Abstract
The goal for this study was to evaluate the effects of daily oral intake of a consumable liquid fermentate containing high-molecular-weight hyaluronan, as well as to perform a basic evaluation of safety and tolerability. A randomized, double-blind placebo-controlled study design was used to examine the effects of oral intake of hyaluronan on chronic pain conditions. Safety assessment included a complete blood count with differential, blood chemistry and electrocardiogram. The study duration was 4 weeks, where three tablespoons (45 mL) product or placebo was ingested during the first 2 weeks, and two tablespoons (30 mL) was consumed during the last 2 weeks. Seventy-eight people between the age of 19 and 71 years enrolled, and 72 people completed the study. Statistical analysis was performed using the two-tailed independent t-test for between-group significance and using the paired t-test for within-group significance. A reduction in pain scores was seen after 2 weeks of consumption of both placebo (P<.1) and active (P<.065) product; the reduction was more pronounced in the group consuming the active test product. Using “within-subject” analysis, a highly significant reduction in chronic pain scores was seen after 2 weeks of consumption of three tablespoons of active product (P<.001), whereas only a mild nonsignificant reduction in pain scores was seen in the placebo group. During the reduced intake for the last 2 weeks of study participation, pain scores showed a slight increase. During the last 2 weeks, a significant increase in the quality of sleep (P<.005) and level of physical energy (P<.05) was seen. The pain reduction during the initial 2 weeks was associated with significant reduction in the use of pain medication (P<.05). Consumption of an oral liquid formula containing high-molecular-weight hyaluronan was associated with relief of chronic pain.
Key Words: : CBC, ECG, metabolic markers, physical energy, skin health
Introduction
Degenerative joint disease and other rheumatic conditions, such as arthritis, are the second most common musculoskeletal diseases among adults,1–3 which represent significant public health issues due to hospitalization costs and loss of quality of life. In the past decade alone, the costs of hip and knee replacement procedures have increased by more than 100%4 and are expected to continue to rise.
Articular cartilage, the hyaline connective tissue lining the surfaces of synovial joints, functions to transmit force across joints and provide a smooth surface for limb movement. However, with age-related wear and tear, the articular cartilage is subject to significant structural, mechanical, and matrix changes consisting of mild fibrillation of the articular surface and a decrease in proteoglycan monomer size and aggregation.5 These changes have been linked to a progressive reduction in the ability of chondrocytes to maintain cartilage homeostasis as a result of decline in mitotic and synthetic activity, to respond to anabolic growth factors, and synthesize cartilage-specific proteoglycan core proteins (CSPCP).6 The overall loss of matrix tensile strength and stiffness that accompany aging result in a restriction of joint movement and loss of mobility, which may lead to the development of degenerative cartilage diseases.
Local inflammatory responses are characteristic of our innate immunity and function to recruit inflammatory cells to the joint and stimulate the inflammatory cascade. However, while acute inflammation is controlled by the rapid production of anti-inflammatory cytokines following the release of proinflammatory cytokines, chronic inflammation results when initiating factors persist or the mechanisms responsible for carrying out the inflammatory response fail.7 Chronic inflammation has been linked to several degenerative human joint diseases and can affect pain thresholds, thus altering the perception of pain and further reducing the range of motion and activity levels.
While acute pain stimulates motor responses to noxious stimuli, chronic pain due to deteriorating joint health results from a combination of insufficient production of collagen and hyaluronan (HA), increased mechanical stress, structural degradation of cartilage and bone, as well as prolonged local inflammatory processes.8 In addition to deteriorating joint health, chronic pain can result in adverse health effects, including an unbalanced posture due to body compensation, restricted range of motion, muscular hypertonicity and reduced muscle strength, and reduced activity levels.5
HA, also referred to as hyaluronic acid, is a high molecular mass polysaccharide consisting of repeating N-acetylglucosamine and glucuronate subunits.9 Depending on the source, such as rooster combs or microbial fermentation, the molecular weights of HA molecules can vary between 104 and 107 Da.10 High- and low-molecular-weight forms of HA have been reported to exhibit distinct biological effects, which may be mediated by their unique bindings with CD44 receptors.11 Many studies have shown that high-molecular-weight HA exhibits anti-inflammatory effects by inhibiting cell proliferation and mobility in vivo.12 Conversely, low-molecular-weight HA has been observed to stimulate cell proliferation and mobility, thus resulting in proinflammatory effects.13
HA has been observed to have anti-inflammatory and analgesic properties in articular joints, which may be attributed to its inhibition of IL-1-induced type 2 collagen mRNA downregulation.14 With age-related changes in articular cartilage and an increase in proinflammatory cytokine and free radical production, the concentration and molecular weight of hyaluronic acid are greatly reduced.15 This affects both the viscosity and elasticity of HA molecules, thus reducing the ability of joints to respond to changes in shear force.12 These changes have been shown to lead to intra-articular inflammation or increased cartilage degradation.
Due to its chondroprotective and shock-absorbing properties in synovial fluid, intra-articular injections of HA have been widely used to treat inflammation-induced or age-related joint pain.12,16,17 However, the effectiveness of this method for treating chronic joint pain is still debated.18–21 For example, although there have been clinical studies reporting relief of pain symptoms lasting ∼6 months, a meta-analysis of 89 trials revealed a clinically insignificant effect on pain scores and an increased risk of serious adverse effects.22 In addition, it has been reported that while the mean intra-articular half-life of injected HA was 20.8 h in normal sheep joints with no intra-articular challenge, the average half-life dropped to 11.5 h in joints with acute inflammation.12 The most recent evidence-based guideline for the treatment of osteoarthritis of the knee does not support the use of viscosupplementation (i.e., injecting HA).23
In contrast to injecting HA, the oral consumption of high-quality HA products has gained interest and has been shown in animal studies to result in absorption into the bloodstream and significant effects on peripheral tissues, such as joints.6,24,25 Specifically, an animal study using radiolabeled high-molecular-weight HA found that small amounts of HA was incorporated into skin, bone, and joint tissue of rats 24 h after oral administration.21 These results challenged the assumption that the large size and molecular weight of HA would restrict systemic uptake and clinical effectiveness. The movement of high-molecular-weight HA into and out of synovial spaces and tissues through lymphatic transport is well-documented, which provides further support for the integration of orally administered HA into systemic circulation and connective tissues.21
Consumable hyaluronan may provide an alternative to the prescription of pharmaceuticals, injected viscosupplementation, or surgical interventions. This study was undertaken to test the efficacy and safety of a liquid oral product containing high-molecular-weight HA. This clinical study collected a core set of data on relief of chronic pain and increased daily functionality in humans consuming the test product. In addition, the study used standard tests to evaluate safety parameters and also asked questions regarding observations (positive and negative) by study participants.
Materials and Methods
Study design
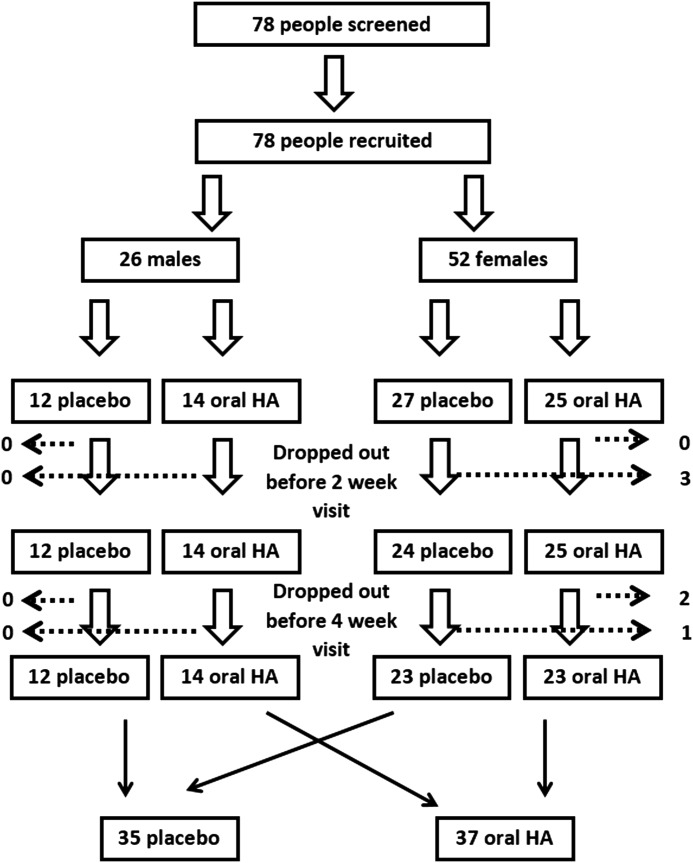
A randomized, double-blind, placebo-controlled clinical study design was used. Seventy-eight people went through screening and were enrolled into the 4-week study upon signing written informed consent, as approved by the Sky Lakes Institutional Review Board (FWA 2603) (Fig. 1). People were excluded from study participation if they had consumed HA-containing nutritional supplements during the month before the study, were taking prednisone, or had done so within 6 months before the study, had known liver or kidney disease, were taking diuretics medication, undergoing intensive medical treatment for diseases such as cancer or viral illness, and if they were undergoing stressful life events that could affect compliance. People on daily pain medication, nondiuretic blood pressure medication, and cholesterol medication were not excluded from participating in the study. People with fibromyalgia or rheumatoid arthritis and other autoimmune diseases were not excluded solely based on the diagnosis. Screening was performed to ensure normal blood chemistry and heart function as measured by a 3-lead electrocardiogram (ECG). After successful screening, participants were randomized to consume either placebo or a liquid oral HA product (Table 1). Follow-up visits were scheduled at 2 and 4 weeks. The study was carried out in Southern Oregon between July and November 2012, at a study location where study participants live and work at an elevation of 1200–1500 m above sea level.
FIG. 1.
Consort flow chart of the study participants. The ratio of males:females was 1:2. Among the female study participants, three females dropped out before the 2-week follow-up visit, and an additional three females dropped out before the 4-week follow-up visit. No males dropped out of the study. A total of 72 people completed the study.
Table 1.
Demographics of Study Participants
| Placebo | Oral HMW HA | P | |
|---|---|---|---|
| Females | 27 | 25 | |
| Age averagea | 50.6±12.5 | 43.5±12.7 | .047 |
| Age range | 22–71 | 19.9–65.6 | |
| BMI averagea | 31.8±6.6 | 31.7±7.1 | .521 |
| BMI range | 18.2–42.3 | 18.9–46.9 | |
| Males | 12 | 14 | |
| Age averagea | 45.4±14.2 | 50.8±15.2 | .357 |
| Age range | 21.8–69.1 | 19.1–65.9 | |
| BMI averagea | 30.8±7.2 | 29.1±4.5 | .486 |
| BMI range | 20.5–49.2 | 21.6–36.8 |
The average±standard deviation is shown.
HMW, high molecular weight; HA, hyaluronan.
Consumables
The active consumable product and placebo were provided by Viscos, LLC. (Fortville, IN, USA). The active product is a liquid microbial fermentate containing high-molecular-weight hyaluronan (HA) at a level of 5 mg/mL and was flavored with sucralose and a mild raspberry flavor. The placebo product had a similar viscosity, sweetness, and flavor, but did not contain the microbial fermentate. The average molecular weight of the HA ranged between 2.5 and 2.8 million Daltons. Study participants were given a measuring spoon with the test products and instructed to consume three tablespoons (45 mL) during the first 2 weeks of the study and two tablespoons (30 mL) during the last 2 weeks of the study.
Pain assessment
For this study, each person's anatomical areas of primary and secondary chronic pain, associated with joint stiffness and reduced function, were identified at the screening visit before study start. This information was used at subsequent visits for scoring each person's main complaints in parallel to the overall pain. At each visit, pain levels for both the primary and secondary areas were scored for “pain at rest” and “pain at use,” using an unmarked 100-mm Visual Analogue Scale (VAS). The VAS was 100 mm without increment marks, where one end was labeled “no pain,” and the other end was labeled “intense pain.” The score was measured on the scale in millimeters and scored in percentage.
Safety assessment
At screening, 2-week, and 4-week follow-up, blood was drawn to perform a complete blood count (CBC) with differential count, a comprehensive metabolic panel (CMP), and Hepatic Function. At screening and 4-week follow up, a 3-lead ECG was performed.
Adherence to study protocol
At each study visit, the study participants went through an interview to monitor adherence to the study protocol. The returned product was weighed to track compliance as it pertains to the consumption of the allocated test product (Table 2).
Table 2.
Compliance Based on Weight of Consumed Product
| Average compliance (%)a | |
|---|---|
| Compliance during Phase 1 (3 tbsp/day) | 84.09 |
| Compliance during Phase 2 (2 tbsp/day) | 86.56 |
| Compliance during entire study | 85.79 |
These %s include all 72 study participants.
Statistical analysis
The number of subjects was based on power calculations based on data from a preliminary open-label pilot study, this study was 90% powered to detect a 10% change. Statistical significance of changes from baseline to later assessments was evaluated by between-groups analysis using the two-tailed independent t-test. Within-subject analysis was performed using the two-tailed paired t-test. Statistical significance was indicated if P<.05.
Results
Reduction of chronic pain
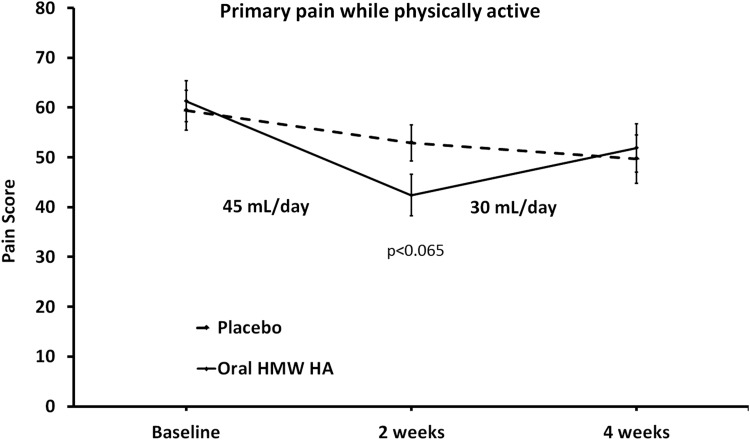
The primary purpose of this 4-week study was to gather data from a placebo-controlled study regarding chronic pain reduction during consumption of oral liquid high molecular weight (HMW) HA. Subgroup analysis included evaluation of pain medication, pain scores at study start, use of antidepressants, and diagnosis of fibromyalgia. Analysis of pain scores for each person's identified primary area of pain from the 63 study participants who were not diagnosed with fibromyalgia syndrome (FMS) (32 in the placebo arm, 31 in the active product arm) showed changes within 2 weeks. A reduction in pain scores was seen for both the placebo group and the group consuming oral HMW HA; however, the reduction was more robust in the group consuming oral HMW HA, reaching a statistical trend both when scoring for “pain when inactive” (P<.1) and when scoring for “pain when physically active” (P<.065) (Fig. 2). Analyzing the data for the oral HMW HA group using within-subject analysis, the pain scores when physically active showed a statistically significant reduction at the 2-week visit (P<.001), followed by a mild increase in pain during the last 2 weeks of the study.
FIG. 2.
The average pain scores are shown for the primary pain when physically active. The primary pain area was identified for each study participant at baseline, and pain in that area tracked throughout the study. The pain level was scored using Visual Analogue Scales (0–100). The data are shown as mean±SEM. After 2 weeks of consumption, the average pain score was lower in the group consuming oral HMW hyaluronan (HA) than in the placebo group (*P<.065). Analyzing the change in pain reduction within the group consuming oral HMW HA reached a high level of statistical significance at 2 weeks (***P<.001). The mild increase seen between 2 and 4 weeks may be a combination of the lower dose consumed during the second phase and also that people had more physical energy and were more physically active.
Among the nine study participants with a physician-confirmed diagnosis of FMS (five in the placebo arm, four in the active product arm), the data suggested a statistical trend at the 4-week follow-up, where reduced pain scores reached borderline significance when compared to the baseline using within-subject analysis (P<.065). This suggested a slower response in people with a physician-confirmed diagnosis of FMS.
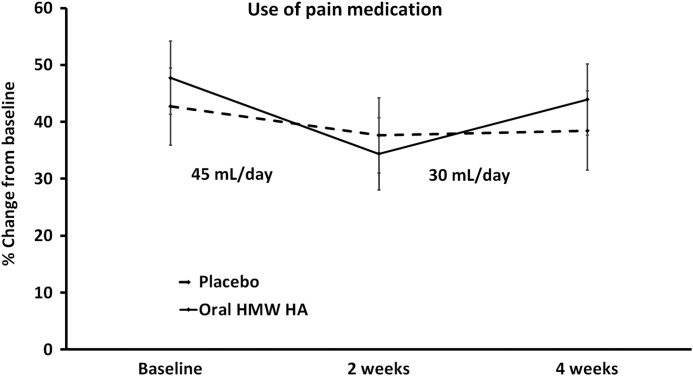
Reduced use of pain medication
The scores for how much people relied on pain medication during the day showed a mild decrease in the placebo group and a more robust decrease in the group consuming oral HMW HA, however, the difference did not reach statistical significance (Fig. 3). Analyzing the reduction within each group using within-subject analysis showed no significant changes in the placebo group, but the decrease was statistically significant (P<.05) in the use of pain medication during the first 2 weeks in the group consuming oral HMW HA, followed by an increase during the last 2 weeks of the study where people had more physical energy. This should also be interpreted in light of the lower dose consumed during the last 2 weeks (two tablespoons) as compared to three tablespoons during the first 2 weeks of the study.
FIG. 3.
The average reliance on pain medication is shown. The reliance on pain medication was scored using Visual Analogue Scales (0–100). The data are shown as mean±SEM. There was no statistically significant difference in the use of pain medication between the two groups, however, the reduction in pain medication seen within the group consuming oral HMW HA reached statistical significance at 2 weeks (*P<0.03). The increase seen between 2 and 4 weeks may be a combination of the lower dose consumed during the second phase and also that people had more physical energy and were more physically active.
Self-reported changes
When subjects were asked to score their quality of sleep and their physical energy level, a significant increase in the self-reported quality of sleep and physical energy levels was seen in the population consuming oral HA, when comparing to the placebo group (P<.03). The improved quality of sleep and physical energy was most distinct during the last 2 weeks of the study, when pain scores had started to increase again, suggesting continued improvement in personal wellness. The improved sleep quality was seen within 2 weeks when compared to baseline for the group consuming oral HMW HA (P<.09), and this improvement reached a high level of significance at 4 weeks (P<.01), data not shown. Self-reported energy levels improved during the last 2 weeks of the study, but did not reach statistical significance (P<.07). When subjects were asked to rate their skin health, statistically significant improvements for skin softness and skin firmness were seen in the group consuming oral HA, but not in the placebo group.
Safety assessment
The safety evaluation during the 4-week study included CBC with differential count, CMP, and 3-lead ECG.
The purpose of the CBC analysis was to examine whether consumption of oral HA would result in changes in blood cell numbers, for example, if allergies were increased as a reaction to ingredients in the product or cell production/death in other ways affected. The CBC data did not show any major changes. All group averages remained within the normal ranges for each parameter (data not shown). An increase in eosinophil numbers was seen for the placebo group, suggesting that some people experienced allergies during the study. No similar increase was seen for the group consuming oral HA; in contrast, a mild decrease in eosinophil numbers was seen in the group consuming oral HMW HA.
The purpose of the CMP was to examine whether consumption of oral HMW HA would result in changes in blood chemistry, for example, if the consumption of oral HMW HA would lead to stress on liver or kidney function (Table 3). The CMP data did not show any major changes and all group averages remained within the normal ranges for each parameter. However, using within-subject analysis, there were statistically significant changes seen for several data sets for the group consuming oral HMW HA. The blood levels of sodium, CO2, blood urea nitrogen, creatinine, and albumin were decreased. These effects were very minute, but using the within-subject analysis (paired two-tailed t-test) reached significance (P<.05).
Table 3.
Comprehensive Metabolic Panel with Hepatic Function
| Placebo | Hyaluronan | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test | Gender | Unit | Min | Max | Week 0 | Week 2 | Week 4 | Week 0 | Week 2 | Week 4 |
| BUN | Male | mg/dL | 6 | 20 | 15.00±3.35 | 14.91±3.30 | 14.45±3.33 | 17.36±5.18 | 17.43±3.88 | 15.00±4.15 |
| Female | mg/dL | 6 | 20 | 15.33±4.43 | 14.04±4.39 | 14.17±4.26 | 13.96±5.17 | 13.43±2.97 | 12.33±3.58 | |
| Creatinine | Male | mg/dL | 0.6 | 1.3 | 0.90±0.13 | 0.85±0.11 | 0.86±0.10 | 0.96±0.08 | 0.92±0.09 | 0.91±0.09 |
| Female | mg/dL | 0.6 | 1.3 | 0.74±0.12 | 0.73±0.11 | 0.73±0.11 | 0.73±0.08 | 0.70±0.08 | 0.69±0.10 | |
| GFR | Male | mL/min/1.7>60 | na | >60 | >60 >60 | >60 >60 | >60 >60 | >60 >60 | >60 >60 | >60 |
| Female | mL/min/1.7>60 | na | >60 | >60 >60 | >60 >60 | >60 >60 | >60 >60 | >60 >60 | >60 | |
| Bun-Creat. | Male | Ratio | 12 | 20 | 17.00±4.73 | 17.69±3.86 | 16.85±4.00 | 18.18±5.37 | 19.16±4.42 | 16.58±4.82 |
| Female | Ratio | 12 | 20 | 21.45±8.09 | 19.37±5.99 | 19.87±7.02 | 18.97±6.52 | 19.39±4.87 | 17.83±4.23 | |
| Glucose | Male | mg/dL | 74 | 106 | 88.27±9.63 | 88.45±11.31 | 90.55±6.70 | 91.50±17.63 | 93.57±18.14 | 91.79±14.57 |
| Female | mg/dL | 74 | 106 | 93.38±26.66 | 90.96±11.78 | 91.04±19.13 | 89.52±14.06 | 88.83±10.32 | 89.43±13.87 | |
| Total protein | Male | g/dL | 6.5 | 8.2 | 7.07±0.43 | 6.84±0.42 | 6.79±0.34 | 6.94±0.44 | 6.88±0.35 | 6.78±0.31 |
| Female | g/dL | 6.5 | 8.2 | 6.83±0.34 | 6.92±0.38 | 6.87±0.39 | 6.83±0.56 | 6.82±0.32 | 6.76±0.37 | |
| Albumin | Male | g/dL | 3.5 | 5 | 4.08±0.21 | 3.98±0.17 | 4.02±0.26 | 4.19±0.31 | 4.18±0.30 | 4.04±0.25 |
| Female | g/dL | 3.5 | 5 | 3.89±0.25 | 3.87±0.27 | 3.87±0.27 | 3.86±0.31 | 3.89±0.36 | 3.84±0.27 | |
| A/G ratio | Male | Ratio | 1.2 | 2.2 | 1.38±0.16 | 1.42±0.17 | 1.48±0.29 | 1.54±0.19 | 1.54±0.18 | 1.51±0.24 |
| Female | Ratio | 1.2 | 2.2 | 1.35±0.20 | 1.30±0.19 | 1.32±0.21 | 1.32±0.20 | 1.36±0.26 | 1.34±0.22 | |
| AST | Male | IU/L | 15 | 41 | 27.91±7.15 | 27.73±9.05 | 27.18±6.59 | 26.21±5.56 | 24.57±6.17 | 25.43±4.78 |
| Female | IU/L | 15 | 41 | 22.58±10.73 | 22.58±12.91 | 21.52±9.59 | 22.13±5.68 | 21.30±5.40 | 20.86±5.32 | |
| ALT | Male | IU/L | 10 | 40 | 38.55±14.98 | 37.18±19.36 | 37.18±18.41 | 26.57±9.10 | 24.50±8.46 | 25.50±7.38 |
| Female | IU/L | 10 | 40 | 22.04±9.65 | 21.25±9.44 | 20.78±9.10 | 20.43±8.52 | 20.26±7.34 | 20.33±9.38 | |
| ALK Phosphatase | Male | IU/L | 38 | 126 | 68.27±13.91 | 64.09±11.56 | 65.36±10.45 | 57.71±7.49 | 57.64±9.47 | 59.29±11.58 |
| Female | IU/L | 38 | 126 | 76.04±22.06 | 77.96±21.42 | 76.57±22.06 | 63.09±14.40 | 62.13±11.83 | 62.43±13.74 | |
| Total bilirubin | Male | IU/L | 0.3 | 1.2 | 1.05±0.55 | 1.11±0.53 | 0.99±0.71 | 0.87±0.20 | 0.91±0.26 | 0.86±0.27 |
| Female | IU/L | 0.3 | 1.2 | 0.75±0.21 | 0.81±0.23 | 0.80±0.31 | 0.79±0.33 | 0.75±0.39 | 0.77±0.23 | |
| Direct bilirubin | Male | mg/dL | 0 | 0.2 | 0.15±0.08 | 0.13±0.06 | 0.12±0.09 | 0.09±0.05 | 0.12±0.04 | 0.10±0.06 |
| Female | mg/dL | 0 | 0.2 | 0.11±0.04 | 0.09±0.07 | 0.10±0.05 | 0.08±0.05 | 0.10±0.10 | 0.08±0.06 | |
BUN, blood urea nitrogen; GFR, glomerular filtration rate.
The glomerular filtration rate was in the normal range (above 60 mL/min/1.73 m2) for all study participants at screening and at both subsequent blood draws.
The ECGs for all study participants were normal at study start (screening visits) as well as at study exits. Thus, no changes to heart function, as measured by the 3-lead ECG, were seen as a result of consumption of oral HMW HA (data not shown).
Discussion
The goal for the study presented here was to evaluate a chronic pain management strategy using oral liquid microbial fermentate containing high-molecular-weight hyaluronic acid as an alternative to injected HA. The data have shown that the oral HMW HA is both efficacious and safe. The daily consumption of oral HMW HA resulted in reduced pain and use of pain medication already after 2 weeks. At that time, in the chosen study design, the daily dose was reduced from three tablespoons (45 mL) to a maintenance dose of two tablespoons (30 mL). During the last 2 weeks of the study, where the maintenance dose was consumed, no further decrease in chronic pain was seen. The self-reported quality of sleep and energy levels continued to increase through the 4-week study, suggesting that the lack of continued reduction in pain scores was due to a combination of the reduced dose of product and increased activity levels. This is typical of chronic pain studies where an initial pain relief is associated with increased physical activity, resulting in either no further change or even a slight increase in pain scores due to the increased activity.8
The noninvasive use of oral high-molecular-weight HA is an attractive intervention and the effectiveness may stem from accumulation of HA in connective tissue. The direct immune modulating properties of HA are multifaceted and may happen through CD44- and/or ICAM-1 signaling pathways.26 With respect to CD44/HA interaction, high- and low-molecular-weight forms of HA have different effects on CD44 clustering, signaling, and downstream cellular behavior such as adhesion.11 The molecular weight of HA has direct impact on whether pro- or anti-inflammatory effects are prominent, including crosstalk between HA-mediated signaling and the COX-2/prostaglandin pathways. Thus, current research has accumulated a convincing volume of documentation of the direct anti-inflammatory properties of high-molecular-weight HA.14
The overall safety data included CBCs with differential counts, CMP of blood markers, and ECG, and showed no significant changes between the two groups. Within the group consuming oral HMW HA, several metabolic markers showed significant changes during the 4 weeks of oral HA consumption. These changes were very small but did reach statistical significance using within-subject analysis and will need further evaluation. Several of these parameters may possibly relate to a better hydration status. Water/fluid consumption was not tracked during this study; it is possible that consumption of oral HMW HA may have led to a higher intake of fluids. Alternatively, the oral HMW HA may have contributed to a reduced level of inflammation, leading to improved function of organs, including the kidneys.
In light of the recent changes in recommended osteoarthritis management, where injectable HA is no longer recommended,23 alternative methods for managing chronic joint pain are in high demand. The data presented here suggest that high-molecular-weight HA offers a noninvasive method for pain management in situations involving moderate chronic joint pain affecting mobility. Future work is warranted on the oral HMW HA and should include detailed assessment of inflammatory status and encompass different study populations, including fibromyalgia patients with longer study duration, younger study population, athletes, and people recovering from acute trauma. It will also be of interest to evaluate the effects of oral HMW HA on its potential effects on inflammatory problems unrelated to joints and mobility.
Acknowledgments
The clinical study was conducted at NIS Labs, an independent contract research laboratory specializing in natural products research. The study was sponsored by Viscos LLC.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Centers for Disease Control and Protection: Prevalence of doctor-diagnosed arthritis-attributable activity information-United States, 2007–2009. MMWR 2010;59:1261–1265 [PubMed] [Google Scholar]
- 2.Gabriel SE, Michaud K: Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther 2009;11:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochbrg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F: Estimates of the prevalence of arthritis and other rheumatic conditions in the United States—Part II. Arthritis Rheum 2008;58:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Bone and Joint Initiative: The Burden of Musculoskeletal Diseases in the United States, Second Edition. American Academy of Orthopaedic Surgeons, Rosemont, IL, 2011, pp. 75–102 [Google Scholar]
- 5.Horton WE, Jr, Bennion P, Yang L: Cellular, molecular, and matrix changes in cartilage during aging and osteoarthritis. J Musculoskelet Neuronal Interact 2006;6:379–381 [PubMed] [Google Scholar]
- 6.Martin JA, Buckwalter JA: Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology 2002;3:257–264 [DOI] [PubMed] [Google Scholar]
- 7.Coussens LM, Werb Z: Inflammation and cancer. Nature 2002;420:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson KF, Ager DM, Landes B, Aruoma OI, Jensen GS: Improvement of joint range of motion (ROM) and reduction of chronic pain after consumption of an ergothioneine-containing nutritional supplement. Prev Med 2012;54(Suppl):S83–S89 [DOI] [PubMed] [Google Scholar]
- 9.Bucci LR, Turpin AA: Will the real hyaluronan please stand up? J Appl Nutr 2004;54:10–33 [Google Scholar]
- 10.Liu L, Liu Y, Li J, Du G, Chen J: Microbial production of hyaluronic acid: current state, challenges and perspectives. Microb Cell Fact 2011;10:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang C, Cao M, Liu H, He Y, Xu J, Du Y, Liu Y, Wang W, Cui L, Hu J, Gao F: The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J Biol Chem 2012;287:43094–43107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noble PW: Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol 2002;21:25–29 [DOI] [PubMed] [Google Scholar]
- 13.Wang YZ, Cao ML, Liu YW, He YQ, Yang CX, Gao F: CD44 mediates oligosaccharides of hyaluronan-induced proliferation, tube formation and signal transfuction in endothelial cells. Exp Biol Med 2011;235:84–90 [DOI] [PubMed] [Google Scholar]
- 14.Masuko K, Murata M, Yudoh K, Kato T, Nakamura H: Anti-inflammatory effects of hyaluronan in arthritis therapy: not just for viscosity. Int J Gen Med 2009;2:77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benke M, Schaffer B: Viscosupplementation treatment of arthritis pain. Curr Pain Headache Rep 2009;13:440–446 [DOI] [PubMed] [Google Scholar]
- 16.Abate M, Pulcini D, Lorio A, Schiavone C: Viscosupplementation with intra-articular hyaluronic acid for treatment of osteoarthritis in the elderly. Curr Pharm Des 2010;16:631–640 [DOI] [PubMed] [Google Scholar]
- 17.Uçar D, Dıraçoğlu D, Süleyman T, Capan N: Intra-articular hyaluronic acid as treatment in elderly and middle-aged patients with knee osteoarthritis. Open Rheumatol J 2013;7:38–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Axe JM, Snyder-Mackler L, Axe MJ: The role of viscosupplementation. Sports Med Arthrosc 2013;21:18–22 [DOI] [PubMed] [Google Scholar]
- 19.McArthur BA, Dy CJ, Fabricant PD, Valle AG: Long term safety, efficacy, and patient acceptability of hyaluronic acid injection in patients with painful osteoarthritis of the knee. Patient Prefer Adherence 2012;6:905–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medical Services Advisory Committee: Intra-articular Viscosupplementation for Treatment of Osteoarthritis of the Knee. Canberra, Australia: MSAC Application 1045, 88, 2003 [Google Scholar]
- 21.Leopold SS, Redd BB, Warme WJ, Wherle PA, Pettis PD, Shott SS: Corticosteroid compared with hyaluronic acid injections for the treatment of osteoarthritis of the knee. A prospective, randomized trial. J Bone Joint Surg Am 2003;85-A:1197–1203 [DOI] [PubMed] [Google Scholar]
- 22.Rutjes AWS, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S: Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med 2012;157:180–191 [DOI] [PubMed] [Google Scholar]
- 23.Jevsevar DS: Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg 2013;21:571–576 [DOI] [PubMed] [Google Scholar]
- 24.Huang SL, Ling PX, Zhang TM: Oral absorption of hyaluronic acid and phospholipid complexes in rats. World J Gastroenterol 2007;13:945–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balogh L, Polyak A, Mathe D, Kiraly R, Thuroczy J, Terez M, Janoki G, Ting Y, Bucci L, Schauss A: Absorption, uptake and tissue affinity of high-molecular-weight hyaluronan after oral administration in rats and dogs. J Agric Food Chem 2008;56:10582–10593 [DOI] [PubMed] [Google Scholar]
- 26.Yatabe T, Mochizuki S, Takizawa M, Chijiiwa M, Okada A, Kimura T, Fujita Y, Matsumoto H, Toyama Y, Okada Y: Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann Rheum Dis 2009;68:1051–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]