Abstract
Atherosclerosis, a major form of cardiovascular disease, has now been recognized as a chronic inflammatory disease. Nonpharmacological means of treating chronic diseases have gained attention recently. We previously reported that sesame oil has anti-atherosclerotic properties. In this study, we have determined the mechanisms by which sesame oil might modulate atherosclerosis by identifying genes and inflammatory markers. Low-density lipoprotein receptor knockout (LDLR−/−) female mice were fed with either an atherogenic diet or an atherogenic diet reformulated with sesame oil (sesame oil diet). Plasma lipids and atherosclerotic lesions were quantified after 3 months of feeding. Plasma samples were used for cytokine analysis. RNA was extracted from the liver tissue and used for global gene arrays. The sesame oil diet significantly reduced atherosclerotic lesions, plasma cholesterol, triglyceride, and LDL cholesterol levels in LDLR−/− mice. Plasma inflammatory cytokines, such as MCP-1, RANTES, IL-1α, IL-6, and CXCL-16, were significantly reduced, demonstrating an anti-inflammatory property of sesame oil. Gene array analysis showed that sesame oil induced many genes, including ABCA1, ABCA2, APOE, LCAT, and CYP7A1, which are involved in cholesterol metabolism and reverse cholesterol transport. In conclusion, our studies suggest that a sesame oil-enriched diet could be an effective nonpharmacological treatment for atherosclerosis by controlling inflammation and regulating lipid metabolism.
Key Words: : cholesterol transport, inflammation, lipid metabolism, sesamin, sesamol
Introduction
Atherosclerosis is a chronic inflammatory disease characterized by the accumulation of lipids and infiltration of leukocytes into the artery wall.1,2 Oxidative stress and inflammation play a pivotal role in every stage of the disease3–6 and both conditions represent targets for intervention. Current therapies for the prevention and treatment of atherosclerosis are unable to target the inflammatory mechanisms involved in the initiation and progression of the disease.7,8
A cardioprotective diet and exercise are important in the prevention and treatment of atherosclerosis. The influence of cooking oil on plasma lipids and atherosclerosis has been one of the most studied topics in cardiovascular nutrition.9–11 Both poly- and monounsaturated fatty acids (PUFAs and MUFAs) have been reported to lower cholesterol,9–11 but the mechanisms by which dietary fat desaturation affects atherosclerosis are unknown. Sesame oil (Sesamum indicum) is rich in both MUFAs and PUFAs. Many studies have identified that sesame oil contains lignans, such as sesamin, sesamolin, and several antioxidant compounds such as sesaminol.12–14 It has been observed that sesamol is able to reduce lipopolysaccharide-induced oxidative stress15 and upregulate phosphatidylinositol 3-kinase/Akt/endothelial nitric oxide synthase pathways.16 Sesame oil plays an important role in controlling hypertension and stress.17,18 Recently, we revealed the close relationship between inflammation and oxidative stress.19 Thus, it would be extremely important and beneficial to determine the effects of sesame oil feeding on inflammation and atherosclerosis in vivo.
Inflammation has been postulated to play a major role in atherosclerosis development.20 Over the past two decades, research has been focused on the role of modified lipoproteins, primarily oxidized LDL (Ox-LDL). Evidence supports the concept that Ox-LDL may be a key antigen in atherosclerosis.21 Sesamin feeding is associated with a reduction of serum lipid levels in rodents, concomitant with an increased fatty acid oxidation22–25 perhaps attributable to activation of peroxisome proliferator-activating receptor (PPAR) pathways. Activation of PPARα has been demonstrated to modulate many aspects of lipoprotein metabolism and inflammation in vitro as well as in animal and human studies.26–29 PPAR-mediated effects include a decrease in lipid accumulation in macrophages by downregulating the SR-A1 receptor and promoting cholesterol efflux by increasing hepatic LDL and SR-B1 receptors. Similarly, activation of the liver X receptor (LXR) pathway would enhance ABCA1, SR-B1, and SREBP, thus enhancing reverse cholesterol transport (RCT) and catabolism.30–31 We previously reported that sesame oil has anti-atherosclerotic properties.32 In this study, we evaluated the effect of sesame oil on inflammation, RCT, and lipid metabolism beyond its ability to reduce lesion formation in low-density lipoprotein receptor (LDLR) knockout mice.
Methods
A detailed description of methods is available in Supplementary Data (Supplementary Data are available online at www.liebertpub.com/jmf).
Animals
Sixty-six 4-week-old female LDLR−/− mice weighing 18–20 g were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and used for the study.
Diet
An atherogenic diet (TD.04287) and atherogenic diet reformulated with sesame oil (TD.04288) were purchased from Harlan Teklad (Madison, WI, USA). The composition of the diet was identical to that described previously.32 The fatty acid composition of sesame oils was analyzed as methyl esters by Varian CP-3380 Gas Chromatography (Varian, Inc., Palo Alto, CA, USA).33,34 The fatty acid and lignan35 composition of the sesame oil used is represented in the Supplementary Table S1.
Collection of plasma and organs
After 15 weeks, mice were fasted overnight and blood, plasma, and tissue samples were collected as described previously32 and stored at −80°C.
Isolation and quantification of aortic lesions
Isolation of the aorta and quantification of aortic lesions were performed as described previously.32,36
Plasma lipid analysis
Plasma lipid profiles were determined by using a Cholestech L*D*X analyzer (Cholestech Corp, Hayaward, CA, USA).
cDNA synthesis and real time-polymerase chain reaction
Total RNA from the liver and aortic tissue was isolated by using Trizol™ reagent. One micro gram of RNA was then reverse transcribed into cDNA using the SuperscriptTM III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). cDNA (50 ng) samples were used to perform the quantitative real-time polymerase chain reaction (PCR) by the iQTM5 iCycler Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) with SYBR Green (Invitrogen). mRNA expression of ABCA1, ABCG1, SRB1, Cyp7a1, NPC1L1, MCP-1, IL-1α, IL-1β, IL-6, IL-4, IL-10, IL-13, Catalase, MnSOD, SRA1, CD36, LXR, pregnane X receptor (PXR), farnesoid X receptor (FXR), MPO, and CD68 with mouse-specific primers was analyzed. The primers for the genes are provided in the Supplementary Table S2.
Global cytokine and gene array
Plasma samples were analyzed by the global cytokine array (Ray Biotech, Inc., Norcross, GA, USA) using the RayBio®Mouse G Series Array three and four glass chip. Liver tissue RNA samples were used for the mouse gene array PAMM-080 (Qiagen, Valencia, CA, USA) analysis.
In vitro oxidation of LDL in the presence of sesamol and sesamin
Lipoproteins from human plasma were isolated as described previously.37 Oxidation of LDL and HDL was performed with 5 μM copper or 0.2 U MPO both in the presence and absence of different concentrations of sesamol and sesamin. The degree of LDL oxidation was assessed by conjugated diene measurement, while the peroxide content was determined by using the leucomethylene blue assay38 and thiobarbituric acid reactive substances.
Statistical analyses
Values are presented as mean±standard deviation (SD), and statistical analyses were performed using Student's t-test and the Wilcoxon matched paired test using Prism Pad software, with P<.05 considered significant.
Results
Body weight analysis in mice
The atherogenic diet contained 17% saturated fat provided by milk, and in the reformulated diet, milk fat was replaced with an equal amount (17%) of sesame oil. A slight, but insignificant, decrease in body weight and liver weight (approximately 15% and 13%, respectively) was observed in sesame oil diet-fed animals (Supplementary Fig. S1A, B).
Reduced plasma lipid levels in sesame oil diet-fed animals
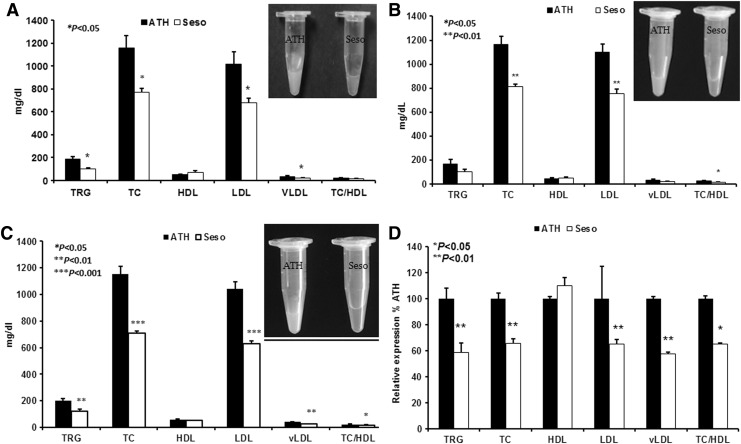
Visual observation of plasma following centrifugation revealed a clear plasma in sesame oil diet-fed animals compared with control animals. This probably reflected a decrease in plasma lipids of sesame oil diet-fed animals. Plasma lipid profile analysis revealed a significant decrease in TRG, TC, LDL cholesterol, and very low-density lipoprotein (VLDL) cholesterol in sesame oil diet-fed animals. A slight increase in HDL cholesterol levels was observed. Figure 1A–C represents the plasma lipid profile of control and sesame oil diet-fed animals from three independent studies. The total cholesterol was significantly reduced in the sesame oil diet-fed animals (P<.05). In animals fed with an atherogenic diet, the cholesterol level was 1158.13±4.37 mg/dL, whereas in the animals fed with a sesame oil diet, the cholesterol level was 763.73±30.7 mg/dL. Triglyceride levels were 184.85±8.35 and 109.3±7.29 mg/dL in control and sesame oil diets, respectively. The results of our study once again confirm that not only plasma cholesterol but also TRG is lowered by sesame oil feeding. There was also a significant reduction in LDL and VLDL cholesterol. On the other hand, HDL cholesterol was elevated in sesame oil-fed animals, suggesting that the effects cannot be attributed to fatty acid composition alone, as none of the PUFA/MUFA-rich dietary oils are known to have such a pronounced effect on HDL. As shown in Figure 1D, a 30–40% reduction in plasma lipid levels (TC, TRG, LDL, and VLDL) was observed in sesame oil diet-fed animals compared with atherogenic diet-fed animals.
FIG. 1.
Reduced plasma lipid levels in sesame oil diet-fed animals. Plasma lipid levels (A-Study I; B-Study II, C-Study III, and D-Aggregated relative fold) in mice fed an atherogenic diet (n=7+6+10) and sesame oil diet (n=8+7+10). Values are represented as mean±SD. *P<.05.
Inhibition of atherosclerotic lesions in sesame oil diet-fed animals
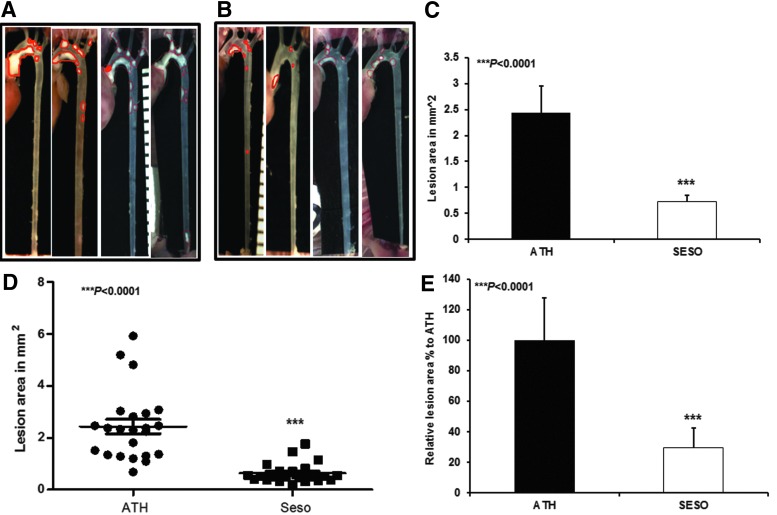
The atherosclerotic lesion formation was analyzed in experimental animals and the extent of lesion formation was quantified by measuring the lesion surface area. We observed a significant reduction in lesion formation in sesame oil diet-fed animals compared with control animals. Figure 2A shows that control animals had prominent lesions in the aortic arch and in some animals it extended up to the abdominal aorta, whereas the sesame oil diet-fed animals had lesser and smaller lesions (Fig. 2B). Quantitation of the lesions also showed that the lesion areas of sesame oil-fed animals were significantly reduced (Fig. 2C). In most of the sesame oil-fed animals (with the exception of three to four animals), the artery was clear compared with control animals. A scatter plot represents the aggregate of the three studies (Fig. 2D). The sesame oil diet reduced lesions by 70% compared with atherogenic diet-fed animals (Fig. 2E; mean±SD (mm2), ***P<.0001).
FIG. 2.
Reduced atherosclerotic lesions in sesame oil diet-fed animals. Representative images of atherosclerotic lesions in (A) high-fat diet-fed animals, (B) sesame oil diet-fed LDLR−/− mice, (C) average lesion area of three independent studies, (D) lesion area of all the animals, and (E) relative lesion area of animals. The values are expressed as mean±SD (mm2). ***P<.0001.
Gene array of mouse livers
The gene array results of our studies for lipoprotein signaling and cholesterol metabolism showed that sesame oil-treated animals exhibited several changes not only in antiatherosclerotic genes but also in genes involved in cholesterol transport and metabolism. As shown in Table 1 (A and B), there was an increase in genes related to RCT and lipid metabolism that included ABCA1, ABCA2, APOE, LCAT, CYP7a1, SR-B1, and LXR. The activation of LXR would have the additional benefit of suppressing tissue factor expression, which plays an important role in thrombus formation after plaque rupture. In contrast, many of the proatherogenic genes were suppressed in sesame oil-fed animals.
Table 1.
Changes in the Levels of Gene Expression in Mouse Livers Between High-Fat Diet and Sesame Oil Diet Groups Were Analyzed by the Global Gene Array
| Gene name | Symbol | ATH/High-fat diet | Seso diet | P value |
|---|---|---|---|---|
| (A) Genes upregulated in cholesterol transport of sesame oil diet-fed animals | ||||
| HDL-associated proteins | ||||
| Apolipoprotein A-I | Apoa1 | 1 | 1.285 | .242 |
| Apolipoprotein D | Apod | 1 | 2.003 | .292 |
| Apolipoprotein F | Apof | 1 | 1.598 | .068 |
| Apolipoprotein L8 | Apol8 | 1 | 1.697 | .110 |
| Cholesterol transporters | ||||
| ATP-binding cassette subfamily A member 1 | Abca1 | 1 | 1.244 | .282 |
| Apolipoprotein A-I | Apoa1 | 1 | 1.285 | .242 |
| Apolipoprotein E | Apoe | 1 | 1.270 | .113 |
| START domain containing 3 | Stard3 | 1 | 1.295 | .126 |
| Cholesterol efflux | ||||
| ATP-binding cassette subfamily A member 1 | Abca1 | 1 | 1.244 | .282 |
| Apolipoprotein E | Apoe | 1 | 1.270 | .113 |
| Reverse cholesterol transport | ||||
| ATP-binding cassette subfamily A member 1 | Abca1 | 1 | 1.244 | .282 |
| Apolipoprotein A-I | Apoa1 | 1 | 1.285 | .242 |
| Apolipoprotein A-II | Apoa2 | 1 | 1.317 | *.041 |
| Apolipoprotein E | Apoe | 1 | 1.270 | .113 |
| Lecithin–cholesterol acyltransferase | Lcat | 1 | 1.225 | .125 |
| Other genes involved in cholesterol transport | ||||
| NPC1-like1 | Npc1l1 | 1 | 1.280 | .567 |
| Oxysterol-binding protein-like 5 | Osbp15 | 1 | 1.605 | .441 |
| (B) Genes upregulated in cholesterol catabolism of sesame oil diet-fed animals | ||||
| Cholesterol catabolism | ||||
| Aldo–Keto reductase family1, member D1 | Akr1d1 | 1 | 1.270 | .318 |
| Apolipoprotein E | Apoe | 1 | 1.270 | .113 |
| Cytochrome P450, family 46, subfamily a, polypeptide 1 | Cyp46a1 | 1 | 1.058 | .926 |
| Cytochrome P450, family 47, subfamily a, polypeptide 1 | Cyp7a1 | 1 | 2.731 | .444 |
| Scavenger receptor class F, member 1 | Scarf1 | 1 | 1.096 | .569 |
| Sorting nexin 17 | Snx 17 | 1 | 1.810 | .422 |
| Cholesterol homeostasis | ||||
| ATP-binding cassette subfamily A member 1 | Abca1 | 1 | 1.244 | .282 |
| Angioprotein-like 3 | Angptl3 | 1 | 1.666 | .130 |
| Apolipoprotein A-I | Apoa1 | 1 | 1.285 | .242 |
| Apolipoprotein A-II | Apoa2 | 1 | 1.317 | *.041 |
| Apolipoprotein E | Apoe | 1 | 1.270 | .113 |
| Lecithin–cholesterol acyltransferase | Lcat | 1 | 1.225 | .125 |
| Low-density lipoprotein receptor | Ldlr | 1 | 1.423 | .360 |
| Ldlr-associated protein 1 | Ldlrap1 | 1 | 1.051 | .588 |
| Proprotein convertase subtilisin/kexin type 9 | Pcsk9 | 1 | 1.064 | .799 |
| Cholesterol biosynthesis | ||||
| Acetyl-Coenzyme A acyltransferase 2 (mitochondrial3-oxoacyl-Coenzyme A thiolase) | Acaa2 | 1 | 1.476 | .059 |
| Cellular nucleic acid-binding protein | Cnbp | 1 | 1.297 | .230 |
| Cytochrome b5 reductase 3 | Cyb5r3 | 1 | 1.537 | *.037 |
| Cytochrome P450, family 51 | Cyp51 | 1 | 1.218 | .212 |
| 24-dehydrocholesterol reductase | Dhcr24 | 1 | 1.212 | .102 |
| Phenylalkylamine Ca+2 antagonist-binding protein | Ebp | 1 | 1.221 | *.014 |
| Farnesyl diphosphate farnesyl transferase 1 | Fdft1 | 1 | 1.419 | *.041 |
| Farnesyl diphosphate synthetase | Fdps | 1 | 1.204 | .712 |
| 3-hydroxy-3-methylgutaryl-Coenzyme A reductase | Hmgcr | 1 | 1.811 | .241 |
| 3-hydroxy-3-methylgutaryl-Coenzyme A synthase 1 | Hmgcs1 | 1 | 1.031 | .778 |
| 3-hydroxy-3-methylgutaryl-Coenzyme A synthase 2 | Hmgcs2 | 1 | 1.235 | .357 |
| Isopenenyl-diphosphate delta isomerase | ldi1 | 1 | 1.287 | .185 |
| Isopenenyl-diphosphate delta isomerase 2 | ldi2 | 1 | 2.988 | .266 |
| Mevalonate decarboxylase | Mvd | 1 | 1.057 | .983 |
| NPC1-like 1 | Npc1l1 | 1 | 1.280 | .567 |
| NAS(P)-dependent steroid dehydrogenase-like | Nsdhl | 1 | 1.936 | .230 |
| Phospho mevalonate kinase | Pmvk | 1 | 1.357 | *.025 |
| Protein kinase, AMP-activated, alpha 2 catalytic subunit | Prkaa1 | 1 | 1.914 | *.031 |
| Transmembrane 7 superfamily member 2 | Tm7sf2 | 1 | 1.519 | .295 |
| Other genes involved in cholesterol metabolism | ||||
| ATP-binding cassette subfamily A member 2 | Abca2 | 1 | 1.036 | .762 |
| Apolipoprotein B | Apob | 1 | 1.053 | .990 |
| Apolipoprotein c3 | Apoc3 | 1 | 1.568 | *.014 |
| Apolipoprotein f | Apof | 1 | 1.598 | .068 |
| Apolipoprotein L8 | Apol8 | 1 | 1.697 | .110 |
| Cytochrome P450, family 7, subfamily b polypeptide 1 | Cyp7b1 | 1 | 2.731 | .444 |
| Chymotrypsin-like elastase family, member 3B | Cela3b | 1 | 1.774 | .525 |
| High-density lipoprotein (HDL)-binding protein | Hdlbp | 1 | 1.041 | .577 |
| Interleukin 4 | Il4 | 1 | 1.674 | .322 |
| Leptin | Lep | 1 | 1.529 | .315 |
| Lipase, hormone sensitive | Lipe | 1 | 1.008 | .966 |
| Membrane-bound transcription factor peptidase, site1 | Mbtps1 | 1 | 1.702 | .354 |
| Nuclear receptor subfamily o, group B, member 2 | Nrob2 | 1 | 1.157 | .597 |
| Nuclear receptor subfamily1, group H, member 4 | Nr1h4 | 1 | 1.728 | *.041 |
| Oxysterol-binding protein-like 1A | Osbpl1a | 1 | 1.181 | .300 |
| Oxysterol-binding protein-like 5 | Osbpl5 | 1 | 1.605 | .441 |
| SRBEF Chaperone | Scap | 1 | 1.293 | *.024 |
| Sterol O-acyl transferase2 | Soat2 | 1 | 1.105 | .418 |
| Sterol regulatory element-binding transcription factor 1 | Srebf1 | 1 | 1.020 | .962 |
| START domain containing 3 | Stard3 | 1 | 1.295 | .126 |
| Very low-density lipoprotein receptor | Vldlr | 1 | 1.180 | .594 |
The results are expressed as fold change between the groups. *P<0.05.
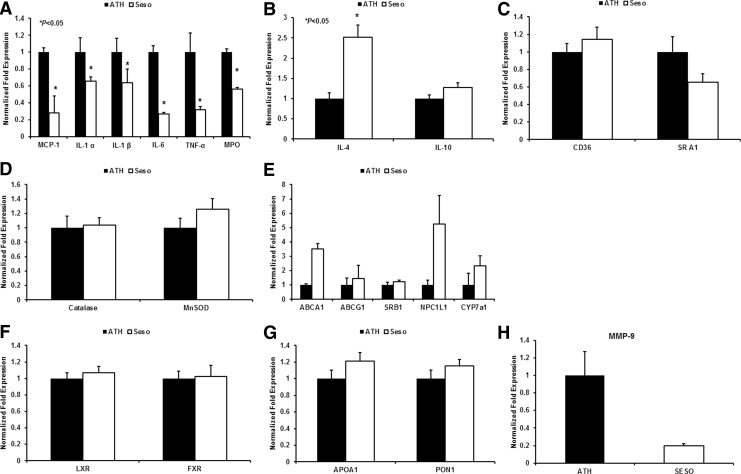
In addition to the global gene array, many of the proinflammatory genes (Fig. 3A; MCP-1, IL-1α, IL-1β, IL-6, TNF-α, and MPO), anti-inflammatory genes (Fig. 3B; IL-4 and IL-10), lipid loading genes (Fig. 3C; CD36 and SRA1), antioxidant genes (Fig. 3D; Catalase and MnSOD), genes involved in RCT and lipid metabolism (Fig. 3E; ABCA1, ABCG1, SRB1, NPC1L1, Cyp7A1), nuclear receptor transcription factors (Fig. 3F; LXR and FXR), genes associated with HDL (Fig. 3G; ApoA1 and PON1), and the matrix metalloproteinase gene (Fig. 3H; MMP9) were analyzed independently by real-time PCR analysis. The pattern of regulation of pro- and anti-inflammatory genes was consistent with the cytokine array analysis. Similarly, expression of genes involved in RCT and lipid metabolism was also consistent with gene array studies. No difference between PXR and PPARα expression was identified (data not shown).
FIG. 3.
Gene analysis of mice liver. The mRNA level of several genes was analyzed in the liver tissue and aorta of LDLr−/− mice after 3 months of feeding with a high-fat diet and the sesame oil diet. Bar diagrams that represent (A) proinflammatory genes, (B) anti-inflammatory genes, (C) scavenger receptors, (D) antioxidant genes, (E) genes involved in RCT and lipid metabolism, (F) nuclear transcription factors, (G) genes associated with HDL, and (H) matrix metalloproteinase were analyzed from the liver tissue. Values are represented as mean±SD. *P<.05.
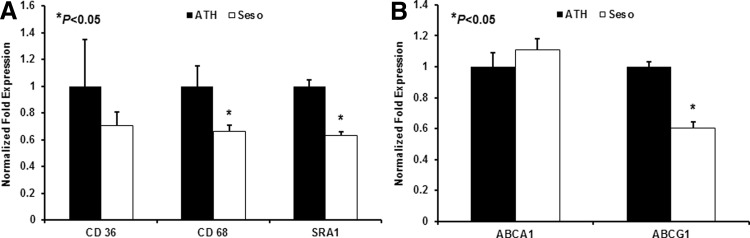
Gene expression in mouse aorta
Aortic gene expressions were also analyzed using real-time PCR. The results showed that sesame oil diet-fed animals had increased mRNA levels of the RCT gene ABCA1, but reduced levels of ABCG1 (Fig. 4A) were observed. Expression of monocyte/macrophage markers and scavenger receptors CD68, SRA1, and CD36 was decreased (Fig. 4B).
FIG. 4.
Gene analysis from mice aorta. The mRNA level of several genes was analyzed in aorta of LDLr−/− mice after 3 months of feeding with a high-fat diet and the sesame oil diet. Bar diagrams that represent (A) scavenger receptors and (B) RCT genes were analyzed from aorta. Values are represented as mean±SD.*P<.05.
Cytokine array
The sesame oil diet caused minimal changes in the profile of 96 inflammation-related proteins as measured by the cytokine array. An analysis of over 96 serum cytokines showed an up- or downregulation of cytokines related to (1) angiogenesis, (2) apoptosis, (3) matrix remodeling, (4) inflammation and immune response, (5) growth promotion, and (6) scavenger function in sesame oil diet-fed animals (Supplementary Fig. S2). Upregulation of proteins involved in the removal of dead cells and growth promotion was observed in sesame oil diet-fed animals. In addition, many of the proinflammatory cytokines were downregulated. Significant changes in the levels of inflammatory mediators, such as MCP-1, IL-1α, IL-6, and RANTES, were noted in sesame oil-fed animals compared with atherogenic diet-fed animals (Table 2), whereas the anti-inflammatory gene such as IL-13 was upregulated.
Table 2.
Changes in the Levels of Inflammatory Mediators in Mouse Plasma Between High-Fat Diet and Sesame Oil Diet Groups
| Relative protein levels compared to ATH, fold difference | ||||
|---|---|---|---|---|
| Protein name | Symbol | ATH/HF diet | Sesame oil diet | P values |
| Downregulated | ||||
| Tyrosine protein kinase | Axl | 1 | 0.548 | *.018 |
| B-lymphocyte chemoattractant | BLC | 1 | 0.759 | *.036 |
| Eotaxin | Eotaxin | 1 | 0.871 | .69 |
| Eotaxin2 | Eotaxin-2 | 1 | 0.818 | .167 |
| TNF superfamily, member 6 | FAS ligand | 1 | 0.975 | .909 |
| Fractalkine | Fractalkine | 1 | 0.886 | .19 |
| Granulocyte macrophage colony-stimulating factor | GM-CSF | 1 | 0.823 | .618 |
| Interferon gamma | IFN-γ | 1 | 0.728 | .348 |
| Insulin growth factor I | IGF-I | 1 | 0.856 | .11 |
| Insulin growth factor II | IGF-II | 1 | 0.965 | .851 |
| Interleukin 1 alpha | IL1-α | 1 | 0.664 | .055 |
| Interleukin 2 | IL2 | 1 | 0.476 | *.034 |
| Interleukin 3 | IL3 | 1 | 0.746 | .364 |
| Interleukin 3 receptor b | IL3 Rb | 1 | 0.918 | .541 |
| Interleukin 6 | IL6 | 1 | 0.894 | .749 |
| Interleukin 7 | IL-7 | 1 | 0.862 | .435 |
| Interleukin 15 | IL-15 | 1 | 0.984 | .904 |
| Interleukin 17B receptor | IL-17B R | 1 | 0.934 | .602 |
| Keratinocyte-derived chemokine | KC | 1 | 0.693 | .323 |
| Monocyte chemotactic protein 1 | MCP-1 | 1 | 0.780 | .21 |
| Monokine induced by gamma interferon | MIG | 1 | 0.951 | .714 |
| Macrophage inflammatory protein 1 alpha | MIP-1-α | 1 | 0.930 | .607 |
| Macrophage inflammatory protein 1 gamma | MIP-1-γ | 1 | 0.955 | .422 |
| Macrophage inflammatory protein 2 | MIP-2 | 1 | 0.706 | .505 |
| Matrix metalloproteinase 2 | MMP-2 | 1 | 0.919 | .595 |
| Matrix metalloproteinase 3 | MMP-3 | 1 | 0.972 | .659 |
| Osteroprotegerin | Osteoprotegerin | 1 | 0.859 | .015 |
| Regulation upon activation normal T cell expressed and presumably secreted | RANTES | 1 | 0.757 | .23 |
| Stem cell factor | SCF | 1 | 0.824 | .435 |
| Stromal cell-derived factor 1 alpha | SDF-1 α | 1 | 0.804 | .459 |
| Thymus and activation-regulated chemokine | TARC | 1 | 0.680 | .228 |
| Thymus-expressed chemokine | TECK | 1 | 0.869 | .416 |
| Tissue inhibitor of metalloproteinase-1 | TIMP-1 | 1 | 0.791 | .154 |
| Tissue inhibitor of metalloproteinase-2 | TIMP-2 | 1 | 0.998 | .972 |
| Tumor necrotic factor alpha | TNF-α | 1 | 0.909 | .774 |
| Soluble tumor necrotic factor receptor 1 | sTNF RI | 1 | 0.698 | .238 |
| Soluble tumor necrotic factor receptor 2 | sTNF RII | 1 | 0.700 | .303 |
| Thrombopoetin | TPO | 1 | 0.881 | *.009 |
| Vascular cell adhesion molecule-1 | VCAM-1 | 1 | 0.953 | .84 |
| Vascular endothelial growth factor | VEGF | 1 | 0.957 | .646 |
| Vascular endothelial growth factor D | VEGF D | 1 | 0.732 | .078 |
| Upregulated | ||||
| Granulocyte colony-stimulating factor | G-CSF | 1 | 1.011 | .933 |
| Macrophage colony-stimulating factor | M-CSF | 1 | 1.049 | .078 |
| Interleukin-1 beta | IL1-beta | 1 | 1.461 | .679 |
| Interleukin 13 | IL13 | 1 | 1.195 | .679 |
| Leptin | Leptin | 1 | 30.348 | .419 |
| Macrophage inflammatory protein 3 beta | MIP-3β | 1 | 1.163 | .707 |
| Macrophage inflammatory protein 3 alpha | MIP-3α | 1 | 1.236 | .203 |
Five samples from each group were analyzed by the Ray Bio cytokine array analysis. The protein levels are expressed as fold change between the groups. *P<0.05.
Antioxidant properties of sesamol and sesamin
Sesame components are suggested to have antioxidant properties, and numerous in vitro systems have documented the ability of sesamol to inhibit oxidative processes. It has been identified that many antioxidants have the capacity to inhibit atherosclerosis, although to our knowledge, there is no evidence to suggest that antioxidants alone can modulate lipid levels. In vitro antioxidant effects of sesamol and sesamin were measured by their ability to inhibit the oxidation of lipoproteins (LDL and HDL) both enzymatically and nonenzymatically. As shown in Supplementary Figures S3 and S4, an increase in lag time of oxidation was observed with increasing concentrations/volume of sesamol and sesamin. Reduced peroxide levels and thiobarbituric reactive substances were observed in the presence of sesamol/sesamin compared with Ox-LDL, as shown in Supplementary Figures S5 and S6. The results also suggest that sesamol and sesamin could delay the oxidation of lipoproteins, but may not change the propagation or total oxidation. However, the ability of sesamol, sesamin, or other nonsaponifiables associated with sesame oil to inhibit lipoprotein oxidation in vivo and hence be antiatherogenic requires further investigation.
Discussion
In the present study, we observed that a sesame oil-containing diet effectively reduced atherosclerotic lesion formation in LDLR−/− female mice as seen in our previous studies with male mice. The plasma levels of TC, TRG, VLDLc, and LDLc were decreased in these animals compared with atherosclerotic diet-fed animals. The plasma HDL level was increased in sesame diet-fed animals. It has been reported that sesame oil can lower lipid levels in serum as well as in the liver of rodents.39 Multiple components of sesame oil, such as MUFA, PUFA, and PPAR ligands, could be responsible for lowering plasma lipids.9,40 Our results corroborate these previous studies. However, the mechanism involved in the effects observed with sesame oil and atherosclerosis is unknown.
The present study has multiple findings. (1) Dietary supplementation with sesame oil for a 15-week period significantly decreases atherosclerotic plaque burden, (2) reduces levels of inflammatory cytokines and gene expressions, and (3) increases expression of genes involved in cholesterol transport and lipid metabolism.
There are at least three major mechanisms by which sesame oil could inhibit atherosclerosis: (1) lowering plasma cholesterol by accelerated catabolism through the oxidation of cholesterol, mediated by cholesterol 7α-hydroxylase or CYP7A1, (2) enhancing RCT mediated by SR-B1 and ABC transporters, such as ABCA1 and ABCG1, and (3) controlling mediators of inflammation. It is likely that all three play a vital role in the effects seen in animals fed with sesame oil.
Several anti-atherogenic genes were upregulated in sesame oil diet-fed animals, including genes in cholesterol transport and metabolism, matrix degrading enzymes, anti-inflammatory chemokines and cytokines, and scavenger receptors (Fig. 3). Thus, the data support our basic contention that sesame oil may have multiple components that could affect the atherogenic process in a number of ways. In peripheral cells, including vascular macrophages, ABCA1 regulates the energy-dependent transport of cholesterol and phospholipids to ApoA-I, the major protein in HDL. Similarly, PPARs are involved in the expression of several inflammatory cytokines and matrix metalloproteinases.
Increased mRNA levels of CD36 in the liver tissue were observed in sesame oil diet-fed animals, whereas no effect was seen with SR-A1. It has been identified that CD36 is activated by PPARs.41 Although the activation of CD36 might be construed as a negative effect, this protein also is involved in fatty acid metabolism,42 and thus, its increase might signify a general increase in fatty acid utilization. Similarly, expression of antioxidant genes such as catalase and Mn-SOD was increased, suggesting that they may be induced because of the generation of oxidized lipids43 or because of PPAR induction.28,44 Reduced mRNA levels of CD36 and SR-A1 were observed in aortic lesions of sesame oil diet-fed animals, suggesting the ability of sesame oil to prevent foam cell formation.
PUFAs are known to increase the conversion of cholesterol to bile acids by way of CYP7A1. Recent studies have shown that the orphan nuclear receptors, FXR and LXR, are negative and positive regulators of CYP7A1 transcription, respectively. Since we observed a trend toward activation of LXR pathways in sesame oil diet-fed animals, it is likely that the conversion of cholesterol to bile acids is enhanced in sesame oil-treated animals.
It has been identified that PUFAs promote RCT through PPARs. Potent ligands for FXR activation are the bile acids, chenodeoxycholic, deoxycholic, and lithocholic acid, while the ligands for LXR activation are oxysterols. We recently reported that oxidized fatty acids mimic bile acids in their properties and could potentially affect the FXR, thus reducing the conversion of cholesterol to bile acids.45 Conversely, the components of sesame oil could activate the LXR and promote RCT. The induction of catalase by sesame oil nonsaponifiables, combined with their direct antioxidant effects, would suppress the negative regulation of the FXR by preventing the formation of oxidized fatty acids (which mimic bile acids), while at the same time promoting the induction of the LXR. Although sesame oil components such as sesamol have not been tested for their ability to induce CYP7A1, such methylene dioxyphenols readily interact with other cytochromes and enhance their synthesis. Thus, a pathway consisting of increased conversion of cholesterol into bile acids would be expected to increase, as well as an increase in RCT mediated by the LXR. The bile acid receptor FXR plays a major role in lipid metabolism, perhaps acting through FGF19 and FGFR4.
MMP9 plays a major role in SMC migration and proliferation during plaque formation. MMP overexpression has been implicated in pathological processes, including atherosclerosis, tumor invasion, and rheumatoid arthritis. Unstable atherosclerotic plaques show a reduced extracellular matrix due to increased MMP secretion mainly from local inflammatory cells. Excessive production of collagenases (MMP-1) and stromelysins (MMP-3) is probably key in plaque rupture. The MMP-1, -3, and -9 promoters contain activator protein-1 (AP-1) sites and an NF-kB-binding site. Sesame oil feeding reduced the expression of MMPs.
The formation of oxidized LDL, adherence of leukocytes (VCAM-1), recruitment of monocytes (MCP-1), smooth muscle cell proliferation, disruption of the plaque (MMPs), and several other steps are influenced by oxidative stress. Hence, each step could be targeted by antioxidants. For example, TNF-α-induced expression of VCAM-1 on endothelial cells is inhibited by the presence of antioxidants. Many inflammatory cytokine genes (IL-1α, IL-6, CSF, and others) are influenced and induced by oxidative stress. Overall, many antioxidants have been shown to affect experimental atherosclerosis. Both sesamol and sesamin have phenolic hydroxyl groups and such compounds usually have potent antioxidant activities. Supplementary Figures S3 and S4 show the in vitro antioxidant effect of sesamol and sesamin as measured by their ability to inhibit the oxidation of LDL both enzymatically and nonenzymatically. As we go from the left to the right of the figure (with increasing concentration of sesamol or sesamin) there is a shift of the curve, suggesting an increase in lag time of oxidation. The top of the curve remains the same showing that propagation, and not the total oxidation, is affected. Thus, sesamol and sesamin could delay the oxidation of lipoproteins. However, these in vitro studies do not reveal whether sesamol or sesamin or other nonsaponifiables associated with sesame oil could be carried in LDL in vivo and whether such antioxidant effects play any role in the oil's in vivo anti-atherogenic effects.
In our study, we also observed reduced body and liver weights of sesame oil diet-fed animals, but they are not significantly different. This might be due to the sesame oil component lignans, which have the ability to activate the body to oxidize more fat and decrease the storage of fat. Yet another possibility is decreased lipogenesis caused by decreasing lipogenic enzymes in the liver metabolism. Sesame seeds and oil are known to benefit the digestive system, relieve constipation, and cause detoxification, which might pave the way for a healthier digestive system and a healthy colon. In addition, increased leptin levels might direct the central nervous system for lessened food intake due to high-energy stores in the adipose. Leptin is a hormone secreted from fat cells, which circulates in the bloodstream and goes to the brain. Increased levels of leptin suggest a satisfactory level of energy storage. The circulating leptin levels reflect the amount of energy stored in adipose tissue and direct the central nervous system to regulate energy homeostasis (either up or down, depending upon the status of storage depots), neuroendocrine function, and metabolism.46 Additional mechanistic insights are needed to determine the role of sesame oil in leptin synthesis as human and animal studies seem to differ, as noted from literature.
Our observations shed light on some of the mechanisms by which a sesame oil-rich diet could influence both the progression and regression of atherosclerotic lesions in a positive manner. Understanding the molecular mechanisms by which sesamol and other similar nonsaponifiables in sesame oil could be anti-atherogenic would open up enormous new areas of research in the dietary prevention of cardiovascular disease.
Supplementary Material
Acknowledgment
This study was supported by National Institutes of Health Grant 5R01AT004106-05.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW: A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 1995;15:1512–1531 [DOI] [PubMed] [Google Scholar]
- 2.Herbert C. Stary: Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol 2000;20;1177–1178 [DOI] [PubMed] [Google Scholar]
- 3.Parthasarathy S, Steinberg D, Witztum JL: The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Annu Rev Med 1992;43:219–225 [DOI] [PubMed] [Google Scholar]
- 4.Steinberg D. Lewis A: Conner Memorial Lecture: oxidative modification of LDL and atherogenesis. Circulation 1997;95:1062–1071 [DOI] [PubMed] [Google Scholar]
- 5.Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D: Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest 1989;84:1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL: Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989;320:915–924 [DOI] [PubMed] [Google Scholar]
- 7.Rader DJ, Daugherty A: Translating molecular discoveries into new therapies for atherosclerosis. Nature 2008;451:904–913 [DOI] [PubMed] [Google Scholar]
- 8.Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, Ruzyllo W, Bachinsky WB, Lasala GP, Tuzcu EM: Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med 2007;356:1304–1316 [DOI] [PubMed] [Google Scholar]
- 9.Mensink RP, Katan MB: Effect of a diet enriched with monounsaturated or polyunsaturated fatty acids on levels of low-density and high-density lipoprotein cholesterol in healthy women and men. N Engl J Med 1989;321:436–441 [DOI] [PubMed] [Google Scholar]
- 10.Parks JS, Rudel LL: Effect of fish oil on atherosclerosis and lipoprotein metabolism. Atherosclerosis 1990;84:83–94 [DOI] [PubMed] [Google Scholar]
- 11.Sanders TA: Olive oil and the Mediterranean diet. Int J Vitam Nutr Res 2001;71:179–184 [DOI] [PubMed] [Google Scholar]
- 12.Grishina NL, Kuznetsov DI: Fatty acid composition of oils from different varieties of sunflower, peanut and sesame. Vopr Pita 1970;29:81–88 [PubMed] [Google Scholar]
- 13.Sengupta A, Roychoudhury SK: Triglyceride composition of Sesamum indicum seed oil. J Sci Food Agric 1976;27:165–169 [DOI] [PubMed] [Google Scholar]
- 14.Egbekun MK, Ehieze MU: Proximate composition and functional properties of full fat and defatted beniseed (Sesamum indicum L.) flour. Plant Foods Hum Nutr 1997;51:35–41 [DOI] [PubMed] [Google Scholar]
- 15.Hou RC, Chen HL, Tzen JT, Jeng KC: Effect of sesame antioxidants on LPS-induced NO production by BV2 microglial cells. Neuroreport 2003;14:1815–1819 [DOI] [PubMed] [Google Scholar]
- 16.Chen PR, Tsai CE, Chang H, Liu TL, Lee CC: Sesamol induces nitric oxide release from human umbilical vein endothelial cells. Lipids 2005;40:955–961 [DOI] [PubMed] [Google Scholar]
- 17.Sankar D, Sambandam G, Ramakrishna Rao M, Pugalendi KV: Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin Chim Acta 2005;355:97–104 [DOI] [PubMed] [Google Scholar]
- 18.Sankar D, Rao MR, Sambandam G, Pugalendi KV: A pilot study of open label sesame oil in hypertensive diabetics. J Medicinal Food 2006;9:408–412 [DOI] [PubMed] [Google Scholar]
- 19.Aluganti Narasimhulu C, Jiang X, Yang Z, Selvarajan K, Parthasarathy S: Is there a connection between oxidative stress and inflammation? In: Chronic Inflammation Molecular Pathophysiology, Nutritional and Therapeutic Interventions (Roy Sashwati, Bagchi Debasis, Raychaudhuri Siba P., eds.). CRC press, Boca Raton, FL, 2013, pp. 138–152 [Google Scholar]
- 20.Ross R: The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801–809 [DOI] [PubMed] [Google Scholar]
- 21.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK: T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A 1995;92:3893–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kok T, Wolters H, Bloks VW, Havings R, Jansen PL, Staels B, Kulpers F: Induction of hepatic ABC transporter expression is part of the PPARalpha-mediated fasting response in the mouse. Gastroenterology 2003;124:160–171 [DOI] [PubMed] [Google Scholar]
- 23.Vosper H, Patel L: The peroxisome proliferator-activated receptor delta promotes lipid accumulation in human macrophages. J Biol Chem 2001;276:44258–44265 [DOI] [PubMed] [Google Scholar]
- 24.Tsuruoka N, Kidokoro A, Matsumoto I, Abe K, Kiso Y: Modulating effect of sesamin, a functional lignan in sesame seeds, on the transcription levels of lipid- and alcohol metabolizing enzymes in rat liver: a DNA microarray study. Biosci Biotechnol Biochem 2005;69:179–188 [DOI] [PubMed] [Google Scholar]
- 25.Zelcer N, Tontonoz P: Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest 2006;116:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM: PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 1998;93:241–252 [DOI] [PubMed] [Google Scholar]
- 27.Feng J, Han J, Pearce SFA, Silverstein RL, Gotto AM, Jr, Hajjar DP, Nicholson AC: Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-gamma. J Lipid Res 2000;41:688–696 [PubMed] [Google Scholar]
- 28.Sulzle A, Hirche F, Eder K: Thermally oxidized dietary fat upregulates the expression of target genes of PPAR alpha in rat liver. J Nutr 2004;134:1375–1383 [DOI] [PubMed] [Google Scholar]
- 29.Malerod L, Sporstøl M, Juvet LK, Mousavi A, Gjøen T, Berg T: Hepatic scavenger receptor class B, type I is stimulated by peroxisome proliferator-activated receptor gamma and hepatocyte nuclear factor 4alpha. Biochem Biophys Res Commun 2003;305:557–565 [DOI] [PubMed] [Google Scholar]
- 30.Naik SU, Wang X, Da Silva JS, et al. : Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation 2006;113:90–97 [DOI] [PubMed] [Google Scholar]
- 31.Tangirala RK, Bischoff ED, Joseph SB: Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci U S A 2002;99:11896–11901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhaskaran S, Santanam N, Penumetcha M, Parthasarathy S: Inhibition of atherosclerosis in low-density lipoprotein receptor-negative mice by sesame oil. J Med Food 2006;9:487–490 [DOI] [PubMed] [Google Scholar]
- 33.Morrison WR, Smith LM: Preparation of fatty acid methyl esters and dimethylacetals from lipids with borontrifluoride–methanol. J Lipid Res 1964;5:600–608 [PubMed] [Google Scholar]
- 34.Garelnabi M, Litvinov D, Parthasarathy S: Evaluation of a gas chromatography method for azelaic acid determination in selected biological samples. N Am J Med Sci 2010;2:397–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hemalatha S, Ghafoorunissa: Lignans and tocopherols in Indian sesame cultivars. JAOCS 2004;81:467–470 [Google Scholar]
- 36.Abramoff MD, Magelhaes PJ, Ram SJ: Image Processing with ImageJ. Biophotonics Int 2004;11:36–42 [Google Scholar]
- 37.Santanam N, Parthasarathy S: Paradoxical actions of antioxidants in the oxidation of low density lipoprotein by peroxidases. J Clin Invest 1995;95:2594–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auerbach BJ, Kiely JS, Cornicelli JA: A spectrophotometric microtiter-based assay for the detection of hydroperoxy derivatives of linoleic acid. Anal Biochem 1991;201:375–380 [DOI] [PubMed] [Google Scholar]
- 39.Ajayi OB, Braimoh J, Olasunkanmi K: Response of hypercholesterolemic rats to Sesamum indicum Linn seed oil supplemented diet. J Life Sci 2012;6:1214–1219 [Google Scholar]
- 40.Staels B, Fruchart JC: Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes 2005;54:2460–2470 [DOI] [PubMed] [Google Scholar]
- 41.Nicholson AC, Hajjar DP: CD36, oxidized LDL and PPAR gamma: pathological interactions in macrophages and atherosclerosis. Vascul Pharmacol 2004;41:139–146 [DOI] [PubMed] [Google Scholar]
- 42.Bonen A, Campbell SE, Benton CR, et al. : Regulation of fatty acid transport by fatty acid translocase/CD36. Proc Nutr Soc 2004;63:245–249 [DOI] [PubMed] [Google Scholar]
- 43.Meilhac O, Zhou M, Santanam N, Parthasarathy S: Lipid Peroxides induce expression of catalase in cultured vascular cells. J Lipid Res 2000;41:1205–1213 [PubMed] [Google Scholar]
- 44.Ammerschlaeger M, Beigel J, Klein KU, Mueller SO: Characterization of the species-specificity of peroxisome proliferators in rat and human hepatocytes. Toxicol Sci 2004;78:229–240 [DOI] [PubMed] [Google Scholar]
- 45.Penumetcha M, Khan-Merchant N, Parthasarathy S: Enhanced solubilization and intestinal absorption of cholesterol by oxidized linoleic acid. J Lipid Res 2002;43:895–903 [PubMed] [Google Scholar]
- 46.Kelesidis T, Kelesidis L, Chou S, Mantzoros CS: Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med 2010;19:152:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.