Abstract
Myostatin (MSTN) is a negative regulator of muscle growth even if some studies have shown a counterintuitive positive correlation between MSTN and muscle mass (MM). Our aim was to investigate the influence of 2 months of resistance training (RT) and diets with different protein contents on plasma MSTN, interleukin 1 beta (IL-1β), interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), and insulin-like growth factor 1 (IGF-1). Eighteen healthy volunteers were randomly divided in two groups: high protein (HP) and normal protein (NP) groups. Different protein diet contents were 1.8 and 0.85 g of protein·kg bw−1·day−1 for HP and NP, respectively. Subjects underwent 8 weeks of standardized progressive RT. MSTN, IGF-1, IL-1β, IL-6, and TNF-α were analyzed before and after the first and the last training sessions. Lean body mass, MM, upper-limb muscle area, and strength were measured. Plasma MSTN showed a significant increase (P<.001) after the last training in the HP group compared with NP group and with starting value. IGF-1 plasma concentration showed a positive correlation with MSTN in HP after the last training (r2=0.6456; P=.0295). No significant differences were found between NP and HP for IL-1β, IL-6, TNF-α, and strength and MM or area. These findings suggest a “paradoxical” postexercise increase of plasma MSTN after 8 weeks of RT and HP diets. This MSTN elevation correlates positively with IGF-1 plasma level. This double increase of opposite (catabolic/anabolic) mediators could explain the substantial overlapping of MM increases in the two groups.
Key Words: : cytokines, diet, exercise, myostatin, nutritional supplement
Introduction
Resistance training (RT) plays a pivotal role in development of muscular hypertrophy and as countermeasure against sarcopenia. Also, protein supply acts on muscle protein balance (i.e., ratio between muscle synthesis and degradation).1 The effects of these two factors on plasma and muscle molecular signals have been widely investigated.2 Exercise may influence simultaneously anabolic (insulin-like growth factor 1 [IGF-1]) and proinflammatory/catabolic (interleukin 1 [IL-1], interleukin 6 [IL-6], and tumor necrosis factor alpha [TNF-α])3 and antianabolic [myostatin (MSTN)] signals. Regarding the latter, it is well known that MSTN is a potent regulator of muscle growth and size. MSTN is a member of the transforming growth factor beta (TGF-β) superfamily of secreted proteins; from MSTN gene a precursor protein is translated and is then processed into a mature protein. Mature MSTN is secreted from muscle into circulation so it can produce its effects both locally and systemically.4 MSTN has showed that (i) it may inhibit striated muscle growth through systemic effects on the IGF-1 axis,5 (ii) it inhibits the Akt-mTOR pathway reducing phosphorylated levels of Aktthr308 and reducing phosphorylation ratio of several downstream signaling proteins,6 (iii) it upregulates the activity of the ubiquitin-proteasoma mechanism via atrogin and Murf,7,8 and (iv) it prevents the generation of new fibers by inhibition of satellite cell replication and translocation, maintaining satellite cells in quiescence and preventing proliferation.9
An opposite role is played by IGF-1 that is a fundamental key for muscle growth. IGF-1 activates many downstream signaling proteins that positively affect protein synthesis.10 RT stimulates IGF-1 production and its downstream signaling protein1 and the same does protein supplementation.11 But, while RT acts on the muscle, muscle, for its part, acts on the whole body. In fact in recent years, a mounting amount of evidence have suggested that contracting muscle can act as a cytokine-producing organ.12 Under this new view, skeletal muscle can be considered an endocrine organ, which by secretion of hormone-like factors (myokines) may influence metabolism.12 Many molecules have been considered as candidates for crosstalk action between muscle and other tissues, for example, interleukin 1 beta (IL-1β), tumor necrosis factor alpha (TNF-α),13 IL-6,12 and MSTN.14 The local production of aforementioned molecules is involved in numerous physiological and pathological processes. One of the most studied is the coordination of the innate and adaptive immune cells' activity. There are many stimuli that can activate production and release of proinflammatory cytokines into circulation: trauma, thermal injury, infection, and so on. While these cytokines might positively affect muscle response after exercise, their chronically elevated values might represent a cause of muscle mass (MM) loss.15 As a matter of fact, the effects are determined less by the cytokines per se than by the modality of production and the organ/tissue-specific response.16
Considering the complex and controversial behavior of MSTN in response to RT and/or protein supplementation4,17–22 and its web-like relations with other muscle signals, we decided to investigate the influence of 2 months of RT and high-protein (HP) or normal-protein (NP) diet on strength performance variables: MM and plasma levels of MSTN, IL-1β, IL-6, TNF-α, and IGF-1; moreover, we aimed to analyze also the acute response of plasma variables just after training session at the beginning and at the end of 8 weeks of experimentation.
Materials and Methods
Subjects
The present study was part of a larger study aimed at investigating the effects of RT and protein supplementation on hormonal status, muscle fiber characteristics, and body composition. Eighteen male volunteers, human movement science undergraduate students (age=24.9±5.3 years), responded to an invitation to participate in the study. Respondents provided written informed consent to participate in the study and were screened for the presence of diseases or conditions that would place them at risk for adverse responses to exercise. Subjects were healthy, nonobese, and nonsmokers, and were not taking any medications and have never engaged in regular RT. All participants were active for 5–6 h/week of various team sports (soccer, volley, and basket). Subjects were first matched in pairs based on age and level of physical activity. Afterward from each pair, one subject was randomly allocated to the HP group or NP group. The study was approved by the Ethical Commission of the Salvatore Maugeri Foundation (Pavia, Italy) in accordance to Helsinki's declaration of 1995 as modified in 2000.
Study design
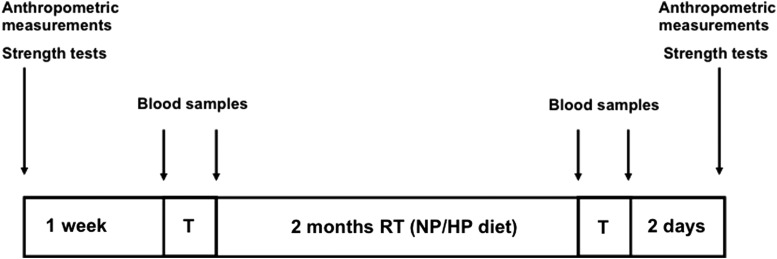
During the first week after recruitment, the subjects underwent scheduled measurements. Blood samples were taken in basal condition (8:00 AM, fasting) and immediately after the first training session. Both the groups followed the same RT program for 8 weeks. During the training period HP group consumed 1.8 g·kg−1·day−1 of protein while NP group consumed 0.85 g·kg−1·day−1 of protein. At the 10th week the same sequence of tests was repeated. A scheme of the study design is shown in Figure 1.
FIG. 1.
Scheme of experimental design. HP, high protein; NP, normal protein; RT, resistance training.
Measurements
Strength test
A pull grip dynamometer (PGD; Kayser Srl, Livorno, Italy) was used to test upper-limb strength. The tool is based on a dynamometer shaped as a handle, designed to be easily fixed to and removed. The PGD handle is mechanically connected to a load cell. A hinged joint allows alignment of force direction with the load cell axis to perform a proper measurement of the pull force. The system is connected to a computer where a specific software records force measurements and drives the testing procedure. In a previous study our group has validated the facility for strength test purposes.23 During tests, PGD was mounted on an handrail and subjects were standing up at 90°. Subjects performed three different trials of maximal voluntary contraction with 3 min of rest between each trial. The best performance was used for data analysis. We have previously demonstrated that during pull grip effort latissimus dorsi, triceps brachii (long head), and posterior deltoid were activated.23 Previous data from our laboratory showed an Intraclass Correlation Coefficient (ICCs) of 0.99 for isometric pull grip.
Body composition
Muscle and fat mass and percentage were assessed by skinfold measurements that are highly reliable means to determine body fat in fit and healthy young individuals.24 We used a software (Fitnext®; Caldogno, Vicenza, Italy) that includes nine skinfolds (triceps, biceps, pectoral, subarmpit, subscapular, iliac crest, mid-abdominal, anterior thigh, and medial calf), six bone circumferences (arm, forearm, waist, hip, thigh, and calf), four bone diameters (elbow, wrist, knee, and ankle), and waistline and hip circumference measurements.25 Anthropometric measurements were performed according to the Anthropometric Standardization Reference Manual.26 Weight was measured to the nearest 0.1 kg using an electronic scale (Tanita BWB-800 Medical Scales, Arlington Heights, IL, USA), and height to the nearest 1 cm using a Harpenden portable stadiometer (Holtain Ltd., Crosswell, United Kingdom). Skinfolds were measured to the nearest 1 mm using a Holtain caliper (Holtain Ltd.), and circumferences to the nearest 0.001 m using an anthropometric tape. All measurements were taken by the same operator (Q.F.P) before and during the study according to standard procedures. Muscle cross-sectional area of the dominant upper limb was assessed by anthropometric measurements and the measurement was validated with magnetic resonance (MR) measurements. MR imaging (MRI) was acquired using a 1.0-T scanner, Philips Panorama (Philips Medical System, Best, The Nederlands), using a dedicated solenoid coil, obtained with axial T1-weighted images (field of view=150 mm, TR=500 msec, TE=20 msec, 2 NSA), oriented along muscle long axis.
Blood analysis
The blood samples were taken from antecubital vein and collected into BD Vacutainers Tubes (SST™ II Advance, REF 367953). After blood sampling, the samples were centrifuged (2254 Relative Centrifugal Force at 4°C using centrifuge J6-MC by Beckman). The serum, so obtained, was aliquoted and stored at −80°C. All samples were analyzed in the same analytical session for each test using the same reagent lot and having coefficent of variation (CV) intraassay <7%. Before the analytical session, the serum samples were thawed overnight at 4°C and then mixed. Analytical characteristics were as follows: both IL-6 and TNF-α and IL-1β were measured using the analyzer IMMULITE One (Medical System S.p.A., Genova, Italy). The test is an immunoassay based on a chemiluminescent revelation. Assay characteristics for IL-6: measuring range=2.0–1000.0 ng/L; analytical sensitivity=2.0 ng/L; between-assay imprecision=88.0–1001.0 ng/L (CV=3.5–6.2%); reference range=0.0–5.9 ng/L. Assay characteristics for TNF-α: measuring range=1.7–1000.0 ng/L; analytical sensitivity=1.7 ng/L; between-assay imprecision=17.0–788.0 ng/L (CV=3.2–6.3%); reference range=0.0–8.1 ng/L. Assay characteristics for IL-1β: measuring range=1.5–1000.0 ng/L; analytical sensitivity=1.5 ng/L; intraassay precision=39–669.0 ng/L (CV=2.8–4.9%); reference range=0.0–5 ng/L. IGF-I was measured using the analyzer Liaison XL (DiaSorin S.p.A, Vercelli, Italy). The test is a sandwich immunoassay based on a chemiluminescent revelation. Assay characteristics for IGF-1: measuring range=3.0–1500.0 ng/mL; analytical sensitivity=3.0 ng/mL; between-assay imprecision=79.7–316.9 ng/mL (CV=5.6–9.6%); the reference range, for this test, depends by age and gender. Plasma MSTN was measured using an ELISA kit (Immunodiagnostik, AG Bensheim, Germany). Assay characteristics: measuring range=0.0–195 ng/mL.
RT protocol
Supervised training sessions were performed in 2 nonconsecutive days/week for the first 2 weeks and in 3 nonconsecutive days/week for the next 6 weeks under qualified exercise trainer supervision and, in particular, during the first 2 weeks all exercises were supervised to ensure correct lifting technique. The exercises used throughout the program were bench press, latissimus pulldowns, seated rows, shoulder press, biceps hammer curls, and dumbbell lying external rotation. During the first week of training, subjects performed 2 sets of 9–11 repetitions at 75–80% 1RM with 2-min breaks between each set in all exercises except for hammer curl (1.5 min) and dumbbell lying external rotation (1 min). Thereafter, from second to fourth week, the training volume was elevated to three sets. At the fifth week the intensity of training was elevated to 80–85% 1RM with three sets of six to eight repetitions. The recovery between each set was 3 min for all exercises except for hammer curl (2 min) and dumbbell lying external rotation (1.5 min). From sixth to eighth week the training volume was elevated to four sets. The participants were instructed to perform the repetitions rapidly (1 sec) during the concentric phase and then return the load through the eccentric (lowering) phase at a more slow and controlled speed (1.5/2 sec). The load was adjusted every week according to the actual number of repetitions performed. A general rule of a 5% adjustment for every two repetitions of deviation from the desired number of repetitions was used or, more generally, weights were adjusted to assure failure on the last or second last repetition.27 Exercise compliance was computed from daily exercise forms filled by the trainers, which were returned to the research staff every week.
Nutritional assessment
Before assigning the HP or NP diet, during a preliminary interview, all participants completed the recall of the previous 24 h. The participants were informed how to record food intake in the 7-day diary. The diaries were collected every week in the course of the study, in order to check the food intake and to exclude a previous HP diet. Afterward, a prescriptive, fixed-menu plan was given to all participants together with explanations of the different kinds of diets. For all diets the total daily caloric intake was divided into 5 meals. The individual daily caloric need was calculated referring to body composition and adjusted for daily activity.28,29 Both NP and HP diets are isocaloric regarding daily energy needs of subjects while the surplus of calories given by protein supplements were substituted by carbohydrates in diet. Protein intake in NP group was 0.85 g/kg of body weight while in HP group it was 1.8 g/kg of body weight. A whey protein supplement (PD Whey 100; Gensan Srl, Ospedaletto, PI, Italy) was given to HP subjects to allow them to reach the desired quantity of protein while a placebo (water with no caloric sweetener) was given with the same modalities to NP subjects. During the warm-up (10 to 15 min prior to the beginning of training session) and 1 h after the end of the training session, the subjects received 250 mL of a beverage containing 15–20 g of protein, for a daily amount of 30–40 g or a placebo. The amino acid composition of the protein supplement was as follows (for 10 g): leucine (1.12 g), isoleucine (0.46 g), valine (0.43 g), methionine (0.2 g), lysine (0.9 g), threonine (0.41 g), phenylalanine (0.3 g), alanine (0.47 g), arginine (0.2 g), aspartic acid (0.95 g), cysteine (0.28 g), glutamic acid (1.37 g), glycine (0.14 g), proline (0.4 g), serine (0.28 g), tyrosine (0.32 g), histidine (0.16 g), and tryptophan (0.3 g). At the end (week 8) of the exercise intervention, the subjects recorded 3-day-weighted dietary records (Thursday–Saturday) to assess potential changes in daily food intake that might have occurred during the intervention period. Food intake records were scrutinized by a dietitian and analyzed with DietNext (GSA-Tea Srl, Caldogno, VI, Italia). Dietary intake was calculated for the entire day. Placebo contains acaloric sweetener and colorant.
Statistical analysis
Baseline differences between the training and control groups for the reported variables were tested using independent-sample Student's t-tests. A two-way repeated measurement analysis of variance (treatments×time) was used. Normality of the data was checked and subsequently confirmed using the Kolmogorov-Smirnov test. The present sample size was selected based on a power analysis. All data were analyzed by using Prism5 GraphPad software (Abacus Concepts, GraphPad Software, San Diego, CA, USA). Statistica software vers. 8.0 (Tulsa, OK, USA) was used to obtain ICC values and for a sample size evaluation. Statistical significance was set at P<.05 for all tests. All values are expressed as means±SEM.
Results
The RT induced significant changes in MM and strength. Maximal isometric strength determined with the PGD showed a significant increase after training in both the groups (NP +8.34%, P<.005 and HP +7.9%, P<.005) without any significant difference between groups. Even if the two groups started from apparently different values of strength (NP vs. HP, 436 vs. 558 N) the unpaired t-test demonstrated no significant differences between basal performance. When merged there was a significant difference in isometric strength comparing pre- and postvalues (from 500.8±42.9 to 541.4±46.4 N, mean±SEM; P=.008). Muscle area of upper limb increased significantly (P=.005) from 45.13±3.3 to 47.94±4.4 cm2 as showed by MRI (Table 1) without any significant difference between dietary variables. These data were in full accordance with the anthropometric measurement as the correlation between the values of muscle area obtained from MRI and those calculated with Fitnext was highly significant (r2=0.88). There were no significant changes in fat mass, while MM showed a significant increase (P=.0003). Again, no significant differences were detectable between diet groups (Table 1).
Table 1.
Values Pre and Post 8 Weeks of Resistance Training and Normal- or High-Protein Diet
| Pre-NP | Post-NP | % Δ SIG | Pre-HP | Post-HP | % Δ SIG | Premerged | Postmerged | % Δ SIG | NP vs. HP | |
|---|---|---|---|---|---|---|---|---|---|---|
| MIP | 436.4±41.52 | 472.8±41.86 | +8.34 | 558.1±68.96 | 602.3±76.05 | +7.9 | 500.8±42.95 | 541.4±46.40 | +8.1 | ns |
| P<.05 | P<.05 | P=.008 | ||||||||
| MA | 44.25±4.64 | 45.4±4.20 | +2.85 | 45.89±4.37 | 47.18±5.00 | +4.45 | 45.13±3.30 | 47.94±4.40 | +3.56 | ns |
| P<.05 | P<.05 | P=.005 | ||||||||
| FM | 12.61±2.02 | 13.77±1.45 | +8.43 | 12.31±1.84 | 11.31±1.49 | −8.41 | 12.46±1.35 | 12.54±1.05 | −0.64 | ns |
| Ns | Ns | ns | ||||||||
| MM | 34.11±1.84 | 35.64±2.20 | +4.48 | 33.44±2.40 | 35.37±2.38 | +5.77 | 33.77±1.47 | 35.5±1.57 | +5.12 | ns |
| P<.05 | P<.005 | P<.0003 |
Data are expressed as mean and standard error of mean. NP versus HP was analyzed through Bonferroni multiple comparison after two-way ANOVA repeated measurements.
ANOVA, analysis of variance; FM, fat mass (expressed in kg); HP, high protein; MA, muscle area (expressed in cm2); MIP, maximal isometric strength at pull grip dynamometer (expressed in Newton); MM, muscle mass (expressed in kg); NP, normal protein; ns, not significant; SIG, significance.
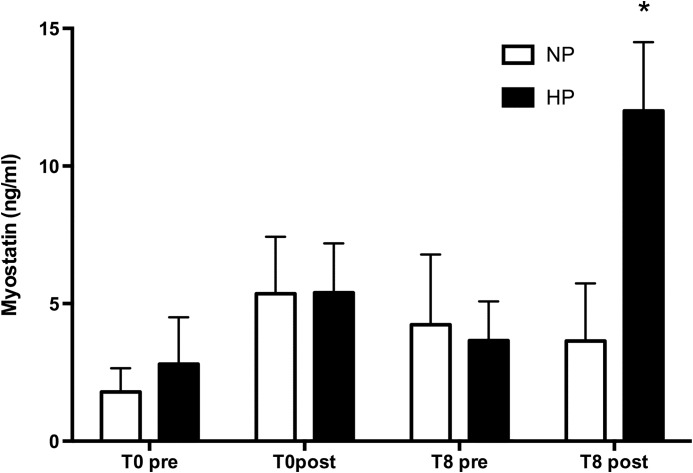
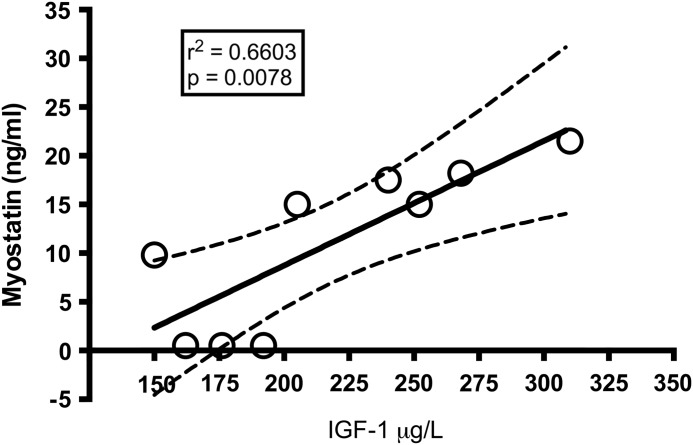
MSTN plasma level showed a significant increase (P=.02) after the last training in the HP group (pretraining session 3.66±1.42 ng/mL, post-training session 12.0±2.5; mean±SEM) while no change was detectable in the NP group (pretraining session 4.23±2.59 ng/mL, post-training session 3.64±2.09 ng/mL; mean±SEM) (Fig. 2). Interestingly, when individual subjects belonging to HP group were considered, IGF-1 values showed a positive correlation with MSTN values after the last training (r2=0.6456; P=.0295) (Fig. 3). No correlation was found with other blood parameters nor with MM and muscle strength changes. IGF-1, IL-1β, and IL-6 showed no significant differences before and after training period while TNF-α showed an interaction only for time effect; that is, we found higher values after 8 weeks both at basal and post-training time points (Table 2) but without any significant differences between different protein intakes or time points.
FIG. 2.
Changes in circulating myostatin at the beginning of experiment (T0) and after 8 weeks of training and diet (T8). Prevalues are obtained in basal conditions, post immediately after training session. *P<.02. Data are expressed as mean and SEM.
FIG. 3.
Correlation between circulating IGF-1 and myostatin. Empty dots represent subjects of HP group. Data are expressed as mean and SEM. IGF-1, insulin-like growth factor 1.
Table 2.
Values Pre and Post a Single Training Session, Before and After 8 Weeks of Resistance Training with Normal- or High-Protein Diet
| T0 pre-NP | T0 post-NP | T0 pre-HP | T0 post-HP | T8 pre-NP | T8 post-NP | T8 pre-HP | T8 post-HP | |
|---|---|---|---|---|---|---|---|---|
| IL-6 | <2.000 | <2.000 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 |
| IL-1β | <1.50 | <1.50 | <1.50 | <1.50 | <1.50 | <1.50 | <1.50 | <1.50 |
| TNF-α | 5.9±0.46 | 7.12±0.88 | 6.44±0.65 | 7.78±0.59 | 15.79±7.4 | 11.34±1.57 | 11.04±2.16 | 10.53±1.06 |
| MSTN | 1.79±0.86 | 5.36±0.58 | 7.29±3.15 | 3.87±1.46 | 4.23±2.55 | 3.64±2.09 | 3.65±1.43 | 12±2.50* |
| IGF-1 | 229.4±20.84 | 223.6±22.30 | 226.9±9.04 | 231.2±12.35 | 216.2±17.31 | 220.4±17.58 | 221.6±14.28 | 222.0±15.04 |
Data are expressed as mean and standard error of mean. Data were analyzed through two-way ANOVA repeated measurements (time×treatment). A Bonferroni multiple comparison showed significance for T8 post.
P=.02 (HP T8 post vs. NP T8 post).
IGF-1, insulin-like growth factor 1 (expressed in ng/mL); IL-1β, interleukin 1 beta (expressed in ng/L); IL-6, interleukin 6 (expressed in ng/L); MSTN, myostatin (expressed in ng/mL); TNF-α, tumor necrosis factor alpha (expressed in ng/L).
Discussion
The main finding of the present study was that a higher protein intake does not cause a greater MM gain compared with normal intake (i.e., 1.8 g of protein·kg−1·day−1 vs. NP group 0.85 g of protein·kg−1·day−1) in healthy young subjects. Our data are in agreement with those of Verdijk30 obtained in elderly but in contrast with others.19,31 One possible explanation for just-discussed results could be found considering the chalone action of MSTN32; indeed, in our experiment the HP group showed an increased IGF-1 response to training with a contemporary increase in MSTN.
As a matter of fact, data available about MSTN behavior in response to RT are somewhat contradictory. For example, some authors found a decrease in serum MSTN levels17,20,33 while others found an increase.34 The differences could be ascribed to the different time points of sampling (immediately after for Willoughby34 and after 48 h for Walker, Jones, and Hulmi17,20,33) suggesting that RT may induce an acute MSTN increase followed by a long-lasting decrease facilitating a hypertrophic adaptation. Since RT has many variables that should be taken into account,35 another possible confounding factor could be different RT protocols that elicit different MSTN effects.36 Peters et al. demonstrated, for example, that eccentric contractions increase MSTN RNA in rat muscles.37 Another variable that could interfere with MSTN response to training is nutrition; surprisingly, reciprocal influence of strength training and nutrition, especially RT and HP diet, on plasma MSTN behavior has been, until now, poorly investigated. A recent article of Hulmi et al. reported that an RT bout acutely decreases MSTN, but only when protein was not supplemented.19 Another study from the same author confirmed that protein ingestion may have a blunted effect on RT-related decrease in MSTN production.11 Our data showed instead a “paradoxical” response of plasma MSTN to HP diet after 8 weeks of RT. Subjects with higher protein intake (1.8 g·kg−1·day−1 of protein) showed a significant increase in blood MSTN immediately after the last training session, while NP group showed no differences. Interestingly IGF-1 in HP showed a significant increase after the last training session while no differences were detected in NP group. MSTN and IGF-1 increase after the last training showed a significant correlation. The parallel increase in MSTN and IGF-1 that have opposite actions on muscle protein balance appears at least curious. But a closer look showed that it is only an apparent paradox. Already in the 1960s, Bullough introduced the concept of chalones, inhibitors of cell growth that provide a negative feedback mechanism to control the size of a specific tissue.38 Our data are in agreement with those of Shyu who suggested a blunting effect of IGF-1 on MSTN expression.39 More in detail Shyu showed that IGF-1 stimulation of cardiac myocytes activates p38 MAPK, a stress kinase, which increases the activity of the transcription factor MEF-2, leading to the transactivation of the MSTN gene.39 This interaction suggests that MSTN acts as a cardiac chalone for IGF-1. This chalone's effect might explain not only the correlation between increase in blood MSTN and IGF-1 but also the substantial overlapping between HP and NP groups in muscle area and strength performance. The simultaneous rise of IGF-1 and MSTN could be carefully explained as an effort to maintain the homeostasis of the organism.
It is known that cytokine production (in particular TNF-α) may affect negatively protein synthesis through activation of nuclear transcription factor NF-kB via the ubiquitin-ligase MuRF1.40 Regarding the investigated inflammatory cytokines (TNF-α, IL-1β, and IL-6) we found no differences between the two groups. It is well known that the body reacts to exercise as it does in the presence of a subclinical inflammatory state. In both conditions (heavy exercise and certain pathological states) pro- and anti-inflammatory cytokines, together with other molecules such as glucocorticoids, are released into circulation. If exercise is of sufficient intensity to stimulate an inflammatory response, then there is a release of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) first and of anti-inflammatory cytokines (IL-4, IL-10, and IL-1) subsequently.3 It is of interest to note that while the effects of endurance, exhaustive exercise on proinflammatory cytokines have been widely investigated,41 few studies have been done instead on RT and cytokines.13,42 Our data showed no changes of circulating IL-1β and IL-6 after a single session of RT; moreover, no changes were detectable also comparing beginning of the training and after 8 weeks of training neither before nor immediately after training session. Our data are in contrast with a recent article of Phillips13 who found a blunted exercise-induced response of circulating IL-6. It should be taken into account, however, that the subjects studied by Phillips were previously sedentary elderly women, and therefore it could be speculated a different age-related response of cytokine to exercise and training. Our data showed an increased rest and post-training TNF-α after 8 weeks of RT without differences between diet treatments. Also other authors have observed a significant elevation of TNF-α, IL-1β, and IL-6 but after 3 h of endurance training.41 Regarding nutrition and cytokines, while many studies have addressed the relationship between carbohydrate supplementation and cytokine production,43 no data are available about long-term protein supplementation, RT, and cytokine production. In this respect our results suggested that 8 weeks of RT could increase both basal and post-training circulating TNF-α without affecting IL-1β and IL-6 and, on the other side, that whey protein supplementation (about 1 g of protein per kg of body weight) has no effect on cytokine response. One caveat should be introduced here.
It is well known that the acidosis produced by an HP diet could affect skeletal muscle protein synthesis44; thus, whether the reduction of pH, due to a higher protein intake, has been influenced by the hypertrophic response to training, therefore, remains uncertain. One limitation of this study was that the subjects were nonexpert RT athletes, we can hypothesize that higher intensity of training will lead to different results in terms of inflammatory cytokines.
In conclusions, taken together our findings indicate a “paradoxical” response of plasma MSTN to HP diet after 8 weeks of RT. It is noteworthy that IGF-1 whose activity is downregulated by MSTN is increased in HP group after the last training and shows a positive significant correlation with MSTN increase. This double increase of opposite mediators could explain the substantial overlapping of MM increases in the two groups. We can argue that HP diet influences metabolic regulation of IGF-1 and MSTN upstream the same pathway. Taken together these data point to a complex and not yet well-understood response of MSTN to RT. The differences found in our study compared with other studies might be explained by the chronic protein supplementation and by the different subjects' basal characteristics (athletes vs. sedentary; young vs. old), but this differences might also reflect the complexity of MSTN release mechanism. The comprehension of such mechanism could help to individuate efficient countermeasures against sarcopenia. More studies are needed to explain this paradoxical behavior that explore also muscle signaling molecules, such as mTOR and Akt.
Acknowledgment
This work was supported by grants from ASI (WP 1B235) (Agenzia Spaziale Italiana–Italian Space Agency).
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- 1.Wilborn CD, Taylor LW, Greenwood M, Kreider RB, Willoughby DS: Effects of different intensities of resistance exercise on regulators of myogenesis. J Strength Cond Res 2009;23:2179–2187 [DOI] [PubMed] [Google Scholar]
- 2.Dodd KM, Tee AR: Leucine and mTORC1: a complex relationship. Am J Physiol Endocrinol Metab 2012;302:E1329–E1342 [DOI] [PubMed] [Google Scholar]
- 3.Moldoveanu AI, Shephard RJ, Shek PN: The cytokine response to physical activity and training. Sports Med 2001;31:115–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott B, Renshaw D, Getting S, Mackenzie R: The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol (Oxf) 2012;205:324–340 [DOI] [PubMed] [Google Scholar]
- 5.Williams NG, Interlichia JP, Jackson MF, Hwang D, Cohen P, Rodgers BD: Endocrine actions of myostatin: systemic regulation of the IGF and IGF binding protein axis. Endocrinology 2011;152:172–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amirouche A, Durieux AC, Banzet S, et al. : Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology 2009;150:286–294 [DOI] [PubMed] [Google Scholar]
- 7.Goncalves MD, Pistilli EE, Balduzzi A, et al. : Akt deficiency attenuates muscle size and function but not the response to ActRIIB inhibition. PLoS One 2010;5:e12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandri M, Barberi L, Bijlsma AY, et al. : Signalling pathways regulating muscle mass in ageing skeletal muscle. The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2013;14:303–323 [DOI] [PubMed] [Google Scholar]
- 9.McFarlane C, Hennebry A, Thomas M, et al. : Myostatin signals through Pax7 to regulate satellite cell self-renewal. Exp Cell Res 2008;314:317–329 [DOI] [PubMed] [Google Scholar]
- 10.Schiaffino S, Mammucari C: Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skeletal Muscle 2011;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hulmi JJ, Kovanen V, Selanne H, Kraemer WJ, Hakkinen K, Mero AA: Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids 2009;37:297–308 [DOI] [PubMed] [Google Scholar]
- 12.Pedersen BK: Muscular interleukin-6 and its role as an energy sensor. Med Sci Sports Exerc 2012;44:392–396 [DOI] [PubMed] [Google Scholar]
- 13.Phillips MD, Flynn MG, McFarlin BK, Stewart LK, Timmerman KL: Resistance training at eight-repetition maximum reduces the inflammatory milieu in elderly women. Med Sci Sports Exerc 2010;42:314–325 [DOI] [PubMed] [Google Scholar]
- 14.McPherron AC: Metabolic Functions of Myostatin and Gdf11. Immunol Endocr Metab Agents Med Chem 2010;10:217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen BK: Muscles and their myokines. J Exp Biol 2011;214(Pt 2):337–346 [DOI] [PubMed] [Google Scholar]
- 16.Shepard RJ, Shek PN: Impact of physical activity and sport on the immune system. Rev Environ Health 1996;11:133–147 [DOI] [PubMed] [Google Scholar]
- 17.Hulmi JJ, Ahtiainen JP, Kaasalainen T, et al. : Postexercise myostatin and activin IIb mRNA levels: effects of strength training. Med Sci Sports Exerc 2007;39:289–297 [DOI] [PubMed] [Google Scholar]
- 18.Hulmi JJ, Kovanen V, Lisko I, Selanne H, Mero AA: The effects of whey protein on myostatin and cell cycle-related gene expression responses to a single heavy resistance exercise bout in trained older men. Eur J Appl Physiol 2008;102:205–213 [DOI] [PubMed] [Google Scholar]
- 19.Hulmi JJ, Tannerstedt J, Selanne H, Kainulainen H, Kovanen V, Mero AA: Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. J Appl Physiol (1985) 2009;106:1720–1729 [DOI] [PubMed] [Google Scholar]
- 20.Walker KS, Kambadur R, Sharma M, Smith HK: Resistance training alters plasma myostatin but not IGF-1 in healthy men. Med Sci Sports Exerc 2004;36:787–793 [DOI] [PubMed] [Google Scholar]
- 21.Willoughby DS: Effects of an alleged myostatin-binding supplement and heavy resistance training on serum myostatin, muscle strength and mass, and body composition. Int J Sport Nutr Exerc Metab 2004;14:461–472 [DOI] [PubMed] [Google Scholar]
- 22.Willoughby DS, Stout JR, Wilborn CD: Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids 2007;32:467–477 [DOI] [PubMed] [Google Scholar]
- 23.Pacelli QF, Paoli A, Zolesi V, Norfini A, Donati A, Reggiani C: Implementation and ground validation of a facility for functional and structural analysis of proximal upper limb muscles in microgravity. Basic Appl Myol 2009;19:77–85 [Google Scholar]
- 24.Hume P, Marfell-Jones M: The importance of accurate site location for skinfold measurement. J Sports Sci 2008;26:1333–1340 [DOI] [PubMed] [Google Scholar]
- 25.Paoli A, Grimaldi K, D'Agostino D, et al. : Ketogenic diet does not affect strength performance in elite artistic gymnasts. J Int Soc Sports Nutr 2012;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohman TG, Roche AF, Martorell R: Anthropometric Standardization Reference Manual. Human Kinetics Books, Champaign, IL, 1991 [Google Scholar]
- 27.Shackelford LC, LeBlanc AD, Driscoll TB, et al. : Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol 2004;97:119. [DOI] [PubMed] [Google Scholar]
- 28.Frankenfield D, Roth-Yousey L, Compher C: Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc 2005;105:775–789 [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Heshka S, Gallagher D, Boozer CN, Kotler DP, Heymsfield SB: Resting energy expenditure-fat-free mass relationship: new insights provided by body composition modeling. Am J Physiol Endocrinol Metab 2000;279:E539–E545 [DOI] [PubMed] [Google Scholar]
- 30.Verdijk LB, Jonkers RA, Gleeson BG, et al. : Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr 2009;89:608–616 [DOI] [PubMed] [Google Scholar]
- 31.Hulmi JJ, Lockwood CM, Stout JR: Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: a case for whey protein. Nutr Metab 2010;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SJ, McPherron AC: Myostatin and the control of skeletal muscle mass. Curr Opin Genet Dev 1999;9:604–607 [DOI] [PubMed] [Google Scholar]
- 33.Jones SW, Hill RJ, Krasney PA, O'Conner B, Peirce N, Greenhaff PL: Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J 2004;18:1025–1027 [DOI] [PubMed] [Google Scholar]
- 34.Willoughby DS: Effects of heavy resistance training on myostatin mRNA and protein expression. Med Sci Sports Exerc 2004;36:574–582 [DOI] [PubMed] [Google Scholar]
- 35.Paoli A, Bianco A: Not all exercises are created equal. Am J Cardiol 2012;109:305. [DOI] [PubMed] [Google Scholar]
- 36.Heinemeier KM, Olesen JL, Schjerling P, et al. : Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol (1985) 2007;102:573–581 [DOI] [PubMed] [Google Scholar]
- 37.Peters D, Barash IA, Burdi M, et al. : Asynchronous functional, cellular and transcriptional changes after a bout of eccentric exercise in the rat. J Physiol 2003;553(Pt 3):947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bullough WS: Mitotic and functional homeostasis: a speculative review. Cancer Res 1965;25:1683–1727 [PubMed] [Google Scholar]
- 39.Shyu KG, Ko WH, Yang WS, Wang BW, Kuan P: Insulin-like growth factor-1 mediates stretch-induced upregulation of myostatin expression in neonatal rat cardiomyocytes. Cardiovasc Res 2005;68:405–414 [DOI] [PubMed] [Google Scholar]
- 40.Sandri M: Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008;23:160–170 [DOI] [PubMed] [Google Scholar]
- 41.Moldoveanu AI, Shephard RJ, Shek PN: Exercise elevates plasma levels but not gene expression of IL-1beta, IL-6, and TNF-alpha in blood mononuclear cells. J Appl Physiol (1985) 2000;89:1499–1504 [DOI] [PubMed] [Google Scholar]
- 42.Donges CE, Duffield R, Drinkwater EJ: Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Med Sci Sports Exerc 2010;42:304–313 [DOI] [PubMed] [Google Scholar]
- 43.Henson DA, Nieman DC, Nehlsen-Cannarella SL, et al. : Influence of carbohydrate on cytokine and phagocytic responses to 2 h of rowing. Med Sci Sports Exerc 2000;32:1384–1389 [DOI] [PubMed] [Google Scholar]
- 44.Dawson-Hughes B, Harris SS, Ceglia L: Alkaline diets favor lean tissue mass in older adults. Am J Clin Nutr 2008;87:662–665 [DOI] [PMC free article] [PubMed] [Google Scholar]