Abstract
Schizophrenia as a mental illness is one of the most serious in the world. Patients with schizophrenia have an increased cardiac mortality rate, but the reasons for this remain unclear. In addition to other factors, the role of impaired autonomic regulation during acute psychosis has become more evident in different studies applying heart rate (HR) variability analyses. But, until now, respiration and cardiorespiratory regulation, which are important for homeostatic control, have not been considered. In this study, short-term cardiorespiratory couplings (CRCs) of 23 unmedicated patients with paranoid schizophrenia (SZO), 20 of their healthy first-degree relatives (REL) and 20 healthy subjects (CON) matched according to age and sex of SZO and REL were investigated by applying high-resolution joint symbolic dynamics (HRJSD) analysis. We found a significantly (p<0.0061) altered HR pattern, respiratory pattern and CRCs in SZO and only marginal alterations for the REL group in comparison with the CON group when we applied HRJSD. These results might be an indication of decreased vagal activity within the brainstem, an altered or suppressed interaction of the brainstem and higher regulatory centres, or panic- and anxiety-related changes in the brainstem associated with the acute psychosis of these patients.
Keywords: joint symbolic dynamics, cardiorespiratory coupling, autonomic nervous system, respiration, heart rate variability, schizophrenia
1. Introduction
Schizophrenia is a severe psychiatric disorder with a lifetime prevalence rate of approximately 1% (USA, 2.2 million people; Germany, 800 000) [1,2]. Furthermore, these patients are reported to have an approximately 20% reduced life expectancy and a relative risk of cardiovascular disease that is up to three times higher than that in the general population [1,3]. Cardiac mortality is assumed to account for 40–45% of all deaths from natural causes for these patients [4]. Possible, and still discussed, causes for these increased cardiac mortality rates are cigarette smoking, obesity leading to dyslipidaemia, insulin resistance and diabetes, hypertension and the effect of antipsychotic medication [1,5]. In addition to these factors, the dysregulation of the autonomic nervous system (ANS) during acute psychosis has become evident for patients with schizophrenia in studies carrying out heart rate variability (HRV) analysis. Studies applying HRV analyses found reduced vagal and increased sympathetic modulation, indicating a cardiac autonomic dysregulation in patients with schizophrenia and partly in their healthy first-degree relatives [6–13]. The pattern of autonomic dysfunction seen in patients and their relatives might indicate underlying disease-inherent genetic vulnerability, especially as it was shown by Busjahn et al. [14] that a genetic dependency of autonomic parameters (HRV) exists. However, any direct association between an altered HRV and the underlying cause of cardiac autonomic imbalance and higher cardiac risk is still elusive and is not yet sufficiently proven and confirmed.
There are only a few studies that have investigated respiration and the cardiorespiratory system in patients with schizophrenia and their relatives [15–19]. These studies found a significantly altered respiratory regulation (variability and dynamics) and a reduced cardiorespiratory coupling (CRC) for patients with schizophrenia but not for their healthy first-degree relatives. Respiration is known to be important in maintaining physiological homeostasis because of the sophisticated interplay between the brainstem and higher centres. The modulation of ventilation by the central nervous system (CNS) is highly complex with primary centres being located in multiple nuclei in the brainstem, medulla and pons. For such a vital physiological function, multiple and redundant brain structures exist that control respiration, whereby the sensorimotor cortex, orbital frontal cortex, limbic lobe, amygdala and hypothalamus are intimately involved in ventilation [20]. Thus, breathing movements are facilitated by a pontine–medullary respiratory network generating rhythmic patterns of inhalation and exhalation [21]. This network originates within the interconnected bilateral columns of the medullary neurons as well as in the ventral respiratory columns and is controlled by inputs from other medullary structures, including the retrotrapezoid nucleus, raphe nuclei and other rostral pontine circuits [15]. Given that respiration is primarily regulated for metabolic and homeostatic purposes in the brainstem but also constantly responds to changes in emotions, such as sadness, anxiety or fear [22], it seems that psychotic states of schizophrenic patients will have a major impact on the cardiorespiratory system. The interactions within the cardiorespiratory system are mainly expressed as respiratory sinus arrhythmia (RSA). RSA describes the rhythmic fluctuation of cardiac cycle intervals in relation to respiration and is expressed as an alternation between inspiratory heart rate (HR) acceleration and expiratory HR deceleration under normal physiological conditions [23,24].
There are only a few studies investigating short-term nonlinear CRCs in patients with schizophrenia and their relatives [15,19]. However, coupling analysis of HR and respiratory frequency (RESP) time series might provide additional relevant clinical information about the complex system of autonomic regulation in patients with schizophrenia and their relatives. For the characterization of linear and nonlinear CRCs, several concepts are available based on Granger causality; nonlinear prediction; entropies; symbolization and phase synchronization that are able to detect direct and indirect couplings between time series [24]. For the characterization of the beat-to-beat changes between HR and RESP time series, the new high-resolution joint symbolic dynamics (HRJSD) analysis approach was applied [25]. HRJSD is based on a redundancy reduction strategy and is characterized by three symbols, a threshold (individual dynamic variability, physiological) for time-series transformation and eight coupling pattern families (resulting in 64 different coupling patterns) which quantify patterns of autonomic regulation. HRJSD seems to be a promising tool to draw conclusions about how and in which way respiration and HR interact and which CRC patterns are the dominating ones in schizophrenic patients and their relatives compared with those of healthy subjects.
The aim of this study was to investigate cardiorespiratory regulation and to quantify short-term nonlinear CRCs in patients with schizophrenia and their healthy first-degree relatives in comparison with healthy subjects by applying the HRJSD analysis approach. We hypothesized that HRJSD indices would reveal alterations in CRC patterns in more detail than other coupling approaches. This might improve the understanding of physiological processes of the cardiorespiratory system in these patients and possibly will improve treatment strategies.
2. Material and methods
(a). Patients
In this study, 23 unmedicated patients with paranoid schizophrenia (SZO), 20 of their healthy first-degree relatives (REL) and 20 healthy subjects (CON) matched according to age and sex were enrolled (table 1). The inclusion criterion for patients was that they had not taken any medication for at least eight weeks. In addition, for all participants, the serum drug levels were controlled for legal (e.g. antipsychotics, antidepressants and benzodiazepines) and illegal (e.g. cannabis) drugs. Prior to our investigations, a clinical ECG was recorded and evaluated by a cardiologist. Diagnosis of paranoid schizophrenia was established when patients fulfilled Diagnostic and Statistical Manual of Mental Disorders, 4th edn (DSM-IV), criteria. Psychotic symptoms were quantified by the positive and negative syndrome scale [26]. The healthy control subjects were recruited from hospital staff, medical students and the local community. A careful interview and clinical investigation were performed for the CON group to exclude any potential psychiatric or other disease as well as any contraindicated medication. The Structured Clinical Interview II and a personality inventory (Freiburger Persönlichkeitsinventar) were additionally applied for the REL and CON groups to detect any possible personality traits or disorders which might influence autonomic function and CRC analyses [27].
Table 1.
Clinical and demographic data of participants. Psychotic symptoms for acute schizophrenia were quantified using the scale for the assessment of positive symptoms (SAPS) and negative symptoms (SANS) and positive and negative syndrome scales (PANSS); n.a., not applicable.
| healthy subjects | healthy first-degree | unmedicated schizophrenic | |
|---|---|---|---|
| data | (CON) | relatives (REL) | patients (SZO) |
| number of participants | 23 | 20 | 23 |
| gender (male/female) | 13/10 | 12/8 | 12/11 |
| age (mean±s.d. in years) | 30.3±9.5 | 31.7±10.7 | 30.4±10.3 |
| PANSS, mean (min–max) | n.a. | n.a. | 85.7 (43–124) |
| SANS, mean (min–max) | n.a. | n.a. | 49.6 (14–81) |
| SAPS, mean (min–max) | n.a. | n.a. | 60.9 (6–108) |
(b). Data recordings and pre-processing
For CRC analysis, a high-resolution short-term ECG (1000 Hz sampling frequency) and synchronized calibrated respiratory inductive plethysmography signal [15] (LifeShirt, Vivometrics, Inc., Ventura, CA, USA) were recorded for 30 min. Investigations were performed between 15.00 and 18.00 in a quiet room which was kept comfortably warm (22–24°C) and began after subjects had rested in the supine position for 10 min. Subjects were asked to relax and to breathe normally to avoid hyperventilation. No further instruction for breathing was given. Subjects were asked explicitly not to talk during the recording.
From each raw data record, the following time series were automatically extracted using in-house software (programming environment Delphi 3):
(1) time series of HR consisting of successive beat-to-beat intervals (BBI) and
(2) time series of respiratory frequency (RESP) as the time intervals between consecutive breathing cycles.
After extraction of the data, these time series were visually inspected and if appropriate re-edited. To obtain synchronized time series, both BBI and RESP were resampled using a linear interpolation method (2 Hz). Afterwards these time series were filtered by applying an adaptive variance estimation algorithm to remove and interpolate seldom occurring ventricular premature beats and artefacts (e.g. movement, electrode noise and extraordinary peaks) [28].
(c). High-resolution joint symbolic dynamics
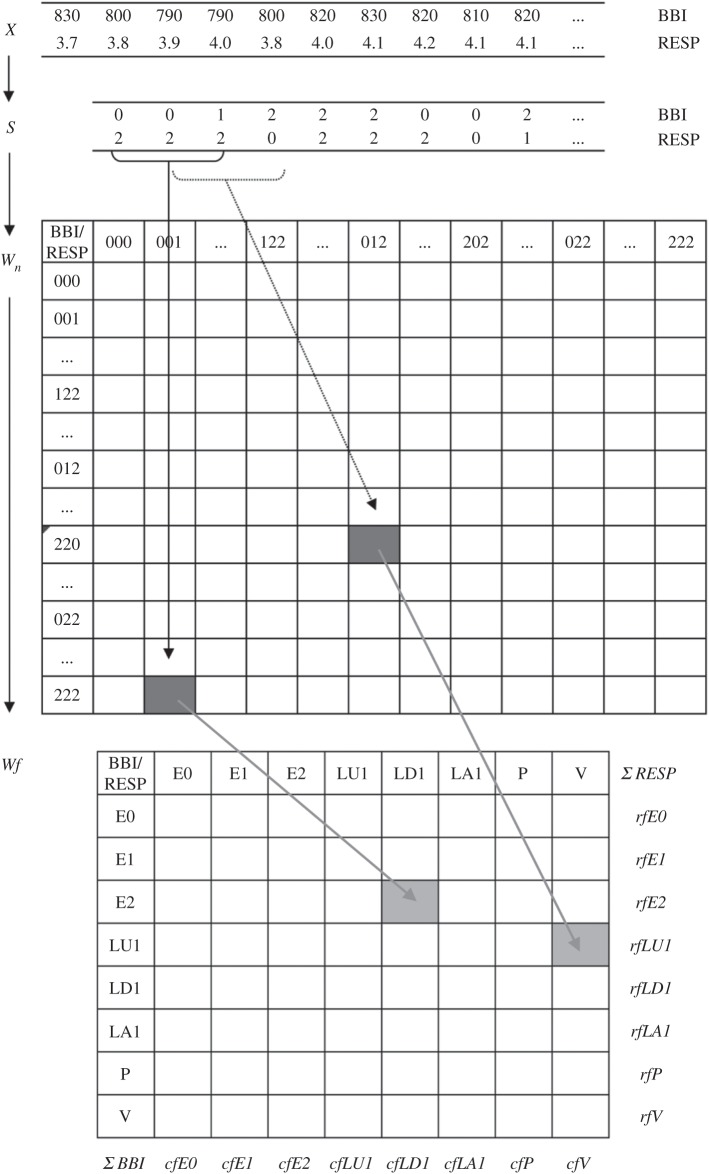
HRJSD was originally introduced [25] to quantify the effects of antipsychotics and their anti-cholinergic effects on nonlinear cardiovascular couplings in acute schizophrenia by means of symbols. The idea of HRJSD is to classify frequent deterministic patterns lasting three beats (symbols). The HRJSD approach enables the classification and characterization of short-term regulatory bivariate coupling patterns that are dominating the interaction generated by the ANS. In this study, we applied the HRJSD as a promising tool to analyse cardiorespiratory system couplings. Recently, HRJSD was enhanced to quantify CRCs [17]. The basic principle of joint symbolic dynamics is to analyse nonlinear couplings between two time series/biosignals based on the analysis of bivariate dynamic processes by means of symbols. Methods that are based on symbolization enable a coarse grain quantitative assessment of short-term dynamics of time series/biosignals. Therefore, the direct analysis of successive signal amplitudes is based on discrete states (symbols) [24]. HRJSD works by transforming both time series (BBI and RESP) into symbol sequences based on their signal amplitudes using a given alphabet A={0,1,2}. Therefore, a bivariate sample vector X of the two time series (BBI and RESP) with xBBI and xRESP is transformed into a bivariate symbol vector S, where n are the nth beat-to-beat values of BBI and RESP, respectively,
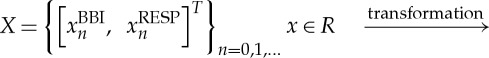 |
2.1 |
and
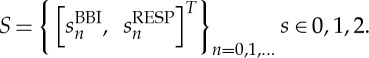 |
2.2 |
For symbol transformation, the bivariate symbol vector S is defined using the following definitions:
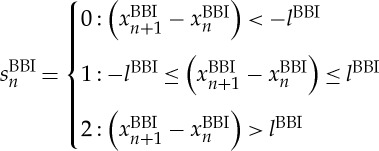 |
2.3 |
and
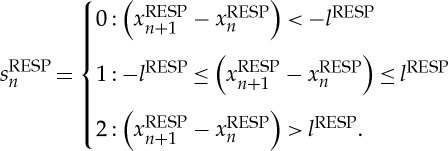 |
2.4 |
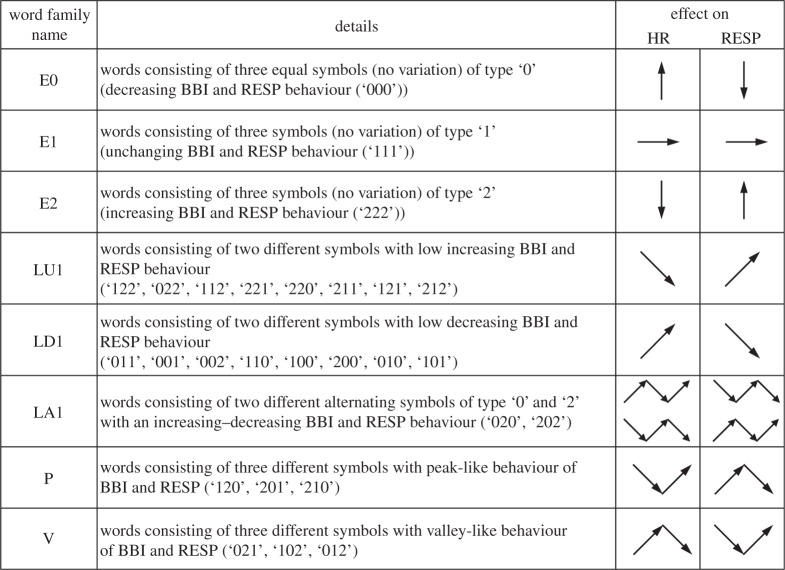
Here, symbol sequences with increasing values are coded as ‘2’, decreasing values are coded as ‘0’ and unchanging (no variability) values are coded as ‘1’. In addition, we applied an adapted threshold l to the individual physiological dynamic variability lBBI and lRESP equal to 25% of the standard deviation of the BBI and RESP time series. After the symbol transformation, the symbol vector S was subdivided into short words (sequences of symbols, bins) wk of length k=3 (figure 1, top). This means that three symbols led to 27 different word types for BBI (wBBI) and RESP (wRESP) time series (word types ranging from: 000,001,…,221,222; figure 1, middle). Then, the word types were sorted into a normalized 27×27 vector matrix Wn ranging from word type (000 000)T to (222 222)T (figure 1, middle). All these single-word types wBBI,RESP (total number of all word type combinations 27×27=729) were afterwards grouped into eight pattern families wf, whereby the sum of probabilities of all single-word family occurrences p(wf) was normalized to 1. These eight pattern families (E0, E1, E2, LU1, LD1, LA1, P and V) represent different aspects of autonomic modulation (strong and weak increase/decrease, no variability, alternations) and were created on a heuristic basis of a minimum of 20 words per bin [29]. Afterwards, these eight pattern families for BBI and RESP were sorted into an 8×8 pattern family density matrix Wf resulting in 64 CRC patterns (figure 1, bottom). For the quantification of these coupling patterns within Wf, the normalized joint probabilities of occurrence were estimated. The pattern definition (figure 2) is as follows:
E0, E1 and E2: Words consisting of three equal symbols (no variation of symbols) of type ‘0’, ‘1’ and ‘2’, respectively.
LU1 and LD1: Words consisting of two different symbols with low increasing behaviour (LU1) and low decreasing behaviour (LD1).
LA1: Words consisting of two different alternating symbols of type ‘0’ and ‘2’ with an increasing–decreasing behaviour.
P and V: Words consisting of three different symbols with peak-like behaviour (P) and with valley-like behaviour (V) [25].
Figure 1.
Basic principle of transformation in HRJSD. Top: transformation of the bivariate sample vector X (BBI,beat-to-beat intervals (ms); RESP,respiratory frequency (s)) into the bivariate symbol vector S (0, decreasing values; 1, equal values; 2, increasing values). Middle: Word distribution density matrix Wn (27× 27). Bottom: Word pattern family distribution density matrix Wf (8×8) with eight pattern families wf, rfBBI row sum of specific word family and cfRESP column sum of specific word family.
Figure 2.
Definition of eight pattern families of HRJSD (HR, heart rate; RESP, respiratory frequency; BBI, beat-to-beat intervals).
As an example, the pattern family ‘E0’ from the BBI time series is coupled with the eight pattern families from RESP as: BBI-E0/RESP-E0, BBI-E0/RESP-E1, BBI-E0/RESP-E2, BBI-E0/RESP-LU1, BBI-E0/RESP-LD1, BBI-E0/RESP-LA1, BBI-E0/RESP-P and BBI-E0/RESP-V. Thus, the pattern family ‘E0’ (BBI-E0/RESP-E0) contains word types that consist only of the ‘0’ symbol. On one hand, this means that BBI decreases over three values and which were therefore coded by ‘0’ three times (representing an increase in the mean HR over three values), whereas, on the other hand, RESP values decrease over three values.
Besides the 64 CRC patterns, eight pattern families for BBI and RESP, we calculated from the matrix Wf the sum of each (n=8) column cfRESP (cfE0, cfE1, cfE2, cfLU1, cfLD1, cfLA1, cfP and cfV) and the sum of each (n=8) row rfBBI (rfE0, rfE1, rfE2, rfLU1, rfLD1, rfLA1, rfP and rfV) (figure 1).
(d). Statistics
The non-parametric Mann–Whitney U-test was performed to evaluate differences in all derived HRJSD indices between SZO and CON, REL and CON, and SZO and REL. Considering the multiple test problem, the necessary local significance level plocal of a single index from an observed m-dimensional parameter space must fulfil Bonferroni's inequality to guarantee (global, pglobal) significance, as follows:
In this study, m=81 HRJSD indices (64 CRC patterns, eight pattern families for BBI and RESP and the Shannon entropy) were calculated and the local significance level was set to plocal=0.00061 considering statistical significance. Descriptive statistics were used to describe basic features of data in terms of mean value (mean) and standard deviation (s.d.).
3. Results
(a). Comparison of patients with schizophrenia with healthy subjects
HRJSD revealed 17 significant CRC patterns between SZO and CON fulfilling the Bonferroni–Holm adjustment (p<0.00061). Four significant respiratory and two significant HR pattern families also showed univariate changes between SZO and CON (table 2, figures 3 and 4). CRCs were quantified by the respiratory pattern families E1, E2, LU1, LD1, LA1, P and V and by the HR patterns families E1, E0, LD1, LU1, P and V, respectively. Thereby, we found that 88% of the CRC patterns, 75% of the respiratory patterns and 50% of the HR patterns were significantly increased in the SZO group in comparison with the CON group.
Table 2.
Significant HRJSD indices from CRC analysis (p<0.00061) between patients with paranoid schizophrenia (SZO), their healthy first-degree relatives (REL) and healthy subjects (CON). Significant discriminating indices are indicated as follows: *CON versus SZO, **CON versus REL, #SZO versus REL. Mean values (mean) and standard deviations (s.d.) are expressed in %.
| CON |
REL |
SZO |
|||
|---|---|---|---|---|---|
| index | p | mean±s.d. | mean±s.d. | mean±s.d. | |
| CRC (RESP-BBI) | RESP-E1/BBI-E1 | * | 2.1±3.0 | 3.5±6.1 | 8.9±8.9 |
| RESP-E1/BBI-V | * | 8.2±2.3 | 7.7±2.9 | 5.1±2.9 | |
| RESP-E2/BBI-LU1 | * | 10.7±4.0 | 8.4±3.5 | 4.8±3.7 | |
| RESP-LU1/BBI-E0 | * | 0.2±0.2 | 0.3±0.3 | 0.5±0.4 | |
| RESP-LU1/BBI-E1 | * | 0.2±0.4 | 0.4±0.6 | 2.1±3.3 | |
| RESP-LU1/BBI-LD1 | * | 1.5±0.8 | 2.5±1.9 | 3.5±1.8 | |
| RESP-LD1/BBI-E1 | * | 0.3±0.5 | 0.6±0.9 | 2.3±3.3 | |
| RESP-LD1/BBI-LD1 | * | 2.3±1.3 | 3.2±1.8 | 3.9±1.7 | |
| RESP-LA1/BBI-LU1 | * | 0.002±0.01 | 0.01±0.05 | 0.1±0.2 | |
| RESP-LA1/BBI-LD1 | * | 0.004±0.02 | 0.02±0.04 | 0.2±0.4 | |
| RESP-LA1/BBI-P | * | 0.0±0.0 | 0.01±0.05 | 0.04±0.1 | |
| RESP-P/BBI-E1 | *, # | 0.002±0.01 | 0.02±0.05 | 0.3±0.4 | |
| RESP-P/BBI-LD1 | * | 0.1±0.1 | 0.2±0.2 | 0.4±0.4 | |
| RESP-P/BBI-V | * | 0.02±0.04 | 0.07±0.1 | 0.1±0.1 | |
| RESP-V/BBI-E1 | * | 0.02±0.05 | 0.04±0.07 | 0.5±1.1 | |
| RESP-V/BBI-LU1 | * | 0.1±0.07 | 0.2±0.2 | 0.6±0.7 | |
| RESP-V/BBI-LD1 | * | 0.1±0.08 | 0.2±0.3 | 0.7±1.1 | |
| RESP | RESP-E2 | * | 15.5±5.6 | 13.9±4.7 | 9.2±4.7 |
| RESP-LA1 | *, # | 0.02±0.05 | 0.07±0.1 | 0.6±0.9 | |
| RESP-P | * | 0.4±0.3 | 0.9±0.7 | 1.5±1.2 | |
| RESP-V | * | 0.4±0.3 | 0.8±0.7 | 2.4±3.1 | |
| BBI | BBI-E1 | * | 3.0±3.9 | 5.1±7.4 | 15.4±17.2 |
| BBI-LU1 | * | 41.6±7.0 | 37.8±6.2 | 31.0±7.5 |
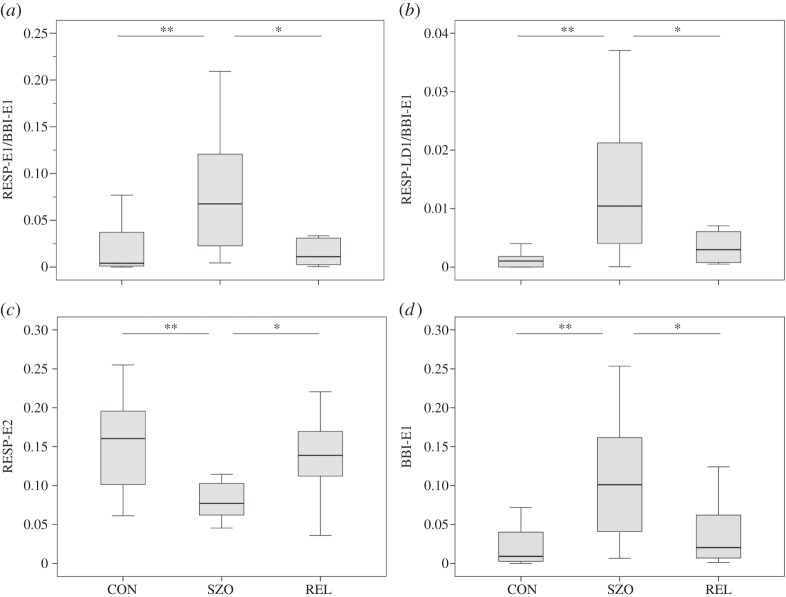
Figure 3.
Box plots of HRJSD indices differentiating healthy control subjects (CON), patients with schizophrenia (SZO) and healthy first-degree relatives (REL). (a) CRC pattern RESP-E1/BBI-E1 indicating reduced HRV in combination with reduced respiratory variability; (b) CRC pattern RESP-LD1/BBI-E1 indicating no variability of HR in combination with weak decreasing respiratory frequency; (c) respiratory pattern family RESP-E2 reflecting strong increasing respiratory frequency; (d) HR pattern family BBI-E1 reflecting no variability of HR. Significant discriminating indices are indicated as follows: *p<0.01 and **p<0.00061. (BBI, beat-to-beat intervals; RESP, respiratory frequency.)
Figure 4.
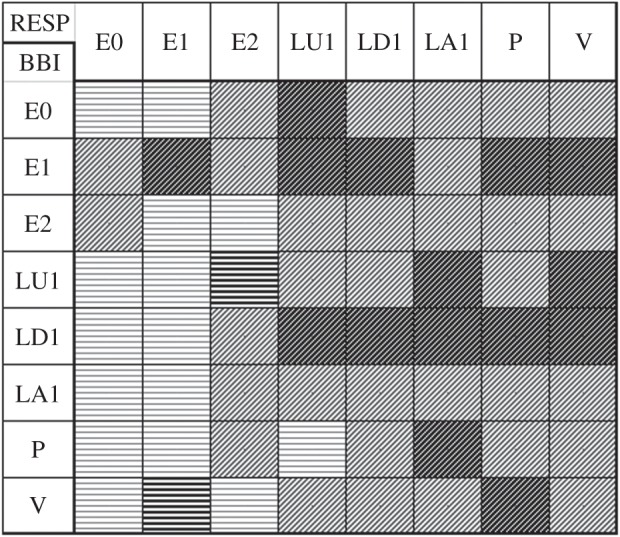
Top view of the HRJSD pattern family distribution density matrix Wf (8×8) for the threshold levels lBBI and lRESP equal to 25% of the standard deviation of the BBI and RESP time series for the comparison of healthy subjects (CON) with schizophrenic patients (SZO). HRJSD indices are as follows. Bold hatched areas are significantly (p<0.00061) increased in SZO. Light hatched areas are increased in SZO (not significant). Areas marked by bold horizontal lines are significantly (p<0.00061) decreased in SZO. Areas marked by light horizontal lines are decreased in SZO (not significant). (BBI, beat-to-beat intervals; RESP, respiratory frequency.)
(b). Comparison of the healthy first-degree relatives of patients with schizophrenia with healthy subjects
HRJSD showed no significantly different CRC patterns as well as no respiratory or HR pattern families between the REL group and the CON group (table 2). However, a trend towards SZO in comparison with the CON group was found regarding the mean values of the HRJSD indices (88% of the CRC patterns, 75% of the respiratory patterns and 50% of the HR patterns were increased in the REL group in comparison with the CON group).
(c). Comparison of patients with schizophrenia with their healthy first-degree relatives
Analysing the differences in CRCs between the SZO group and the REL group revealed only one significantly different CRC pattern (RESP-P/BBI-E1) and only one significantly different respiratory family pattern (RESP-LA1) between the SZO group and the REL group (table 2). Here, we found a comparable trend, as for the SZO group versus the CON group, that 88% of the CRC patterns, 75% of the respiratory patterns and 50% of the HR patterns were increased in the SZO group in comparison with the REL group.
4. Discussion
Applying HRJSD, we found significantly changed HR, respiratory frequency and CRC in patients with schizophrenia, but only marginally changed values in healthy first-degree relatives, in comparison with healthy subjects. As a unique feature of the HRJSD approach in contrast to other coupling approaches, we could clearly identify altered cardiorespiratory physiological regulatory patterns generated by the ANS in patients with schizophrenia. Our results highlight that patients with schizophrenia had a higher number of cardiorespiratory patterns that were less predominant but therefore more widely distributed in comparison with the CON group, indicating decreased CRC in SZO.
On one hand, as can be clearly seen in figure 4, CRC in the SZO group was mainly characterized by a larger amount of increased short-term weak increasing/decreasing, alternating and fluctuating respiratory pattern families (LU1, LD1, LA1, P, V; 82%) and invariable respiratory frequency (E1, 12%). On the other hand, CRC in the SZO group was mainly dominated by a larger amount of increased short-term weak increasing/decreasing and invariable HR pattern families (LU1, LD1, E1, 76%). It seems to be that respiratory regulation is more complex and more strongly influences the CRC pattern in the SZO group. It can be further shown that CRC is strongly affected by reduced HRV in combination with reduced respiratory variability (RESP-E1/BBI-E1, figure 5c). The SZO group exhibited a more random CRC with less strong rhythmic fluctuation of the cardiac cycle intervals in relation to respiration, indicating a decreased RSA in those patients.
Figure 5.
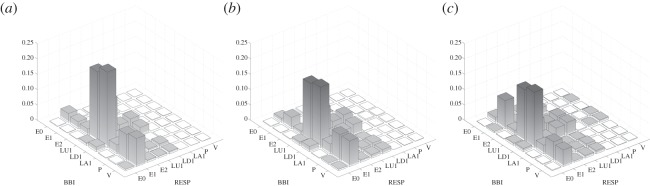
Three-dimensional plots of the HRJSD pattern family distribution density matrix Wf (8×8) for the threshold levels lBBI and lRESP equal to 25% of the standard deviation of the BBI and RESP time series for healthy subjects (a), healthy first-degree relatives (b) and schizophrenic patients (c). (BBI, beat-to-beat intervals; RESP, respiratory frequency.)
Porta et al. [30] investigated healthy subjects and found a progressive increase in stable BBI patterns as a function of the magnitude of the sympathetic activity during the graded head-up tilt test. This finding supports our interpretation that the increased presence of stable BBI patterns in patients with schizophrenia might be due to an increased sympathetic drive to the heart. Bär et al. [15] investigated the interdependency of cardiorespiratory interactions (cross-conditional entropy (CCE), classical joint symbolic dynamics (JSD) [31]) in control subjects in comparison with patients with schizophrenia and their relatives. They observed an impaired CRC revealed by an increased uncoupling function (CCE) and complexity of cardiorespiratory interactions (Shannon entropy of JSD) in patients with schizophrenia. They suggested that their findings were consistent with decreased vagal activity within the brainstem or its suppression from higher regulatory centres [15,32] and assumed that descending projections from the central nucleus of the amygdaloid complex or other limbic/paralimbic regions might be responsible for these findings [33]. However, they were not able to characterize how these changed interactions were caused by the ANS. In a further study by Schulz et al. [24], short-term nonlinear bivariate CRCs in the SZO group in comparison with the CON group applying the classical JSD approach (symbol 1=increases, symbol 0=decreases and equilibrium) were investigated. They found that RESP-related word types were highly significantly altered between the SZO and CON groups and that two prominent RESP word types (111,000) occurred mostly in combination with all other HR word types in both the SZO and CON groups. They assumed that their results pointed to an increased complexity of short-term cardiorespiratory regulation and that the specific occurrences of predominant RESP patterns could be further considered as a marker for a restricted RSA. Furthermore, Porta et al. [34] showed for healthy subjects that a decrease in cardiopulmonary coupling was linked to the degree of sympathetic activation and to a reduction of RSA during a graded head-up tilt test. This might further support our interpretation about a loss of cardiorespiratory interactions in patients with schizophrenia in relation to their higher sympathetic drive.
Respiration is not only primarily regulated for metabolic and homeostatic purposes in the brainstem but also constantly responds to changes in emotions, such as sadness, happiness, anxiety and fear. As a result, the final respiratory output can be understood as a complex interaction between the brainstem and higher centres, including the limbic system and cortical structures [22]. These centres (e.g. limbic system, amygdala and hypothalamus) are closely associated with regulation of mood and it is thought that there are reciprocal and interconnected interactions between respiration and emotional states [20]. Masaoka & Homma [35,36] found that respiratory patterns and respiratory frequency changed dramatically and were related to personality anxiety. They assumed that their findings may provide important evidence that the respiratory function (respiratory patterns, respiratory frequency) are related to personality anxiety. They further demonstrated that an increase in the respiratory frequency is not related to metabolic factors and is consistent with a mechanism involving the limbic system modulating respiratory drive [37].
Boiten et al. [38] have shown that respiration patterns reflect the general extent of the emotional response that is linked to emotional situations. Furthermore, it has been shown [39] that there is intriguing evidence suggesting pathophysiological relationships among dyspnoea, hyperventilation and panic anxiety. The symptoms of panic attacks and pulmonary disease overlap, so that panic anxiety can reflect underlying cardiopulmonary disease and dyspnoea can reflect an underlying anxiety disorder [39]. Our findings regarding the cardiorespiratory system of patients with schizophrenia could be disease-inherent characteristics and might reflect arousal during the psychosis stage in these patients. This is supported by the study of Peupelmann et al. [19], who found a close interrelation between positive symptoms and respiratory patterns in schizophrenia. It is known that panic attacks are common among individuals with schizophrenia and that they are associated with higher rates of other co-occurring mental disorders, service utilization and suicidality [40]. Owing to the link between the CNS and respiration, the slightest changes in respiratory functioning may add some background symptoms of panic and anxiety in any disorder [20]. Therefore, we hypothesize that the impairments of the cardiorespiratory system in the SZO group may be the result of panic-related changes in the brainstem during the acute psychotic state.
Considering the healthy first-degree relatives of patients with schizophrenia in comparison with healthy subjects, we found no significant CRC, HR and respiratory pattern families differentiating both groups. This is in accordance with other findings. Bär et al. [15] observed only changes of respiration in schizophrenic patients and assumed that this might reflect arousal in acutely ill patients. Schulz et al. [18] found significantly altered respiratory regulation (variability and dynamics) and reduced CRC in patients with schizophrenia but not in their healthy first-degree relatives. This is quite interesting because it was demonstrated that comparable changes of reduced HRV patterns in patients with schizophrenia and healthy first-degree relatives seems to be evident [6,12,13,15]. These findings might further point to an underlying disease-inherent genetic vulnerability of the cardiovascular system in patients with schizophrenia and their relatives but not for their cardiorespiratory system.
We also found one significant CRC pattern (RESP-P/BBI-E1) and one significant respiratory family pattern (RESP-LA1), both decreased in the REL group compared with the SZO group. The CRC pattern RESP-P/BBI-E1 means no variability of HR in combination with fast alterations of increased and subsequently decreased respiratory frequency (peak-like behaviour). These results might come from the changed HR regulation in the REL group that was proved in different studies where the REL group revealed a comparable pattern of reduced HRV to that in the SZO group. The mean value of invariable HR pattern (BBI-E1, 5.1±7.4) of the REL group was closer to that of the CON group (3.0±3.9), leading to no significant (but in trend) differences between these groups, but were further away from the mean values of the SZO group (15.4±17.2), leading to this significant difference; this seems to be mainly the result of the altered HRV in the REL group and not of respiratory variability. However, we would like to especially emphasize that the REL group have fast alterations of increased and subsequently decreased (RESP-P) and alternating (RESP-LA1) respiratory frequency. This result may be due to the fact that the measurement procedure is stressful for the REL group or that they were worried about their diseased relatives (SZO) who require comprehensive clinical treatment.
The changes within the cardiorespiratory system in schizophrenia seem to be disease-inherent characteristics and might reflect arousals during the psychosis stage in acutely ill schizophrenic patients. Because their relatives are obviously not in the same emotional and psychotic state it seems to be more evident that the alterations of CRC found in the SZO group are closely connected to the emotions such as sadness, happiness, anxiety and fear that appear during this disease. However, this could not be proved within this study and has to be therefore investigated in other studies applying, for example, functional imaging to analyse cortex activity in connection with different behavioural situations. At the moment, we cannot draw a clear physiological interpretation about the impairments of the different underlying regulatory mechanisms in schizophrenia regarding respiration. However, a genetic background for the alterations of the cardiovascular system is likely and has to be investigated in more detail in further studies. This assumption is supported by other studies where a genetic dependency of HRV was proven in healthy twins [14]. Further support of a genetic background in patients with schizophrenia and their relatives was shown recently in a large cohort study by Meda et al. [41], who investigated the brain's default mode network (DMN) that is highly heritable in 296 patients with schizophrenia, 179 of their unaffected first-degree relatives and 324 healthy subjects by applying independent component analysis (ICA) and parallel ICA (para-ICA). The study subjects underwent a resting-state functional MRI scan and were analysed by a two-stage approach (ICA, para-ICA) to identify DMNs, to test their biomarker and/or endophenotype status and to identify imaging–genetic relationships. They observed both unique and shared impairments in functional connectivity across the cohort of patients with schizophrenia and showed that these impairments were selectively familiar only for the unaffected first-degree relatives of patients with schizophrenia, whereby genes regulating specific neurodevelopment/transmission processes primarily mediated DMN disconnectivity. They also showed that selective nodes within the DMN are differentially affected and are modestly heritable among patients and unaffected relatives.
For the future, it seems to be necessary and of high interest to perform follow-up studies for relatives. As a consequence, one could determine whether the possible genetic disposition will lead to an increased incidence of schizophrenia within healthy relatives and whether such a pathological development could be predicted at an earlier stage [12].
The recently introduced HRJSD approach based on a redundancy reduction strategy and the grouping of single-word types into eight pattern families enables a detailed description and quantification of CRCs. The idea of HRJSD is to classify frequent deterministic patterns lasting three beats (symbols), as already proposed for univariate HR time series introduced by Porta et al. [42]. The HRJSD approach enables the classification and characterization of short-term cardiovascular and cardiorespiratory regulatory bivariate coupling patterns which are dominating the interaction generated by the ANS. As a quite new feature in contrast to the classical JSD approach or other coupling approaches [24], HRJSD emphasizes a clear characterization of how the couplings are composed of the different regulatory aspects of the ANS. The proposed HRJSD approach creates a bridge between univariate and bivariate symbolic analyses, allowing the quantification and classification of deterministic regulatory bivariate coupling patterns depending on the experimental conditions [25].
In conclusion, we demonstrated an altered HR pattern, respiratory pattern and CRC in patients with schizophrenia and only marginal changes for their healthy first-degree relatives in comparison with healthy subjects by applying HRJSD. These findings might be based on a decreased vagal activity within the brainstem, an altered or suppressed interaction of the brainstem and higher regulatory centres or panic- and anxiety-related changes in the brainstem due to acute psychosis in these patients. Patients with schizophrenia revealed CRC patterns which were characterized as less predominant but more widely distributed than those in healthy subjects, indicating a decreased CRC in schizophrenia. The HRJSD approach enables a more detailed description of the short-term cardiorespiratory physiological regulatory mechanisms in schizophrenia.
Ethics statement
The study complied with the Declaration of Helsinki as all participants gave written informed consent to a protocol approved by the local ethics committee of the University Hospital Jena. Patients and relatives were informed that refusal to participate in this study would not affect future treatment.
Funding statement
This work was partly supported by grants from the Deutsche Forschungsgemeinschaft (DFG-VO 505/8-2), the Thuringian Ministry of Economy, Labour and Technology and the European Fund for Regional Development (EFRE) TMBWAT/TAB 2011 FE 9092 and by grants from the University of Applied Sciences Jena, Germany.
References
- 1.Hennekens CH, Hennekens AR, Hollar D, Casey DE. 2005. Schizophrenia and increased risks of cardiovascular disease. Am. Heart J. 150, 1115–1121. ( 10.1016/j.ahj.2005.02.007) [DOI] [PubMed] [Google Scholar]
- 2.Jablensky A. 1995. Schizophrenia: recent epidemiologic issues. Epidemiol. Rev. 17, 10–20. [DOI] [PubMed] [Google Scholar]
- 3.Brown S, Inskip H, Barraclough B. 2000. Causes of the excess mortality of schizophrenia. Br. J. Psychiatry 177, 212–217. ( 10.1192/bjp.177.3.212) [DOI] [PubMed] [Google Scholar]
- 4.Koponen H, Alaräisänen A, Saari K, Pelkonen O, Huikuri H, Raatikainen MJP, Savolainen M, Isohanni M. 2008. Schizophrenia and sudden cardiac death: a review. Nord. J. Psychiatry 62, 342–345. ( 10.1080/08039480801959323) [DOI] [PubMed] [Google Scholar]
- 5.Straus SM, Bleumink GS, Dieleman JP, van der Lei J, Jong GW, Kingma JH, Sturkenboom MCJM, Stricker BHC. 2004. Antipsychotics and the risk of sudden cardiac death. Arch. Intern. Med. 164, 1293–1297. ( 10.1001/archinte.164.12.1293) [DOI] [PubMed] [Google Scholar]
- 6.Bär KJ, et al. 2010. Autonomic dysfunction in unaffected first-degree relatives of patients suffering from schizophrenia. Schizophr. Bull. 36, 1050–1058. ( 10.1093/schbul/sbp024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bär KJ, Boettger MK, Koschke M, Schulz S, Chokka P, Yeragani VK, Voss A. 2007. Non-linear complexity measures of heart rate variability in acute schizophrenia. Clin. Neurophysiol. 118, 2009–2015. ( 10.1016/j.clinph.2007.06.012) [DOI] [PubMed] [Google Scholar]
- 8.Bar KJ, Letzsch A, Jochum T, Wagner G, Greiner W, Sauer H. 2005. Loss of efferent vagal activity in acute schizophrenia. J. Psychiatr. Res. 39, 519–527. ( 10.1016/j.jpsychires.2004.12.007) [DOI] [PubMed] [Google Scholar]
- 9.Toichi M, Kubota Y, Murai T, Kamio Y, Sakihama M, Toriuchi T, Inakuma T, Sengoku A, Miyoshi K. 1999. The influence of psychotic states on the autonomic nervous system in schizophrenia. Int. J. Psychophysiol. 31, 147–154. ( 10.1016/S0167-8760(98)00047-6) [DOI] [PubMed] [Google Scholar]
- 10.Chang JS, Yoo CS, Yi SH, Hong KH, Oh HS, Hwang JY, Kim S-G, Ahn YM, Kim YS. 2009. Differential pattern of heart rate variability in patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 991–995. ( 10.1016/j.pnpbp.2009.05.004) [DOI] [PubMed] [Google Scholar]
- 11.Valkonen-Korhonen M, Tarvainen MP, Ranta-Aho P, Karjalainen PA, Partanen J, Karhu J, Lehtonen J. 2003. Heart rate variability in acute psychosis. Psychophysiology 40, 716–776. ( 10.1111/1469-8986.00072) [DOI] [PubMed] [Google Scholar]
- 12.Voss A, Schulz S, Baer KJ. 2010. Linear and nonlinear analysis of autonomic regulation of heart rate variability in healthy first-degree relatives of patients with schizophrenia. In Proc. IEEE Conf. Engineering in Medicine and Biology Society, Buenos Aires, Argentina, 31 August–4 September 2010, pp. 5395–5398. Piscataway, NJ: IEEE. [DOI] [PubMed] [Google Scholar]
- 13.Castro MN, et al. 2009. Heart rate variability response to mental arithmetic stress is abnormal in first-degree relatives of individuals with schizophrenia. Schizophr. Res. 109, 134–140. ( 10.1016/j.schres.2008.12.026) [DOI] [PubMed] [Google Scholar]
- 14.Busjahn A, et al. 1998. Angiotensin-converting enzyme and angiotensinogen gene polymorphisms and heart rate variability in twins. Am. J. Cardiol. 81, 755–760. ( 10.1016/S0002-9149(97)01019-9) [DOI] [PubMed] [Google Scholar]
- 15.Bär KJ, Rachow T, Schulz S, Bassarab K, Haufe S, Berger S, Koch K, Voss A. 2012. The phrenic component of acute schizophrenia—a name and its physiological reality. PLoS ONE 7, 33459 ( 10.1371/journal.pone.0033459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz S, Bär KJ, Voss A. 2012. Cardiovascular and cardiorespiratory coupling in unmedicated schizophrenic patients in comparison to healthy subjects. In Proc. IEEE Conf. Engineering in Medicine and Biology Society, San Diego, CA, 28 August–1 September 2012, pp. 3664–3667. Piscataway, NJ: IEEE. [DOI] [PubMed] [Google Scholar]
- 17.Steffen S, Karl-Jurgen B, Haueisen J, Andreas V. 2013. Quantification of cardiorespiratory coupling in acute schizophrenia applying high resolution joint symbolic dynamics. In Proc. Computing in Cardiology Conf. (CinC), Zaragoza, Spain, 22–25 September 2013, pp. 101–104. Piscataway, NJ: IEEE. [Google Scholar]
- 18.Schulz S, Bar KJ, Voss A. 2012. Respiratory variability and cardiorespiratory coupling analyses in patients suffering from schizophrenia and their healthy first-degree relatives. Biomed. Tech. (Berl.) 57, 1044 ( 10.1515/bmt-2012-4336) [DOI] [Google Scholar]
- 19.Peupelmann J, Boettger MK, Ruhland C, Berger S, Ramachandraiah CT, Yeragani VK, Bär K. 2009. Cardio-respiratory coupling indicates suppression of vagal activity in acute schizophrenia. Schizophr. Res. 112, 153–157. ( 10.1016/j.schres.2009.03.042) [DOI] [PubMed] [Google Scholar]
- 20.Weiden PJ, Weiden M. 2010. Schizophrenia and respiratory symptoms: a serious, but overlooked, comorbidity. CNS Spectr. 15, 10–13.20414160 [Google Scholar]
- 21.Ramirez JM, Richter DW. 1996. The neuronal mechanisms of respiratory rhythm generation. Curr. Opin. Neurobiol. 6, 817–825. ( 10.1016/S0959-4388(96)80033-X) [DOI] [PubMed] [Google Scholar]
- 22.Homma I, Masaoka Y. 2008. Breathing rhythms and emotions. Exp. Physiol. 93, 1011–1021. ( 10.1113/expphysiol.2008.042424) [DOI] [PubMed] [Google Scholar]
- 23.Eckberg DL. 2003. The human respiratory gate. J. Physiol. 548, 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz S, Adochiei F-C, Edu I-R, Schroeder R, Costin H, Bar K-J, Voss A. 2013. Cardiovascular and cardiorespiratory coupling analyses: a review. Phil. Trans. A 371, 20120191 ( 10.1098/rsta.2012.0191) [DOI] [PubMed] [Google Scholar]
- 25.Schulz S, Tupaika N, Berger S, Haueisen J, Bar K-J, Voss A. 2013. Cardiovascular coupling analysis with high-resolution joint symbolic dynamics in patients suffering from acute schizophrenia. Physiol. Meas. 34, 883–901. ( 10.1088/0967-3334/34/8/883) [DOI] [PubMed] [Google Scholar]
- 26.Kay SR, Fiszbein A, Opler LA. 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276. ( 10.1093/schbul/13.2.261) [DOI] [PubMed] [Google Scholar]
- 27.LeBlanc J, Ducharme MB, Thompson M. 2004. Study on the correlation of the autonomic nervous system responses to a stressor of high discomfort with personality traits. Physiol. Behav. 82, 647–652. ( 10.1016/j.physbeh.2004.05.014) [DOI] [PubMed] [Google Scholar]
- 28.Wessel N, Voss A, Malberg H, Ziehmann C, Voss HU, Schirdewan A, Meyerfeldt U, Kurths J. 2000. Nonlinear analysis of complex phenomena in cardiological data. Z. Herzschr. Elektrophys. 11, 159–173. ( 10.1007/s003990070035) [DOI] [Google Scholar]
- 29.Voss A, et al. 1996. The application of methods of non-linear dynamics for the improved and predictive recognition of patients threatened by sudden cardiac death. Cardiovasc. Res. 31, 419–433. ( 10.1016/0008-6363(96)00008-9) [DOI] [PubMed] [Google Scholar]
- 30.Porta A, Tobaldini E, Guzzetti S, Furlan R, Montano N, Gnecchi-Ruscone T. 2007. Assessment of cardiac autonomic modulation during graded head-up tilt by symbolic analysis of heart rate variability. Am. J. Physiol. Heart Circ. Physiol. 293, 702 ( 10.1152/ajpheart.00006.2007) [DOI] [PubMed] [Google Scholar]
- 31.Baumert M, Walther T, Hopfe J, Stepan H, Faber R, Voss A. 2002. Joint symbolic dynamic analysis of beat-to-beat interactions of heart rate and systolic blood pressure in normal pregnancy. Med. Biol. Eng. Comput. 40, 241–245. ( 10.1007/BF02348131) [DOI] [PubMed] [Google Scholar]
- 32.Williams LM, et al. 2004. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am. J. Psychiatry 161, 480–489. ( 10.1176/appi.ajp.161.3.480) [DOI] [PubMed] [Google Scholar]
- 33.Hadziefendic S, Haxhiu MA. 1999. CNS innervation of vagal preganglionic neurons controlling peripheral airways: a transneuronal labeling study using pseudorabies virus. J. Auton. Nerv. Syst. 76, 135–145. ( 10.1016/S0165-1838(99)00020-X) [DOI] [PubMed] [Google Scholar]
- 34.Porta A, Bassani T, Bari V, Tobaldini E, Takahashi ACM, Catai AM, Montano N. 2012. Model-based assessment of baroreflex and cardiopulmonary couplings during graded head-up tilt. Comput. Biol. Med. 42, 298–305. ( 10.1016/j.compbiomed.2011.04.019) [DOI] [PubMed] [Google Scholar]
- 35.Masaoka Y, Homma I. 1997. Anxiety and respiratory patterns: their relationship during mental stress and physical load. Int. J. Psychophysiol. 27, 153–159. ( 10.1016/S0167-8760(97)00052-4) [DOI] [PubMed] [Google Scholar]
- 36.Masaoka Y, Homma I. 1999. Expiratory time determined by individual anxiety levels in humans. J. Appl. Physiol. 86, 1329–1336. [DOI] [PubMed] [Google Scholar]
- 37.Masaoka Y, Homma I. 2001. The effect of anticipatory anxiety on breathing and metabolism in humans. Respir. Physiol. 128, 171–177. ( 10.1016/S0034-5687(01)00278-X) [DOI] [PubMed] [Google Scholar]
- 38.Boiten FA, Frijda NH, Wientjes CJ. 1994. Emotions and respiratory patterns: review and critical analysis. Int. J. Psychophysiol. 17, 103–128. ( 10.1016/0167-8760(94)90027-2) [DOI] [PubMed] [Google Scholar]
- 39.Smoller JW, Pollack MH, Otto MW, Rosenbaum JF, Kradin RL. 1996. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am. J. Respir. Crit. Care Med. 154, 6–17. ( 10.1164/ajrccm.154.1.8680700) [DOI] [PubMed] [Google Scholar]
- 40.Goodwin R, Lyons JS, McNally RJ. 2002. Panic attacks in schizophrenia. Schizophr. Res. 58, 213–220. ( 10.1016/S0920-9964(01)00373-5) [DOI] [PubMed] [Google Scholar]
- 41.Meda SA, et al. 2014. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc. Natl Acad. Sci. USA 111, 2066 ( 10.1073/pnas.1313093111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porta A, Guzzetti S, Montano N, Furlan R, Pagani M, Malliani A, Cerutti S. 2001. Entropy, entropy rate, and pattern classification as tools to typify complexity in short heart period variability series. IEEE Trans. Biomed. Eng. 48, 1282–1291. ( 10.1109/10.959324) [DOI] [PubMed] [Google Scholar]