Abstract
microRNAs are endogenous noncoding RNA molecules of ∼22 nucleotides that regulate gene function by modification of target mRNAs. Due to tissue specific of miR-133a and miR-1/206 for skeletal muscles, we investigated the role of miR-133a and miR-1/206 in promoting the differentiation of the C2C12 cells. The results show that directly transfecting mature miR-133a, miR-1/206, or combinations (miR-1 and miR-206, miR-1 and miR-133a, and miR-133a and miR-206) into C2C12 cells, respectively, for 5 days induces formation of myogenic progenitor cells. Overexpression of miR-133a and miR-206 in C2C12 cells greatly improved multinucleated myotube formation. microRNA-133a (miR-133a) is highly expressed during human muscle development. Using bioinformatics, we identified one putative miR-133a binding site within the 3′-untranslated region of the mouse Foxl2 mRNA. The expression of Foxl2 was shown to be downregulated by subsequent western blot analysis.
Introduction
MicroRNAs (miRNAs) are endogenous noncoding RNA molecules of ∼22 nucleotides in length that play important regulatory roles in metabolism, cell signaling, function, decay, migration, apoptosis, dedifferentiation, and transdifferentiation by targeting the cleavage or translational repression activities of mRNAs (Bartel, 2004; Krol et al., 2010; Shenoy and Blelloch, 2012). These molecules regulate metabolism and cell function via a range of mechanisms (Alvarez-Garcia and Miska, 2005; Rooij et al., 2006; Brase et al., 2010; Krol et al., 2010), and have recently been involved in muscle biology (Chen et al., 2006; Kim et al., 2006; Callis et al., 2007; Eisenberg et al., 2008; Takaya et al., 2009; Chen et al., 2010a, Ge and Chen, 2011; Han et al., 2011; Koutsoulidou et al., 2011; Xie et al., 2011; Xu et al., 2012). Multiple miRNAs were found to be specifically expressed or highly abundant in skeletal and/or cardiac muscles, and form a distinct muscle miRNA group (Kim et al., 2006; Ge and Chen, 2011). MiR-133a may attenuate apoptosis of myocardiocytes by targeting CASP9 in ischemic postconditioning protection (He et al., 2011). Two miRNAs (miR-1 and miR-133) are highly expressed in both skeletal and cardiac muscles, whereas miR-206 is expressed only in skeletal muscle (Chen et al., 2006; Kim et al., 2006; Ge and Chen, 2011). Expression levels of miR-133a and miR-1/206 increased during the latter stages of human fetal muscle development (Koutsoulidou et al., 2011). MiR-1 and miR-206 regulate the transition of skeletal muscle satellite cells from proliferation to differentiation by repressing Pax7 (Chen et al., 2010a), while miR-133 enhances myoblast proliferation by repressing SRF and miR-1 promotes myogenesis by targeting histone deacetylase 4 (HDAC4) (Chen et al., 2006). However, miR-133 has also been shown to directly promote differentiation by downregulating the alternative splicing factor neuronal polypyrimidine tract-binding protein (nPTB) (Boutz et al., 2007), and miR-133a targets uncoupling protein 2 (UCP2) that regulates energy expenditure and thermogenesis in various organisms and negatively impacts myoblast differentiation (Chen et al., 2008). MiR-206 was found to be expressed differentially in female Piedmontese and Friesian cattle (Miretti et al., 2011). The miR-1/miR-206 and miR-133a families are the most extensively studied myogenic miRNAs (Williams et al., 2009; Ge and Chen, 2011). However, no study has addressed whether mature miRNA mimics can promote myoblast differentiation. A recent study indicated that mature miRNAs have the ability to cause transdifferentiation of human fibroblasts into neurons and reprogram cells to pluripotency (Miyoshi et al., 2011; Yoo et al., 2011). In this study, we attempted to decipher the biological function of miR133a in the differentiation of C2C12 cells. Mature miR-133a, miR-1/206, or combinations (miR-1 and miR-206, miR-1 and miR-133a, and miR-133a and miR-206) were transfected into C2C12 cells and their effects on differentiation to myogenic progenitor cells were investigated. We also investigated forkhead transcriptional factor 2 (Foxl2), a possible target of miR-133a, which is a potential target for the treatment of muscle disease.
Materials and Methods
Reagents
Culture reagents, molecular biology products, Opti-MEM® Reduced Serum Medium, and horse serum (HS) were purchased from Gibco. Lipofectamine® RNAiMAX and Lipofectamine™ 2000 transfection reagent were from Invitrogen. MirVana® miRNA mimics (hsa-miR-1, hsa-miR-206, and hsa-miR-133a) and the negative control were from Applied Biosystems (Life Technologies). SYBR® Premix Ex Taq II and PrimeScript RT reagent kit were from Takara. Antibody MYH1/2/4 (MY-32) sc-58797 and goat anti-mouse IgG-FITC sc-2010 were from Santa Cruz Biotechnology. FOXL2 antibody was from Boster Biotechnology. Foxl2 siRNA (sense: 5′-GCGUCGUGAACUCCUACAAT T-3′, antisense: 5′-UUGUAGGAGUUCACGACGCTT-3′) was from Shanghai Jima. Dual-Glo® Luciferase Assay System was from Promega. MiRNA real-time detection kit was from Applied Biosystems.
Cell culture
C2C12 cells were cultured in growth medium comprising Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% fetal bovine serum and incubated at 37°C in a humidified incubator containing 5% CO2. HEK-293 cells were grown under the same conditions. When C2C12 cells reached a high confluency the medium was changed to differentiation medium comprising DMEM supplemented with 2% HS to induce differentiation (Kato et al., 2009).
Transient transfection of miRNA mimic
According to the Lipofectamine RNAiMAX Transfection Reagent protocol, 10 nM of miRNAs was reverse transfected into C2C12 cells in six-well plates at a density of 2.5×104 cells/well. After culturing for 48 h at 37°C with 5% CO2, miRNAs were forward transfected into C2C12 cells for a second time and incubated for 72 h prior to immunodetection.
Real-time polymerase chain reaction assay
Total RNA was extracted from C2C12 cells with Trizol reagent (Invitrogen), and 2.0 μg of samples was reverse transcribed using the PrimeScript RT reagent kit (Takara) according to manufacturer's instructions. Quantitative real-time PCR (RT-PCR) was performed using an SYBR Premix Ex Taq II PCR kit (Takara) with specific primers listed in Table 1. In each analysis, ∼100 ng of DNA was used for amplification by PCR using a 40-cycle program in an ABI7900 fast instrument.
Table 1.
Primers Used for Real-Time Polymerase Chain Reaction
| Primer name | Forward sequence (5′-3′) | Reverse sequence (5′-3′) | Product length (bp) |
|---|---|---|---|
| GAPDH | AAGGTGAAGGTCGGAGTCAAC | GGGGTCATTGATGGCAACAATA | 102 |
| MyoD | CGCCATCCGCTATATCGAGG | CTGTAGTCCATCATGCCGTCG | 186 |
| MyHC | GCTGACTGATCGAGACAACCA | CCTCTAGGGTTCCCTGCATTTT | 161 |
| MyoG | CCTGCTCAGCTCCCTCAACC | CAGCCGTGAGCAGATGATCCC | 170 |
| Caldesmon | TGGAGGTGAATGCCCAGAAC | GAAGGCGTTTTTGGCGTCTTT | 138 |
| MAML1 | GCCAGCAACAGGCCGCTGTAA | GGCAGCAGAGGACCCTGTGAACT | 242 |
| Foxl2 | ACAACACCGGAGAAACCAGAC | CGTAGAACGGGAACTTGGCTA | 145 |
Muscle protein immunodetection
Cells treated in six-well plates were washed with phosphate-buffered saline (PBS) for 10 min, fixed with 4% paraformaldehyde in PBS for 10 min, and treated in 0.5% Triton X-100 for 10 min at 37°C. After blocking with 1% bovine serum albumin (BSA) in PBS for 30 min, cells were incubated with primary antibody for 16 h at 4°C: using a 1:50 dilution of the anti-MyHC. The secondary antibody (fluorescein-conjugated anti-mouse and anti-rabbit; 1:100 dilution) in PBS containing 1% BSA was added and incubated for 1 h at 37°C. After washing with PBS for 10 min, nuclei were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) for 2 min at room temperature. Images were subjected to fluorescence microscopy. For each well, 10 fields covering the whole well were imaged and green-fluorescence-positive cells and total cells with DAPI staining were counted.
Plasmid construction
To construct the luciferase/Foxl2 3′UTR wild-type reporter construct, mouse Foxl2 3′UTR including seed sequences (sense: 5′-CTAGTGTAGC TTGTTGTTTGGGGGACCAAATTTTCTAGAGAGAACTAA-3′; antisense: 5′-AGCTTTAGTTCTCTCTAGAAAATTTGGTCCCCCAAACAACAA GCTACA-3′) was synthesized by Invitrogen. SpeI and HindIII restriction sites are underlined. Double-stranded oligonucleotide was synthesized in annealing buffer at 95°C for 5 min and 37°C for 1 h. The synthesized product was ligated into the SpeI and HindIII sites downstream from the firefly luciferase reporter vector pMIR-REPOTR (Promega). Seed regions were mutated to remove all complementarity to nucleotides 2–6 of miR-133a by artificial mutation.
Luciferase reporter assays
The 3′-untranslated region (UTR) of mouse Foxl2 was artificially synthesized and individually cloned into the pMIR-REPORT vector (Ambion). Seed regions were mutated in intermediate 5 complementarity nucleotides of miR133a. HEK-293 cells were cotransfected with 20 ng of firefly luciferase reporter vector and 5 ng of the control vector containing Renilla luciferase, pRL-TK (Promega), using Lipofectamine 2000 (Invitrogen) in 96-well plates. Each transfection was carried out in four wells. For each well, 50 nM of miR-133a precursor molecule (Ambion) or a negative control precursor miRNA (Ambion) was cotransfected with the reporter constructs. Luciferase assays were performed 24 h after transfection using the Dual-Glo Luciferase Assay System (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity.
Western blot analysis
C2C12 cells at a density of 1×105 were seeded and grown in DMEM containing 10% fetal bovine serum in six-well plates for 24 h. After transfection for 48 h, cells were washed with cold PBS and subjected to lysis buffer (62.5 mM Tris-Cl, 2% SDS, 10% glycine, 50 mM DTT, and 0.1% bromophenol blue). Cell lysates containing equal amounts of protein were separated by 10% SDS-PAGE and electrotransferred to nitrocellulose membranes. Membranes were blocked with buffer containing 5% nonfat milk in PBS and 0.1% Tween 20 for 2 h and then incubated overnight at 4°C with primary antibody. After washing with PBS containing 0.1% Tween 20, membranes were incubated with peroxidase-conjugated secondary antibodies and developed using an enhanced SuperSignal West Pico Chemiluminent Substrate detection kit (Pierce). GAPDH was used as a loading control.
Statistical analysis of real-time PCR data
Statistical analysis was performed as described (Livak and Schmittgen, 2001). ΔCt was calculated as the Ct (muscle protein) − Ct (GAPDH). Data analysis was performed using the 2−ΔΔCt method. Mean±SD was used to measure intrasample variation. A p<0.05 was considered statistically significant. All experiments were independently repeated at least three times.
Results
Transfection of C2C12 cells with miR-133a and miR-1/206 induced changes in muscle-associated gene expression
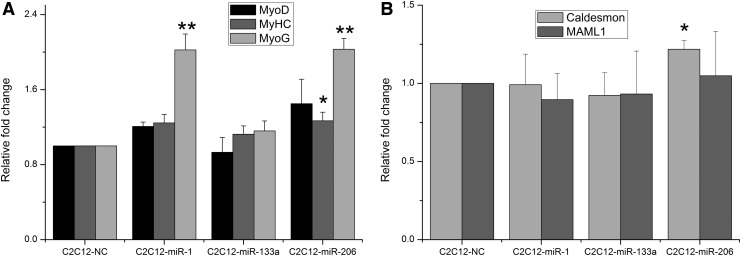
To verify whether mature miR-133a and miR-1/206 mimics are able to induce muscle-associated gene expression, C2C12 cells were transfected with these miRNA mimics for 48 h. Results showed that myogenin (MyoG) was upregulated 2.02±0.17-fold (p<0.01) in the C2C12-miR-1 cells and 2.03±0.12-fold (p<0.01) in the C2C12-miR-206 cells. In contrast, MyoG was unaffected in the C2C12-miR-133a cells. Myogenic differentiation-1 (MyoD) gene expression did not alter significantly between the control and C2C12-miR-1, C2C12-miR-1-206, and C2C12-miR-133a cells. Expression of myosin heavy chain (MyHC) gene was upregulated 1.268±0.09-fold (p<0.05) in the C2C12-miR-206 cells, but not in the C2C12-miR-1 and C2C12-miR-133a cells (Fig. 1A). Caldesmon expression level was upregulated (1.22±0.06-fold, p<0.05) in the C2C12-miR-206 cells but relatively unchanged in the C2C12-miR-1 and C2C12-miR-133a cells. Mastermind-like-1 (MAML1) expression was unchanged in all transfected lines (Fig. 1B). Expression was normalized against C2C12-NC in all cases.
FIG. 1.
Expression of muscle-associated gene determined by quantitative RT-PCR 48 h after transfection of C2C12 with miRNA-1, miRNA-133a, and miRNA-206. (A) Expression levels of MyoD, MyHC, and MyoG gene mRNAs in C2C12-miR-1, C2C12-miR-133a, and C2C12-miR-206 cells. (B) Expression levels of Caldesmon and MAML1 in C2C12-miR-1, C2C12-miR-133a, and C2C12-miR-206 cells. The error bars represent the data in triplicates. Results are displayed as mean±SEM, **p<0.01; *p<0.05. miRNA, microRNA; RT-PCR, real-time polymerase chain reaction.
Overexpression of miR-133a and miR-1/206 caused differentiation of C2C12 cells to muscle precursor cells
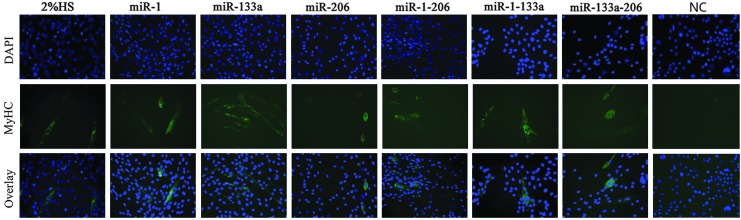
The specific effects of the miRNA mimics on C2C12 cells were also investigated. Strong myogenic differentiation was observed in transfected C2C12 cells 5 days after induction of differentiation, as demonstrated by multinucleated myotube formation and MyHC expression (Fig. 2). Further, miR-133a, miR-1, miR-206, and the combinations miR-1-206, miR-1-133a, and miR-133a-206 were able to induce the differentiation of C2C12 cells into myocytes.
FIG. 2.
Induction of differentiation of C2C12 cells by miRNAs in vitro. C2C12 cells were transfected with NC miRNA, miR-1, miR-133a, miR-206, and the combinations miR-1-206, miR-1-133a, and miR-133a-206 for 5 days. Myogenic differentiation was detected by immunostaining for MyHC expression. C2C12 cells treated with 2% HS were used as a control. Nuclei were stained with DAPI. Scale bars=20 μm. HS, horse serum.
The effects of the miRNAs on human amnion (FL) cells were also studied. Five days after transfection of FL cells with the miRNA mimics, no MyHC-positive cells or fused cells were observed using light microscopy (data not shown), which suggests that only miRNAs could not alter the differentiation fate of FL cells.
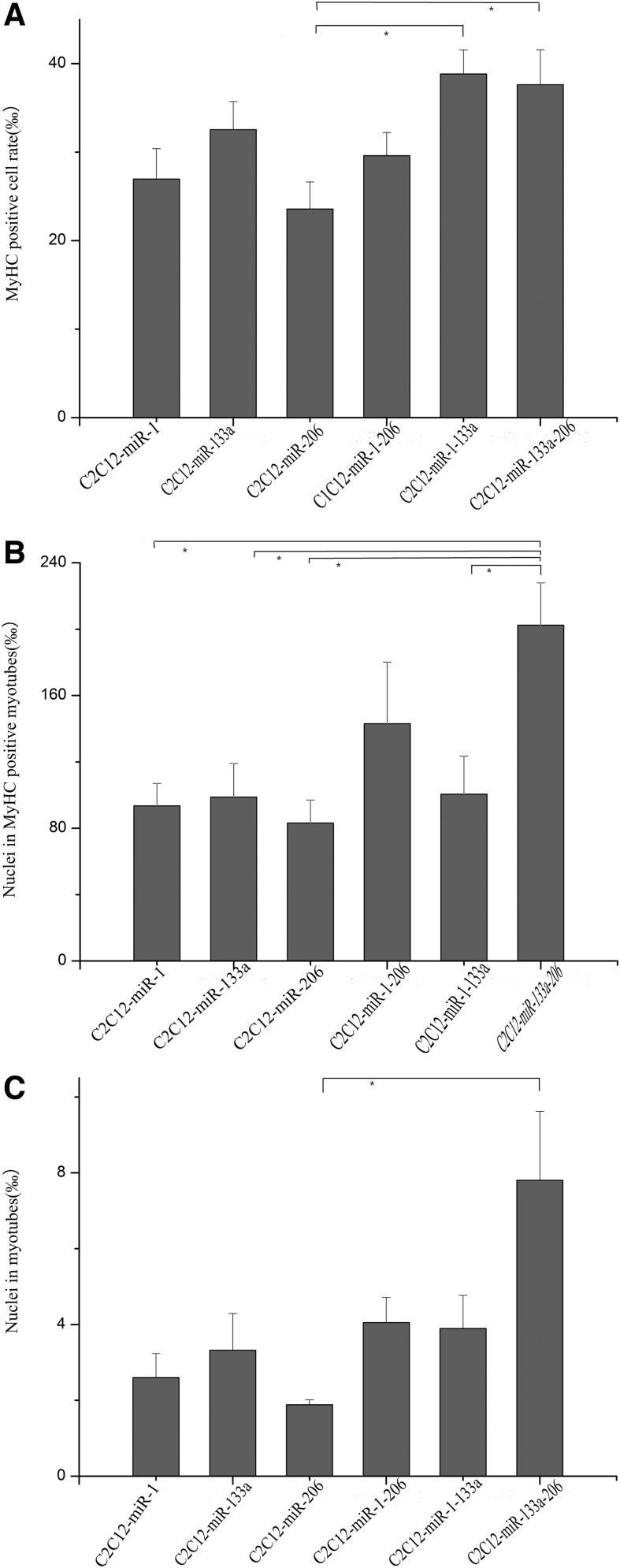
The MyHC-positive cell rate and the fusion rate in differentiation of muscle precursor cells
The fusion index is calculated as the percentage of nuclei in fused myotubes out of the total number of nuclei in each microscopic field. Myotubes with two or more nuclei were defined as fused myotubes. A positive cell rate refers to the percentage of MyHC-positive cells out of the total cells, and the positive fusion index is calculated as the percentage of nuclei in fused myotubes out of the total number of MyHC-positive cells.
The MyHC-positive cell rate was essentially identical in miR-1-, miR-133a-, miR-206-, miR-1-133a-, miR-1-206-, and miR-133a-206-induced C2C12 cell lines. However, C2C12-miR-206 cells exhibited much lower MyHC expression (29.60‰±2.62‰) than C2C12-miR-1-133a (38.84‰±2.73‰, p<0.05) and C2C12-miR-133a-206 (37.63‰±3.96‰, p<0.05) (Fig. 3A). Five days after induction, myogenic differentiation was observed in transfected cell lines, as demonstrated by multinucleated myotube formation and MyHC expression. The miR-1-206, miR-1-133a, and miR-133a-206 lines exhibited increased multinucleated myotube formation. The high positive fusion index score of the miR-133a-206 cell line (202.41‰±25.49‰) was particularly striking compared with the other cell lines (C2C12-miR-1, 93.54‰±13.47‰; C2C12-miR-133a, 98.74‰±20.35‰; C2C12-miR-206, 83.10‰±13.95‰; C2C12-miR-1-133a, and 100.48‰±22.98‰; p<0.05, Fig. 3B). These cells also exhibited a markedly higher nuclei fusion rate (7.80‰±1.82‰) compared with the other cell lines (C2C12-miR-206, 1.88‰±0.13‰; p<0.05, Fig. 3C). Together these results show that mature miRNAs induced differentiation of C2C12 cells into myogenic progenitor cells, and the combined use of miR-133a and miR-206 greatly enhanced multinucleated myotube formation.
FIG. 3.
Relative MyHC-positive cell rate and the fusion rate of C2C12 cells transfected with miRNAs. (A) Quantitative analysis of MyHC-positive cell rate at 5 days. (B) Quantitative analysis of positive fusion event of myoblasts at 5 days. (C) Quantitative analysis of fusion event of myoblasts at 5 days. Error bars indicate SEM of 10 microscopic fields from three independent experiments. *p<0.05. Scale bars=20 μm.
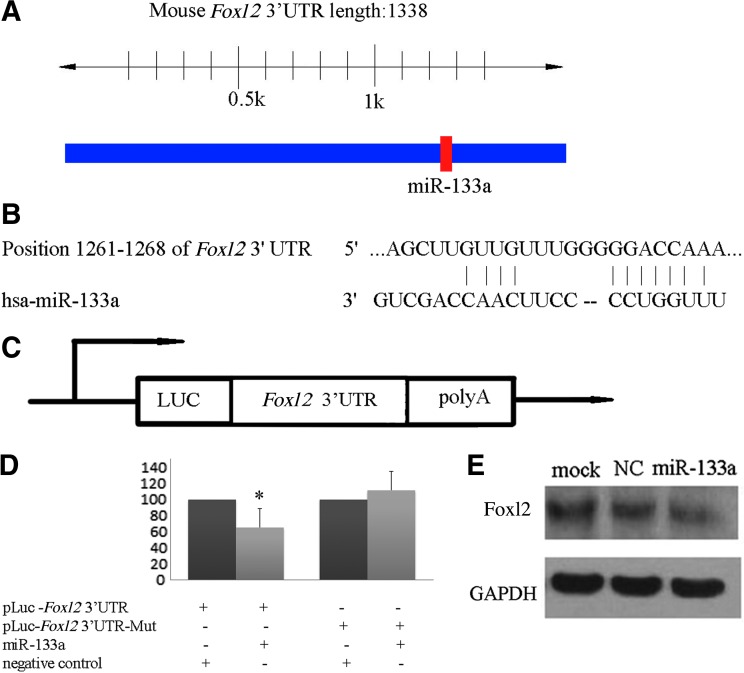
Foxl2 is a target of miR-133a
After demonstrating a role for mature miR133a in differentiation of C2C12 cells, the cellular mechanisms underlying miR133a-mediated cell proliferation were investigated. TargetScan, microRNA, and PicTar were used to predict potential target of miR-133a. One potential binding site of miR-133a was predicted in the mouse Foxl2 3′UTR (Fig. 4A). An alignment of the predicted miR-133a target sites and miR-133a and the conserved 7-bp seed sequence for miR-133a:mRNA pairing are shown (Fig. 4B). To investigate the possible regulation of Foxl2 through this predicted binding sites, we synthesized the Foxl2 3′UTR sequence and inserted it downstream of the firefly luciferase coding region in the pMIRLuc vector (Fig. 4C). Mutants with the putative binding sites were prepared as described previously in the “Materials and Methods” section. Introduction of miR-133a into HEK293 cells with the wild-type 3′UTR (pLuc-Foxl2 3′UTR) caused significant inhibition of luciferase activity compared with negative control (Fig. 4D). Mutations of the binding sites (using mutant vector pLuc-Foxl2 3′UTR-Mut) completely abolished the ability of miR-133a to regulate luciferase expression (Fig. 4D). These results indicated that Foxl2 was a potential target of miR-133a. To further confirm that miR-133a was indeed responsible for the regulation of Foxl2, C2C12 cells were transfected with the miR-133a or negative control. Western blot analysis confirmed that Foxl2 expression was reduced when transfected with miR-133a (Fig. 4E).
FIG. 4.
Foxl2 is a direct target of miR-133a. (A) Predicted miR-133a binding sites in Foxl2 3′UTR. Specific locations of the binding sites were marked with red color, and Foxl2 3′UTR was marked with blue color. (B) Alignment between the predicted miR-133a target sites and miR-133a is shown. The conserved, 7-bp seed sequence for miR-133a:mRNA pairing is also indicated. (C) Diagram depicting the pMIR luciferase reporter constructs, containing a cytomegalovirus (CMV) promoter, which was utilized to verify the putative miR-133a binding sites. Luc, luciferase; poly A, poly(A) tail. (D) HEK293 cells were cotransfected with miR-133a, pLuc-FOXL2 3′UTR, along with a pRL-TK reporter plasmid. After 24 h, the luciferase activity was measured. Values are presented as relative luciferase activity after normalization to Renilla luciferase activity. The data are expressed as the mean value±S.E. (error bars) of the results obtained from three independent experiments. *Differences in luciferase activity between miR-133a and negative control transfected cells were significant, p<0.05. (E) Foxl2 expression levels in C2C12 cells after transfection with miR-133a were determined by western blot analysis. As compared with the NC miRNA, miR-133a expression reduced the levels of Foxl2 in C2C12 cells. GAPDH was used as an internal control. Foxl2, forkhead transcriptional factor 2; UTR, untranslated region.
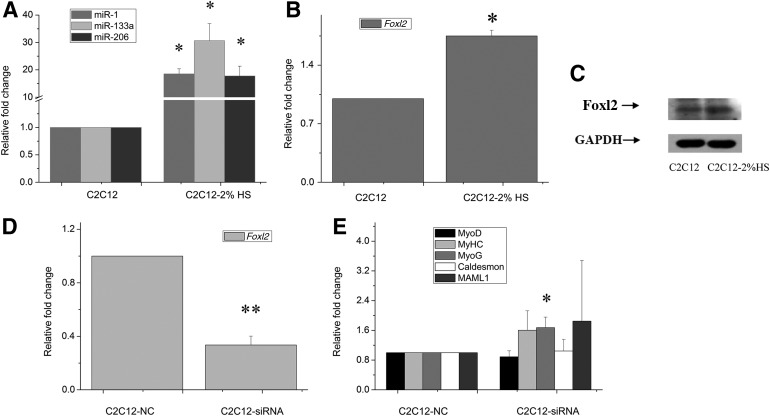
Expression changes of miRNAs and Foxl2 after C2C12 cells were treated by 2% HS
We identified the expression changes of miR-1, miR-133a, miR-206, and Foxl2 after C2C12 cells were treated by 2% HS for 5 days. The results indicated that the levels of these three miRNAs increased significantly (miR-1, 18.5±1.8-fold; miR-133a, 30.7±6.2-fold; and miR-206, 17.75±3.5-fold; p<0.05, Fig. 5A). Further, the Foxl2 mRNA increased 1.75±0.07-fold (p<0.05, Fig. 5B) and protein expression also increased (Fig. 5C).
FIG. 5.
Expression of miRNAs and Foxl2 after using 2% HS differentiation of C2C12 cells for 5 days and Foxl2 knockdown by siRNA. (A) Quantitative analysis of miR-1, miR-133a, and miR-206 by real-time PCR. (B) Quantitative analysis of Foxl2 by real-time PCR. (C) Foxl2 protein expression. (D) Quantitative analysis of Foxl2 by real-time PCR. (E) Quantitative analysis of MyoD, MyHC, MyoG, Caldesmon, and MAML1 by real-time PCR. **p<0.01, *p<0.05.
Foxl2 knockdown
Finally, we examined muscle-associated gene expression changes after Foxl2 was knocked down. Foxl2 siRNA was transfected into C2C12 for 48 h, and the Foxl2 mRNA expression was knocked down by more than 60% (C2C12-siRNA, 0.34±0.06-fold; p<0.01, Fig. 5D). Results show that MyoG mRNA expression level was upregulated compared with negative control (MyoG, 1.67±0.28-fold; p<0.05, Fig. 5E). But other muscle-associated gene expression levels were unchanged. This result was consistent with C2C12 cells transfected with miRNA-1 or miRNA-206. We also transfected siRNA into C2C12 cells for two times and the Foxl2 inhibition time up to 5 days. The final results remain unchanged. And we had not observed the formation of multinucleated myotube.
Discussion
The great discovery of miRNAs has revolutionized both cell biology and medical science. The rapid progress on miRNAs in the past few years illustrates the huge potential of miRNAs for the diagnosis and treatment of various diseases in the near future. A wealth of literature has focused on the role of miRNAs in human cancer (Sun et al., 2010; Ha, 2011; Fabbri, 2013; Heidi et al., 2013), cardiovascular disease (Rooij et al., 2006; Ha, 2011; Han et al., 2011; Limana et al., 2011; Xu et al., 2012; Nishizawa and Suzuki, 2013), muscle disorders (Eisenberga et al., 2007; Cordes et al., 2009; Lee et al., 2009; Mohamed et al., 2011; Xie et al., 2011), and skeletal myogenesis (Puri et al., 2001; Chen et al., 2006; Chen et al., 2010a). Recently, it was reported that miR-1 and miR-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7 (Chen et al., 2010b). MiR-1 and miR-133 may play important roles in the myocardial differentiation of mouse embryonic stem cells (Takaya et al., 2009). The cardiac-enriched miRNAs, including miR-1, miR-133, and miR-208, as well as the ubiquitous miR-23a and miR-199b, play major roles in the development of cardiac hypertrophy. Whereas miR-21, miR-199a, miR-210, and miR-494 have been shown to be critical for adaptation and survival during hypoxia/ischemia in myocytes (Han et al., 2011).
Exciting new discoveries revealed the role that miRNAs may play in regenerative medicine. Not only are they involved in cell fate transitions, they also participate in numerous molecular signaling pathways and cellular processes (Ma et al., 2012; Jin et al., 2013). While transcription factors can transdifferentiate cells (Takahashi and Yamanaka, 2006; Huangfu et al., 2008; Kim et al., 2009), they must be introduced into the cell as DNA or highly modified mRNAs. In contrast, miRNAs can be introduced into cells in their mature form relatively easily, providing a potentially more tractable alternative to transcription factors in clinic (Brase et al., 2010; Krol et al., 2010; Ha, 2011; Miyoshi et al., 2011; Shenoy and Blelloch, 2012).
Overexpression of miR-133a and miR-1/206 during myogenesis has been profiled extensively by many research groups. However, the ability of these three mature miRNA mimics to promote differentiation has not been reported. In this study, miR133a and miR-1/206 promoted differentiation of C2C12 cells into myogenic progenitor cells, and combinations of miRNAs enhanced multinucleated myotube formation, particularly miR-133a and miR-206. These three miRNAs also stimulated the expression of MyoD, MyHC, and MyoG in FL cells, but did not cause cells to transdifferentiate into muscle precursor cells, indicating that other factors are involved in muscle cell differentiation.
Using bioinformatics, we computationally identified the Foxl2 mRNA 3′UTR as a potential target of miR-133a, and showed experimentally that miR-133a reduced Foxl2 expression in C2C12 cells by western blot. Foxl2 is a particularly interesting target due to its involvement in many types of diseases, including blepharophimosis syndrome (Jiang et al., 2013) and ovarian granulosa cell tumors (Benayoun et al., 2012). Foxl2 is also mutated in granulosa cell tumors in males (Lima et al., 2012). In addition, we provided evidence that miR-133a reduced Foxl2 expression in C2C12 cells. While Foxl2 and miRNA133a increased during the differentiation of C2C12 cells upon 2% HS treatment. This may mean that the HS and miRNA regulate differentiation in different ways. Also, this phenomenon may be due to the weaker ability of miRNA133a downregulating Foxl2 expression than HS upregulating Foxl2 expression. Foxl2 knockdown caused MyoG upregulation in C2C12. But MyoD, MyHC, Caldesmon, and MAML1 expression levels changed little. This result was consistent with C2C12 cells transfected with miRNA-1 or miRNA-206. But we had not observed the formation of multinucleated myotube after C2C12 cells were transfected by siRNA for 5 days. The possible reason may be that myogenic differentiation was a complicated process and many factors are involved in this process. Just downregulating the expression of Foxl2 was not enough to affect C2C12 differentiation process. And more studies should be done to clarify the mechanism of the relationship among the miRNAs, HS, and C2C12 differentiation.
In conclusion, transfection of mature miR-133a, miR-1/206, and the combinations miR-1-133a, miR-1-206, and miR-133a-206 caused the differentiation of C2C12 into myogenic progenitor cells. Overexpression of miR-133a and miR-206 greatly improved multinucleated myotube formation. Further, Foxl2 was identified as a target of miR-133a. The miRNAs characterized in this work have potential therapeutic applications and may be useful for identifying other targets for treating muscle diseases. Since this differentiation approach does not require vector-based gene transfer and leaves no residual vector DNA, it holds significant potential for biomedical research and regenerative medicine. The targeting of Foxl2 also shows promise, but further study is needed to elucidate how miR-133a influences differentiation through Foxl2 gene and long-term effects of these mature miRNAs on cells, especially regarding potential clinical application.
Acknowledgments
This work was supported by grants from the National Science and Technology Major Project (No. 2012ZX10002006), Key Technologies R&D Program of Zhejiang Province (No. 2012C03SA170003), and Hangzhou Key Technologies R&D Program (No. 20122513A49). The authors thank members of our laboratories for discussion and support.
Disclosure Statement
No competing financial interests exist.
References
- Alvarez-Garcia I., and Miska E.A. (2005). MicroRNA functions in animal development and human disease. Development 132,4653–4662 [DOI] [PubMed] [Google Scholar]
- Bartel D.P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116,281–297 [DOI] [PubMed] [Google Scholar]
- Benayoun B.A., Anttonen M., L'Hôte D., Bailly-Bechet M., Andersson N., Heikinheimo M., et al. (2012) Adult ovarian granulosa cell tumor transcriptomics: prevalence of FOXL2 target genes misregulation gives insights into the pathogenic mechanism of the p.Cys134Trp somatic mutation. Oncogene 32,2739–2746 [DOI] [PubMed] [Google Scholar]
- Boutz P.L., Chawla G., Stoilov P., and Black D.L. (2007). MicroRNAs regulate the expression of the alternative splicing factor nPTB during muscle development. Gene Dev 21,71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brase J.C., Wuttig D., Kuner R., and Sültmann H. (2010). Serum microRNAs as non-invasive biomarkers for cancer. Mol Cancer 9,306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis T.E., Chen J.F., and Wang D.Z. (2007). MicroRNAs in skeletal and cardiac muscle development. DNA Cell Biol 26,219–225 [DOI] [PubMed] [Google Scholar]
- Chen J.F., Mandel E.M., Thomson M.J., Wu Q.L., Callis T.E., Hammond S.M., et al. (2006). The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38,228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.F., Tao Y.Z., Li J., Deng Z.L., Yan Z., Xiao X., et al. (2010). microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol 190,867–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Wang K.H., Chen J.N., Guo J.G., Yin Y., Cai X., et al. (2008). In vitro evidence suggests that miR-133a-mediated regulation of uncoupling protein 2 (UCP2) is an indispensable step in myogenic differentiation. J Biol Chem 284,5362–5369 [DOI] [PubMed] [Google Scholar]
- Cordes K.R., Sheehy N.T., White M., Berry E., Morton S.U., Muth A.N., et al. (2009). miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460,705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg I., Alexanderb M.S., and Kunkela L.M. (2008). miRNAS in normal and diseased skeletal muscle. J Cell Mol Med 13,2–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberga I., Eranb A., Nishino I., Moggio M., Lamperte C., et al. (2007). Distinctive patterns of microRNA expression in primary muscular disorders. Proc Natl Acad Sci U S A 104,17016–17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M. (2013). MicroRNAs and cancer: towards a personalized medicine. Curr Mol Med 13,751–756 [DOI] [PubMed] [Google Scholar]
- Ge Y.J., and Chen J. (2011). MicroRNAs in skeletal myogenesis. Cell Cycle 10,441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T.Y. (2011). MicroRNAs in human diseases: from cancer to cardiovascular disease. Immune Netw 11,135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M.Y., Toli J., and Abdellatif M. (2011). MicroRNAs in the cardiovascular system. Curr Opin Cardiol 26,181–189 [DOI] [PubMed] [Google Scholar]
- He B., Xiao J., Ren A.J., Zhang Y.F., Zhang H., Chen M., et al. (2011). Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J Biomed Sci 18,22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidi D., Anna G., Stefan G., Mali S.D., Christina C., Andrea S., et al. (2013). The shaping and functional consequences of the microRNA landscape in breast cancer. Nature 497,378–382 [DOI] [PubMed] [Google Scholar]
- Huang F.D., Osafune K., Maehr R., Guo W., Eijkelenboom A., Chen S., et al. (2008). Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 26,1269–1275 [DOI] [PubMed] [Google Scholar]
- Jiang H., Huang X.H., Su Z.G., Rao L.B., Wu S.S., Zhang T., et al. (2013). Genetic analysis of the forkhead transcriptional factor 2 gene in three Chinese families with blepharophimosis syndrome. Mol Vis 19,418–423 [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Tymen S.D., Chen D., Fang Z.J., Zhao Y., Dragas D., et al. (2013). MicroRNA-99 family targets AKT/mTOR signaling pathway in dermal wound healing. PLoS One 8,e64434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Miyaki S., Yokoyama S., Omori S., Inoue A., Horiuchi M., et al. (2009). Real-time functional imaging for monitoring miR-133 during myogenic differentiation. Int J Biochem Cell Biol 41,2225–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Kim C.H., Moon J.I., Chung Y.G., Chang M.Y., Han B.S., et al. (2009). Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 4,472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.K., Lee Y.S., Sivaprasad U., Malhotra A., and Dutta A. (2006). Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol 174,677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsoulidou A., Mastroyiannopoulos N.P., Furling D., Uney J.B., and Phylactou L.A. (2011). Expression of miR-1, miR-133a, miR-133b and miR-206 increases during development of human skeletal muscle. BMC Dev Biol 11,34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J., Loedige I., and Filipowicz W. (2010). The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11,597–610 [DOI] [PubMed] [Google Scholar]
- Lee T.H., Song S.H., Kim K.L., Yi J.Y., Shin G.H., Kim J.Y., et al. (2009). Functional recapitulation of smooth muscle cells via induced pluripotent stem cells from human aortic smooth muscle cells. Circ Res 106,120–128 [DOI] [PubMed] [Google Scholar]
- Lima J.F., Jin L., De Araujo A.R.C., Erikson-Johnson M.R., Oliveira A.M., Sebo T.J., et al. (2012). FOXL2 mutations in granulosa cell tumors occurring in males. Arch Pathol Lab Med 136,825–828 [DOI] [PubMed] [Google Scholar]
- Limana F., Esposito G., D'Arcangelo D., Carlo A.D., Guido S.R., et al. (2011). HMGB1 attenuates cardiac remodelling in the failing heart via enhanced cardiac regeneration and miR-206-mediated inhibition of TIMP-3. PLoS One 6,e19845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., and Schmittgen T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25,402–408 [DOI] [PubMed] [Google Scholar]
- Ma D.L., Tao X.C., Gao F., Fan C.J., and Wu D.Q. (2012). miR-224 functions as an onco-miRNA in hepatocellular carcinoma cells by activating AKT signaling. Oncol Lett 4,483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miretti S., Martignani E., Taulli R., Bersani F., Accornero P., and Baratta M.A. (2011). Differential expression of microRNA-206 in skeletal muscle of female Piedmontese and Friesian cattle. Vet J 190,412–413 [DOI] [PubMed] [Google Scholar]
- Miyoshi N., Ishii H., Nagano H., Haraguchi N., Dewi D.L., Kano Y., et al. (2011). Reprogramming of mouse and human cells to pluripotency using mature MicroRNAs. Cell Stem Cell 8,633–638 [DOI] [PubMed] [Google Scholar]
- Mohamed J.S., Hajira A., Li Z.L., Paulin D., and Boriek A.M. (2011). Early growth responsive protein-1 induces desmin null airway smooth muscle hypertrophy through MicroRNA-26a. J Biol Chem 286,43394–43404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa T., and Suzuki H. (2013). The role of microRNA in gastric malignancy. Int J Mol Sci 14,9487–9496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P.L., Iezzi S., Stiegler P., Chen T.T., Schiltz R.L., Muscat G.E.O., et al. (2001). Class I histone deacetylases sequentially interact with MyoD and pRb during skeletal myogenesis. Mol Cell 8,885–897 [DOI] [PubMed] [Google Scholar]
- Rooij E.V., Sutherland L.B., Liu N., Williams A.H., McAnally J., et al. (2006). A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A 103,18255–18260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy A., and Blelloch R. (2012). microRNA induced transdifferentiation. F1000 Biol Rep 4,3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.G., Liao R.X., Qiu J., Jin J.Y., Wang X.X., Duan Y.Z., et al. (2010). Microarray-based analysis of microRNA expression in breast cancer stem cells. J Exp Clin Cancer Res 29,174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126,663–676 [DOI] [PubMed] [Google Scholar]
- Takaya T., Ono K., Kawamura T., Takanabe R., Kaichi S., Morimoto T., et al. (2009). MicroRNA-1 and MicroRNA-133 in spontaneous myocardial differentiation of mouse embryonic stem cells. Circ J 73,1492–1497 [DOI] [PubMed] [Google Scholar]
- Williams A.H., Liu N., van, Rooij E., and Olson E.N. (2009). MicroRNA control of muscle development and disease. Curr Opin Cell Biol 21,461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C.Q., Huang H.R., Sun X., Guo Y., Hamblin M., Raquel P., et al. (2011). MicroRNA-1 regulates smooth muscle cell differentiation by repressing Kruppel-like factor 4. Stem Cells Dev 20,205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.F., Ding Y.J., Shen Y.W., Xue A.M., Xu H.M., Luo C.L., et al. (2012). MicroRNA-1 represses Cx43 expression in viral myocarditis. Mol Cell Biochem 362,141–148 [DOI] [PubMed] [Google Scholar]
- Yoo A.S., Sun A.X., Li L., Shcheglovitov A., Portmann T., Li Y., et al. (2011). MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476,228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]