Abstract
p-Synephrine, the primary protoalkaloid in the extract of bitter orange and other citrus species, has gained interest due to its lipolytic activity in adipose tissues. We previously found that p-synephrine stimulates glucose consumption via AMP-activated protein kinase (AMPK) in L6 skeletal muscle cells. This study investigated the effect of p-synephrine on glucose production and lipid accumulation in H4IIE rat liver cells. Glucose production was increased in H4llE cells that were incubated in glucose-free medium but decreased dose dependently (1–100 μM) with p-synephrine treatment. Protein levels of glucose-6-phosphatase (G6Pase) and phosphoenol pyruvate carboxykinase (PEPCK) were also decreased by treatment (4 h) with p-synephrine. Antagonists against α- and β-adrenergic receptors (phentolamine and propranolol) and other inhibitors against signaling molecules did not interrupt p-synephrine-induced suppression in glucose production. However, H7 (an inhibitor of serine/threonine kinases PKA, PKC, and PKG) significantly blocked p-synephrine-induced suppression of glucose production and further increased basal glucose production. Unlike the suppressive effect on glucose production, p-synephrine failed to affect palmitic acid-induced cytoplasmic lipid accumulation. Protein levels of fatty acid synthase (FAS) and phosphorylation levels of AMPK and ACC were not changed by p-synephrine. Altogether, p-synephrine can suppress glucose production but does not affect lipid accumulation in H4IIE liver cells.
Key Words: : glucose production, H4IIE liver cells, lipid accumulation, p-synephrine
Introduction
p-Synephrine, the principal protoalkaloid in the peel of Citrus aurantium, is widely used in weight management. It increases basal metabolic rate and lipolysis and also suppresses appetite.1,2 Traditional Chinese medicine also has prescribed bitter orange, including C. aurantium, for various digestive problems such as indigestion, diarrhea, and dysentery and as an expectorant for respiratory problems.3 Although the issue of safety of p-synephrine (including adverse cardiovascular effects) has been raised,4 a recent review summarized the results of more than 20 studies involving ∼360 subjects who consumed p-synephrine and concluded that p-synephrine did not produce significant adverse events such as increases in heart rate or blood pressure and did not alter electrocardiographic data, serum chemistry, blood cell counts, or urinalysis.5
p-Synephrine is one of three different isomeric forms (ortho, o-; meta, m-; and para, p-). p-Synephrine has affinity for α-adrenergic receptors, and it can be applied to treat hypotension.6 m-Synephrine, also named phenylephrine, is widely employed as an intranasal decongestant.7
Although a number of studies have focused on p-synephrine's lipolytic activity, little is known about its role in glucose metabolism in skeletal muscle and liver, the main target tissue in the maintenance of blood glucose levels. p-Synephrine's lipolytic activity has been found mostly in adipose tissue and not in other tissues, including liver tissue. Our previous study reported that p-synephrine stimulates glucose uptake in L6 skeletal muscle cells, and that the activation of AMP-activated protein kinase (AMPK) is important in this process.8
Hepatic glucose production and muscular glucose uptake are two key processes that control plasma glucose levels; therefore, those two processes represent the main targets to treat diabetic hyperglycemia. This study investigated whether p-synephrine alters lipid accumulation and glucose production in H4IIE rat liver cells. We also tried to characterize signaling properties underlying p-synephrine's functions. Our results show that p-synephrine suppresses basal glucose production but not palmitic acid-induced lipid accumulation. We, therefore, suggest that p-synephrine has a potent antihyperglycemic activity by lowering hepatic glucose production as well as muscular glucose uptake.
Materials and Methods
Cells
H4IIE rat hepatoma cells were obtained from the Korean Cell Line Bank (Seoul, Korea) and maintained in Dulbecco's minimal essential medium (DMEM, 1 g/L glucose) with 10% fetal bovine serum (FBS). H4IIE cells were plated in multiwell culture plates and incubated in serum-free DMEM overnight. Cells were washed twice with Dulbecco's phosphate-buffered saline (D-PBS) and again incubated in glucose-free DMEM (GFM) supplemented with 2 mM pyruvate and 20 mM lactate for 30 min before treatment with reagents.
Materials
p-Synephrine, DMEM, D-PBS, trypsin-EDTA solution, palmitic acid, wortmannin, BAPTA-AM, PD98059, SB202190, SP600125, H-7, H-89, KT5823, phentolamine (PA), propranolol (PP), 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), and compound-C were purchased from Sigma Chemical Corp. (St. Louis, MO, USA). FBS was obtained from Life Technologies, Inc. (Rockville, MD, USA). Protein kinase C inhibitor (GF109203x) was purchased from Biomol (Plymouth Meeting, PA, USA). Polyclonal antibodies against phosphoenol pyruvate carboxykinase (PEPCK), fatty acid synthase (FAS), and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); polyclonal antibodies against phospho-AMPK, phospho-ACC were obtained from Millipore (Billerica, MA, USA); and glucose-6-phosphatase (G6Pase) was from Abcam (Boston, MA, USA). Electrophoresis reagents (including Bis-Tris gels, running buffer, and polyvinylidenedifluoride [PVDF] membranes) were obtained from Invitrogen (Carlsbad, CA, USA).
Glucose production
H4IIE cells (5×105 cells/mL) were plated in 24-well tissue culture plates. Confluent cells were incubated in serum-free, low-glucose (1 g/L) DMEM overnight. The cells were washed twice with D-PBS and then further incubated in GFM containing lactate (20 mM) and pyruvate (2 mM). After treatment with insulin (100 nM) or p-synephrine (1–100 μM) for 24 h, glucose concentration in medium was measured. To investigate signaling properties of p-synephrine in terms of glucose production, H4IIE cells were treated with pharmacological antagonists (PA, PP) against adrenoceptors and inhibitors (KT5823, PKCi, GF109203x, H89) of intracellular signaling proteins in the presence of p-synephrine. Glucose concentration was measured with a glucose assay reagent (Asan set Glucose; Asan Pharm, Whaseong, Korea) based on the glucose oxidase method.
Analysis of cell viability
Cell viability was assayed by MTT analysis in 24-well tissue culture plates as previously described.9 Treatment with p-synephrine (1–100 μM) was done for 72 h, whereas treatment with PA and PP (1–25 μM) was done for 24 h. After treatment, culture medium was removed from the wells, and 0.5 mL of MTT reagent (Sigma Chemical Corp.) at a concentration of 1 mg/mL in D-PBS was added to each well. After 30 min of incubation at 37°C, the MTT reagent in D-PBS was removed and then the blue-colored formazan product was solubilized in 0.5 mL of 2-propanol for 20 min. The absorbance of converted dye was measured at a wavelength of 570 nm with a microplate reader (Sunrise, Tecan, Austria).
Western blotting
Confluent H4IIE cells in six-well plates were preincubated in serum-free DMEM overnight, washed twice with D-PBS, and then further incubated with GFM and p-synephrine (50 μM) containing GFM for 30 min, for 4 and 24 h. After treatment, the cells were lysed in an ice-cold lysis buffer (50 mM Tris-HCl, 1% nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM sodium orthovanadate, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 mM aprotinin, 1 mM leupeptin, and 1 mM pepstatin A). Equal amounts (10–20 μg) of protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 4–20% polyacrylamide gels and electrotransferred onto PVDF membranes. The membranes were incubated in blocking buffer (5% nonfat dry milk in Tris-buffered saline [TBS]-0.1% Tween-20 [TBS-T]) for 1 h at room temperature, after which they were probed with different primary antibodies (1:200–1:1000). After a series of washes, the membranes were further incubated with the respective horseradish peroxidase-conjugated secondary antibodies (1:5000–1:10,000). The signal was detected with the enhanced chemiluminescence detection system (WEST-ZOL plus, Intron, Korea).
Triglyceride measurement and Nile red staining
Concentrations of cytoplasmic triglyceride were measured with Cleantech TG-S kit (Asan Pharm). Briefly, 2×106 cells were homogenized with 0.2 mL 5% Triton X-100. The cell lysates were heated for 2–5 min at 90°C until the Triton X-100 became cloudy and cooled down to room temperature. After two to three repeats of heating and cooling, the lysates were centrifuged at 17,000 g for 5 min. Supernatants were used for triglyceride assay through a kit protocol. Otherwise intracellular lipid droplets were stained with the fluorescent dye, Nile red.10 After treatments, cells were washed with D-PBS and stained for 15 min with 200 ng/mL Nile red in 2% acetone. Cells were then washed with D-PBS to remove excess staining, observed under a fluorescent microscope (IX70; Olympus, Tokyo, Japan), and photographed with a digital camera (DP-70; Olympus).
Statistics
The results are represented as mean±standard error. The significance of the differences among groups was determined using Student's t-test. P<.05 was considered statistically significant.
Results
We examined the direct effect of p-synephrine on glucose production in cultured rat H4IIE hepatoma cells. Glucose production by H4IIE cells in GFM was remarkably suppressed by treatment with insulin (100 nM) for 24 h and also suppressed by treatment with p-synephrine (1–100 μM) in a dose-dependent manner (Fig. 1A). Fifty percent reduction in glucose production was observed in the middle of 25–100 μM p-synephrine. Thus, 50 μM p-synephrine was used in the next experiments. Protein levels of PEPCK and G6Pase, two key players in gluconeogenesis, were also decreased by treatment with 50 μM p-synephrine for 4 h (Fig. 1B). However, the p-synephrine-induced decrease in the levels of two proteins was restored after 24 h in the presence of p-synephrine, comparable to the control group (GFM). In the same experiments, an insulin (100 nM)-induced decrease in the levels of PEPCK and G6Pase was observed only after 24 h of treatment (data not shown). Treatment with p-synephrine (1–100 μM) did not affect cell viability (Fig. 1C).
FIG. 1.
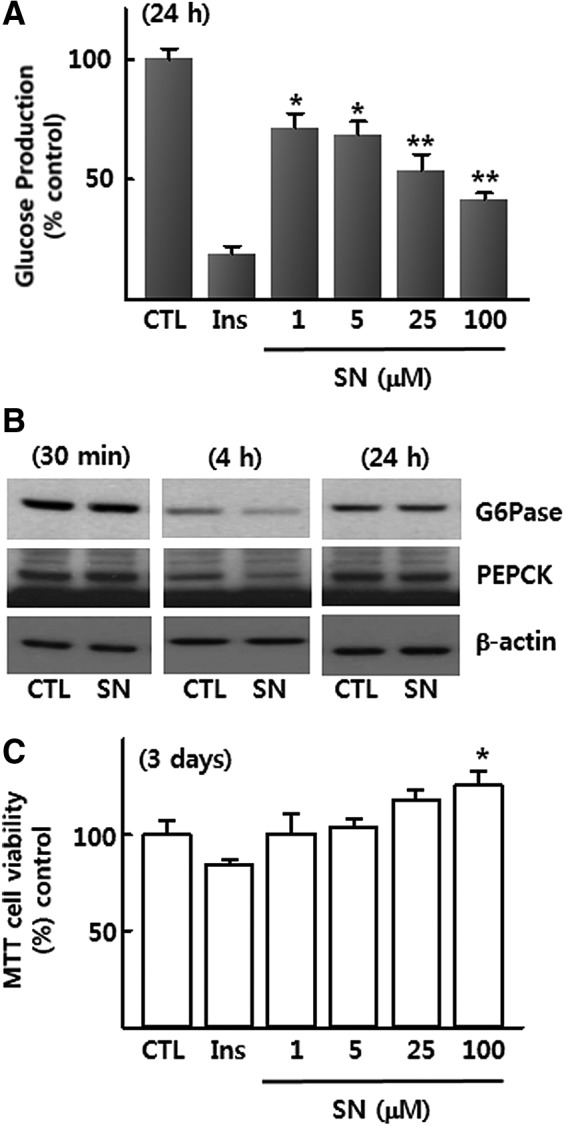
(A) Effect of p-synephrine (1–100 μM) and insulin (100 nM) on glucose production in H4llE cells after 24 h of treatment. (B) Effect of p-synephrine (50 μM) on the regulation of G6Pase and PEPCK proteins after 30 min, 4, and 24 h of treatment. (C) Effect of p-synephrine (1–100 μM) and insulin (100 nM) on H4llE cells' viability after 3 days of treatment. Data were expressed as mean±SE from three independent experiments. *P<.05, **P<.01 versus control (without p-synephrine). CTL, control; G6Pase, glucose-6-phosphatase; Ins, insulin (100 nM); PEPCK, phosphoenol pyruvate carboxykinase; SE, standard error; SN, p-synephrine.
We next tested whether the inhibition of adrenoceptors can affect the p-synephrine-induced suppression of glucose production in H4IIE cells. H4IIE cells were treated for 24 h with PA and PP, two specific antagonists against α-adrenocetors and β-adrenoceptors respectively. PA (25 μM) and PP (5 and 25 μM) suppressed glucose production in GFM (Fig. 2A), suggesting that glucose production in GFM is mediated by the stimulation of α-adrenoceptors and β-adrenoceptors. A cell viability study showed that all concentrations of PA and a low concentration of PP (5 μM) did not cause any change in MTT reactivity. However, higher concentration (25 μM) significantly reduced H4llE cells viability to <50% (Fig 2C). Neither PP nor PA blocked the p-synephrine-induced decrease in glucose production. On the contrary, treatment with 25 μM PA and PP augmented the degree of the p-synephrine-induced decrease in glucose production (Fig. 2B). These results indicate that p-synephrine does not activate α- and β-adrenoceptors to suppress hepatic glucose production.
FIG. 2.
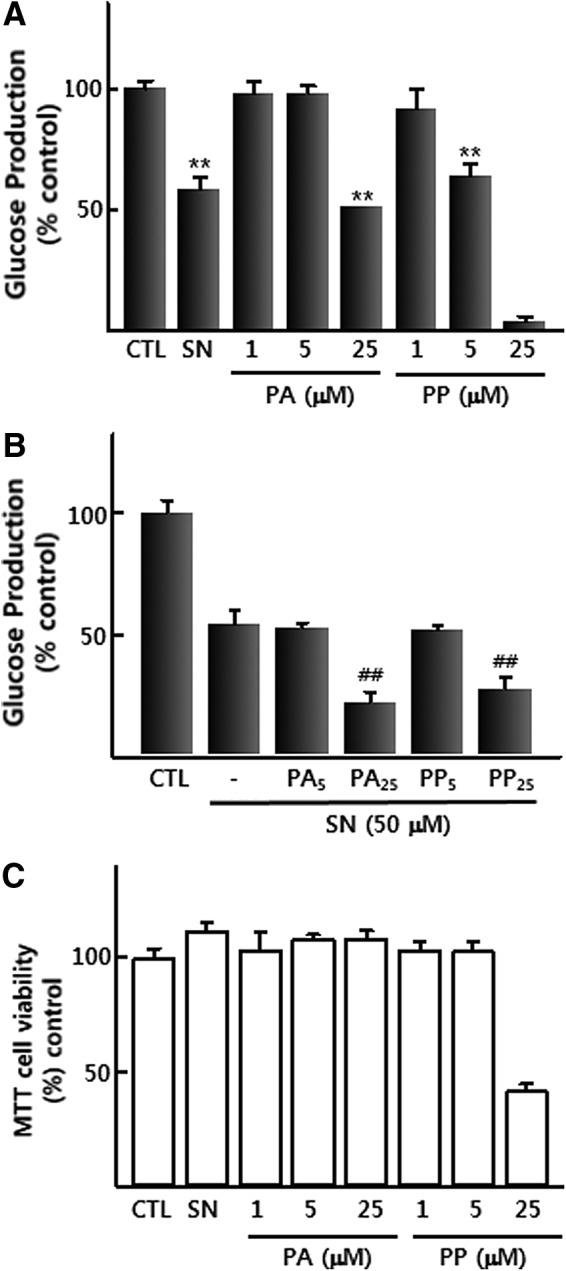
(A) Effect of p-synephrine (50 μM), PA, and PP (1–25 μM) on glucose production in H4llE cells after 24 h of treatment. (B) Effect of PA and PP (5–25 μM) with and without p-synephrine (50 μM) on glucose production in H4llE cells after 24 h of treatment. (C) Effect of PA and PP (1–25 μM) on the viability of H4llE cells after 24 h of treatment. Data were expressed as mean±SE from three independent experiments. **P<.01 versus control (without p-synephrine), ##P<.01 versus p-synephrine-alone. CTL, control; SN, p-synephrine; PA, phentolamine; PP, propranolol.
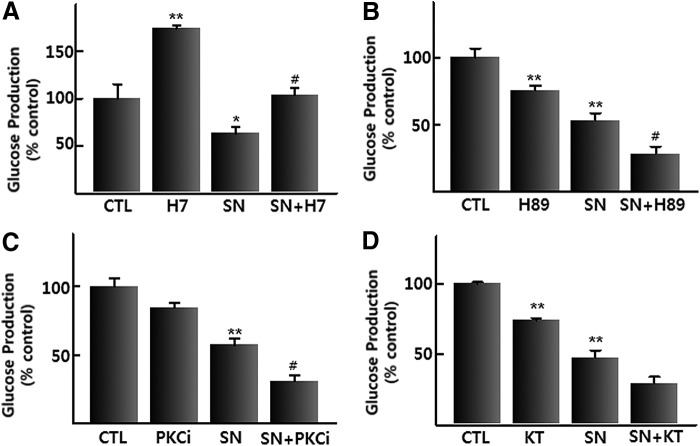
So far, little information is available on which intracellular signaling molecules are mobilized by p-synephrine treatment. Given the suppression of glucose production by p-synephrine, we next examined the effects of various inhibitors against intracellular signaling molecules on the p-synephrine-induced decrease in glucose production. Treatment with inhibitors against various signaling proteins and calcium chelator (BAPTA-AM) was unable to block the p-synephrine-induced decrease in glucose production (data not shown). Only one inhibitor (H-7) against serine/threonine kinase significantly increased the basal glucose production and blocked the p-synephrine-induced decrease in glucose production (Fig. 3A). Since H-7 can inhibit various serine/threonine kinases, including protein kinase A (PKA), protein kinase C (PKC), and protein kinase G (PKG), highly selective inhibitors (KT5823/PKG, GF109203x/PKC, and H89/PKA) were used to test which specific serine/threonine kinase can block the p-synephrine-induced decrease in glucose production. Concentrations of KT5823 (200 nM), PKCi (500 nM), and H89 (5 μM) used in experiments were determined to inhibit each kinase with minimal effects on other kinase. Unexpectedly, all three kinase inhibitors failed to block the p-synephrine-induced decrease in glucose production (Fig. 3B–D). From these results, novel serine/threonine kinase(s) other than PKA, PKC, and PKG are expected to mediate the p-synephrine-induced decreases in glucose production in H4IIE cells.
FIG. 3.
Effect of inhibitors of serine/threonine kinases on p-synephrine-induced suppression of glucose production in H4IIE cells. (A) H7, (B) H89, (C) PKCi, and (D) KT. Data were expressed as mean±SE from three independent experiments. *P<.05, **P<.01 versus control (without p-synephrine). #P<.5 versus p-synephrine-alone. CTL, control; SN, p-synephrine; KT, KT5823 (200 nM); PKCi, PKC inhibitor, GF109203x (500 nM); H89 (5 μM).
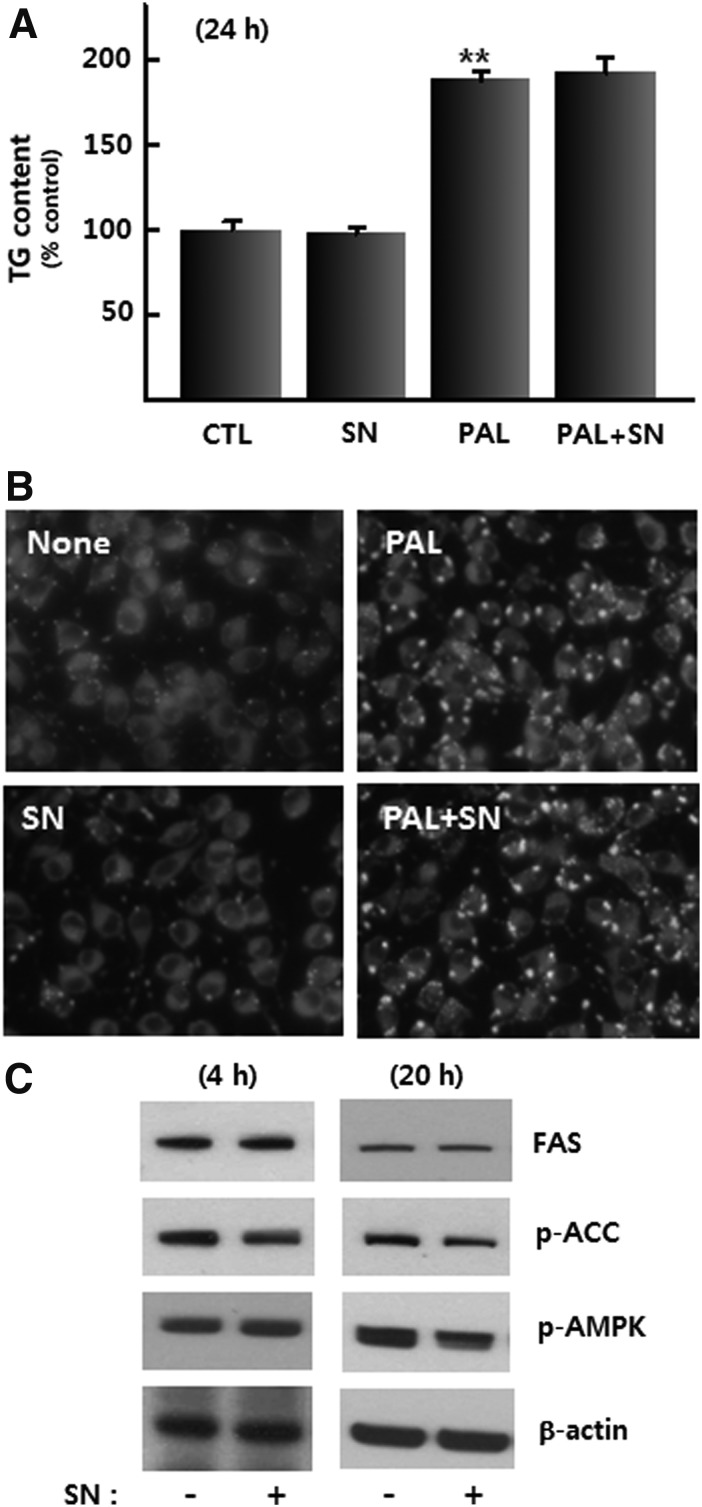
Various studies indicate the lipolytic activity of p-synephrine in adipose tissues of animals and humans.6 However, little is known about the effect of p-synephrine on hepatic lipid metabolism. Since it can be assumed that p-synephrine can stimulate lipolysis or suppress lipid accumulation in liver cells as well as adipose cells, experiments were designed to investigate the effect of p-synephrine on saturated free fatty acid-induced intracellular lipid accumulation in H4IIE cells. Addition of 0.1 mM palmitic acid increased the amount of triglyceride (1.9-fold over the control) within H4IIE cells (Fig. 4A). However, treatment with 50 μM p-synephrine for 24 h did not affect the basal- or palmitic acid induced increase in the amount of intracellular triglyceride (Fig. 4A). Staining with Nile red, a lipid-specific fluorescent dye, showed the same results (Fig. 4B). The amounts of FAS and phospho-acetyl-CoA carboxylase (pACC), key enzymes for increasing triglyceride, were not altered by p-synephrine treatment for 24 h (Fig. 4C).
FIG. 4.
(A) Effect of 24 h of treatment with p-synephrine (50 μM) and PA (0.1 mM) on triglyceride content in H4llE cells after. (B) Lipid droplet profile after p-synephrine (50 μM) and palmitic acid (0.1 mM) treatment. (C) Effect of p-synephrine (50 μM) on the regulation of FAS, p-ACC, and p-AMPK proteins after 4 and 20 h of treatment. Data were expressed as mean±SE from three independent experiments. **P<.01 versus control (without palmitic acid). CTL, control; FAS, fatty acid synthase; SN, p-synephrine; PAL, palmitic acid.
AMPK has a variety of metabolic functions in glucose and lipid metabolism.11 In L6 skeletal muscle cells, p-synephrine stimulates glucose uptake via AMPK.8 However, AMPK was not stimulated by p-synephrine in H4IIE liver cells (Fig. 4C). These results indicate that p-synephrine has little effect on lipid metabolism in liver cells.
Discussion
In this study, p-synephrine stimulated glucose production in cultured liver cells through serine/threonine kinase(s) other than PKA, PKC, and PKG. Although p-synephrine is a well-known lipolytic reagent in adipose tissues, our results show little effect of p-synephrine on hepatic lipid metabolism.
Previous studies showed the diverse effects of citrus extracts on antioxidant enzyme and lipid profiles,12 anti-inflammatory potential,13 and serum concentrations of glucose and insulin.14 However, little is known about the specific functions of active compounds in citrus extracts in hepatic glucose metabolism.
Our unpublished data show that the extract of citrus peel decreases glucose production and PEPCK protein levels in H4IIE cells. The liver is an important metabolic organ for maintaining plasma glucose and lipid levels within physiological ranges. Usually, hepatic glucose production is increased during the prolonged fasting state by activating the breakdown of glycogen (glycogenolysis) and the synthesis of glucose (gluconeogenesis) to supply the body with energy.15 However, de novo glucose synthesis persists despite a high blood glucose concentration in Diabetes Mellitus or insulin resistance.16 Thus, a series of studies have tried to develop agents from various natural products to lower hepatic glucose production. Epigallocatechin gallate (green tea),17 polyphenolic compounds from Artemisia dracunculus L,18 naringenin (citrus fruit),19 and ginsenoside20 can decrease glucose production in various hepatoma cell models. The anti-gluconeogenic activity of each compound might be mediated by mimicking insulin receptor signaling, stimulating AMPK activity, or through other unknown mechanisms although the biochemical hallmark of each compound is the suppression of PEPCK1.17–20
p-Synephrine has a similar structure to adrenergic agonists such as adrenaline, noradrenaline, ephedrine, and amphetamine. Both m- and p-synephrine activate adrenoceptors.21 In adipocytes, the lipolytic effect of p-synephrine may occur via the activation of β3-adrenoceptors.22,23 However, little is known about the role of adrenoceptors in the p-synephrine-induced suppression of hepatic glucose production. Different kinds of adrenergic receptors are expressed in liver cells and are involved in glycogenolysis and gluconeogenesis.24,25 Since p-synephrine can activate adrenoceptors in liver cells, it was assumed that p-synephrine may stimulate glucose production over the control group (GFM). However, p-synephrine unexpectedly suppressed the basal glucose production and the protein levels of PEPCK and G6Pase (Fig. 1A, B). The prolonged treatment with p-synephrine (100 μM) for 3 days did not induce any cytotoxicity (Fig. 1C). These results suggest the metabolic role of p-synephrine to be the suppression of hepatic glucose production without any adverse cytotoxic effects. The next experiments aimed at comparing the effects on the suppression of glucose production in H4IIE cells of p-synephrine with those of antagonists against adrenoceptors.
In the absence of any extracellular ligands or adrenergic agonists in GFM, the basal glucose production was dose dependently suppressed by antagonists against α-adrenocetors (PA) and β-adrenoceptors (PP). This means that the basal activity of adrenoceptors is stimulated by a glucose-deprivation stress per se, followed by an increase in hepatic glucose production. When H4IIE cells were treated with antagonists, PP suppressed glucose production at a lower dose (5 μM) than PA (25 μM). A higher dose (25 μM) of PP remarkably reduced MTT reactivity <50% of the control, implying the cytoprotective potential of β-adrenoceptors under the glucose-deprivation stress. In addition, p-synephrine-induced suppression of glucose production was not changed at lower doses (5 μM), or more exaggerated at higher doses (25 μM) of PA and PP.
Since both p-synephrine, an agonist of adrenoceptors, and antagonists against α-adrenocetors (PA) and β-adrenoceptors (PP) suppressed glucose production in liver cells, it is uncertain which signaling molecules or intracellular environments mediate the suppression of glucose production by p-synephrine.
Follow-up experiments were designed to identify signaling pathways that mediate p-synephrine's suppression of glucose production. Neither pharmacological inhibitors for PI3K, AMPK, MAPK, p38MAPK, and JNK nor calcium chelator BAPTA-AM blocked the p-synephrine's suppression of glucose production. Only H7, an inhibitor of serine/threonine kinases, including PKA, PKC, and PKG, significantly increased the basal glucose production and restored glucose production suppressed by p-synephrine. KT5823 is a potent, specific inhibitor of PKG (Ki=234 nM) that affects the activity of PKA (Ki>10 μM) and PKC (Ki>4 μM) only at higher doses.26 GF109203x is a potent and selective PKC inhibitor (IC50=0.02 μM).27 H89 is a PKA inhibitor (IC50=135 nM) that does not affect PKC and PKG.28 Concentrations of KT5823 (200 nM), PKCi (500 nM), and H89 (5 μM) used in experiments were determined to inhibit each kinase with minimal effects on other kinases. However, the decreased glucose production by p-synephrine was not restored by any specific inhibitors for PKA, PKC, or PKG. AMPK is a possible signaling component for suppressing hepatic glucose production.20 However, our data show that p-synephrine does not stimulate AMPK (Fig. 4C). To reduce hepatic glucose production, insulin receptor-mediated signaling pathways and LKB1-AMPK signaling pathways are properly operated. In insulin resistance or diabetes mellitus, insulin receptor signaling is restricted. Therefore, other glucose-lowering agents need to be introduced. A number of natural products or their components that activate AMPK have been introduced as pharmaceutical candidates for various diseases, including Diabetes Mellitus and obesity.29 Although the mechanism of action of p-synephrine in reducing hepatic glucose production is not yet understood, its potent glucose-lowering activity has been scientifically proved. Furthermore, p-synephrine has been found to be cytoxicologically safe.
This study showed that p-synephrine did not modify FFA-induced lipid accumulation within H4IIE cells. AMPK was also unaffected by p-synephrine. We previously found that p-synephrine stimulated glucose consumption as well as AMPK activity, the inhibition of AMPK blocked the translocation of glucose transporter 4 (Glut4) to the plasma membrane, and the increase in glucose consumption was stimulated by p-synephrine in L6 skeletal muscle cells.8 Previous studies have demonstrated the lipolytic activity of p-synephrine in adipose tissues of animals and humans.6 In adipocytes, the lipolytic effect of p-synephrine may occur via the activation of β3-adrenoceptors3.22,23 β3-adrenoceptors are expressed mainly in adipose tissue, and are also found in the gallbladder, urinary bladder, and brown adipose tissue.30 Other tissues, including skeletal muscles, do not express β3-adrenoceptors.30 The rarity of β3-adrenoceptors in the liver explains why p-synephrine does not affect intracellular lipid content. Although many natural products or their components can stimulate AMPK and lipolysis in the liver as well as in adipose tissue, p-synephrine might be excluded from the group of compounds having lipolytic activity in the liver.
Accordingly, we suggest that the main metabolic role of p-synephrine is the stimulation of glucose uptake in skeletal muscle cells and the suppression of hepatic glucose production in liver cells. We do not yet understand which signaling components or cytoplasmic environments are responsible for the suppression of hepatic glucose production by p-synephrine. Certain novel serine/threonine kinase(s), other than PKA, PKC, and PKG, potentially underlying p-synephrine's metabolic function need further investigation.
Acknowledgment
This work was supported by a National Research Foundation of Korea (NRFK) grant (2010-0022036).
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Haaz S, Fontaine KR, Cutter G, Limdi N, Perumean-Chaney S, Allison DB: Citrus aurantium and synephrine alkaloids in the treatment of overweight and obesity: an update. Obes Rev 2006;7:79–88 [DOI] [PubMed] [Google Scholar]
- 2.Stohs SJ, Shara M: A review of the safety and efficacy of Citrus aurantium in weight management. In: Obesity: Epidemiology, Pathophysiology, and Prevention (Bagchi D, Preuss HG, eds.). CRC Press, Boca Raton, FL, USA, 2007, pp. 371–382 [Google Scholar]
- 3.Blumenthal M: Bitter orange peel and synephrine. Part 1. Whole Foods 2004;77–79 [Google Scholar]
- 4.Stohs SJ, Preuss HG, Shara M: The safety of Citrus aurantium (bitter orange) and its primary protoalkaloid p-synephrine. Phytother Res 2011;25:1421–1428 [DOI] [PubMed] [Google Scholar]
- 5.Stohs SJ, Preuss H, Shara M: A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine. Int J Med Sci 2012;9:527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fugh-Berman A, Myers A: Citrus aurantium, an ingredient of dietary supplements marketed for weight loss: current status of clinical and basic research. Exp Biol Med 2004;229:698–704 [DOI] [PubMed] [Google Scholar]
- 7.Rossato LG, Costa VM, Limberger RP, Bastos ML, Remião E: Synephrine: from trace concentrations to massive consumption in weight-loss. Food Chem Toxicol 2011;49:8–16 [DOI] [PubMed] [Google Scholar]
- 8.Hong N, Cui Z, Kang H, Lee D, Lee Y, Park D: p-Synephrine stimulates glucose consumption via AMPK in L6 skeletal muscle cells. Biochem Biophys Res Commun 2012;418:720–724 [DOI] [PubMed] [Google Scholar]
- 9.Mosmann T: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63 [DOI] [PubMed] [Google Scholar]
- 10.Phillip G, Mayer E, Fowler S: Nile red: A selective fluorescent stain for intracellular lipid droplets. J Cell Biol 1985;100:965–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadowaki T, Yamauchi T: Adiponectin receptor signaling: a new layer to the current model. Cell Metab 2011;13:123–124 [DOI] [PubMed] [Google Scholar]
- 12.Deyhim F, Lopez E, Gonzales J, Patil BS: Citrus juice modulates antioxidant enzymes and lipid profiles in orchidectomized rats. J Med Food 2006;9:422–426 [DOI] [PubMed] [Google Scholar]
- 13.La VD, Zhao L, Epifano F, Genovese S, Grenier D: Anti-inflammatory and wound healing potential of Citrus auraptene. J Med Food 2013;16:961–964 [DOI] [PubMed] [Google Scholar]
- 14.Parmar HS, Kar A: Medicinal values of fruit peels from Citrus sinensis, Punica granatum, and Musa paradisiaca with respect to alterations in tissue lipid peroxidation and serum concentration of glucose, insulin, and thyroid hormones. J Med Food 2008;11:376–381 [DOI] [PubMed] [Google Scholar]
- 15.Raddatz D, Ramadori G: Carbohydrate metabolism and the liver: actual aspects from physiology and disease. Z Gastroenterol 2007;45:51–62 [DOI] [PubMed] [Google Scholar]
- 16.Quinn PG, Yeagley D: Insulin regulation of PEPCK gene expression: a model for rapid and reversible modulation. Curr Drug Targets Immune Endocr Metabol Disord 2005;5:423–437 [DOI] [PubMed] [Google Scholar]
- 17.Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK: Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem 2002;277:34933–34940 [DOI] [PubMed] [Google Scholar]
- 18.Govorko D, Logendra S, Wang Y, Esposito D, Komarnytsky S, Ribnicky D, Poulev A, Wang Z, Cefalu WT, Raskin I: Polyphenolic compounds from Artemisia dracunculus L. inhibit PEPCK gene expression and gluconeogenesis in an H4IIE hepatoma cell line. Am J Physiol Endocrinol Metab 2007;293:E1503–E1510 [DOI] [PubMed] [Google Scholar]
- 19.Purushotham A, Tian M, Belury MA: The citrus fruit flavonoid naringenin suppresses hepatic glucose production from Fao hepatoma cells. Mol Nutr Food Res 2009;53:300–307 [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Yuan HD, Chung SH: Ginsenoside Rg1 suppresses hepatic glucose production via AMP-activated protein kinase in HepG2 cells. Biol Pharm Bull 2010;33:325–328 [DOI] [PubMed] [Google Scholar]
- 21.Stohs SJ, Preuss HG, Shara M: A review of the receptor-binding properties of p-synephrine as related to its pharmacological effects. Oxid Med Cell Longev 2011;2011:482973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arch JR: β(3)-Adrenoceptor agonists: potential, pitfalls and progress. Eur J Pharmacol 2002;440:99–107 [DOI] [PubMed] [Google Scholar]
- 23.Tsujita T, Takaku T: Lipolysis induced by segment wall extract from Satsuma mandarin orange (Citrus unshu Mark). J Nutr Sci Vitaminol (Tokyo) 2007;53:547–551 [DOI] [PubMed] [Google Scholar]
- 24.Saitoh Y, Ui M: Stimulation of glycogenolysis and gluconeogenesis by epinephrine independent of its beta-adrenergic function in perfused rat liver. Biochem Pharmacol 1976;25:841–845 [DOI] [PubMed] [Google Scholar]
- 25.Reinhart PH, Taylor WM, Bygrave FL: Studies on alpha-adrenergic-induced respiration and glycogenolysis in perfused rat liver. J Biol Chem 1982;257:1906–1912 [PubMed] [Google Scholar]
- 26.Burkhardt M, Glazova M, Gambaryan S, Vollkommer T, Butt E, Bader B, Heermeier K, Lincoln TM, Walter U, Palmetshofer A: KT5823 inhibits cGMP-dependent protein kinase activity in vitro but not in intact human platelets and rat mesangial cells. J Biol Chem 2000;275:33536–33541 [DOI] [PubMed] [Google Scholar]
- 27.Lee SK, Stern PH: Divergent effects of protein kinase C (PKC) inhibitors staurosporine and bisindolylmaleimide I (GF109203X) on bone resorption. Biochem Pharmacol 2000;60:923–926 [DOI] [PubMed] [Google Scholar]
- 28.Lochner A, Moolman JA: The many faces of H89. Cardiovasc. Drug Rev 2006;24:261–274 [DOI] [PubMed] [Google Scholar]
- 29.Ruderman NB, Carling D, Prentki M, Cacicedo JM: AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest 2013;123:2764–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krief S, Lönnqvist F, Raimbault S, Baude B, Van Spronsen A, Arner P, Strosberg AD, Ricquier D, Emorine LJ: Tissue distribution of beta 3-adrenergic receptor mRNA in man. J Clin Invest 1997;91:344–349 [DOI] [PMC free article] [PubMed] [Google Scholar]