosure. Behaviour. 1984;91:1–23. [Google Scholar]
- Donahue R.J., Muschamp J.W., Russo S.J., Nestler E.J., Carlezon W.A. Effects of striatal ΔFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol. Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson Z.R., Young L.J. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Elliott E., Ezra-Nevo G., Regev L., Neufeld-Cohen A., Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat. Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- Engh A.L., Beehner J.C., Bergman T.J., Whitten P.L., Hoffmeier R.R., Seyfarth R.M., Cheney D.L. Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus) Proc. Biol. Sci. 2006;273:707–712. doi: 10.1098/rspb.2005.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts H.G.J., De Ruiter A.J.H., Koolhaas J.M. Differential lateral septal vasopressin in wild-type rats: correlation with aggression. Horm. Behav. 1997;31:136–144. doi: 10.1006/hbeh.1997.1375. [DOI] [PubMed] [Google Scholar]
- Ferguson J.N., Young L.J., Insel T.R. The neuroendocrine basis of social recognition. Front. Neuroendocr. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- File S.E., Hyde J.R. Can social interaction be used to measure anxiety? Br. J. Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füchsl A.M., Neumann I.D., Reber S.O. Stress resilience: a low-anxiety genotype protects male mice from the consequences of chronic psychosocial stress. Endocrinology. 2013;155:117–126. doi: 10.1210/en.2013-1742. [DOI] [PubMed] [Google Scholar]
- Gadek-Michalska A., Bugajski J. Repeated handling, restraint, or chronic crowding impair the hypothalamic-pituitary-adrenocortical response to acute restraint stress. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2003;54:449–459. [PubMed] [Google Scholar]
- Galea L.A., McEwen B.S. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience. 1999;89:955–964. doi: 10.1016/s0306-4522(98)00345-5. [DOI] [PubMed] [Google Scholar]
- Gamallo A., Villanua A., Trancho G., Fraile A. Stress adaptation and adrenal activity in isolated and crowded rats. Physiol. Behav. 1986;36:217–221. doi: 10.1016/0031-9384(86)90006-5. [DOI] [PubMed] [Google Scholar]
- Gattermann R., Fritzsche P., Neumann K., Al-Hussein I., Kayser A., Abiad M., Yakti R. Notes on the current distribution and the ecology of wild golden hamsters (Mesocricetus auratus) J. Zool. 2001;254:359–365. [Google Scholar]
- Geraghty, A.C., Muroy, S., Zhao, S., Kriegsfeld, L.J., Bentley, G.E., Kaufer, D., 2013. Chronic stress causes an increase in RFRP expression and leads to reproductive dysfunction in the adult female rat. Program No 37504III1 2013 Neurosci Meet Plan Wash DC Soc Neurosci 2013 Online.
- Germann P.G., Kohler M., Ernst H., Baumgart H., Mohr U. The relation of amyloidosis to social stress induced by crowding in the Syrian hamster (Mesocricetus auratus) Z. Für Vers. 1990;33:271–275. [PubMed] [Google Scholar]
- Getz L.L., Carter C.S., Gavish L. The mating system of the prairie vole, Microtus ochrogaster: field and laboratory evidence for pair-bonding. Behav. Ecol. Sociobiol. 1981;8:189–194. [Google Scholar]
- Getz L.L., Hofmann J.E. Social organization in free-living prairie voles, Microtus ochrogaster. Behav. Ecol. Sociobiol. 1986;18:275–282. [Google Scholar]
- Glasper E.R., DeVries A.C. Social structure influences effects of pair-housing on wound healing. Brain Behav. Immunol. 2005;19:61–68. doi: 10.1016/j.bbi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Lovic V., Ward G.R., Wainwright P.E., Fleming A.S. Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behavior and emotionality in female rats. Dev. Psychobiol. 2001;38:11–32. doi: 10.1002/1098-2302(2001)38:1<11::aid-dev2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Gouin J.-P., Carter C.S., Pournajafi-Nazarloo H., Glaser R., Malarkey W.B., Loving T.J., Stowell J., Kiecolt-Glaser J.K. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010;35:1082–1090. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E., Tanapat P., McEwen B.S., Flügge G., Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves F.C., Hennessy M.B. Comparison of the effects of the mother and an unfamiliar adult female on cortisol and behavioral responses of pre- and postweaning guinea pigs. Dev. Psychobiol. 2000;36:91–100. [PubMed] [Google Scholar]
- Grippo A.J., Carter C.S., McNeal N., Chandler D.L., LaRocca M.A., Bates S.L., Porges S.W. 24-Hour autonomic dysfunction and depressive behaviors in an animal model of social isolation: implications for the study of depression and cardiovascular disease. Psychosom. Med. 2011;73:59–66. doi: 10.1097/PSY.0b013e31820019e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A.J., Ihm E., Wardwell J., McNeal N., Scotti M.-A.L., Moenk D.A., Chandler D.L., LaRocca M.A., Preihs K. The effects of environmental enrichment on depressive and anxiety-relevant behaviors in socially isolated prairie voles. Psychosom. Med. 2014;76:277–284. doi: 10.1097/PSY.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A.J., Lamb D.G., Carter C.S., Porges S.W. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol. Psychiatry. 2007;62:1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A.J., Sgoifo A., Mastorci F., McNeal N., Trahanas D.M. Cardiac dysfunction and hypothalamic activation during a social crowding stressor in prairie voles. Auton. Neurosci. 2010;156:44–50. doi: 10.1016/j.autneu.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A.J., Trahanas D.M., Zimmerman R.R., Porges S.W., Carter C.S. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A.J., Wu K.D., Hassan I., Carter C.S. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety. 2008;25:E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosenick L., Clement T.S., Fernald R.D. Fish can infer social rank by observation alone. Nature. 2007;445:429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Hall F.S. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit. Rev. Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Hall J.C. Some conditions of anxiety extinction. J. Abnorm. Soc. Psychol. 1955;51:126–132. doi: 10.1037/h0046917. [DOI] [PubMed] [Google Scholar]
- Haller J., Fuchs E., Halász J., Makara G.B. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res. Bull. 1999;50:33–39. doi: 10.1016/s0361-9230(99)00087-8. [DOI] [PubMed] [Google Scholar]
- Harvey P.W., Chevins P.F.D. Crowding during pregnancy delays puberty and alters estrous cycles of female offspring in mice. Experientia. 1987;43:306–308. doi: 10.1007/BF01945561. [DOI] [PubMed] [Google Scholar]
- Hatch A.M., Wiberg G.S., Zawidzka Z., Cann M., Airth J.M., Grice H.C. Isolation syndrome in the rat. Toxicol. Appl. Pharmacol. 1965;7:737–745. doi: 10.1016/0041-008x(65)90132-8. [DOI] [PubMed] [Google Scholar]
- Heidbreder C.A., Weiss I.C., Domeney A.M., Pryce C., Homberg J., Hedou G., Feldon J., Moran M.C., Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs, S.C., Koob, G.F., 2006. Application of experimental stressors in laboratory rodents. Curr Protoc Neurosci Editor Board Jacqueline N Crawley Al Chapter 8:Unit8.4. [DOI] [PubMed]
- Hellemans K.G.C., Benge L.C., Olmstead M.C. Adolescent enrichment partially reverses the social isolation syndrome. Dev. Brain Res. 2004;150:103–115. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hennessy M.B., Hornschuh G., Kaiser S., Sachser N. Cortisol responses and social buffering: a study throughout the life span. Horm. Behav. 2006;49:383–390. doi: 10.1016/j.yhbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hennessy M.B., Maken D.S., Graves F.C. Consequences of the presence of the mother or unfamiliar adult female on cortisol, ACTH, testosterone and behavioral responses of periadolescent guinea pigs during exposure to novelty. Psychoneuroendocrinology. 2000;25:619–632. doi: 10.1016/s0306-4530(00)00014-7. [DOI] [PubMed] [Google Scholar]
- Herzog C.J., Czéh B., Corbach S., Wuttke W., Schulte-Herbrüggen O., Hellweg R., Flügge G., Fuchs E. Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience. 2009;159:982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Hofmann H.A., Beery A.K., Blumstein D.T., Couzin I.D., Earley R.L., Hayes L.D., Hurd P.L., Lacey E.A., Phelps S.M., Solomon N.G., Taborsky M., Young L.J., Rubenstein D.R. An evolutionary framework for studying mechanisms of social behavior. Trends Ecol. Evol. 2014 doi: 10.1016/j.tree.2014.07.008. http://linkinghub.elsevier.com/retrieve/pii/S0169534714001608 Available at: (accessed 25.08.14) [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J., Smith T.B., Layton J.B. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler C.M., Ryabinin A.E. Social partners prevent alcohol relapse behavior in prairie voles. Psychoneuroendocrinology. 2014;39:152–157. doi: 10.1016/j.psyneuen.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman K.L., Solomon M.B., Janicki M., Harmon A.C., Lin S.M., Israel J.E., Jasnow A.M. Conditioned defeat in male and female syrian hamsters. Horm. Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Jylhä P., Isometsä E. The relationship of neuroticism and extraversion to symptoms of anxiety and depression in the general population. Depress Anxiety. 2006;23:281–289. doi: 10.1002/da.20167. [DOI] [PubMed] [Google Scholar]
- Kaiser S., Kirtzeck M., Hornschuh G., Sachser N. Sex-specific difference in social support–a study in female guinea pigs. Physiol. Behav. 2003;79:297–303. doi: 10.1016/s0031-9384(03)00091-x. [DOI] [PubMed] [Google Scholar]
- Katz P.S., Lillvis J.L. Reconciling the deep homology of neuromodulation with the evolution of behavior. Curr. Opin. Neurobiol. 2014;29:39–47. doi: 10.1016/j.conb.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Kawachi I., Kennedy B.P. Income inequality and health: pathways and mechanisms. Health Serv. Res. 1999;34:215–227. [PMC free article] [PubMed] [Google Scholar]
- Kelly A.M., Goodson J.L. Social functions of individual vasopressin–oxytocin cell groups in vertebrates: What do we really know? Front. Neuroendocrinol. 2014;35:512–529. doi: 10.1016/j.yfrne.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Kikusui T., Winslow J.T., Mori Y. Social buffering: relief from stress and anxiety. Philos. Trans. R. Soc. B Biol. Sci. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.A. Social relations of the domestic guinea pig living under semi-natural conditions. Ecology. 1956;37:221–228. [Google Scholar]
- Kirby E.D., Geraghty A.C., Ubuka T., Bentley G.E., Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc. Natl. Acad. Sci. U.S.A. 2009;106:11324–11329. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby E.D., Muroy S.E., Sun W.G., Covarrubias D., Leong M.J., Barchas L.A., Kaufer D. Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. eLife. 2013;2:e00362. doi: 10.7554/eLife.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.-M., Hellhammer D.H. The “Trier Social Stress Test” – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y., Kikusui T., Takeuchi Y., Mori Y. Partner's stress status influences social buffering effects in rats. Behav. Neurosci. 2004;118:798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- Klein S.L., Taymans S.E., DeVries A.C., Nelson R.J. Cellular immunity is not compromised by high serum corticosterone concentrations in prairie voles. Am. J. Physiol-Regul. Integr. Comp. Physiol. 1996;271:R1608–R1613. doi: 10.1152/ajpregu.1996.271.6.R1608. [DOI] [PubMed] [Google Scholar]
- Koolhaas J.M., De Boer S., De Rutter A., Meerlo P., Sgoifo A. Social stress in rats and mice. Acta Physiol. Scand. Suppl. 1996;640:69–72. [PubMed] [Google Scholar]
- Kotrschal A., Ilmonen P., Penn D.J. Stress impacts telomere dynamics. Biol. Lett. 2007;3:128–130. doi: 10.1098/rsbl.2006.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H.A. The August Krogh principle: “For many problems there is an animal on which it can be most conveniently studied”. J. Exp. Zool. 1975;194:221–226. doi: 10.1002/jez.1401940115. [DOI] [PubMed] [Google Scholar]
- Krishnan V. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lacey E.A., Braude S.H., Wieczorek J.R. Burrow sharing by colonial tuco-tucos (Ctenomys sociabilis) J. Mammal. 1997;78:556–562. [Google Scholar]
- Latané B. Gregariousness and fear in laboratory rats. J. Exp. Soc. Psychol. 1969;5:61–69. [Google Scholar]
- Latané B., Eckman J., Joy V. Shared stress and interpersonal attraction. J. Exp. Soc. Psychol. 1966;1(Suppl. 1):80–94. [Google Scholar]
- Lee A., Clancy S., Fleming A.S. Mother rats bar-press for pups: effects of lesions of the MPOA and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav. Brain Res. 1999;100:15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- Lepore S.J., Allen K.A., Evans G.W. Social support lowers cardiovascular reactivity to an acute stressor. Psychosom. Med. 1993;55:518–524. doi: 10.1097/00006842-199311000-00007. [DOI] [PubMed] [Google Scholar]
- Lieberwirth C., Wang Z. The social environment and neurogenesis in the adult mammalian brain. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00118. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3347626/ Available at: (accessed 01.08.14.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A., Sharma S., Pearson D., Plotsky P.M., Meaney M.J. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lucas M., Ilin Y., Anunu R., Kehat O., Xu L., Desmedt A., Richter-Levin G. Long-term effects of controllability or the lack of it on coping abilities and stress resilience in the rat. Stress. 2014:1–8. doi: 10.3109/10253890.2014.930430. [DOI] [PubMed] [Google Scholar]
- Lukas M., Toth I., Reber S.O., Slattery D.A., Veenema A.H., Neumann I.D. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons D.M., Parker K.J., Schatzberg A.F. Animal models of early life stress: implications for understanding resilience. Dev. Psychobiol. 2010;52:616–624. doi: 10.1002/dev.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabry K.E., Streatfeild C.A., Keane B., Solomon N.G. avpr1a length polymorphism is not associated with either social or genetic monogamy in free-living prairie voles. Anim. Behav. 2011;81:11–18. doi: 10.1016/j.anbehav.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald D., Mathews F., Berdoy M. Ecologically-based Management of Rodent Pests. Australian Centre for International Agricultural Research; Canberra: 1999. The behaviour and ecology of Rattus norvegicus: from opportunism to kamikaze tendencies. [Google Scholar]
- Madison D.M., Fitzgerald R.W., McShea W.J. Dynamics of social nesting in overwintering meadow voles (Microtus pennsylvanicus): possible consequences for population cycling. Behav. Ecol. Sociobiol. 1984;15:9–17. [Google Scholar]
- Madison D.M., McShea W. Seasonal changes in reproductive tolerance, spacing, and social organization in meadow voles: a microtine model. Am. Zool. 1987;27:899–908. [Google Scholar]
- Magariños A.M., McEwen B.S., Flügge G., Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J. Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S.F., Watkins L.R. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Marchleswska-Koj A. Sociogenic stress and rodent reproduction. Neurosci. Biobehav. Rev. 1997;21:699–703. doi: 10.1016/s0149-7634(96)00021-8. [DOI] [PubMed] [Google Scholar]
- Margerum L. Effects of semi-natural environmental conditions on phenotypic plasticity in Rattus norvegicus. eScholarship. 2013 http://escholarship.org/uc/item/60w8q88v Available at: (accessed 01.08.14.) [Google Scholar]
- Martin L.J., Tuttle A.H., Mogil J.S. The interaction between pain and social behavior in humans and rodents. Curr. Top. Behav. Neurosci. 2014:1–18. doi: 10.1007/7854_2014_287. http://link.springer.com/chapter/10.1007/7854_2014_287 Springer Berlin Heidelberg. Available at: (accessed 01.08.14) [DOI] [PubMed] [Google Scholar]
- Martinez M., Calvo-Torrent A., Pico-Alfonso M.A. Social defeat and subordination as models of social stress in laboratory rodents: a review. Aggress. Behav. 1998;24:241–256. [Google Scholar]
- McEwen B.S. Brain on stress: how the social environment gets under the skin. Proc. Natl. Acad. Sci. U.S.A. 2012;109(Suppl. 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Magarinos A.M. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum. Psychopharmacol. Clin. Exp. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- McNeal N., Scotti M.-A.L., Wardwell J., Chandler D.L., Bates S.L., LaRocca M., Trahanas D.M., Grippo A.J. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Auton. Neurosci. 2014;180:9–16. doi: 10.1016/j.autneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meerlo P., Overkamp G.J.F., Daan S., Van Den Hoofdakker R.H., Koolhaas J.M. Changes in behaviour and body weight following a single or double social defeat in rats. Stress. 1996;1:21–32. doi: 10.3109/10253899609001093. [DOI] [PubMed] [Google Scholar]
- Mendoza S.P., Coe C.L., Lowe E.L., Levine S. The physiological response to group formation in adult male squirrel monkeys. Psychoneuroendocrinology. 1978;3:221–229. doi: 10.1016/0306-4530(78)90012-4. [DOI] [PubMed] [Google Scholar]
- Meyerhoff D.J., Mon A., Metzler T., Neylan T.C. Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep. 2014;37:893–900. doi: 10.5665/sleep.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C., Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Mogil J.S., Chanda M.L. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Morris W.N., Worchel S., Bois J.L., Pearson J.A., Alan C., Samaha G.M., Wachtler J., Wright S.L. Collective coping with stress: group reactions to fear, anxiety, and ambiguity. J. Pers. Soc. Psychol. 1976;33:674–679. doi: 10.1037//0022-3514.33.6.674. [DOI] [PubMed] [Google Scholar]
- Moy S.S., Nadler J.J., Young N.B., Perez A., Holloway L.P., Barbaro R.P., Barbaro J.R., Wilson L.M., Threadgill D.W., Lauder J.M., Magnuson T.R., Crawley J.N. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav. Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayasu T., Ishii K. Effects of pair-housing after social defeat experience on elevated plus-maze behavior in rats. Behav. Process. 2008;78:477–480. doi: 10.1016/j.beproc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Naragon-Gainey K., Rutter L.A., Brown T.A. The interaction of extraversion and anxiety sensitivity on social anxiety: evidence of specificity relative to depression. Behav. Ther. 2014;45:418–429. doi: 10.1016/j.beth.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Neumann I.D. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J. Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Nyuyki K.D., Beiderbeck D.I., Lukas M., Neumann I.D., Reber S.O. Chronic subordinate colony housing (CSC) as a model of chronic psychosocial stress in male rats. PLoS ONE. 2012;7:e52371. doi: 10.1371/journal.pone.0052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir A.G., Gessel A., Zheng D.-J., Phelps S.M. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm. Behav. 2012;61:445–453. doi: 10.1016/j.yhbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir A.G., Phelps S.M., Sorin A.B., Wolff J.O. Social but not genetic monogamy is associated with greater breeding success in prairie voles. Anim. Behav. 2008;75:1143–1154. [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neurosci. Biobehav. Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Palanza P., Gioiosa L., Parmigiani S. Social stress in mice: gender differences and effects of estrous cycle and social dominance. Physiol. Behav. 2001;73:411–420. doi: 10.1016/s0031-9384(01)00494-2. [DOI] [PubMed] [Google Scholar]
- Panksepp J.B., Lahvis G.P. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent C.I., Del Corpo A., Cameron N.M., Meaney M.J. Maternal care associates with play dominance rank among adult female rats. Dev. Psychobiol. 2013;55:745–756. doi: 10.1002/dev.21070. [DOI] [PubMed] [Google Scholar]
- Parent C.I., Meaney M.J. The influence of natural variations in maternal care on play fighting in the rat. Dev. Psychobiol. 2008;50:767–776. doi: 10.1002/dev.20342. [DOI] [PubMed] [Google Scholar]
- Parker K.J., Buckmaster C.L., Lindley S.E., Schatzberg A.F., Lyons D.M. Hypothalamic-pituitary-adrenal axis physiology and cognitive control of behavior in stress inoculated monkeys. Int. J. Behav. Dev. 2012;36 doi: 10.1177/0165025411406864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne A.P., Swanson H.H. Agonistic behaviour between pairs of hamsters of the same and opposite sex in a neutral observation area. Behaviour. 1970;36:260–269. [PubMed] [Google Scholar]
- Peña C.J., Neugut Y.D., Calarco C.A., Champagne F.A. Effects of maternal care on the development of midbrain dopamine pathways and reward-directed behavior in female offspring. Eur. J. Neurosci. 2014;39:946–956. doi: 10.1111/ejn.12479. [DOI] [PubMed] [Google Scholar]
- Perkeybile A.M., Griffin L.L., Bales K.L. Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster) Front. Behav. Neurosci. 2013;7:21. doi: 10.3389/fnbeh.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., Grunwald N., Rümmele P., Endlicher E., Lechner A., Neumann I.D., Obermeier F., Reber S.O. Chronic psychosocial stress increases the risk for inflammation-related colon carcinogenesis in male mice. Stress. 2011;15:403–415. doi: 10.3109/10253890.2011.631232. [DOI] [PubMed] [Google Scholar]
- Peuler J.D., Scotti M.-A.L., Phelps L.E., McNeal N., Grippo A.J. Chronic social isolation in the prairie vole induces endothelial dysfunction: implications for depression and cardiovascular disease. Physiol. Behav. 2012;106:476–484. doi: 10.1016/j.physbeh.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps S.M., Campbell P., Zheng D.-J., Ophir A.G. Beating the boojum: comparative approaches to the neurobiology of social behavior. Neuropharmacology. 2010;58:17–28. doi: 10.1016/j.neuropharm.2009.06.043. [DOI] [PubMed] [Google Scholar]
- Puzserova A., Slezak P., Balis P., Bernatova I. Long-term social stress induces nitric oxide-independent endothelial dysfunction in normotensive rats. Stress Amst. Neth. 2013;16:331–339. doi: 10.3109/10253890.2012.725116. [DOI] [PubMed] [Google Scholar]
- Pyter L.M., Weil Z.M., Nelson R.J. Latitude affects photoperiod-induced changes in immune response in meadow voles (Microtus pennsylvanicus) Can. J. Zool. 2005;83:1271–1278. [Google Scholar]
- Quirici V., Faugeron S., Hayes L.D., Ebensperger L.A. The influence of group size on natal dispersal in the communally rearing and semifossorial rodent, Octodon degus. Behav. Ecol. Sociobiol. 2011;65:787–798. [Google Scholar]
- Reiss D., Wolter-Sutter A., Krezel W., Ouagazzal A.-M. Effects of social crowding on emotionality and expression of hippocampal nociceptin/orphanin FQ system transcripts in mice. Behav. Brain Res. 2007;184:167–173. doi: 10.1016/j.bbr.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Resendez S.L., Kuhnmuench M., Krzywosinski T., Aragona B.J. -Opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. J. Neurosci. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross H.E., Young L.J. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocr. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruis M.A.W., te Brake J.H.A., Buwalda B., De Boer S.F., Meerlo P., Korte S.M., Blokhuis H.J., Koolhaas J.M. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Ruscio M.G., Sweeny T., Hazelton J., Suppatkul P., Sue Carter C. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm. Behav. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Hypercortisolism among socially subordinate wild baboons originates at the CNS level. Arch. Gen. Psychiatry. 1989;46:1047–1051. doi: 10.1001/archpsyc.1989.01810110089012. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M., Meaney M.J., McEwen B.S. The development of the glucocorticoid receptor system in the rat limbic brain. III. Negative-feedback regulation. Brain Res. 1985;350:169–173. doi: 10.1016/0165-3806(85)90261-5. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M., Romero L.M., Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schmidt M.V., Trümbach D., Weber P., Wagner K., Scharf S.H., Liebl C., Datson N., Namendorf C., Gerlach T., Kühne C., Uhr M., Deussing J.M., Wurst W., Binder E.B., Holsboer F., Müller M.B. Individual stress vulnerability is predicted by short-term memory and AMPA receptor subunit ratio in the hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 2010;30:16949–16958. doi: 10.1523/JNEUROSCI.4668-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney M., Goldman B.D., Forger N.G. Breeding status affects motoneuron number and muscle size in naked mole-rats: recruitment of perineal motoneurons? J. Neurobiol. 2006;66:1354–1364. doi: 10.1002/neu.20314. [DOI] [PubMed] [Google Scholar]
- Sgoifo A., Carnevali L., Grippo A.J. The socially stressed heart. Insights from studies in rodents. Neurosci. Biobehav. Rev. 2014;39:51–60. doi: 10.1016/j.neubiorev.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Sgoifo A., Koolhaas J., De Boer S., Musso E., Stilli D., Buwalda B., Meerlo P. Social stress, autonomic neural activation, and cardiac activity in rats. Neurosci. Biobehav Rev. 1999;23:915–923. doi: 10.1016/s0149-7634(99)00025-1. [DOI] [PubMed] [Google Scholar]
- Sgoifo A., Pozzato C., Meerlo P., Costoli T., Manghi M., Stilli D., Olivetti G., Musso E. Intermittent exposure to social defeat and open-field test in rats: acute and long-term effects on ECG, body temperature and physical activity. Stress Int. J. Biol. Stress. 2002;5:23–35. doi: 10.1080/102538902900012387. [DOI] [PubMed] [Google Scholar]
- Sherman P.W., Jarvis J.U.M., Alexander R.D. Princeton University Press; Princeton, New Jersey: 1991. The Biology of the Naked Mole-rat. [Google Scholar]
- Siegrist J., Marmot M. Health inequalities and the psychosocial environment—two scientific challenges. Soc. Sci. Med. 2004;58:1463–1473. doi: 10.1016/S0277-9536(03)00349-6. [DOI] [PubMed] [Google Scholar]
- Silk J.B., Beehner J.C., Bergman T.J., Crockford C., Engh A.L., Moscovice L.R., Wittig R.M., Seyfarth R.M., Cheney D.L. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Simpson J., Kelly J.P. The impact of environmental enrichment in laboratory rats—behavioural and neurochemical aspects. Behav. Brain Res. 2011;222:246–264. doi: 10.1016/j.bbr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A., Marmot M.G., Adler N.E. Does subjective social status predict health and change in health status better than objective status? Psychosom. Med. 2005;67:855–861. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- Smith A.S., Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge R.E. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods. 2014 doi: 10.1038/nmeth.2935. http://www.nature.com/doifinder/10.1038/nmeth.2935 Available at: (accessed 13.05.14.) [DOI] [PubMed] [Google Scholar]
- Späni D., Arras M., König B., Rülicke T. Higher heart rate of laboratory mice housed individually vs in pairs. Lab. Anim. 2003;37:54–62. doi: 10.1258/002367703762226692. [DOI] [PubMed] [Google Scholar]
- Stanton M.A., Mann J. Early social networks predict survival in wild bottlenose dolphins. PLoS ONE. 2012;7:e47508. doi: 10.1371/journal.pone.0047508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr-Phillips E.J., Beery A.K. Natural variation in maternal care shapes adult social behavior in rats. Dev. Psychobiol. 2014;56:1017–1026. doi: 10.1002/dev.21182. [DOI] [PubMed] [Google Scholar]
- Stranahan A.M., Khalil D., Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat. Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztainberg Y., Chen A. An environmental enrichment model for mice. Nat. Protoc. 2010;5:1535–1539. doi: 10.1038/nprot.2010.114. [DOI] [PubMed] [Google Scholar]
- Szyf M., Weaver I., Meaney M. Maternal care, the epigenome and phenotypic differences in behavior. Reprod. Toxicol. Elmsford. N. 2007;24:9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Taylor G.T. Fear and affiliation in domesticated male rats. J. Comp. Physiol. Psychol. 1981;95:685–693. [Google Scholar]
- Taylor S.E. Tend and befriend: biobehavioral bases of affiliation under stress. Curr. Dir. Psychol. Sci. 2006;15:273–277. [Google Scholar]
- Taylor S.E., Klein L.C., Lewis B.P., Gruenewald T.L., Gurung R.A., Updegraff J.A. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol. Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Taymans S.E., DeVries A.C., DeVries M.B., Nelson R.J., Friedman T.C., Castro M., Detera-Wadleigh S., Carter C.S., Chrousos G.P. The hypothalamic-pituitary-adrenal axis of prairie voles (Microtus ochrogaster): evidence for target tissue glucocorticoid resistance. Gen. Comp. Endocrinol. 1997;106:48–61. doi: 10.1006/gcen.1996.6849. [DOI] [PubMed] [Google Scholar]
- Teichman Y. Predisposition for anxiety and affiliation. J. Pers. Soc. Psychol. 1974;29:405–410. doi: 10.1037/h0035920. [DOI] [PubMed] [Google Scholar]
- Thorsteinsson E.B., James J.E., Elizabeth M. Effects of video-relayed social support on hemodynamic reactivity and salivary cortisol during laboratory-based behavioral challenge. Health Psychol. 1998;17:436–444. doi: 10.1037//0278-6133.17.5.436. [DOI] [PubMed] [Google Scholar]
- Tornatzky W., Miczek K.A. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol. Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Tramullas M., Dinan T.G., Cryan J.F. Chronic psychosocial stress induces visceral hyperalgesia in mice. Stress Amst. Neth. 2012;15:281–292. doi: 10.3109/10253890.2011.622816. [DOI] [PubMed] [Google Scholar]
- Tzanoulinou S., Riccio O., de Boer M.W., Sandi C. Peripubertal stress-induced behavioral changes are associated with altered expression of genes involved in excitation and inhibition in the amygdala. Transl. Psychiatry. 2014;4:e410. doi: 10.1038/tp.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valzelli L. The “isolation syndrome” in mice. Psychopharmacologia. 1973;31:305–320. doi: 10.1007/BF00421275. [DOI] [PubMed] [Google Scholar]
- Van Hasselt F.N., Tieskens J.M., Trezza V., Krugers H.J., Vanderschuren L.J.M.J., Joëls M. Within-litter variation in maternal care received by individual pups correlates with adolescent social play behavior in male rats. Physiol. Behav. 2012;106:701–706. doi: 10.1016/j.physbeh.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Veenema A.H. Toward understanding how early-life social experiences alter oxytocin- and vasopressin-regulated social behaviors. Horm. Behav. 2012;61:304–312. doi: 10.1016/j.yhbeh.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Veenema A.H., Meijer O.C., de Kloet E.R., Koolhaas J.M., Bohus B.G. Differences in basal and stress-induced HPA regulation of wild house mice selected for high and low aggression. Horm. Behav. 2003;43:197–204. doi: 10.1016/s0018-506x(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Veenema A.H., Reber S.O., Selch S., Obermeier F., Neumann I.D. Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology. 2008;149:2727–2736. doi: 10.1210/en.2007-1469. [DOI] [PubMed] [Google Scholar]
- Veeraiah P., Noronha J.M., Maitra S., Bagga P., Khandelwal N., Chakravarty S., Kumar A., Patel A.B. Dysfunctional glutamatergic and γ-aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression. Biol. Psychiatry. 2014;76:231–238. doi: 10.1016/j.biopsych.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Virgin C.E., Sapolsky R.M. Styles of male social behavior and their endocrine correlates among low-ranking baboons. Am. J. Primatol. 1997;42:25–39. doi: 10.1002/(SICI)1098-2345(1997)42:1<25::AID-AJP2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ward I.L., Ward O.B., Winn R.J., Bielawski D. Male and female sexual behavior potential of male rats prenatally exposed to the influence of alcohol, stress, or both factors. Behav. Neurosci. 1994;108:1188–1195. doi: 10.1037//0735-7044.108.6.1188. [DOI] [PubMed] [Google Scholar]
- Weaver I.C.G., Cervoni N., Champagne F.A., D'Alessio A.C., Sharma S., Seckl J.R., Dymov S., Szyf M., Meaney M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Westenbroek C., Snijders T.A.B., den Boer J.A., Gerrits M., Fokkema D.S., Ter Horst G.J. Pair-housing of male and female rats during chronic stress exposure results in gender-specific behavioral responses. Horm. Behav. 2005;47:620–628. doi: 10.1016/j.yhbeh.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Wilkinson M.B., Dias C., Magida J., Mazei-Robison M., Lobo M., Kennedy P., Dietz D., Covington H., Russo S., Neve R., Ghose S., Tamminga C., Nestler E.J. A novel role of the WNT-dishevelled-GSK3β signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.R., Catania K.C., Carter C.S. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm. Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Wilson J.H. A conspecific attenuates prolactin responses to open-field exposure in rats. Horm. Behav. 2000;38:39–43. doi: 10.1006/hbeh.2000.1600. [DOI] [PubMed] [Google Scholar]
- Wohleb E.S., Hanke M.L., Corona A.W., Powell N.D., Stiner L.M., Bailey M.T., Nelson R.J., Godbout J.P., Sheridan J.F. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G.E., Shors T.J. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S.K., Walker H.E., Valentino R.J., Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff J.A., Lacey E.A., Bentley G.E., Kriegsfeld L.J. Effects of social environment on baseline glucocorticoid levels in a communally breeding rodent, the colonial tuco-tuco (Ctenomys sociabilis) Horm. Behav. 2013;64:566–572. doi: 10.1016/j.yhbeh.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Woodruff T.K., Kibbe M.R., Paller A.S., Turek F.W., Woolley C.S. Commentary: “Leaning in” to support sex differences in basic science and clinical research. Endocrinology. 2014;155:1181–1183. doi: 10.1210/en.2014-1068. [DOI] [PubMed] [Google Scholar]
- Wu M.V., Shamy J.L., Bedi G., Choi C.-W.J., Wall M.M., Arango V., Boldrini M., Foltin R.W., Hen R. Impact of social status and antidepressant treatment on neurogenesis in the baboon hippocampus. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2014;39:1861–1871. doi: 10.1038/npp.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne-Edwards K.E., Lisk R.D. Differential effects of paternal presence on pup survival in two species of dwarf hamster (Phodopus sungorus and Phodopus campbelli) Physiol. Behav. 1989;45:465–469. doi: 10.1016/0031-9384(89)90059-0. [DOI] [PubMed] [Google Scholar]
- Yee J.R., Cavigelli S.A., Delgado B., McClintock M.K. Reciprocal affiliation among adolescent rats during a mild group stressor predicts mammary tumors and lifespan. Psychosom. Med. 2008;70:1050–1059. doi: 10.1097/PSY.0b013e31818425fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K.A., Liu Y., Wang Z. The neurobiology of social attachment: a comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2008;148:401–410. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L.J. Frank A. Beach Award. Oxytocin and vasopressin receptors and species-typical social behaviors. Horm. Behav. 1999;36:212–221. doi: 10.1006/hbeh.1999.1548. [DOI] [PubMed] [Google Scholar]
- Young L.J., Wang Z. The neurobiology of pair bonding. Nat. Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zucker I., Beery A.K. Males still dominate animal studies. Nature. 2010;465:690. doi: 10.1038/465690a. [DOI] [PubMed] [Google Scholar]
- Zucker R.A., Manosevitz M., Lanyon R.I. Birth order, anxiety, and affiliation during a crisis. J. Pers. Soc. Psychol. 1968;8:354–359. doi: 10.1037/h0025574. [DOI] [PubMed] [Google Scholar]