Abstract
Significance: Open fractures are fractures in which the bone has violated the skin and soft tissue. Because of their severity, open fractures are associated with complications that can result in increased lengths of hospital stays, multiple operative interventions, and even amputation. One of the factors thought to influence the extent of these complications is exposure and contamination of the open fracture with environmental microorganisms, potentially those that are pathogenic in nature.
Recent Advances: Current open fracture care aims to prevent infection by wound classification, prophylactic antibiotic administration, debridement and irrigation, and stable fracture fixation.
Critical Issues: Despite these established treatment paradigms, infections and infection-related complications remain a significant clinical burden. To address this, improvements need to be made in our ability to detect bacterial infections, effectively remove wound contamination, eradicate infections, and treat and prevent biofilm formation associated with fracture fixation hardware.
Future Directions: Current research is addressing these critical issues. While culture methods are of limited value, culture-independent molecular techniques are being developed to provide informative detection of bacterial contamination and infection. Other advanced contamination- and infection-detecting techniques are also being investigated. New hardware-coating methods are being developed to minimize the risk of biofilm formation in wounds, and immune stimulation techniques are being developed to prevent open fracture infections.

Samir Mehta, MD
Scope and Significance
Open fractures occur when bone is exposed through skin as a result of bone breaking through skin or wound penetration with fractured bone exposure. While multiple factors may influence open fracture rate, a recent study reported an incidence of 30.7/105/year.1 Open fractures have multiple causes, often occur in extremities, and are most severe in lower legs and feet (Fig. 1A–C). Infection rates also vary, but have been reported as 2.3% with effective antibiotic treatment.2 Because of their severity, open fractures are associated with complications, including longer hospital stays, multiple operative interventions, and amputations (average amputation lifetime healthcare costs over $500,000.).3
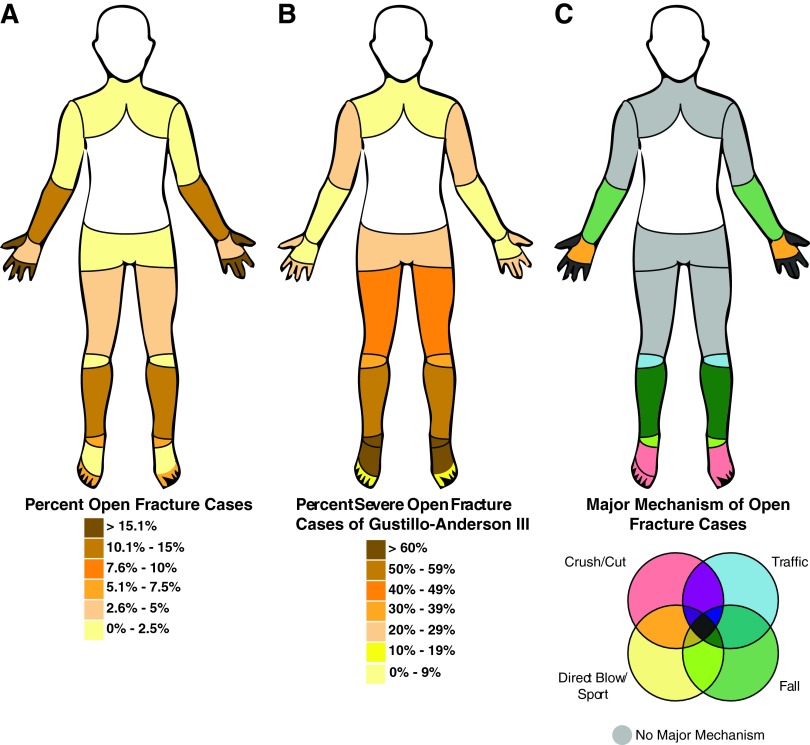
Figure 1.
Open fracture rate, severity, and mechanistic cause statistics. Open fracture rates and statistics, grouped by anatomical site, from a recent report by Court-Brown et al.1 The information represents a collection of 2386 open fracture cases recorded at the Royal Infirmary of Edinburgh between 1995 and 2009. The data suggest the majority of open fracture cases occurred on the distant extremities (A). The most severe open fractures (GA Type III) occurred on the lower extremities, especially the lower legs and feet (B). The distant extremities were characterized by major open fracture mechanisms, which have been grouped into four categories for easier visualization (C). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Translational Relevance
Microbes are known to complicate open fracture healing through infections and biofilm formation, as well as potentially playing roles in nonunion/malunion cases.4 Further, while the importance of microbes in open fracture healing is accepted as significant, there is still little known about how or what microbes affect these wounds, or how we can use microbes diagnostically to predict complications and better inform treatment. The goal of current research is to address the deficiencies in the current paradigms of open fracture care, and to improve prevention and treatment of open fracture infection.
Clinical Relevance
Microbial contamination and infection are common concerns in all wound care scenarios, but open fractures are at a higher risk for infection and other microbe-related complications. Open fractures are often the result of high-energy events that result in severe bone and soft tissue damage, thereby leading to significant risk of infection. Open fracture care focuses on effective management, especially in the early stages, with the goal of minimizing complications caused by microbial contamination.
Discussion
Current concepts in open fracture care and infectious risk minimization
Open fractures are at a high risk for infection and other complications, and the steps taken during initial treatment have a significant impact on the overall outcome. This impact has been evidenced as a decrease in infections and other complications as the result of effective fracture classification, prophylactic antibiotic administration, early debridement and irrigation, and proper fracture fixation. In this section we will further discuss the importance of initial wound management, highlighting the current concepts in open fracture care and the standard treatments, both prophylactic and therapeutic, for infections during open fracture healing.
Open fracture classification and diagnosis
The initial description and evaluation of the wound is important for informing downstream actions and standardizing descriptive measures among the medical professional community. Several classification methods have been proposed for the description and evaluation of open fractures. The most frequently quoted and widely used scheme was first described by Gustilo and Anderson,5 and later modified to its current form by Gustilo et al. (Table 1).6 This classification system involves the intraoperative scoring of open fractures from one to three in ascending order of severity, with a Type I injury involving a small soft tissue wound, Type II involving a large wound with little soft tissue damage, and Type III involving extensive soft tissue damage. Examples of Type II and Type III severity are shown in Fig. 2. Type III wounds are further subcategorized into three subgroups, with Type IIIa fractures having extensive soft tissue damage with adequate soft tissue coverage, Type IIIb fractures having extensive soft tissue damage requiring transfer of soft tissue to cover the defect, and Type IIIc being the most severe due to extensive arterial damage requiring vascular repair. The severity of the open fracture, as scored by the Gustilo and Anderson classification system, is associated with the rate of infection and therefore has prognostic value.5–7
Table 1.
The Gustilo and Anderson classification scheme
| Classification Score | Wound Size (cm) | Soft Tissue Damage |
|---|---|---|
| Type I | <1 | Minimal |
| Type II | >1 | Minimal |
| Type III | ||
| A | >1 | Extensive damage with adequate coverage |
| B | >1 | Extensive damage with inadequate coverage |
| C | >1 | Extensive damage, inadequate coverage, and extensive arterial and vascular damage |
Figure 2.
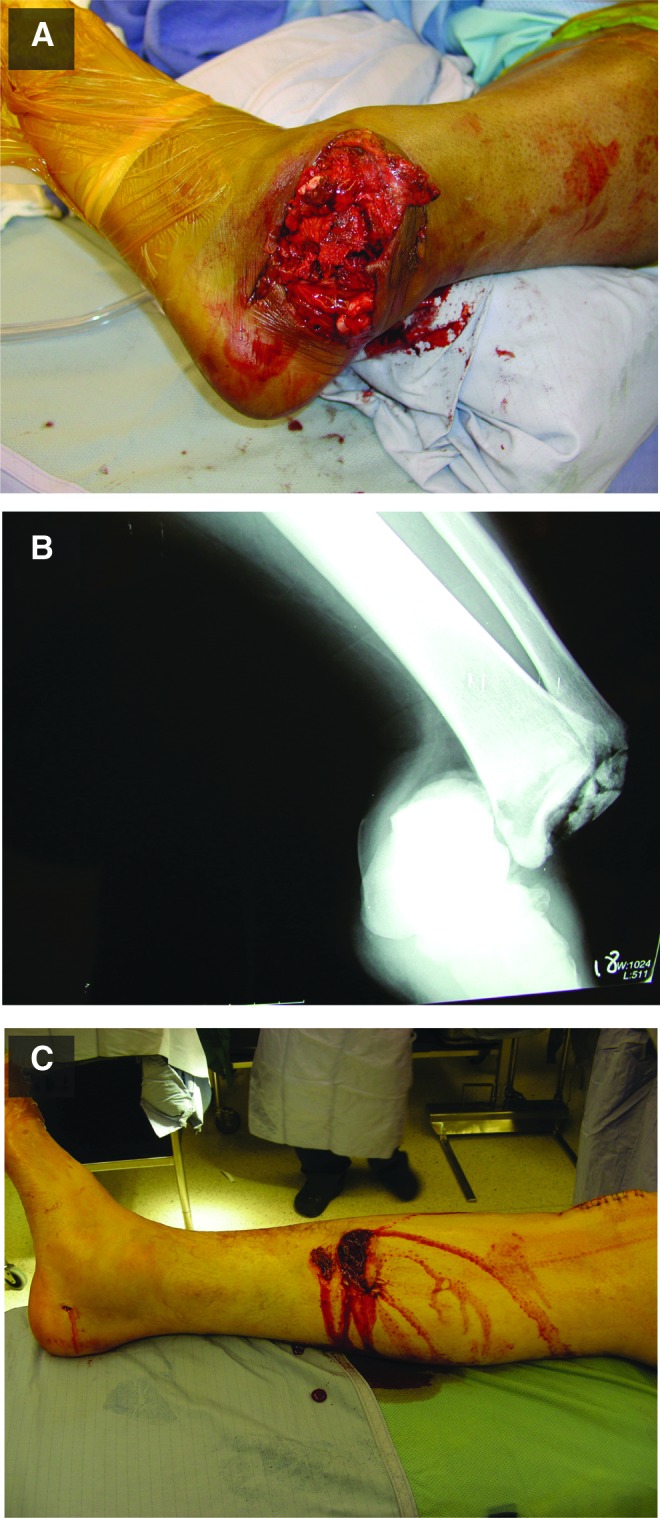
Examples of Gustilo–Anderson wound severities. An example of a Gustilo–Anderson Type III open fracture that exhibits extensive soft tissue damage with minimal coverage (A). (B) An X-ray image of the wound in (A). (C) A Type II open fracture with minimal soft tissue damage. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Oestern and Tscherne proposed a classification system based on fracture type and soft tissue damage for both open and closed fractures (Table 2).8 Additionally, the Association for the Study of Internal Fixation (translated from the German “Arbeitsgemeinschaft für Osteosynthesefragen” and abbreviated as the AO Foundation) has published a classification system that is designed to provide information about both the soft tissue and bone damage of the open fracture (Table 3).9,10 This scheme considers the damage done to skin, the muscle tissues and tendons, and neurovascular system, overall making this a comprehensive and accurate classification scheme.9,10 While both classification methods are valuable, the Gustilo and Anderson classification scheme remains the most widely used due to its simplicity and familiarity.
Table 2.
Tscherne classification scheme for open fractures
| Grade | Soft Tissue Damage | Mechanism of Injury | Associated Contamination | Other Considerations |
|---|---|---|---|---|
| Grade 1 | Minimal | Indirect trauma | Minimal | Small puncture wound without skin contusion |
| Grade 2 | Moderate | Direct trauma | Moderate | Small skin and soft tissue contusions |
| Grade 3 | Extensive | Farming accidents, gunshot wounds, and compartment syndrome | Extensive | Arterial and/or neural injuries |
| Grade 4 | Extensive | Subtotal and total amputation | Extensive |
Adapted from Rüedi and Murphy10 and Moore D (www.orthobullets.com/trauma/1002/tscherne-classification).
Table 3.
AO classification of open fractures
| Affected Tissue | Classification Score | Damage Severity |
|---|---|---|
| Skin | IO 1 | Minimal with skin breakage from inside out |
| IO 2 | Minimal with skin breakage from outside in (<5 cm) | |
| IO 3 | Moderate with skin breakage from outside in (>5 cm) | |
| IO 4 | Extensive with full-thickness contusion, abrasion, open degloving, and skin loss | |
| IO 5 | Extensive with severe degloving | |
| Muscle/Tendon | MT 1 | None |
| MT 2 | Minimal and local | |
| MT 3 | Moderate | |
| MT 4 | Extensive with muscle defect and tendon laceration | |
| MT 5 | Extensive with wide zone of injury and compartment syndrome | |
| Neurovasculature | NV 1 | None |
| NV 2 | Isolated | |
| NV 3 | Local | |
| NV 4 | Extensive with vascular injury | |
| NV 5 | Extensive with subtotal or total amputation |
Prophylactic antibiotic administration
Once an open fracture has been identified and loosely classified in the resuscitation bay or emergency room (typing of open fractures is most accurate in the operating room), treatment with antibiotics is initiated to minimize the risk for infection. Obvious gross contamination is also removed at this time. These treatments are performed as early as possible after the traumatic event in order to minimize infection and other complications.
Infections and bacterially related complications are important concerns when treating open fractures. In fact, the Gustilo and Anderson study of 1976 reported positive initial bacterial cultures in 70.3% of the 158 prospectively observed open fractures.5 Because bacterial colonization was strongly associated with open fractures, orthopedic professionals accepted, without evidence, that prophylactic antibiotics would lower the risk of wound infection. Attempts to address the utility of prophylactic antibiotics yielded weak and conflicting results until 1974, when Patzakis et al. reported a reduction in open fracture wound infections from 13.9% in patients without antibiotic treatment, to 2.3% when patients were treated with cephalothin antibiotics.2 This study strongly supported the need for prophylactic antibiotic use. The study also illustrated the importance of understanding the administered antibiotics because, while cephalothin antibiotics were significantly effective in reducing infection rate, the patients treated with penicillin and streptomycin did not show a significant reduction in infection rate (a nonsignificant reduction from 13.9% in nontreated patients to 9.7% in penicillin and streptomycin treated).2 Patzakis et al. used antibiotic resistance culture techniques to show this was at least partially due to penicillin and streptomycin resistance. Other studies have since highlighted other important considerations when deciding an antibiotic regimen.
Deciding an appropriate antibiotic course requires an understanding of the bacteria most likely to colonize wounds. Both Patzakis et al. and Gustilo and Anderson found that, when culturing wound infections, staphylococci (specifically coagulase positive staphylococci such as Staphylococcus aureus) were the most commonly isolated organisms.2,5 Because these bacteria appeared to be the most likely causes of infections, they suggested that prophylactic antibiotics should target Gram positive bacteria, and most especially staphylococci. The benefits of prophylactic antibiotic use against Gram positive bacteria have since been supported by other series.11–13 While research supports the benefits of prophylactic antibiotics that target Gram positive bacteria, there is insufficient evidence to support the prophylactic use of Gram negative antibiotics.9,13,14 As Gram negative bacteria become more prevalent in open fracture infections, including Acinetobacter baumannii and Pseudomonas aeruginosa, research addressing the prophylactic benefits of Gram negative antibiotics will become increasingly important.
Another concern is the increasing threat of acquisition of antibiotic resistance by bacteria. The continued emergence of methicillin-resistant S. aureus (MRSA) has brought new considerations to prophylactic antibiotic treatment of open fractures.14 Various rates of MRSA colonization of the nares have been reported, with a high rate of 7.4% in healthy university students in 2009,15 and general rates being around 1–2.5%.16–20 MRSA colonization in the nares, axilla, and groin has been suggested to increase the risk of MRSA infection at surgical wound sites,14,21 and nasal decolonization treatments, paired with antibiotic prophylaxis, have been shown to reduce the risk of MRSA infection in some cases.22 Although the benefits of prophylactic antibiotic regimens that target MRSA have not yet been established, this will likely continue to be an important consideration as surgeons decide the best prophylactic antibiotic regimens to administer.14
In addition to MRSA, there is also an increasing concern about other antibiotic-resistant bacteria, including Acinetobacter, Klebsiella, Pseudomonas, and Enterobacter, which are present in open fractures23 and are known to be potentially infectious agents of open wounds.24–26 Notably, pan-resistant strains of the significant hospital pathogen A. baumannii have emerged, developing resistance to colistin, the drug of last resort.27 Antibiotic-susceptible and -resistant A. baumannii infections have continued to increase in prevalence over the past decades, both in military and civilian settings.28–30 Because the antibiotic-resistant profiles of A. baumannii and other potentially antibiotic-resistant bacteria can vary geographically, orthopedic clinicians must consider the local potentially pathogenic bacteria and the local antibiotic-resistance profiles associated with those bacteria, as has been suggested for treating open fracture A. baumannii infections.25
While it is important to predict what prophylactic antibiotics will be most effective, it is also important to understand the ideal administration timeline. This timeline includes the ideal gap length between patient presentation and antibiotic administration, and duration of antibiotic administration. Most surgeons agree that prophylactic antibiotic treatment should be started as soon as possible.7,9,14 The key study by Patzakis and Wilkins showed that the most important treatment in preventing open fracture infection is prophylactic antibiotic administration.7 The group showed that the patients who were treated prophylactically with antibiotics within 3 h were less likely to develop infection, and this timeframe is still used today.
While ideal time to administration is straightforward, ideal duration of therapy is less clear. One study has suggested that antibiotic treatment should be continued for 3 days after initiation,31 while another study argues that 24 h is no less effective than 72 h.32 Currently, authors advise that antibiotic treatment should be continued for at least 24 h, and may be continued for up to 72 h.9,13,14 A concern with longer antibiotic administration times (i.e., 72 h) is that the increased exposure may promote antibiotic resistance among the bacterial populations, which has been shown to occur in some cases.9,13 Further research will be required to provide definitive responses to these concerns.
Debridement and irrigation
In addition to prophylactic antibiotic administration, wound debridement and irrigation are important procedures for preventing open fracture infection. The goal of open fracture surgical debridement is the excision of environmental debris, devitalized soft tissue, and bone, as well as irrigation of the wound to reduce bacterial load. The three major considerations are ideal timing between injury and debridement, the extent of debridement, and the irrigation materials to be used.
The recommended time to debridement after injury is dictated by the “6-h rule.” The 6-h rule is an orthopedic rule of thumb that claims that, to be effective, open fracture debridement should be conducted within 6 h after the injury. While this rule is widely accepted, little scientific evidence supports it. The 6-h rule was started by Friedrich, who utilized a guinea pig model and reported decreased risk of infection when contaminated soft tissue wounds were debrided <6 h after contamination. This suggested that debridement earlier than 6 h resulted in lower infectious risk.33 Recent literature, including human studies, has not supported the Friedrich claims, and while further study is needed, it seems that there is no increased risk of infection in delayed debridement cases.9,34 Despite these recent findings, most surgeons recommend immediate debridement of highly contaminated types II and III open fractures.9
Irrigation is an important supplement for aggressive debridement of necrotic tissue and particulate matter because it further removes particulate debris and bacteria from the wound. The specifics of what materials should be used, and to what extent irrigation should be performed, remain a topic of debate. These discussions focus on whether soaps, antiseptics, or antibiotics should be included in the irrigation saline, and whether pulsatile lavage should be used. Pulsatile lavage is a point of concern because it may drive bacteria further into soft tissue and cause microscopic damage to the soft tissue, and thereby impede healing and increase the risk for infection.
Most surgeons irrigate open fracture wounds using sterile saline alone, saline in combination with soap, saline in combination with antiseptic chemicals, or saline in combination with antibiotics. Although one might intuitively think that chemical additives would eliminate more bacteria and decrease the risk of infection, they are found to be ineffective. Antiseptic compounds are known to destroy bacteria, but studies have yielded conflicting results regarding their beneficial effect on wound healing and infection rate compared to saline solutions alone.35,36 In fact, antiseptics may be toxic to the human host cells, which could limit their efficacy due to host damage.35,36 Like antiseptics included in saline, the beneficial effects of human wound irrigation using antibiotics with saline has been unconvincing.35,37
Unlike antiseptics and antibiotics that destroy bacteria, soaps facilitate the physical removal of bacteria. Studies suggest that the use of soap with saline is just as, if not more, effective compared to antibiotic saline solutions.37–39 Because antibiotic and antiseptic use in saline can add additional cost to treatment, may promote antibiotic resistance, and may harm the human host tissue, orthopedic clinicians recommend the use of soap with saline when irrigating wounds.9
The pressure used in open fracture irrigation is just as important as the solutions used. The benefits of low-pressure gravity irrigation or high-pressure pulsatile lavage in open fractures remain a point of discussion. While high-pressure pulsatile lavage seems attractive because it is thought to better remove entrenched bacteria and debris, the high pressure may push bacteria further into the tissue. High-pressure irrigation may also heighten the risk of healing complications because it damages the surrounding human tissue. Although bacteria and debris may be more effectively removed from wounds using high-pressure pulsatile lavage,40 others have argued that high-pressure pulsatile lavage does in fact push bacteria further into tissue and increases the numbers of bacteria retained in the wound.41 Additionally, there has been significant research to suggest that high-pressure pulsatile lavage damages human tissue, thereby increasing risks for complications, infections, and delayed healing.41,42 Together, the effects of high-pressure irrigation are seen as more destructive than helpful, and low-pressure irrigation is recommended.9
Volume of irrigation solution used is also an important consideration. In 2001, Anglen proposed increasing volumes of irrigation for more severe wounds. Given the availability of 3-L irrigation bags, he proposed 3 L for Type I fractures, 6 L for Type II fractures, and 9 L for Type III fractures.35 This is the method currently used by most surgeons.
Internal and external fracture fixation devices
After the initial treatment of the wound, attention turns to fracture reduction and fixation. Not only is fracture reduction (anatomical realignment of fracture fragments) important for proper bone union and healing, stabilization of the fractured bones limits soft tissue damage. Anatomic reduction mediates the inflammatory response, decreases hematoma volume and dead space, and allows for tissue revascularization.
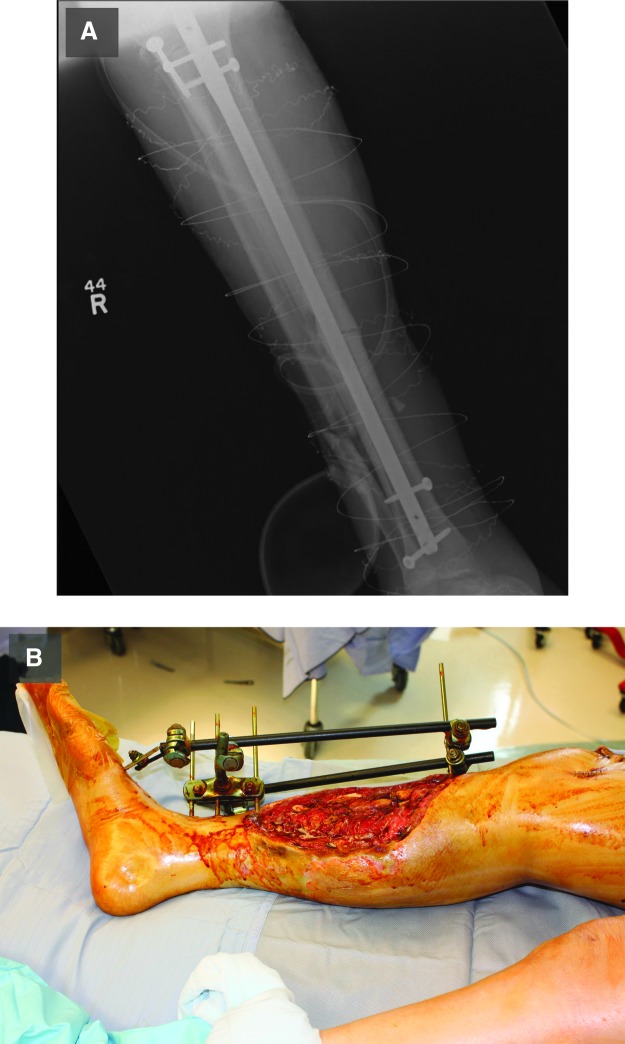
The utilization and efficacy of various fracture fixation techniques differs based on the anatomical location and severity of the injury. There are three general methods to fixing fractures: plate fixation, intramedullary (IM) nailing, and external fixation. Examples of IM nailing and external fixation are shown in Fig. 3. Both plate fixation and IM nailing are internal fixation approaches, while external fixation is external, as the name suggests. Due to the high rates of complications and concerns for periosteal blood supply damage associated with plate fixation methods, they have been largely replaced by IM nailing and external fixation techniques for lower extremity diaphyseal fractures.43 However, plates are still commonly used for periarticular fractures and open fractures of the radius and ulna, as it becomes more important to get an anatomic reduction. For example, internal plate fixation has been shown to be more effective than external fixation in the treatment of distal radius fractures.44
Figure 3.
Example of fracture fixation techniques and hardware. An example of an intramedullary nail used to fix an open diaphyseal tibia fracture (A). The fibula was also fractured but it was not fixed because it is not a weight-bearing bone (A). An example of an external fixation device being used to fix an open tibial fracture (B). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The external fixation technique involves the insertion of threaded pins into the fractured long bones through the skin (Fig. 3B). These screws are attached to external hardware that provides stable fracture fixation. The advantages of the external fixation approach are that it allows for rapid fracture stabilization, avoids placement of internal hardware, and minimizes further soft tissue damage by placing screws outside the zone of injury. Pin-track infections, concerns about fracture malalignment, and poor patient compliance limit its use for definitive fixation. External fixation is now more commonly used for temporary fixation of fractures while the surgeon awaits the soft tissues to recover, eventually converting to internal fixation.
IM nailing is an internal fixation approach for long-bone fractures, in which a titanium or stainless steel rod is placed into the reamed or unreamed medullary canal of a long bone (Fig. 3A). This rod is secured in place and serves as an internal scaffold around which bone can heal. The advantage to the IM nailing technique is that it offers effective bone fixation that maintains length, alignment, and rotation, and also allows for earlier weight bearing. Though reamed femoral nailing is the gold standard for closed femoral shaft fractures,45 concerns about infection risk in open fractures have been raised.46 However, two prospective randomized trials do not show a significant increase in infection risk when using a reamed, locked IM nail for treatment of open tibial shaft fractures.47,48 With different technical advantages to each fracture fixation technique, the surgeon must take into account fracture pattern and soft tissue injury when deciding which method will best provide a positive functional outcome.43,49
Treatment of fracture and soft tissue infections
Following initial classification, prophylactic antibiotic administration, surgical debridement and irrigation, and fracture stabilization, the open fracture wound may still become infected. In this case the patient is most often treated with intravenous antibiotics to suppress and eliminate the infection. Depending on the nature of the infection (the severity, location, and depth), the fracture fixation hardware may be left in place until the fracture heals, and will be removed after healing. If the infection is more severe, then the hardware may have to be removed, the wound will be debrided in addition to local antibiotic administration, and the hardware will be reinstalled after the infection has been cleared. Another common infectious concern is the formation of biofilms, which can occur rapidly on medical devices as well as host substrates like bone.
Ongoing research and the future of open fracture care
Although there are many established treatment paradigms in place for open fractures, infections and other complications remain a present threat. Research to improve these treatments remains ongoing. Now that we have discussed the more established concepts in current open fracture care, we are going to move our focus toward the ongoing dilemmas facing open fractures and infection prevention/treatment, and the research aimed at finding solutions.
Detection of bacterial contamination at time of injury
One of most comprehensive problems in management and treatment of open fractures is identification and quantification of microbial contamination at time of injury. Identifying microbial biomarkers indicative of complication risk would also better inform open fracture management. Surveillance cultures at the time of presentation have little value in predicting what organism will cause a downstream infection. One study that illustrated this limited predictive value of surveillance cultures was a prospective clinical study by Valenziano et al.50 The group collected swabs from open fractures upon patient presentation to the hospital (before antibiotic intervention), obtained aerobic and anaerobic cultures from the samples, and examined correlations between the cultures and the patient progressions to infection. Only 24% of the surveillance cultures resulted in growth. Additionally, 77% of the infected wounds yielded negative cultures, and none of the cultured organisms matched the infectious organisms. This suggested an inability of surveillance cultures to reliably predict the infectious organisms of open fractures. This inability of surveillance cultures to accurately predict the infectious organism has been supported by other studies.50–53
While surveillance cultures have limited value in predicting downstream infectious organisms, some studies have suggested a value in surveillance culture bacterial load quantification. This was recently addressed in a retrospective study conducted by Burns et al. in a combat environment. The group took a similar approach to that mentioned previously, by attempting to find correlations between surveillance cultures taken during initial wound debridement and the later development of infections. Burns et al. found that the positive surveillance cultures were not able to accurately predict the infecting organism, as has been shown before. However, 38.7% of the culture-positive patients went on to infection, while only 11.5% of the culture-negative patients developed infection, and this correlation between a positive bacterial load culture and progression to infection was found to be significant. This therefore suggested that quantitative bacterial culturing may have limited value in predicting general infection. Other studies have also demonstrated the value of quantifying bacterial loads of wounds for general infection prediction, either through the use of quantitative Gram staining or more commonly through the use of quantitative culturing.53–56 The samples used for these bacteria quantifications were either wound swabs, wound effluent, or debrided tissue.
The timing of sample collection for quantification, such as whether the sample is collected before or after debridement, may be important and this may explain some different results reported in the literature. In a study by Merritt, the surveillance cultures for bacterial load were shown to have predictive value when taken as the patient was leaving the operating room (after the wound was debrided, irrigated, and cleaned), but not when taken as the patient entered the operating room (the sample was taken during debridement).56 This suggested that the timing of surveillance culture sampling may be important. Although this study was conducted many years ago, the importance of sampling timing will likely remain a point for further investigation.
Advances in molecular analysis of bacterial contamination at time of injury
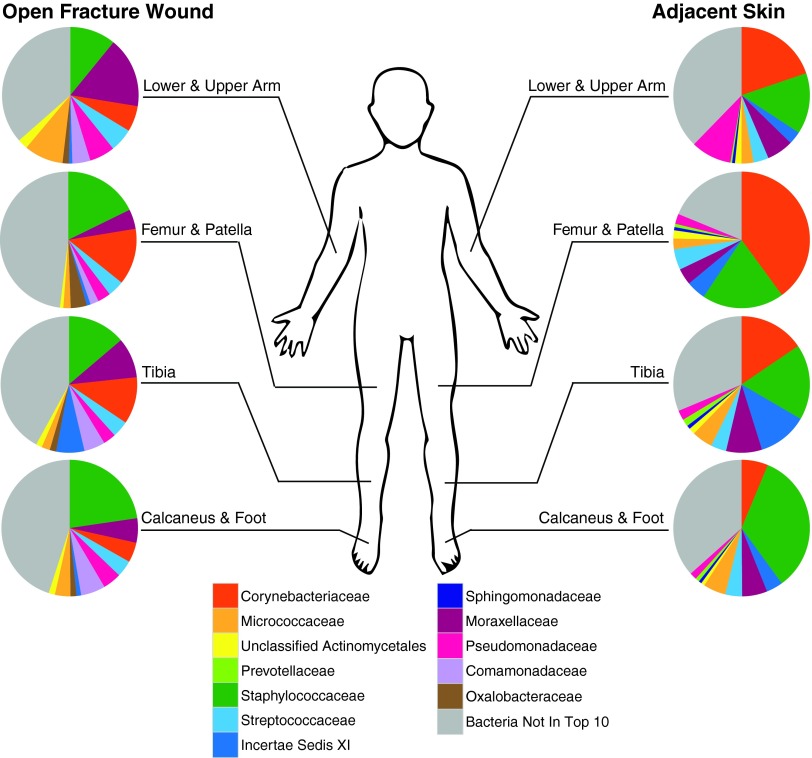
The advent of next-generation sequencing platforms, with increased throughput and decreased costs, has enabled approaches that do not rely on cultures for bacterial identification. Based on the DNA sequence of the prokaryote-specific 16S small subunit ribosomal RNA (rRNA) gene, culture-independent sequencing methods eliminate biases associated with cultures. Our group recently reported an ongoing pilot study that is using such approaches to understand the bacteria associated with open fractures.23 This study utilized high-throughput sequencing of the bacterial 16S rRNA gene to characterize 30 open fractures, and was able to correlate specific bacterial taxa and community dynamics with time points and other clinical factors, including the anatomical wound location and patient progression to healing complications. The data can also be used to visualize the differences in bacterial communities between anatomical sites, and between the wound and healthy skin, at presentation of the patient to the emergency room (Fig. 4). It also shows that healthy skin communities are dominated by Corynebacteriaceae and Staphylococcaceae bacteria, while the wound communities are not strongly dominated by any particular bacteria (Fig. 4). Because this was a pilot study, the prognostic value of certain bacterial abundances or community compositions were not addressed, but this will be an obvious next step as more patients are enrolled and as more follow-up information is collected until the end of each patient's healing process. Overall, this study is allowing for more robust, detailed studies of the communities associated with open fractures.
Figure 4.
Bacterial communities associated with open fractures at emergency room presentation. The bacterial communities of open fracture wounds (left) and their corresponding adjacent, unaffected skin (right), as reported by Hannigan et al.23 The communities were grouped into four anatomical categories. The top 10 bacterial families, calculated as median relative abundance across all samples, were calculated for the wound and skin groups. The bacterial communities upon patient presentation to the emergency room are shown. The skin communities are primarily dominated by Corynebacteriaceae and Staphylococcaceae, while the wound communities are less dominated by these or other bacteria. The wound and skin communities differ from each other at the same anatomical locations, and the different anatomically located communities also differ within the wound and skin categories. The bacteria labels are listed in the legend near the figure bottom. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Just as it is important to understand the specific bacteria that contaminate open fractures and cause infectious complications, it is also important to understand the ecology of open fracture wound bacterial communities. Up to this point, individual-cultured bacteria have been primarily considered either harmful or potentially pathogenic. In fact, not all bacteria are harmful, and some can be beneficial. Having a better understanding of these harmful and beneficial groups will improve further therapeutic development.
Changes in the human microbiome have been associated with a multitude of inflammatory diseases and states, including inflammatory bowel disease, acne vulgaris, and atopic dermatitis.57,58 In these cases, disease states are associated with alterations in the bacterial community structure, an alteration referred to as “dysbiosis.” Together, these examples illustrate that the entire microbial community, not just the potentially pathogenic or opportunistic microorganisms, influences host–microbe homeostasis. Further, commensal bacteria are thought to promote health in many ways, including competitive inhibition of potentially opportunistic and/or pathogenic microorganisms, educating and modulating the host immune response, and through the production of compounds that inhibit growth of potential pathogens, such as antimicrobial peptides. Work toward understanding the open fracture microbiome, and the beneficial and harmful bacteria in that community, remains ongoing.
Perhaps one of the most basic culture-independent, molecular methods is the estimation of bacterial load using quantitative polymerase chain reaction techniques. This method involves the quantification of the 16S rRNA gene sequences present in a wound swab or other sample type. This method is also ideal as a basic starting point because it does not require any sequencing of the bacterial genome, as primers are designed to regions of the 16S rRNA gene that are conserved throughout a broad range of prokaryotic taxa. Although this method is used in research laboratories,4,23 it has not yet been implemented in clinical settings.
Another high-throughput approach to understanding host–microbe homeostasis in traumatic injury was reported in a recent study by Chromy et al., who investigated the utility of global protein profiling approaches for identifying host biomarkers.59 Wound effluent was collected prior to, and shortly after, surgical debridement. Twenty-five proteins were significantly differentially expressed between uneventful healing and complicated healing groups, many with established roles in regulating inflammatory and immune responses. For example, increased expression of complement C3 protein was associated with dehisced wounds, a similar finding to a chronic wound model in which complement genes were upregulated.60 Excessive complement activation can be damaging to the host and has been linked to myriad inflammatory and autoimmune conditions.61 Although these identified host biomarkers need further validation in open fracture settings (only one open fracture was included in the study of 19 patients with severe traumatic injury), this general approach is promising as a readout of the host immune response and may enable identification of protein biomarkers with predictive and/or prognostic value.
Techniques and methods for contamination eradication
While it will be important to continue to improve methods for diagnosing contaminated wounds and predicting their outcomes, it will also be important to improve methods for eradicating contamination from open fracture wounds. Open fracture infection is currently prevented through the minimization of contamination, often practiced as aggressive wound debridement, irrigation, and prophylactic antibiotic administration. The use of local antibiotic therapy for severe open fractures (Type IIIB and Type IIIC) has been shown to reduce the incidence of infection in a series of 1,085 open fractures.62 Ostermann et al. used aminoglycoside-impregnated polymethylmethacrylate (PMMA) beads to provide high local concentrations of antibiotics. Because PMMA is not bioabsorbable, the length of implantation remains controversial and requires retrieval. Bioabsorbable antibiotic delivery vehicles may eliminate the need for reoperation and removal.63
Assessment of open fractures for infection
Just as it is important to accurately diagnose bacterial contamination at the time of injury, it is also important to accurately assess wounds for infection. Although it may seem the assessment of infection should be obvious, this remains a difficult procedure. In fact, a series of studies that began in 1995 showed that, in cases of otitis media, inflammatory and bacterial cells could be observed by microscopy, the presence of bacteria could be confirmed by 16S rRNA gene quantification, and the presence of live bacteria could be confirmed by mRNA quantification, but the majority of bacterial cultures remained negative.64 Due to these inaccuracies, culture methods alone are not sufficient to properly diagnose an infection. In fact, no single method is sufficient for infection diagnosis and multiple methods must be used for proper diagnosis.64–66 Methods for the assessment of infections include repeated measurements of immune-related markers (i.e., C-reactive protein and erythrocyte sedimentation rate), culturing, histopathology, X-ray imaging (diffuse periosteal reaction, fracture delayed union or nonunion, or loosening of pins indicates potential infection), nuclear imaging of 99mTc accumulation, and computed tomography, including magnetic resonance imaging and positron emission tomography methods.64–66 Because of the level of specialty required, infectious disease teams will often coordinate with the orthopedic team, when available, to identify and provide the most appropriate treatment. An overview of the mentioned methods for detecting open fracture bacterial contamination and infection can be found in Table 4.
Table 4.
Methods for detecting open fracture microbial contamination/infection
| Method | References | Details | |
|---|---|---|---|
| Contamination | Surveillance culturing | 50 | Isolation of microbes on artificial media, followed by identification using biochemical and molecular techniques. Surveillance cultures are thought to have little value in predicting infections. |
| DNA sequencing | 23 | The sequencing of taxonomically/phylogenetically informative genes to allow for bacterial community identification. This method is in development and is not used clinically. | |
| qPCR detection | 4,23 | Quantification of microbial load using qPCR of conserved microbial genes. This method is not widely used for clinical prophylaxis of open fractures. | |
| Host protein biomarker identification | 59 | Some host proteins have been identified as potential biomarkers for predicting open fracture healing complications. Many of these proteins have established roles in the immune response, and may be clinically useful upon further investigation. | |
| Infection | Immune-related marker | 65,66 | Identification of host biomarkers, such as secondary rises in C-reactive protein levels, provides good support that a wound or implanted device is infected. |
| Quantitative and qualitative culturing | Culturing of tissue samples can provide insight into what potentially pathogenic microbes are present at a site suspected of being infected, and can be used to predict the most prominent microbes. This can help in the prediction of the infecting organism itself. | ||
| Histopathology | Histopathological examination of tissue sample infiltration by inflammatory cells can provide evidence for infection. | ||
| X-Ray imaging | X-ray evidence of hardware implant nail loosening or widening of the fracture gap both suggest the presence of infection, although this alone is not definitive. | ||
| Nuclear imaging | Nuclear imaging showing a lack of 99mTc accumulation, which indicates dead or devascularized bone, is suggestive of infection. | ||
| Computed tomography | MRI allows for detection of soft tissue abnormalities, but is not effective for areas around metallic implants. PET and PET-CT scanning systems are able to address this deficiency in MRI approaches by allowing for assessment of accumulation of compounds, such as FDG, which indicate potential infection. |
FDG, fluorine 18 fluorodeoxyglucose; MRI, magnetic resonance imaging; PET, positron emission tomography; PET-CT, positron emission tomography-computed tomography; qPCR, quantitative polymerase chain reaction.
Prevention of biofilm formation on hardware
A specific infectious interest to orthopedic clinicians is the prevention of biofilms on fracture fixation hardware. Biofilms are complex communities of bacteria that create extracellular polymers that allow them to adhere to each other, as well as to implanted devices. Biofilms are a particular concern in open fractures, as well as other implant settings, because they are difficult to eradicate. Most antibiotics are unable to penetrate into biofilms, thereby weakening the primary line of attack. Biofilms also make the enclosed bacteria resistant to most effects of the host immune system. Additionally, the close proximity of biofilms creates an environment that promotes horizontal gene transfer, including transfer of antibiotic resistance and other virulence factor genes.67 Culture identification of microorganisms forming a biofilm is challenging, as those microbes forming the biofilm rely on microbe–microbe interactions, and are thus difficult to isolate as individual planktonic colonies. Additionally, biofilms are usually polymicrobial, and are often collections of Gram positive and negative bacteria, which makes their culture identification and treatment particularly difficult.
Biofilms are polymicrobial and maintain a “supragenome” that is necessary for the overall biofilm survivability.68 This means that biofilms are complex communities of bacteria that, together, express the genes needed for biofilm formation and maintenance, but no single bacteria has all of the required genes; the genetic burden is shared among the community.68 Because of the metagenomic synergy, bacterial diversity, horizontal gene transfer, and overall genomic diversity associated with biofilms, almost any bacteria is capable of forming a biofilm. All of these factors contribute to the difficulty in treating biofilm infections. Overall, the best approach is to prevent biofilm formation in the first place.
Open fractures are at a higher risk for biofilm infections compared with closed fractures, likely because they have a greater burden of contamination and deficient immune responses. Incidence of biofilm formation after open fracture internal fixation may exceed 30%.65 Prevention of biofilm formation is important because biofilms can delay healing, propagate complications, and increase treatment costs. While there are multiple methods to prevent biofilm formation, including prophylactic antibiotics and accurate detection of potential biofilm-forming bacteria (both discussed previously), we will focus on hardware coatings that can deter or prevent bacterial adhesion and biofilm formation.
Biofilm-prevention studies are conducted on many different types of devices, but recently the group of Williams et al. reported an effective antimicrobial coating that was tested in a type IIIB open fracture sheep model.69 The coating reported in this study was an active release compound (meaning the coating continuously releases the antimicrobial compounds into the surrounding tissue) that was composed of silicone polymer and an active release antimicrobial agent called cationic steroid antimicrobial-13. Williams et al. found that their coated fracture fixation devices prevented 100% of infections when challenged with biofilm inocula in the open fracture sheep model, and 100% of the uncoated devices went on to infection. This particular coat shows promise and warrants further investigation.
Other coat-based approaches to preventing biofilm formation include the use of antisense molecules that can target and silence bacterial virulence factor genes, the use of quorum sensing inhibitors, and even coating with bacteriophages (viruses that only target and destroy bacteria), which are capable of penetrating biofilms.67 Additionally, the use of ultrasound or electric currents may be effective in disrupting biofilms to allow for antibiotic or antimicrobial compound penetrance.67 The use of external fixation devices as discussed previously, when possible, is another way to reduce the risk of biofilm formation. External fixation devices can potentially reduce the risk for infection because the pins are placed outside of the zone of injury, because they have a smaller surface area, and because they are never permanent. Because pin-site infections are common, these devices' values are also limited.
Difficulties in elimination of infection and biofilm destruction without sacrificing construct stability
When biofilm infections do occur on internal fracture fixation hardware, the treatment must balance the risks of fostering infection with the benefits of fracture stability. As mentioned earlier, treatment of biofilms is particularly difficult because the structure protects the bacteria from antibiotics and host immune responses. The choice to remove hardware to treat a potential hardware biofilm infection depends largely on the state of bone healing. If the patient's bone has sufficiently healed, then the hardware is removed and the patient is treated with antibiotics. The case becomes more difficult when the bone has not sufficiently healed.
If the patient's bone has failed to heal, then the surgeon must make a decision as to whether the hardware should remain until the bone has healed, or to remove the hardware, treat the infection (often with local antibiotics actively released by a PMMA vehicle), and install new hardware to stabilize the fracture after the infection has cleared. This can be a difficult choice and in many cases an infectious disease specialist is consulted.
Increased risks of infection due to deficient immune responses in open fractures
One of the major ways open fracture wounds are left more susceptible to infection is their deficient immune response. While there are multiple deficiencies in the local immune responses after an open fracture, one of the deficiencies is decreased function of T helper 1 (TH1) lymphocytes. TH1 lymphocytes are important modulators of the cellular immune response, as well as the production of complement-fixing antibodies.70 This deficiency in TH1 lymphocytes has been linked to the reduced ability of open fractures to resist infections, and attempts to restore TH1 function in open fractures have resulted in increased resistance to infection in animal models.70,71 This knowledge has led some groups to attempt to prevent open fracture infection by modulating the immune system.
In 2012, a group led by Boyce et al. attempted to therapeutically modulate the immune response in an open fracture rat model, in which the rats' femurs were fractured using a custom apparatus.70 The group used IL-12 to modulate the immune response because IL-12 is known to play a role in naive T lymphocyte differentiation into TH1 lymphocytes, which would therefore stimulate the wounds' immune response and address their deficiency in TH1 lymphocytes. After femur fracture, the group inoculated the wounds with clinical isolates of S. aureus and treated the rat wounds with percutaneous injections of placebo, IL-12, ampicillin antibiotic, or a combination of IL-12 and antibiotic. The group found that, although the antibiotic treatment was more effective than IL-12 alone in preventing infection, the combination of IL-12 and antibiotic was more effective than the antibiotic treatment alone. This suggests that the use of IL-12, in combination with antibiotic treatment of open fractures, may improve the wound's resistance to infection. While this was only an animal model study, it warrants further investigation into using immune-modulating cytokines to improve the efficiency of prophylactic antibiotic, or other antibiotic treatments.
The same group, led by Li et al., also investigated the efficacy of coating implant devices with IL-12 to prevent biofilm formation and infection by stimulating the immune system as described previously.71 The group used the rat femur fracture model and S. aureus bacterial challenge model as described previously. Metallic wires were used as IM nails at the fracture sites. Half of the rats received wires coated with IL-12 and the other half received uncoated wires. The results showed that rats who received IL-12-coated wires had significantly lower rates of infection, and those rats also had better bone quality and improved healing as assessed by three blinded, orthopedic surgeons. This report supports the benefits of IL-12 as coatings on implant devices, such as IM nails, and warrants further investigation.
Conclusions and perspectives
Due to exposure to the external environment, the extended duration of required healing, and suppressed immune responses, open fractures are at significant risk for infectious complications. A major focus of current open fracture care is minimization of this infectious risk. During initial treatment, infectious risk of the open fracture is reduced by properly categorizing the wound, treating the patient with prophylactic antibiotics, debriding and irrigating the wound, and stabilizing the fracture with appropriate hardware.
The early detection of bacterial contaminants continues to be a focus of current research. Unfortunately, contemporary surveillance culture methods are unable to reliably predict the bacteria that will lead to infection, often because the cultured bacteria are not the same bacteria present at the time of infection. There is still a need to accurately predict which patients will move on to develop infections of particular bacteria, and researchers will likely continue to investigate potential methods for making such predictions.
Timing of sample collection will likely play a role in the success of biomarker discovery for infecting bacteria. Timing of sample collection, such as whether the sample was taken before or after surgical debridement, is a potentially significant factor in whether or not the detected bacteria will lead to downstream infections. Further, existing studies have focused on detecting potentially infectious organisms upon presentation, or shortly thereafter, but often fail to assess the potentially infectious organisms colonizing the wound at later times. This may be important because the bacteria present at the wound site at later times may be more significant to causing infection than bacteria at presentation. Improved culture-independent techniques, such as protein biomarker identification and 16S rRNA gene sequencing, will improve diagnostic and prognostic abilities and give greater power to future studies that investigate these issues.
As biofilms continue to complicate open fracture care by establishing persistent infections of implanted hardware and host tissue substrates, researchers will likely continue to develop new methods to prevent and eradicate them. Promising methods include the coating of hardware devices with actively released antibiotics, antisense molecules, quorum sensing inhibitors, bacteriophages, and immune-system-stimulating cytokines. Effective alternative methods to antibiotic treatment for established biofilm infections, such as bacteriophage therapy, need to be further explored because antibiotics poorly penetrate biofilms.
Summary
Open fracture wounds are at an increased risk for developing infections and other related complications. Current treatment paradigms aim to minimize infectious risks by effectively categorizing the wounds, treating the patients with prophylactic antibiotics, effectively debriding and irrigating the wounds, and appropriately fixing the fractures. While these treatment methods are well established in modern practice, many therapeutic details remain a point of discussion, such as the prophylactic benefits of Gram negative antibiotics.
Despite the efficacy of contemporary treatment paradigms, current research is continuing to address the deficiencies in current care methods. This research includes the use of culture-independent techniques, including bacterial DNA sequencing and protein biomarker detection, for assessing open fracture contamination or infection. Improved methods are also being developed for the removal of contamination and treatment of infection. Biofilm formation on fracture fixation hardware is a major concern, and techniques are being developed to prevent these infections, including various hardware coating techniques. One such coating technique aims to stimulate the antibacterial immune response, and this is also being developed as a compound to be administered with antibiotics to improve their overall efficacy. Additionally, treatments involving immune system stimulation are being developed to address the local deficient immune responses of open fractures.
TAKE-HOME MESSAGES.
• Open fractures are at an increased risk for infection and related complications.
• Current treatment aims to prevent infectious complications through wound categorization, prophylactic antibiotic administration, debridement and irrigation, and effective fracture fixation.
• Culture-based methods offer poor prognostic value, leading to current research on culture-independent methods.
• Biofilm infection is a significant concern in open fracture treatment, and different hardware coating treatments are under development.
• Treatments involving immune system stimulation are being developed to address the local deficient immune responses of open fractures.
Abbreviations and Acronyms
- IM
intramedullary
- MRSA
methicillin-resistant Staphylococcus aureus
- PMMA
polymethylmethacrylate
- rRNA
ribosomal RNA
- TH1
T helper 1
- qPCR
quantitative polymerase chain reaction
Acknowledgments and Funding Sources
The authors thank the members of the Elizabeth Grice Laboratory, as well as the members of the Department of Orthopaedic Surgery at the Hospital of University of Pennsylvania, for their underlying contributions. G.D.H. is supported by the Department of Defense through the National Defense Science and Engineering Graduate Fellowship Program.
Author Disclosure and Ghostwriting
The authors do not declare any conflicts of interest. The authors do not declare any ghostwriter contributions.
About the Authors
Geoffrey D. Hannigan is a PhD candidate at the University of Pennsylvania in the Cell & Molecular Biology: Gene Therapy and Vaccines training program. G.D.H. is a member of EAG's laboratory and actively collaborates with SM and the Orthopaedic Surgery Department of the Hospital of the University of Pennsylvania. Nicholas Pulos is a graduate of the University of Pennsylvania School of Medicine and is currently a resident in the Department of Orthopaedic Surgery at the Hospital of the University of Pennsylvania. Elizabeth A. Grice is Assistant Professor of Dermatology at the University of Pennsylvania Perelman School of Medicine. She received her doctorate in Human Genetics in 2006 from Johns Hopkins University, and did her postdoctoral fellowship at the National Institutes of Health. Her research interests focus on genomic and metagenomic analyses of cutaneous host–microbe interactions. Samir Mehta is an Assistant Professor of Orthopaedic Surgery at the Perelman School of Medicine at the University of Pennsylvania. He is also the chief of the Orthopaedic Trauma and Fracture Service at the Hospital of the University of Pennsylvania. After completing his undergraduate work at Northwestern, he received his MD from Temple University and completed his postgraduate training in orthopedic surgery at the University of Pennsylvania and Harborview Medical Center.
References
- 1.Court-Brown CM, Bugler KE, Clement ND, Duckworth AD, McQueen MM. The epidemiology of open fractures in adults. A 15-year review. Injury 2012;43:891–897 [DOI] [PubMed] [Google Scholar]
- 2.Patzakis MJ, Harvey JP, Jr, Ivler D. The role of antibiotics in the management of open fractures. J Bone Joint Surg Am 1974;56:532–541 [PubMed] [Google Scholar]
- 3.MacKenzie EJ, Jones AS, Bosse MJ, et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg Am 2007;89:1685–1692 [DOI] [PubMed] [Google Scholar]
- 4.Gille J, Wallstabe S, Schulz AP, Paech A, Gerlach U. Is non-union of tibial shaft fractures due to nonculturable bacterial pathogens? A clinical investigation using PCR and culture techniques. J Orthop Surg Res 2012;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am 1976;58:453–458 [PubMed] [Google Scholar]
- 6.Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma 1984;24:742–746 [DOI] [PubMed] [Google Scholar]
- 7.Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop Relat Res 1989:36–40 [PubMed] [Google Scholar]
- 8.Oestern HJ, Tscherne H, eds. Pathophysiology and classification of soft tissue injuries associated with fractures. In: Fractures with Soft Tissue Injuries. Berlin: Springer-Verlag, 1984:1–9 [Google Scholar]
- 9.Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhai JL, Mehta S. Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg 2010;18:10–19 [DOI] [PubMed] [Google Scholar]
- 10.Rüedi TP, Murphy WM. AO Principles of Fracture Management. Stuttgart, New York, Davos Platz, Switzerland: Thieme, AO Pub., 2000 [Google Scholar]
- 11.Braun R, Enzler MA, Rittmann WW. A double-blind clinical trial of prophylactic cloxacillin in open fractures. J Orthop Trauma 1987;1:12–17 [DOI] [PubMed] [Google Scholar]
- 12.Bergman BR. Antibiotic prophylaxis in open and closed fractures: a controlled clinical trial. Acta Orthop Scand 1982;53:57–62 [DOI] [PubMed] [Google Scholar]
- 13.Hauser CJ, Adams CA, Jr, Eachempati SR; Council of the Surgical Infection S. Surgical Infection Society guideline: prophylactic antibiotic use in open fractures: an evidence-based guideline. Surg Infect 2006;7:379–405 [DOI] [PubMed] [Google Scholar]
- 14.Saveli CC, Belknap RW, Morgan SJ, Price CS. The role of prophylactic antibiotics in open fractures in an era of community-acquired methicillin-resistant Staphylococcus aureus. Orthopedics 2011;34:611–616; quiz 617. [DOI] [PubMed] [Google Scholar]
- 15.Rohde RE, Denham R, Brannon A. Methicillin resistant Staphylococcus aureus: carriage rates and characterization of students in a Texas university. Clin Lab Sci 2009;22:176–184 [PubMed] [Google Scholar]
- 16.Graham PL, 3rd, Lin SX, Larson EL. A U.S. population-based survey of Staphylococcus aureus colonization. Ann Intern Med 2006;144:318–325 [DOI] [PubMed] [Google Scholar]
- 17.Gorwitz RJ, Kruszon-Moran D, McAllister SK, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis 2008;197:1226–1234 [DOI] [PubMed] [Google Scholar]
- 18.Morita JE, Fujioka RS, Tice AD, et al. Survey of methicillin-resistant Staphylococcus aureus (MRSA) carriage in healthy college students, Hawai'i. Hawaii Med J 2007;66:213–215 [PubMed] [Google Scholar]
- 19.Rackham DM, Ray SM, Franks AS, Bielak KM, Pinn TM. Community-associated methicillin-resistant Staphylococcus aureus nasal carriage in a college student athlete population. Clin J Sport Med 2010;20:185–188 [DOI] [PubMed] [Google Scholar]
- 20.Chen AF, Schreiber VM, Washington W, Rao N, Evans AR. What is the rate of methicillin-resistant Staphylococcus aureus and Gram-negative infections in open fractures? Clin Orthop Relat Res 2013;471:3135–3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shukla S, Nixon M, Acharya M, Korim MT, Pandey R. Incidence of MRSA surgical-site infection in MRSA carriers in an orthopaedic trauma unit. J Bone Joint Surg Br 2009;91:225–228 [DOI] [PubMed] [Google Scholar]
- 22.Schweizer M, Perencevich E, McDanel J, et al. Effectiveness of a bundled intervention of decolonization and prophylaxis to decrease Gram positive surgical site infections after cardiac or orthopedic surgery: systematic review and meta-analysis. BMJ 2013;346:f2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannigan GD, Hodkinson BP, McGinnis K, et al. Culture-independent pilot study of microbiota colonizing open fractures and association with severity, mechanism, location, and complication from presentation to early outpatient follow-up. J Orthop Res 2014;32:597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mody RM, Zapor M, Hartzell JD, et al. Infectious complications of damage control orthopedics in war trauma. J Trauma 2009;67:758–761 [DOI] [PubMed] [Google Scholar]
- 25.Glass GE, Barrett SP, Sanderson F, Pearse MF, Nanchahal J. The microbiological basis for a revised antibiotic regimen in high-energy tibial fractures: preventing deep infections by nosocomial organisms. J Plast Reconstr Aesthet Surg 2011;64:375–380 [DOI] [PubMed] [Google Scholar]
- 26.Johnson EN, Burns TC, Hayda RA, Hospenthal DR, Murray CK. Infectious complications of open type III tibial fractures among combat casualties. Clin Infect Dis 2007;45:409–415 [DOI] [PubMed] [Google Scholar]
- 27.Snitkin ES, Zelazny AM, Montero CI, et al. Genome-wide recombination drives diversification of epidemic strains of Acinetobacter baumannii. Proc Natl Acad Sci U S A 2011;108:13758–13763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lockhart SR, Abramson MA, Beekmann SE, et al. Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol 2007;45:3352–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaynes R, Edwards JR; National Nosocomial Infections Surveillance S. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 2005;41:848–854 [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease C and Prevention. Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002–2004. MMWR Morb Mortal Wkly Rep 2004;53:1063–1066 [PubMed] [Google Scholar]
- 31.Zalavras CG, Patzakis MJ, Holtom PD, Sherman R. Management of open fractures. Infect Dis Clin North Am 2005;19:915–929 [DOI] [PubMed] [Google Scholar]
- 32.Dellinger EP, Caplan ES, Weaver LD, et al. Duration of preventive antibiotic administration for open extremity fractures. Arch Surg 1988;123:333–339 [DOI] [PubMed] [Google Scholar]
- 33.Friedrich PL. Die aseptische versorgung frischer wundern. Arch Klin Chir 1898:288–310 [Google Scholar]
- 34.Schenker ML, Yannascoli S, Baldwin KD, Ahn J, Mehta S. Does timing to operative debridement affect infectious complications in open long-bone fractures? A systematic review. J Bone Joint Surg Am 2012;94:1057–1064 [DOI] [PubMed] [Google Scholar]
- 35.Anglen JO. Wound irrigation in musculoskeletal injury. J Am Acad Orthop Surg 2001;9:219–226 [DOI] [PubMed] [Google Scholar]
- 36.Penn-Barwell JG, Murray CK, Wenke JC. Comparison of the antimicrobial effect of chlorhexidine and saline for irrigating a contaminated open fracture model. J Orthop Trauma 2012;26:728–732 [DOI] [PubMed] [Google Scholar]
- 37.Anglen JO. Comparison of soap and antibiotic solutions for irrigation of lower-limb open fracture wounds. A prospective, randomized study. J Bone Joint Surg Am 2005;87:1415–1422 [DOI] [PubMed] [Google Scholar]
- 38.Anglen J, Apostoles PS, Christensen G, Gainor B, Lane J. Removal of surface bacteria by irrigation. J Orthop Res 1996;14:251–254 [DOI] [PubMed] [Google Scholar]
- 39.Bhandari M, Adili A, Schemitsch EH. The efficacy of low-pressure lavage with different irrigating solutions to remove adherent bacteria from bone. J Bone Joint Surg Am 2001;83-A:412–419 [DOI] [PubMed] [Google Scholar]
- 40.Dirschl DR, Duff GP, Dahners LE, Edin M, Rahn BA, Miclau T. High pressure pulsatile lavage irrigation of intraarticular fractures: effects on fracture healing. J Orthop Trauma 1998;12:460–463 [DOI] [PubMed] [Google Scholar]
- 41.Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res 2005;439:27–31 [DOI] [PubMed] [Google Scholar]
- 42.Boyd JI, 3rd, Wongworawat MD. High-pressure pulsatile lavage causes soft tissue damage. Clin Orthop Relat Res 2004:13–17 [DOI] [PubMed] [Google Scholar]
- 43.Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhai JL, Mehta S. Open tibial shaft fractures: II. Definitive management and limb salvage. J Am Acad Orthop Surg 2010;18:108–117 [DOI] [PubMed] [Google Scholar]
- 44.Esposito J, Schemitsch EH, Saccone M, Sternheim A, Kuzyk PR. External fixation versus open reduction with plate fixation for distal radius fractures: a meta-analysis of randomised controlled trials. Injury 2013;44:409–416 [DOI] [PubMed] [Google Scholar]
- 45.Canadian Orthopaedic Trauma Society. Nonunion following intramedullary nailing of the femur with and without reaming. Results of a multicenter randomized clinical trial. J Bone Joint Surg Am 2003;85-A:2093–2096 [PubMed] [Google Scholar]
- 46.Wiss DA, Stetson WB. Unstable fractures of the tibia treated with a reamed intramedullary interlocking nail. Clin Orthop Relat Res 1995:56–63 [PubMed] [Google Scholar]
- 47.Keating JF, O'Brien PJ, Blachut PA, Meek RN, Broekhuyse HM. Locking intramedullary nailing with and without reaming for open fractures of the tibial shaft. A prospective, randomized study. J Bone Joint Surg Am 1997;79:334–341 [DOI] [PubMed] [Google Scholar]
- 48.Finkemeier CG, Schmidt AH, Kyle RF, Templeman DC, Varecka TF. A prospective, randomized study of intramedullary nails inserted with and without reaming for the treatment of open and closed fractures of the tibial shaft. J Orthop Trauma 2000;14:187–193 [DOI] [PubMed] [Google Scholar]
- 49.Hutchinson AJ, Frampton AE, Bhattacharya R. Operative fixation for complex tibial fractures. Ann R Coll Surg Engl 2012;94:34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valenziano CP, Chattar-Cora D, O'Neill A, Hubli EH, Cudjoe EA. Efficacy of primary wound cultures in long bone open extremity fractures: are they of any value? Arch Orthop Trauma Surg 2002;122:259–261 [DOI] [PubMed] [Google Scholar]
- 51.Lee J. Efficacy of cultures in the management of open fractures. Clin Orthop Relat Res 1997:71–75 [DOI] [PubMed] [Google Scholar]
- 52.Fischer MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting, and intramedullary nailing in patients who have a fracture of the tibial shaft with extensive soft-tissue injury. J Bone Joint Surg Am 1991;73:1316–1322 [PubMed] [Google Scholar]
- 53.Burns TC, Stinner DJ, Mack AW, et al. ; Skeletal Trauma Research C. Microbiology and injury characteristics in severe open tibia fractures from combat. J Trauma Acute Care Surg 2012;72:1062–1067 [DOI] [PubMed] [Google Scholar]
- 54.Sen RK, Murthy N, Gill SS, Nagi ON. Bacterial load in tissues and its predictive value for infection in open fractures. J Orthop Surg 2000;8:1–5 [DOI] [PubMed] [Google Scholar]
- 55.Cooney WP, 3rd, Fitzgerald RH, Jr, Dobyns JH, Washington JA., 2nd Quantitative wound cultures in upper extremity trauma. J Trauma 1982;22:112–117 [DOI] [PubMed] [Google Scholar]
- 56.Merritt K. Factors increasing the risk of infection in patients with open fractures. J Trauma 1988;28:823–827 [DOI] [PubMed] [Google Scholar]
- 57.Hannigan GD, Grice EA. Microbial ecology of the skin in the era of metagenomics and molecular microbiology. Cold Spring Harb Perspect Med 2013;3:a015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet 2012;13:260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chromy BA, Eldridge A, Forsberg JA, et al. Wound outcome in combat injuries is associated with a unique set of protein biomarkers. J Transl Med 2013;11:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grice EA, Snitkin ES, Yockey LJ, et al. Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proc Natl Acad Sci U S A 2010;107:14799–14804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 2010;11:785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ostermann PA, Seligson D, Henry SL. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J Bone Joint Surg Br 1995;77:93–97 [PubMed] [Google Scholar]
- 63.Zalavras CG, Patzakis MJ, Holtom P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Orthop Relat Res 2004:86–93 [DOI] [PubMed] [Google Scholar]
- 64.Costerton JW, Post JC, Ehrlich GD, et al. New methods for the detection of orthopedic and other biofilm infections. FEMS Immunol Med Microbiol 2011;61:133–140 [DOI] [PubMed] [Google Scholar]
- 65.Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis 2006;19:349–356 [DOI] [PubMed] [Google Scholar]
- 66.Trampuz A.Zimmerli W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 2006;37Suppl 2:S59–S66 [DOI] [PubMed] [Google Scholar]
- 67.Vergidis P, Patel R. Novel approaches to the diagnosis, prevention, and treatment of medical device-associated infections. Infect Dis Clin North Am 2012;26:173–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolcott R, Costerton JW, Raoult D, Cutler SJ. The polymicrobial nature of biofilm infection. Clin Microbiol Infect 2013;19:107–112 [DOI] [PubMed] [Google Scholar]
- 69.Williams DL, Haymond BS, Beck JP, et al. In vivo efficacy of a siliconecationic steroid antimicrobial coating to prevent implant-related infection. Biomaterials 2012;33:8641–8656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyce BM, Lindsey BA, Clovis NB, et al. Additive effects of exogenous IL-12 supplementation and antibiotic treatment in infection prophylaxis. J Orthop Res 2012;30:196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li B, Jiang B, Boyce BM, Lindsey BA. Multilayer polypeptide nanoscale coatings incorporating IL-12 for the prevention of biomedical device-associated infections. Biomaterials 2009;30:2552–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]