Abstract
Significance: Humans are under constant bombardment by various stressors, including psychological anxiety and physiologic injury. Understanding how these stress responses influence the innate immune system and the skin microbiome remains elusive due to the complexity of the neuroimmune and stress response pathways. Both animal and human studies have provided critical information upon which to further elucidate the mechanisms by which mammalian stressors impair normal wound healing and/or promote chronic wound progression.
Recent Advances: Development of high-throughput genomic and bioinformatic approaches has led to the discovery of both an epidermal and dermal microbiome with distinct characteristics. This technology is now being used to identify statistical correlations between specific microbiota profiles and clinical outcomes related to cutaneous wound healing and the response to pathogenic infection. Studies have also identified more prominent roles for typical skin commensal organisms in maintaining homeostasis and modulating inflammatory responses.
Critical Issues: It is well-established that stress-induced factors, including catecholamines, acetylcholine, and glucocorticoids, increase the risk of impaired wound healing and susceptibility to infection. Despite the characterization of the cutaneous microbiome, little is known regarding the impact of these stress-induced molecules on the development and evolution of the cutaneous microbiome during wound healing.
Future Directions: Further characterization of the mechanisms by which stress-induced molecules influence microbial proliferation and metabolism in wounds is necessary to identify altered microbial phenotypes that differentially influence host innate immune responses required for optimal healing. These mechanisms may yield beneficial as targets for manipulation of the microbiome to further benefit the host after cutaneous injury.

Katherine A. Radek, PhD
Scope and Significance
Complications and costs associated with wound care, particularly chronic wounds with complicated infections, are extremely detrimental to healthcare from both a financial and psychosocial perspective. Host stress responses promote endocrine and metabolic changes within the wound microenvironments that directly impact the metabolic requirements and pathogenicity of various microorganisms.1–6 Further increasing the complexity, these host–pathogen interactions intersect several fields of research involving neuroimmunology, neuroendocrinology, and microbiology. Alterations in the physiologic stress responses allow pathogens to circumvent host innate immune responses by modification of membrane components or virulence factors that promote their survival.7,8 We will highlight emerging evidence indicating that the interplay between the skin microbiome and host stress responses directly impacts innate immunity, which has direct consequences on normal and pathologic wound repair.4,9 The objective of this review is to broaden the existing paradigm of how stress-related molecules may influence host innate immune mechanisms, and to highlight the idea that bacteria can utilize these factors to augment their pathogenic potential following wounding.
Translational Relevance
Cutaneous microbial commensals and pathogens encounter numerous microenvironments, which change rapidly and robustly during wounding and healing. Consequently, microbes must physiologically respond accordingly to successfully promote host innate immune responses (e.g., commensals) or pathologic infection (e.g., primary or opportunistic pathogens). Elucidation of the mechanisms by which stress mediators influence the cutaneous microbiome is expected to promote the development of therapeutics, such as topical pharmacologic or molecular targets, to block or promote the production of stress mediators. Our group has demonstrated that cholinergic antagonists applied during stress improve epidermal barrier function, augment antimicrobial responses, and reduce bacterial survival.10,11
Clinical Relevance
Steroids and other neuroendocrine therapies are common treatments for numerous conditions, but they also impact the bacterial microbiome by manipulating local and systemic host stress molecules.2,3,12 Aberrant use of these drugs can promote pathologic wound healing and more significant infections. Further, the manipulation of tissue planes and the transposition of tissue are common during surgical procedures, which promote the introduction of new microbial communities (e.g., bacterial, viral, or fungal) to skin regions and wound sites. Consequently, alterations in the local microbiome due to current pharmacologic and surgical practices can suppress or augment skin innate immune responses and inhibit normal wound repair.
Background
Overview of skin innate immune responses, stress mediators, and the microbiome
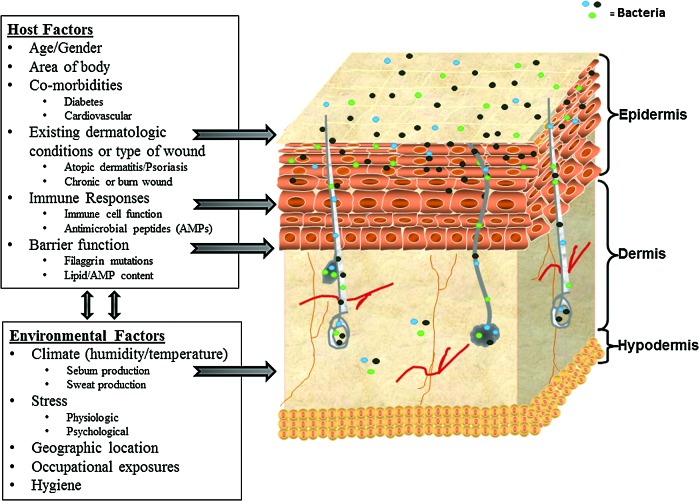
Human skin is comprised of ∼2 m2 of innumerable invaginations and areas with variable temperature, pH, humidity, antimicrobial peptide (AMP) composition, and lipid content. Consequently, these diverse areas provide unique and variable niches for commensal and pathogenic microorganisms. Interactions between the microbial inhabitants and the host innate immune system are an integral part of normal skin function and wound repair. Several groups have demonstrated significant variations in the composition of the microbiome at sites on the human body, depending on the physiology and anatomy of the site, and variations within the same site of different individuals.4,9,13–17 Nerve density may also play a role in bacterial inhabitance, as dry areas of the skin, such as the shins and dorsal forearms, have a relatively low number of neurons and therefore a decrease in neuropeptides.18 Further, the bacterial populations at certain sites have unique characteristics that allow them to survive specifically in those environments, and some of these characteristics are even beneficial to the host (Table 1). As shown in Fig. 1, host factors as well as environmental factors act as stressors to shape unique niches necessary for the survival and virulence of commensal and pathogenic microorganisms.
Table 1.
Members of the microbiome have both beneficial and detrimental effects on cutaneous homeostasis, which dictates their role as a commensal microorganism or pathogen in uninjured skin or a wound microenvironment
| Bacteria | Good | Bad | Effects of Stress Mediators |
|---|---|---|---|
| Staphylococcus epidermidis | Stimulates keratinocyte production of host AMPs, such as hBD2 and hBD3, and produces its own AMPs, including PSMγ and PSMδ.10,18 | Causative agent of hospital-acquired infections associate with medical devices.3,53 | Glucocorticoids decrease the effects of super antigen activated T cells and inhibit staphylococcal exotoxin-induced T-cell proliferation, cytokine release.3 |
| Propionibacterium acnes | Fatty acids generated by lipase activities may slow/inhibit growth of other microorganisms.49,67 | Associated with pathogenesis of acne and a number of other opportunistic infections.67 | Cortisol and steroids significantly exacerbate inflammation associated with P. acnes via TLR2 stimulation.2,49 |
| Pseudomonas aeruginosa | Produces pseudomonic acid A, which kills staphylococcal and streptococcal pathogens.2 | Most common cause of chronic and acute burn wound infections.6 | Norepinephrine increases expression of the attachment factor PA-1 of P. aeruginosa and increases biofilm formation.2,7 |
| Accelerates epithelialization and neovascularization in acute wounds.6,36 | |||
| Staphylococcus aureus | Produces bacteriocins such as staphylococcin 462, which can inhibit growth of other. | Commonly associated with infectious skin conditions, such as folliculitis and abscesses. | Norepinephrine increases ability to steal iron from host and therefore increases ability to form biofilms.2,55 |
| S. aureus strains.67 | Produces superantigen toxins that can trigger staphylococcal toxic shock syndrome.67 | ||
| Corynebacterium jeikeium | Manganese acquisition inhibits Mg-dependent superoxide dismutase, which may function to prevent oxidative damage to epidermal tissue.68 | Causes infections in immune-compromised patients, in conjunction with underlying malignancies, on implanted medical devices and in skin-barrier defects.68 | Reduced expression of transcriptional regulators involved in C. jeikeium carbohydrate metabolism due to a less versatile sugar metabolism; variations in the number of metalloregulatory sensors such that pathogenic C. jeikeium predominantly import metal ions directly from host during hypoglycemic or ionic stress responses.2,68 |
| Group A Streptococcus | Surface-expressed streptokinase sequesters and activates host plasminogen in the epidermis, which leads to keratinocyte chemotaxis, suppression of cell proliferation, and potential re-epithelialization of wounds.68 | Associated with numerous infections, such as “strep throat,” impetigo, cellulitis, erysipelas, and necrotizing fasciitis.59 | Catecholamines enhance growth likely by increasing iron availability.2,68 |
Stress mediators can alter bacterial physiology and increase virulence. This shift from a nonpathogenic to a pathogenic state can result in delayed or stalled wound healing responses and infection.
AMP, antimicrobial peptide; hBD, human β-defensin; PSM, phenol-soluble modulin; TLRs, toll-like receptors.
Figure 1.
Multiple host and environmental factors influence the composition of the microbiome. The schematic illustrates the multiple locations of microbe inhabitance and summarizes the influences of the host and external environment. Systemic stress and exogenous stress molecules have been shown to significantly alter skin barrier permeability function, lipid and antimicrobial peptide (AMP) composition, and wound repair processes.9,16–18,20,31,35,63–65 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
The skin participates in a mutualistic and complex relationship with a diverse repertoire of microorganisms. Despite the significant diversity seen in the microbiome, the skin is able to discriminate between commensal and pathogenic microbes and maintain normal barrier homeostasis and limit inflammatory responses. Resident skin cells are constantly sampling the inhabiting microbes in the epidermis and dermis via pattern recognition receptors (PRRs). Toll-like receptors (TLRs) are major PRRs that recognize pathogen-associated molecular patterns (PAMPs). PAMPs include nucleic acids, lipoproteins, peptidoglycan, and lipoteichoic acid (LTA) from Gram-positive bacteria; lipopolysaccharide and flagellin from Gram-negative bacteria; and portions of fungal cell walls.9,19 The portion of the immune system activated and how these changes are regulated differentiates a commensal organism from a potential pathogen. AMPs are fundamental components of the innate immune system that directly kill microbes by destabilization and disruption of the cellular membranes. AMPs also stimulate and augment TLR pathways, induce chemokine production and chemotactic activity, and modulate dendritic and/or T cell function to promote wound healing and maintain skin barrier homeostasis (Table 2).20–22
Table 2.
Members of the microbiome modulate the host immune response via toll-like receptors
| Bacteria | Interactions with TLRs |
|---|---|
| S. epidermidis | Modulates TLR3-dependent inflammation by initiating a TLR2-mediated crosstalk mechanism to suppress inflammation.20,34 |
| Induces keratinocytes to express endogenous AMPs through a TLR2-dependent mechanism.20,34 | |
| S. aureus | Induction of hBD3 gene expression is TLR2 dependent.20,34 |
| Bacterial lipoproteins and lipoteichoic acid serve as TLR2/6 or TLR2/2 agonists.53 | |
| Propionibacterium acnes | Colonizes sebaceous glands and stimulates keratinocytes to release inflammatory cytokines via TLR2 activation.49 |
| Escherichia coli | Flagellin from the bacteria triggers TLR5 in keratinocytes and induces expression of psoriasin (S100A7c).15 |
| Listeria monocytogenes | TLR2 is required for rapid inflammasome activation in response to infection.64 |
| Mycobacterium leprae | PAMPs from Mycobacteria are capable of altering the expression levels of TLR2 and TLR1.65 |
These interactions are crucial for maintaining tissue homeostasis and limiting pathologic inflammation. TLR recognition of specific microbial targets allows the host to differentiate between normal inhabitants of the microbiome and invasive pathogens.
PAMPs, pathogen-associated molecular patterns.
Physiological (e.g., metabolic disease, inherent skin pathologies, etc.) and psychological (e.g., depression, perceived stress, etc.) stressors can modulate communication between the host and microbiome to impair wound healing and/or promote pathologic infection. Three major pathways of the stress response include catecholamines (i.e., epinephrine and norepinephrine) via adrenergic stimulation, glucocorticoids (i.e., cortisol) via activation of the hypothalamic-pituitary-adrenal (HPA) axis, and cholinergic stimulation via acetylcholine.
Although a multitude of conditions affect the interplay between host and stress, certain diseases predispose patients to the development of altered stress responses, impaired wound healing, and chronic wounds. Diabetes, peripheral vascular disease, immune deficiency (or suppression), and advanced aged are routinely associated with such alterations.23–25 Diabetes markedly reduces the reaction of HPA axis to hypoglycemia, as suggested by lower than expected increases in adrenocorticotropic hormone (ACTH), corticosterone, and epinephrine.26 Further, foot ulcers in diabetic patients demonstrate a lack of substance P+nerve endings and a reduced distribution of calcitonin-gene-related peptide+nerves in the skin.24 Advanced age also significantly affects the body's response to stress. For example, the heightened hypothalamic response to an acute psychosocial stressor observed in young men notably decreased with age.27 Patients taking systemic glucocorticoids may have global immune suppression, which in turn, diminishes fibroblast proliferation, alters collagen synthesis, reduces wound contraction, and causes incomplete formation of granulation tissue.28,29 All of these comorbid conditions play a key role in determining the response of the host to additional stressors, such as acute wounding or the persistence of chronic wounds. Further, these individuals are more likely to exhibit an unhealthy lifestyle, which include poor nutrition, inadequate sleep, insufficient exercise habits, and a greater propensity for use and/or abuse of alcohol and cigarettes. Collectively, the interplay between these comorbid factors and unhealthy habits exacerbates these detrimental effects on wound healing and likely, the wound microbiome.
It has been demonstrated by our lab and others that stress and stress-derived hormone agonists or antagonists (e.g., glucocorticoids, acetylcholine, dopamine, histamine, and catecholamines) have a profound effect on cutaneous barrier function, wound healing, and susceptibility to skin infection.1,4,11,47,53 Activation of immune responses can reduce norepinephrine levels in the spleen, increase plasma corticosterone levels, and induce proinflammatory cytokines (i.e., interleukin [IL]-6 and IL-8) after periods of acute stress. Importantly, lymphocytes and keratinocytes, as well as neuronal cells, express functional α- and β-adrenergic receptors, muscarinic and nicotinic acetylcholine receptors (nAChRs), and glucocorticoid receptors, and produce numerous neurotransmitters (i.e., substance P, epinephrine, norepinephrine, catestatin, etc.; Table 3)33,34 with the capacity to suppress or enhance innate immune responses [reviewed in Radek18]. This data indicates that the skin microbiome must be regulated by dermatotopography, as well as the concentration and activity of neuropeptides and endogenous stress hormones secreted by resident cells of the epidermis and dermis during stress. Thus, it is critical to elucidate how microorganisms exploit host stress responses to benefit their survival and pathogenic ability in acute and chronic wounds.
Table 3.
Host stress mediators have the capacity to modulate the microbiome
| Stress Mediator | Modes of Action | Location of Synthesis | Regulator(s) | Receptor(s) | Effect on Microbiome |
|---|---|---|---|---|---|
| Cortisol | Stimulates gluconeogenesis, suppresses the immune system, aids with metabolism | Zona fasciculata of the adrenal cortex and epidermal keratinocytes | Production controlled by corticotropin-releasing hormone and ACTH | Glucocorticoid receptor | Alters susceptibility to group A Streptococcus pyogenes skin infections32 |
| Epinephrine | Vasoconstrictor and vasodilator, increases heart rate, bronchodilator, stimulates glycogenolysis, triggers lipolysis | Chromaffin cells of the adrenal medulla and epidermal keratinocytes | Synthesis stimulated by ACTH and the sympathetic nervous system, synthesized primarily from tyrosine | Adrenergic receptors (i.e., α1, α2, β1, β2) | Increases growth of human oral bacteria implicated in periodontal disease7 |
| Norepinephrine | Responsible for vigilant concentration, increases vascular tone, increases heart rate, underlies the “fight-or-flight” response | Chromaffin cells of the adrenal medulla and epidermal keratinocytes | Origin of activation pathway in the brain stem (locus coeruleus), synthesized primarily from tyrosine, must be released from synaptic vesicles to function | Adrenergic receptors (i.e., α1, α2, β1, β2) | Acts as a potent stimulant for bacterial attachment to gut tissues7 |
| Acetylcholine | Major neurotransmitter in the autonomic nervous system, activates skeletal muscle; in the central nervous system, tends to cause antiexcitatory actions | Cholinergic neurons, immune cells, and epidermal keratinocytes | Synthesized by choline acetyltransferase from choline and acetyl-CoA; acetylcholinesterase converts it into inactive metabolites | nAChR and muscarinic acetylcholine receptors | Augments susceptibility to infection by group A Streptococcus and S. aureus18 |
| Catestatin | Vasodilator, functional AMP, exhibits potent catecholamine release-inhibitory activity, stimulates histamine release | Chromaffin cells of the adrenal medulla and epidermal keratinocytes (derived from chromogranin A) | Costored and coreleased with catecholamines from adrenal chromaffin cells and adrenergic neurons | nAChR antagonist; also active in some receptor-independent manners | Exhibits antimicrobial activity against Gram-positive and Gram-negative bacteria in the skin63 |
| Substance P | Functions as a neurotransmitter, neuromodulator of nociception, and AMP; has proinflammatory effects; regulator of anxiety and stress; vasodilator | Secreted by nerves and inflammatory cells | Intense peripheral stimulation, allergens, histamine, prostaglandins, and leukotrienes induce release of substance P | Neurokinin 1 receptor | Indirectly regulates Pseudomonal infections of the cornea64 |
| α-Melanocyte stimulating hormone | Stimulates production of melanin; regulator of appetite, metabolism, and sexual behavior; anti-inflammatory mediator | Intermediate lobe of the pituitary gland, epidermal keratinocyes | Generated from precursor hormone proopiomelanocortin; proteolytic cleavage catalyzed by prohormone convertases | Melanocortin receptors (MC1, MC3, MC4, MC5) | Effective against S. aureus and its biofilms64 |
Several stress factors are synthesized primarily in the adrenal glands and select neurons through a variety of pathways. Each mediator utilizes a specific receptor(s) to trigger a diverse local and systemic response. Together, these responses collectively influence the microbiome in multiple regions of the skin.
ACTH, adrenocorticotropic hormone; nAChRs, nicotinic acetylcholine receptors.
Discussion of Findings and Relevant Literature
Modulation of the microbiome and innate immunity by stress mediators
Stress and skin pathology
The capacity of host neuroendocrine-derived stress mediators to directly influence the skin microbiome and perpetuate delayed wound healing and skin infection is poorly understood. When the balance between the skin and microbiome is altered, skin disorders, infection, and impaired wound healing tend to occur. Atopic dermatitis is a classic illustration of the importance of balance between the host innate immune responses and the microbiome. Atopic dermatitis flares are linked to an increased proportion of Staphylococcus aureus within the bacterial microbiome and higher prevalence of pathologic S. aureus infection, which is accompanied by reduced AMP responses to skin injury and bacterial challenge.35 In parallel, diabetic and chronic wounds exhibit a similar predominance of S. aureus in the epidermal microbiome; these changes are associated with excess inflammation and delayed wound healing responses.19,36–38 Further, we recently determined that significant changes in the human bacterial microbiome occur in both the burn margin as well as distal, uninjured skin sites after burn injury, which is characterized by an increase in the abundance of Gram-negative bacteria.* Thus, the presence of primary disease combined with acute skin injury has the potential to elicit acute or prolonged stress responses, which likely influence wound repair processes by modulating both host innate immune response and the microbiome.
Stress has been shown to suppress AMP production and localization in the epidermis, impair barrier permeability function, and increase susceptibility to infection.10,11,26 Glucocorticoids, a key component of the stress response, have been found to decrease keratinocyte proliferation, impair epidermal differentiation, increase epidermal barrier permeability, and decrease cathelin-related AMP and mouse β-defensin-3 antimicrobials in mouse skin. These defects in skin innate immune response and barrier function caused by glucocorticoids ultimately resulted in a greater susceptibility to Streptococcus infections and delayed healing. These defects were reversed by the presence of glucocorticoid antagonists, which highlights a potential use for antagonists to improve wound healing.3,26
Glucocorticoids can also exert direct effects on several aspects of wound repair. It is well known that systemic steroids inhibit wound repair via suppression of cellular wound responses, such as fibroblast proliferation and collagen synthesis. Moreover, it has been shown that corticosteroids suppress important growth factors (i.e., keratinocyte growth factor [KGF], transforming growth factor β1, and multiple from the fibroblast growth factor family) involved in all stages of wound healing.39 Synthetic glucocorticoids and endogenous cortisol indirectly affect wound progression via their propensity to induce hyperglycemia by counteracting the effects of insulin, which is observed in diabetic patients and those with other metabolic disorders. Hyperglycemia is known to delay wound healing, increase the risk of infection, and alter immune cell function.29,39 Numerous factors can lead to hyperglycemia and/or impaired cortisol production, including prolonged stress, exogenous steroids, pharmacologic agents, and pathologic alterations in the HPA axis (i.e., Addison's disease, Cushing's syndrome, adrenal fatigue, and thyroid dysfunction).
Human mast cell degranulation by neuropeptides has only been observed in cutaneous mast cells, further demonstrating the intricate relationship between the cutaneous and neuronal immune systems. Other stress hormones and neuropeptides may initiate or block various inflammatory processes, as many neuropeptides facilitate both an inflammatory and stress response, and have been described elsewhere [reviewed in Naik et al.17].
Catecholamines in wound healing and bacterial virulence
Catecholamines are another key component of the stress response, and elicit divergent effects on immunity and wound healing. β-Adrenergic agonists have a negative effect on chemotaxis of human neutrophils in an in vitro wound model; however, β-adrenergic antagonists have been shown to expedite closure of scratch wounds in human keratinocyte cultures after 2–5 days and enhance keratinocyte migration in culture.26,30 Catecholamines not only affect the host, but can also influence bacterial behavior. Catecholamines increase the ability of multiple bacteria to adhere to host tissues, increase proliferation, and increase virulence, particularly in Pseudomonas aeruginosa, S. aureus, and Staphylococcus epidermidis.2,7 Thus, the effect of the stress response may be twofold: to dampen the host response to infection and to augment the microenvironment for proliferation and survival of pathogenic bacteria. It is attractive to speculate that some microorganisms might utilize this host dynamic to enhance their pathogenic potential, a concept that should be investigated in the future. Evaluating local or systemic levels of catecholamines in patients with nonhealing wounds or in experimental wound models may give insight into the potential mechanisms for impaired wound healing responses and bacterial virulence, which may identify a new therapeutic target for nonhealing wounds.
Although catecholamines are an important component of the stress response, cholinergic signaling also plays a crucial role in stress and innate immunity and will be discussed in the next heading.
Acetylcholine receptors in epidermal innate immune function and skin infection
Cholinergic signaling via the nicotinic receptor is associated with the physiologic stress response and exerts a negative effect on the epithelial barrier and innate immunity.1,11 Cathelicidin and β-defensins, AMPs important for innate immunity, were reduced after stimulation of the α7 nAChR.10,11 Epidermal permeability was elevated and the structural integrity of the epidermis was compromised after topical stimulation of the nAChRs with nicotine.10 Most epithelia, including the oral mucosa, gut, and lung, similarly respond to nAChR activation through immunosuppression of immune cells.40,41 The combined defects in epidermal barrier function and dampened response to infection may increase dissemination of bacteria from initial sites of infection to distal organs or tissues.
Nicotine is known to exert several effects on wound healing and wound infection. Topical application of nicotine in mouse and cellular models has been shown to alter local blood flow and oxygenation, stimulate angiogenesis, and keratinocyte motility.25 Abstinence from smoking in human subjects was found to restore inflammation, characterized by greater inflammatory cell and macrophage infiltration into skin wounds, but did not have an effect on cellular proliferation.42 Currently, there are no topical preparations approved for human wound treatment, nor any current Food and Drug Administration (FDA) approved nAChR agonists or antagonists for wound healing or infection. One clinical trial was designed to use the topical application of nicotine for diabetic foot ulcers, but was terminated for undisclosed reasons.43 In a separate clinical trial that resulted in publication, transdermal nicotine (e.g., nicotine patches, 25 mg/day that corresponds to ∼25 cigarettes daily) had no effect on human incisional wound dehiscence as compared with a placebo patch. However, smokers exhibited a significantly greater rate of wound infection (defined as purulent discharge with or without wound dehiscence or erythema indicative of cellulitis), which was reduced by 4 weeks of abstinence from smoking, as compared with never-smokers.44,45 One caveat to this study is that the effects of nicotine derived from smoking are unclear due to the multitude of other bioactive compounds in tobacco smoke, and that these compounds have the potential to overstimulate the sympathetic nervous system. However, it has been shown that smoking also promotes significant alterations in wound closure, cutaneous blood flow and oxygenation, vascular integrity, and immune cell alterations.25,29 Our laboratory has demonstrated that the activation of the epidermal cholinergic system via psychological stress or topical nicotine could augment the susceptibility to infection by Group A Streptococcus and S. aureus in a mouse and keratinocyte model, which was attributed to the dampened AMP response to infection and TLR2 activation.11 This suggests that activation of nAChRs likely plays a significant role in modulating wound healing and infection responses. However, stress is a powerful modulator of the human wound healing and infection response via crosstalk between the cholinergic, HPA, and adrenergic pathways, which remains largely unexplored.
Bacterial AMPs and host TLR interactions: potential impact of stress on microbial antimicrobial activity
S. epidermidis is one of the most common bacterial species in the human skin microbiome and, until recently, it was unknown that this bacterium may play a role in the suppression of skin inflammation during wound repair.38,46,47 Recent studies determined that S. epidermidis may benefit the skin by generating AMPs and LTA, and was also found to modulate inflammatory responses in keratinocytes through crosstalk between TLR2 and TLR3 and modulation of its downstream signaling molecule, tumor necrosis factor receptor-associated factor 1.35,46 In addition, phenol-soluble modulins (PSMs), particularly PSMγ and PSMδ, found on human epidermis originate from S. epidermidis and increase AMP expression in differentiated keratinocytes.15,22,46,48 This suggests that differentiated keratinocytes, via TLR modulation, are tolerant of S. epidermidis as opposed to other bacteria present on the skin.46 The ability of S. epidermidis to modulate innate immune responses illustrates the complexity of the interactions between the microbiome and host defenses.
TLRs play a variety of roles, including production of inflammatory cytokines, stimulating dendritic cell maturation, and induction of phagocytosis by macrophages.14,15,38,47 TLR2 gene expression in keratinocytes was observed to be markedly altered in vitro by glucocorticoids. Moreover, glucocorticoids further enhance TLR2 gene expression in combination with bacteria or inflammatory cytokines.49 Altered TLR expression during stress could alter the balance between the microbiome and host defenses leading to pathologic inflammation and infection as TLRs also play a large role in the host interaction with the microbiome. Table 2 illustrates the interactions of select members of the microbiome and TLRs.4,14–16,19,35,37,38,47,50,51 The continuous research in the field of microbial endocrinology will hopefully unify clinicians and basic scientists in an effort to expand this unexplored area in the context of wound healing and infection as well as elucidate how host stress mediators influence bacterial antimicrobial mechanisms and the host's response to commensal versus pathogenic bacteria.
Stress mediators and biofilm formation in wound healing
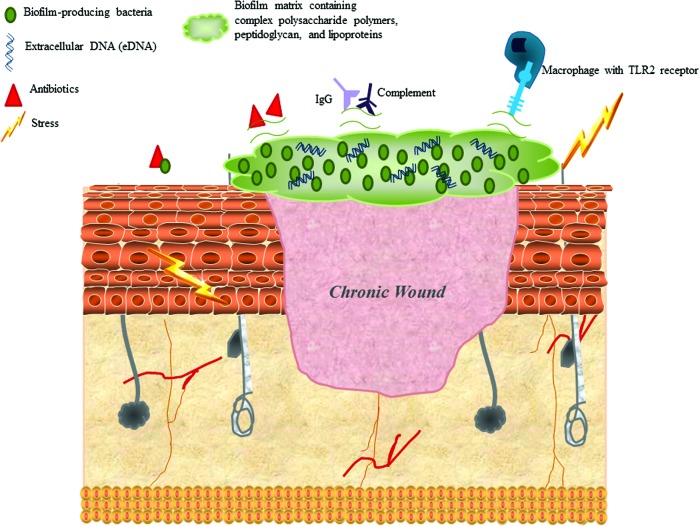
Biofilms are the predominate form in which bacteria are found in chronic wounds, and the bacteria in biofilms have distinct characteristics from planktonic bacteria. Recent evidence has shown that 60% of chronic wounds are colonized by bacteria in a biofilm, as compared with 6% of acute wounds.6 The physical characteristics of the exopolysaccharide layer of the biofilm and the metabolic changes of the bacteria afford them resistance to antimicrobials and immunity to host defenses (Fig. 2).8,52 Stress response mediators allow multiple staphylococcal species to access host iron and may contribute to biofilm formation. Escherichia coli and P. aeruginosa have demonstrated an increase in adherence factors in the presence of catecholamines that led to an increase in biofilm formation.7,18 Many bacteria have the ability to form biofilms; however, the most well studied and important to chronic wound healing are S. aureus, S. epidermidis, and P. aeruginosa.8,52–54
Figure 2.
Biofilm characteristics that are influenced by stress and allow bacteria to proliferate and survive. The most common biofilm-forming bacteria include Enterococcus faecalis, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus viridans, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa. The biofilm matrix consists of complex polysaccharide polymers, peptidoglycan, lipoproteins, and extracellular DNA (eDNA), which may interfere with optimal engagement of potential ligands, such as toll-like receptors (TLRs). Some biofilms impair IgG and complement deposition, resulting in increased resistance to opsonization and phagocyte-mediated killing. Bacterial cells in the biofilm are in a different physiological status compared with planktonic cells, which minimizes sensitivity to antibiotics that target active cell processes. The biofilm matrix may also represent a diffusion barrier for some types of antibiotics. Stress is known to increase formation of biofilms in S. aureus, S. epidermidis, and E. coli by increasing adherence factors, altering iron availability, and enhancing virulence. Glucocorticoids and catecholamines alter host cytokine and proinflammatory response to biofilms and modulate bacterial metabolism. These modifications ultimately block the recognition of bacterial proteins by macrophage TLRs and impair bacterial clearance. These stress mediators also enhance the expression of bacterial surface proteins (e.g., adhesion molecules), which interferes with the interactions between host IgG and complement with bacterial targets.66 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
P. aeruginosa is known to express genes encoding quorum-sensing ligands and virulence factors differently when in a biofilm compared to the planktonic state.6 Within a polymicrobial biofilm, P. aeruginosa enhances methicillin-resistant S. aureus USA300 virulence, and is associated with detrimental effects on wound healing. Wounds with polymicrobial biofilms showed a significant delay in wound healing as well as decreased expression of KGF1 compared with wounds with single-species biofilm infection.6 Evidence from a recent study using an in vivo rabbit model demonstrates the importance of biofilm increase in resistance to standard treatment.55 Recent evidence however shows that treatment with a combination of debridement and topical bacteriophage application may be effective in destroying biofilms and improving wound healing.17,21,55–58 Bacteriophage studies conducted in animal models reduced the biofilm burden and improved wound healing significantly.21,55,56,58 Interestingly, tests performed in humans demonstrated the safety of bacteriophage treatment, as well as its effectiveness in the treatment of chronic infections associated with biofilms. Developing and taking advantage of all the possible treatments for biofilms is critical, especially when stress is a factor. Because stress mediators have such great potential to influence biofilm formation, increase virulence, and enhance the adhesion of common biofilm-forming pathogens, clinicians must be open to the use of alternative strategies to prevent biofilm formation early on in the wound care process. Therefore, traditional methods of treatment may be considered less effective if certain individuals exhibit a more robust composition of stress mediators or neuropeptides in the local wound environment. Novel therapeutics, such as bacteriophage treatment, may prove to be advantageous in certain subsets of wound care patients.21,55,57
The change in the microbiome seen in biofilms is likely a critical component of amplifying and perpetuating the inflammation that is characteristic of the chronic wound microenvironment.14,16,38,59,60 There are clinical and animal model data that illustrate that the colonization of wounds by bacteria in biofilms can prevent epithelial growth and cause aberrant inflammatory responses (Fig. 3).15,19,51,59 Bacteria and bacterial components can directly stimulate a powerful immune response characterized by an influx of neutrophils and macrophages, which can be harmful to the wound environment.15,16,61 Ineffective phagocytosis results in tissue damage, due to the release of reactive oxygen species, proinflammatory cytokines, and enzyme activity. This results in pathologic inflammation, ultimately destroying structural integrity of the surrounding tissue, and the generation of necrotic tissue.15,16,38,51,61 Collectively, this process perpetuates the formation of bacterial biofilms in wounds. Recent evidence from a mouse model demonstrated that P. aeruginosa present in a biofilm had different characteristics compared with planktonic bacteria, and that genetically different hosts have divergent responses to biofilm-related infection, better clearance of infection, and higher levels of IL-1β.5,60 Biofilms are a key aspect that links the role of the microbiome in modulating chronic wound progression, as the bacteria that colonize chronic wounds are vastly different compared with acute or healing wounds and the “normal” microbiome of the individual.14,16,38,54 Therefore, genetic or phenotypic differences in regards to systemic and local stress responses, along with local neuropeptide composition, may give us insight into why some individuals can control pathogenic bacteria and why others develop biofilms in chronic wounds.
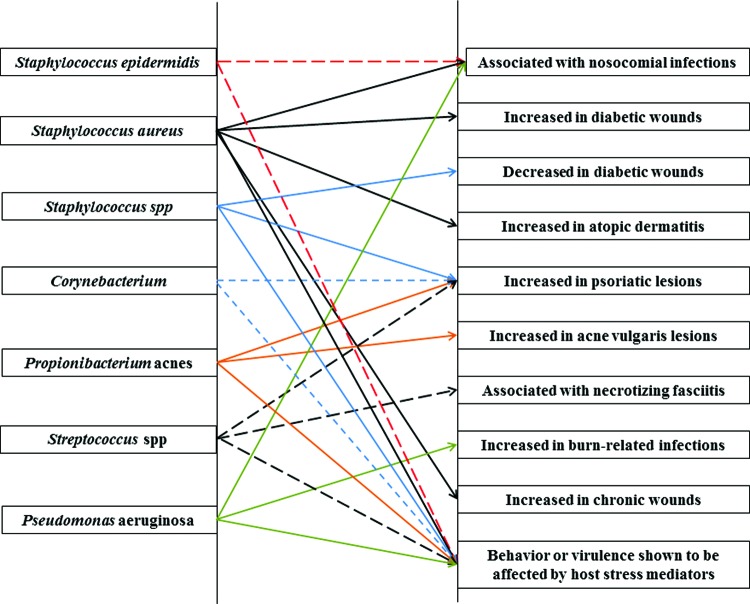
Figure 3.
Associations between common bacteria in the “normal” skin microbiome and various skin diseases or wounds. (red dashed line=S. epidermidis; black solid line=S. aureus; blue solid line=Staphylococcus spp; blue dashed line=Corynebacterium; orange solid line=Proprionibacterium acnes; black dashed line=Streptococcus spp.; green solid line=P. aeruginosa).9,16–18,20,35,53,63 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Dermal microbiome
Recent evidence suggests that the microbiome is not contained to the epidermal surface and that the dermis, subcutaneous, and adipose sites may also have a unique microbiome.4 This evidence further complicates the relationship between the host and microbiome, and hosts may have multiple interactions with multiple distinctly different microbiomes.4 We hypothesize that microbiomes below the stratum corneum and the epidermis also play a role in host pathophysiology, as other studies have demonstrated multiple signaling pathways that the epidermal microbiome affects. The epidermal microbiome likely has profound effects on the inflammatory response, wound healing, and in the development of chronic wounds. Therefore the dermal and subcuticular microbiome may also affect similar pathways.4 The possibility of significant interactions between the host and the deeper microbiome is suggested by the demonstration of altered cutaneous T-cell functions via IL-1 by commensal bacteria, and evidence that disruption of normal interactions between the host and the skin microbiome is associated with skin disorders that affect cells deeper than the top layers of the epidermis.4 Stress would likely have an effect on the dermal microbiome due to the extravasation of circulating catecholamines and cortisol into the dermis, as well as the presence of autonomic nerves and secreted neuropeptides.18 For example, nerve density varies significantly between locations in the skin, whereas dry areas of the skin, such as the shins and dorsal forearms, have a relatively low number of neurons. The low concentration of neurons correlates to a decrease in neuropeptides and neurotransmitters available to stimulate Langerhans and mast cells.18 Consequently, this may contribute to the increase in diversity seen in these sites. Langerhans cells are associated with the autonomic nervous system (ANS). Neuropeptides from the ANS directly stimulate Langerhans cells and increase cutaneous inflammation. Elimination of the nerve fibers associated with Langerhans cells significantly decreases inflammation associated with local inflammatory stimuli.18 In addition to immune cells, dermal innervation may also participate in the regulation of endothelial permeability and proinflammatory cytokine release, as well as in the synthesis of extracellular matrix components by fibroblasts. Therefore, the implications for stress mediators in the epidermal and subepidermal regions of the skin are profound, yet fundamentally unexplored, in the context of microbiome dynamics. This notion is critical to the normal wound healing process, where such displacement of bacterial communities (e.g., epidermal-dermal-subcutaneous transposition of skin) occurs during surgical procedures, involving skin grafting, free flaps, and tunneling pedicles for coverage of wounds, as well as with implanted hardware susceptible to biofilm formation.
Burn injury alters the local and distal microbiome
Changes in the microbiome are associated with alterations in the host's regulation of inflammation, skin disorders, and changes in wound healing. Further evidence that suggests that a delicate balance in the host condition and microbiome composition exists was illustrated in recent unpublished data from our lab. We observed dramatic differences between the microbiome of human skin control samples and the donor site skin samples from burn patients. These changes occur rapidly, as samples were taken at time of skin grafting, which usually occurs between 3 and 5 days postburn. These alterations may be due to changes in the host's production of AMPs, cytokines, inflammation, or other systemic changes that have an impact on the local microbiome. Our lab has recently demonstrated that burn injury alters the barrier function of distal, unburned skin in mice.62 Distal, unburned skin exhibited an increased permeability and pH, as well as a redistribution of epidermal lipids. Donor skin also was observed to have altered AMP and protease activity, while Kallikrein-related peptidase 5 and 7 gene expression was decreased at 24 h and the overall protease activity was increased after burn injury. Distal, nonburned skin also demonstrated a diminished capacity to inhibit the growth of several potential skin pathogens, including S. aureus and P. aeruginosa.62 In comparison to burn wounds, which undergo routine dressing changes and daily application of topical antimicrobials, the samples from donor skin sites were not routinely treated with topical antibiotics or antiseptics, they experienced no mechanical stresses, and no interventions were done that would affect the microbiome locally. These observed changes highlight the importance of the interactions between the microbiome and the human epidermis and dermis after burn or traumatic injury. Previous observations in our lab have demonstrated impaired epidermal barrier function of distal, unburned skin, as well as lung and bladder tissues, which was partly attributed to an enhanced production of local and systemic stress mediators following burn injury.18 Although the current mechanisms are unknown, we hypothesize that cytokines, chemokines, AMPs, and TLRs will be integral in the underlying neural-cutaneous communication. These findings illustrate the potential impact of a systemic influence on the local microbiome and the possible effects that inflammation likely has on the microbiome composition.
Summary and Future Directions
The microbiome influences multiple aspects of the host's interaction with the environment, including maintenance of the normal barrier and protection from potential pathogens. Further, pleiotropic effects of stress-induced molecules on several cell types and, likely, numerous bacterial genera present a challenge in designing appropriate experiments intended to define potential mechanisms for impaired wound healing mediated by stress. It is well established that stress exacerbates several symptoms associated with chronic wounds and skin pathologies, and chronic wounds clearly exhibit a distinct bacterial flora as compared with acute wounds (Fig. 4).15,16,38,61 Further, our unpublished observations revealed that burn injury to one site of the body dramatically alters the microbiome at sites for potential donor skin intended for grafting procedures. This suggests that the physiologic stress response to injury can directly influence the microbiome in distal sites, which may lead to wound healing complications following grafting or other surgical procedures. Understanding the bacteria in the microbiome and their effects on wound healing may alter the way we think about wound care, and invites further investigation into how altering the microbiome may influence wound healing. Further characterization of the mechanisms by which stress-induced molecules influence microbial proliferation and metabolism in wounds is necessary to identify altered microbial phenotypes that differentially influence host innate immune responses required for optimal healing. This host-microbial profile that comprised of host stress-molecules and microbial markers (i.e., virulence factors or metabolic factors) can potentially be used to identify those patients at risk for delayed healing or microbial infection and yield potential therapeutics. Identification of patients who possess a stress molecule and microbiome profile that places them at high risk for developing wound infections could allow for earlier intervention. The human host–microbiome–stress molecule interactions need more investigation and translation of insights gained from animal models.
Figure 4.
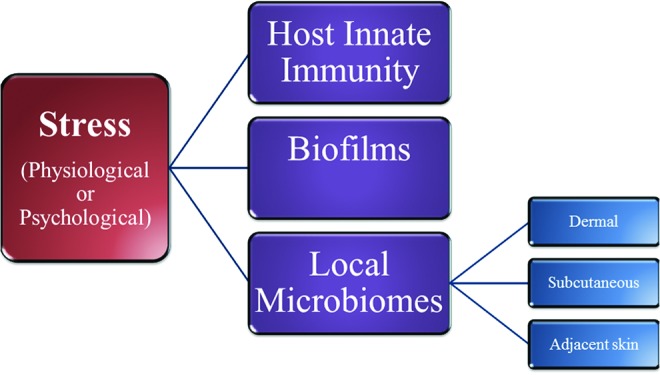
Stress mediators influence the wound microbiome. Stress mediators (i.e., cortisol, catecholamines, acetylcholine, neuropeptides, etc.) directly promote alterations in the host innate immune response, the formation of biofilms, and the formation/dynamics of the various skin microbiomes. The interplay between these factors ultimately determines both the composition of the wound microbiome, as well as the stagnation or progression of wound healing responses.9,17,20,35,53,63,67,68 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
TAKE-HOME MESSAGES.
• The local microbiome can suppress or augment skin innate immune responses and inhibit normal wound repair.
• Recent advances in genomics and bioinformatics have led to the discovery of both a superficial and dermal microbiome with distinct characteristics within the bacterial inhabitants.
• Human skin participates in a mutualistic and complex relationship with a diverse repertoire of microorganisms that comprise the microbiome.
• Stress responses and the endocrine and metabolic changes within wound microenvironments increase the risk of impaired wound healing and susceptibility to infection.
• Manipulation of the microbiome to benefit healing after cutaneous injury is a potential target for future research endeavors.
• Cholinergic antagonists applied topically during stress improve the epidermal barrier and augment host innate antimicrobial response.
• Sixty percent of chronic wounds are colonized by bacteria in biofilms, compared with only 6% of acute wounds. Differences observed in the host response to the same pathogen may give us insight into why individuals eradicate pathogenic bacteria and why others develop biofilm-laden chronic wounds.
• The local microbiome is affected by systemic factors; inflammation plays a key role in the composition of the microbiome.
Abbreviations and Acronyms
- ACTH
adrenocorticotropic hormone
- AMP
antimicrobial peptide
- ANS
autonomic nervous system
- hBD
human β-defensin
- HPA
hypothalamic-pituitary-adrenal
- IL
interleukin
- KGF1
keratinocyte growth factor 1
- LTA
lipoteichoic acid
- nAChRs
nicotinic acetylcholine receptors
- PAMPs
pathogen-associated molecular patterns
- PRRs
pattern recognition receptors
- PSM
phenol-soluble modulin
- TLRs
toll-like receptors
Acknowledgments and Funding Sources
This work was supported by the National Institutes of Health grants awarded to K.A.R. (NIAMS R01-AR061497) and R.L.G. (NIGMS T32-GM008750), and the Dr. Ralph and Marian C. Falk Medical Research Trust.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the listed authors. No ghostwriters were used to write this article.
About the Authors
Casey J. Holmes, MD, is a surgical resident participating in a 2-year NIH T32 research training fellowship in the Burn Shock Trauma Research Institute of the Loyola University Chicago Health Sciences Division. He received his medical education from the University of Nebraska Medical Center. Jennifer K. Plichta, MD, is a surgical resident and former NIH T32 trainee in the Burn Shock Trauma Research Institute of the Loyola University Chicago Health Sciences Division. Richard L. Gamelli, MD, is the Robert J. Freeark Professor of Surgery, Director of the Burn and Shock Trauma Research Institute, and the Medical Director of the Burn Unit at the Loyola University Chicago Health Sciences Division. Katherine A. Radek, PhD, is a tenure-track Assistant Professor in the Burn and Shock Trauma Research Institute at the Loyola University Chicago Health Sciences Division. Dr. Radek is also a member of the Wound Healing Society and Society for Investigative Dermatology. Dr. Radek's research focuses on the role of stress and epidermal acetylcholine receptors in modulating cutaneous innate immune responses to wounding and infection.
In unpublished observations in 2013, 16S rRNA gene sequencing was used for microbiome analyses in urine specimens from burn patient and control subjects at Loyola University Medical Center, Burn Shock Trauma Research Institute (BSTRI).
References
- 1.Curtis BJ, Radek KA. Cholinergic regulation of keratinocyte innate immunity and permeability barrier integrity: new perspectives in epidermal immunity and disease. J Invest Dermatol 2012;132:28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandrini SM, Shergill R, Woodward J, et al. Elucidation of the mechanism by which catecholamine stress hormones liberate iron from the innate immune defense proteins transferrin and lactoferrin. J Bacteriol 2010;192:587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi E-H, Demerjian M, Crumrine D, et al. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am J Physiol Regul Integr Comp Physiol 2006;291:R1657–R1662 [DOI] [PubMed] [Google Scholar]
- 4.Nakatsuji T, Chiang HI, Jiang SB, Nagarajan H, Zengler K, Gallo RL. The microbiome extends to subepidermal compartments of normal skin. Nat Commun 2013;4:1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trøstrup H, Thomsen K, Christophersen LJ, et al. Pseudomonas aeruginosa biofilm aggravates skin inflammatory response in BALB/c mice in a novel chronic wound model. Wound Repair Regen 2013;21:292–299 [DOI] [PubMed] [Google Scholar]
- 6.Pastar I, Nusbaum AG, Gil J, et al. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS One 2013;8:e56846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freestone PPE, Sandrini SM, Haigh RD, Lyte M. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol 2008;16:55–64 [DOI] [PubMed] [Google Scholar]
- 8.Whiteley M, Bangera MG, Bumgarner RE, et al. Gene expression in Pseudomonas aeruginosa biofilms. Nature 2001;413:860–864 [DOI] [PubMed] [Google Scholar]
- 9.Grice E, Segre J. The skin microbiome. Nat Rev Microbiol 2011;9:244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis BJ, Plichta JK, Blatt H, Droho S, Griffin TM, Radek KA. Nicotinic acetylcholine receptor stimulation impairs epidermal permeability barrier function and recovery and modulates cornified envelope proteins. Life Sci 2012;91:1070–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radek KA, Elias PM, Taupenot L, Mahata SK, O'Connor DT, Gallo RL. Neuroendocrine nicotinic receptor activation increases susceptibility to bacterial infections by suppressing antimicrobial peptide production. Cell Host Microbe 2010;7:277–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krakauer T, Buckley M. Dexamethasone attenuates staphylococcal enterotoxin B-induced hypothermic response and protects mice from superantigen-induced toxic shock. Antimicrob Agents Chemother 2006;391–395 Available at: http://aac.asm.org/content/50/1/391.short (accessed May22, 2014) [DOI] [PMC free article] [PubMed]
- 13.Christensen GJM, Brüggemann H. Bacterial skin commensals and their role as host guardians. Benef Microbes 2013;5:1–15 [DOI] [PubMed] [Google Scholar]
- 14.Frank DN, Wysocki A, Specht-Glick DD, et al. Microbial diversity in chronic open wounds. Wound Repair Regen 2009;17:163–172 [DOI] [PubMed] [Google Scholar]
- 15.Grice E, Segre J. Interaction of microbiome and the innate immune response in chronic wounds. Adv Exp Med Biol 2012;946:55–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grice EA, Snitkin ES, Yockey LJ, Bermudez DM, Liechty KW, Segre JA. Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proc Natl Acad Sci U S A 2010;107:14799–14804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naik S, Bouladoux N, Wilhelm C, et al. Compartmentalized control of skin immunity by resident commensals. Science (New York, NY) 2012;337:1115–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radek KA. Antimicrobial anxiety: the impact of stress on antimicrobial immunity. J Leukoc Biol 2010;88:263–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scales BS, Huffnagle GB. The microbiome in wound repair and tissue fibrosis. J Pathol 2013;229:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai Y, Gallo RL. Toll-like receptors in skin infections and inflammatory diseases. Infect Disord Drug Targets 2008;8:144–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakatsuji T, Gallo RL. Dermatological therapy by topical application of non-pathogenic bacteria. J Invest Dermatol 2014;134:11–14 [DOI] [PubMed] [Google Scholar]
- 22.Radek KA, Gallo RL. Amplifying healing: the role of antimicrobial peptides in wound repair. Inflammation 2010;1:223–229 [Google Scholar]
- 23.Gibran NS, Jang Y-C, Isik FF, et al. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res 2002;108:122–128 [DOI] [PubMed] [Google Scholar]
- 24.Galkowska H, Olszewski WL, Wojewodzka U, Rosinski G, Karnafel W. Neurogenic factors in the impaired healing of diabetic foot ulcers. J Surg Res 2006;134:252–258 [DOI] [PubMed] [Google Scholar]
- 25.Sørensen LT, Jørgensen S, Petersen LJ, et al. Acute effects of nicotine and smoking on blood flow, tissue oxygen, and aerobe metabolism of the skin and subcutis. J Surg Res 2009;152:224–230 [DOI] [PubMed] [Google Scholar]
- 26.Aberg KM, Radek KA, Choi E-H, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest 2007;117:3339–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudielka B, Buske-Kirschbaum A, Hellhammer D, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology 2004;29:83–98 [DOI] [PubMed] [Google Scholar]
- 28.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res 2012;49:35–43 [DOI] [PubMed] [Google Scholar]
- 29.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pullar CE, Rizzo A, Isseroff RR. beta-Adrenergic receptor antagonists accelerate skin wound healing: evidence for a catecholamine synthesis network in the epidermis. J Biol Chem 2006;281:21225–21235 [DOI] [PubMed] [Google Scholar]
- 31.Eijkelkamp N, Engeland CG, Gajendrareddy PK, Marucha PT. Restraint stress impairs early wound healing in mice via alpha-adrenergic but not beta-adrenergic receptors. Brain Behav Immun 2007;21:409–412 [DOI] [PubMed] [Google Scholar]
- 32.Rojas I-G, Padgett DA, Sheridan JF, Marucha PT. Stress-induced susceptibility to bacterial infection during cutaneous wound healing. Brain Behav Immun 2002;16:74–84 [DOI] [PubMed] [Google Scholar]
- 33.Radek KA, Lopez-Garcia B, Hupe M, et al. The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J Invest Dermatol 2008;128:1525–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Augustyniak D, Nowak J, Lundy FT. Direct and indirect antimicrobial activities of neuropeptides and their therapeutic potential. Curr Protein Peptide Sci 2012;13:723–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai Y, Nardo AD, Nakatsuji T, et al. Commensal bacteria regulate TLR3-dependent inflammation following skin injury. Nat Med 2009;15:1377–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liechty KW, Grice EA, Snitkin ES, Yockey LJ, Bermudez DM, Sequencing NC. Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proc Natl Acad Sci U S A 2010;107:14799–14804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanno E, Kawakami K, Ritsu M, et al. Wound healing in skin promoted by inoculation with Pseudomonas aeruginosa PAO1: The critical role of tumor necrosis factor-α secreted from infiltrating neutrophils. Wound Repair Regen 2011;19:608–621 [DOI] [PubMed] [Google Scholar]
- 38.Gontcharova V, Youn E, Sun Y, Wolcott RD, Dowd SE. A comparison of bacterial composition in diabetic ulcers and contralateral intact skin. Open Microbiol J 2010;4:8–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brauchle M, Fässler R, Werner S. Suppression of keratinocyte growth factor expression by glucocorticoids in vitro and during wound healing. J Invest Dermatol 1995;105:579–584 [DOI] [PubMed] [Google Scholar]
- 40.Giebelen IAJ, Leendertse M, Florquin S, van der Poll T. Stimulation of acetylcholine receptors impairs host defence during pneumococcal pneumonia. Eur Respir J 2009;33:375–381 [DOI] [PubMed] [Google Scholar]
- 41.Pullan RD, Rhodes J, Ganesh S, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med 1994;330:811–815 [DOI] [PubMed] [Google Scholar]
- 42.Sørensen LT, Toft B, Rygaard J, Ladelund S, Teisner B, Gottrup F. Smoking attenuates wound inflammation and proliferation while smoking cessation restores inflammation but not proliferation. Wound Repair Regen 2010;18:186–192 [DOI] [PubMed] [Google Scholar]
- 43.ClinicalTrials.gov 2007. http://clinicaltrials.gov/ct2/show/NCT00316537?term=nicotine+AND+wounds&rank=2 (accessed April15, 2014)
- 44.Sørensen L. Wound healing and infection in surgery. The clinical impact of smoking and smoking cessation: a systematic review and meta-analysis. Arch Surg 2012;147:373–383 [DOI] [PubMed] [Google Scholar]
- 45.Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg 2003;238:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai Y, Cogen AL, Radek KA, et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol 2010;130:2211–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect 2004;6:1382–1387 [DOI] [PubMed] [Google Scholar]
- 48.Nakatsuji T, Gallo R. Antimicrobial peptides: old molecules with new ideas. J Invest Dermatol 2012;132:887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shibata M, Katsuyama M, Onodera T, Ehama R, Hosoi J, Tagami H. Glucocorticoids enhance Toll-like receptor 2 expression in human keratinocytes stimulated with Propionibacterium acnes or proinflammatory cytokines. J Invest Dermatol 2009;129:375–382 [DOI] [PubMed] [Google Scholar]
- 50.Laato M, Niinikoski J, Lundberg C, Gerdin B. Inflammatory reaction and blood flow in experimental wounds inoculated with Staphylococcus aureus. Eur Surg Res 1988;20:33–38 [DOI] [PubMed] [Google Scholar]
- 51.Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008;58:185–206 [DOI] [PubMed] [Google Scholar]
- 52.Schierle CF, De la Garza M, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen 2009;17:354–359 [DOI] [PubMed] [Google Scholar]
- 53.Otto M. Staphylococcus epidermidis—“the accidental” pathogen. Nat Rev Microbiol 2009;7:555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin JM, Zenilman JM, Lazarus GS. Molecular microbiology: new dimensions for cutaneous biology and wound healing. J Invest Dermatol 2010;130:38–48 [DOI] [PubMed] [Google Scholar]
- 55.Seth AK, Geringer MR, Nguyen KT, et al. Bacteriophage therapy for Staphylococcus aureus biofilm-infected wounds: a new approach to chronic wound care. Plast Reconstr Surg 2013;131:225–234 [DOI] [PubMed] [Google Scholar]
- 56.Mendes JJ, Leandro C, Corte-Real S, et al. Wound healing potential of topical bacteriophage therapy on diabetic cutaneous wounds. Wound Repair Regen 2013;21:595–603 [DOI] [PubMed] [Google Scholar]
- 57.Rhoads DD, Wolcott RD, Kuskowski MA, Wolcott BM, Ward LS, Sulakvelidze A. Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J Wound Care 2009;18:237–238, 240–243. [DOI] [PubMed] [Google Scholar]
- 58.Knoll BM, Mylonakis E. Antibacterial bioagents based on principles of bacteriophage biology: an overview. Clin Infect Dis 2014;58:528–534 [DOI] [PubMed] [Google Scholar]
- 59.Meneghin A, Hogaboam C. Infectious disease, the innate immune response, and fibrosis. J Clin Invest 2007;117:530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Percival S. Biofilms and bacterial imbalances in chronic wounds: anti-Koch. Int Wound J 2010;7:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Costello EK, Lauber CL, Hamady M, Fierer N, Jeffrey I, Knight R. Bacterial community variation in human body habitats across space and time. Science 2013;326:1694–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plichta JK, Droho S, Curtis BJ, Patel P, Gamelli R, Radek KA. Local burn injury impairs epithelial permeability and antimicrobial peptide barrier function in distal unburned skin. Crit Care Med 2014;42:e420– e431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li M, Cha DJ, Lai Y, Villaruz AE, Sturdevant DE, Otto M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol Microbiol 2007;66:1136–1147 [DOI] [PubMed] [Google Scholar]
- 64.Jones CL, Weiss DS. TLR2 signaling contributes to rapid inflammasome activation during F. novicida infection. PLoS One 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krutzik SR, Ochoa MT, Sieling PA, et al. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat Med 2003;9:525–532 [DOI] [PubMed] [Google Scholar]
- 66.Hanke ML, Kielian T. Deciphering mechanisms of staphylococcal biofilm evasion of host immunity. Front Cell Infect Microbiol 2012;2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanford JA, Gallo RL. Functions of the skin microbiota in health and disease. Semin Immunol 2013;25:370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cogen A, Nizet V, Gallo R. Skin microbiota: a source of disease or defence? Br J Dermatol 2008;158:442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]