Abstract
African American women have disproportionately high prevalence rates of HIV and cervical cancer. HIV-infected women are significantly less likely to obtain recommended cervical cancer screenings than HIV-uninfected women. The purpose of this study was to examine sociocultural and structural factors associated with cervical cancer screening among HIV-infected African American in Alabama. The PEN-3 Model and the Health Belief Model were used as theoretical frameworks. In-depth interviews were conducted with twenty HIV-infected African American women to identify perceptions, enablers, and nurturers, perceived susceptibility, perceived severity, and perceived benefits related to cervical cancer and screening. The most common positive perceptions, enablers, and nurturers that contributed to cervical cancer screening included internal motivation and awareness of the importance of HIV-infected women getting Pap tests due to their weakened immune system. Negative perceptions, enablers, and nurturers included lack of knowledge about cervical cancer and screening, and lack of perceived susceptibility to cervical cancer. The results of this study can be used to guide the development of culturally relevant cervical cancer and screening education interventions aimed at increasing cervical cancer screening adherence among HIV-infected African American women.
Introduction
As the HIV/AIDS epidemic enters its 34th year, persons living with HIV/AIDS still face a high risk of co-morbidities such as invasive cervical cancer, which has been defined as an AIDS-related malignancy.1,2 The results of several studies suggest that the increased prevalence of cervical intraepithelial neoplasia (CIN) among HIV-infected women is associated with lower CD4 counts characterizing the progression of HIV disease.3–6 Despite advances in antiretroviral therapy and overall associated increases in CD4 counts, prevalence rates for CIN and cervical cancer in HIV-infected women have not declined.2,4 With increased life expectancies due to the success of antiretroviral therapy, HIV-infected women are at especially high risk for pre-invasive cervical disease, cervical disease progression, cervical lesions that require excisional therapy, persistent or recurrent precursor abnormalities after treatment, and, ultimately, invasive cervical cancer.1,5–12
The intersection between human papillomavirus (HPV) and HIV co-infection is particularly problematic for African American women, who are disproportionately affected by HIV/AIDS, particularly in the Deep South region of the US.13,14 African American women have the highest incidence of HIV infection among women in the US, and are also at high risk for invasive cervical cancer and associated mortality.15–18 In Alabama, approximately 74% of all new HIV/AIDS cases among females are among African American women.19 Findings from the Women's Interagency HIV Study (WIHS) show that HIV-infected women who participated in regular cervical cancer screening programs had a higher rate of CIN and other cervical abnormalities than a comparison group of HIV-negative wom
Due to the increased risk of cervical disease among HIV-infected women, more frequent cervical cancer screenings are recommended for HIV-infected women than for HIV-negative women.21 Women living with HIV disease should have two cervical cancer screenings, 6 months apart within the first year of HIV diagnosis, followed by annual screenings if initial results are negative.11,22
Recent research suggests that HPV vaccines are safe and immunogenic in HIV-infected women, however, vaccination against HPV will not prevent cervical cancer in women already infected with HPV.23,24 Therefore, in the foreseeable future, cervical cancer screening remains the primary strategy to prevent cervical cancer by detecting cervical lesion early in HIV-infected women. However, African American women living with HIV disease are less likely to adhere to the recommended screening intervals and follow-up on abnormal Pap test results.25–28 Research suggests that factors such as older age, low educational attainment, lack of financial resources, and tobacco use are associated with lower cervical cancer screening among HIV-infected African American women.25,29–31
Studies have shown that the sociocultural environment influences the knowledge, beliefs, and values that influence decision-making associated with health behaviors such as participation in cervical cancer screening.32 Cervical cancer rates are higher in resource-constrained settings that limit the available resources supportive of a woman adhering to screening recommendations.33 However, there is limited research focused on the sociocultural and structural factors that enable and hinder HIV-infected African American women's uptake of cervical cancer screening. Therefore, the purpose of this study was to examine the sociocultural and structural factors associated with cervical cancer screening among HIV-infected African American in Alabama.
Methods
Theoretical framework
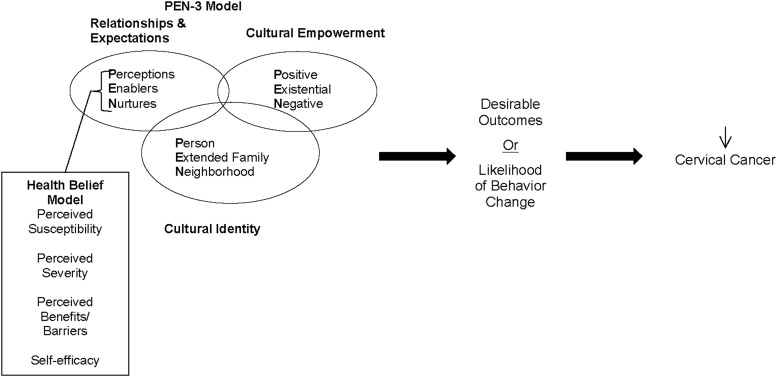
The PEN-3 and Health Belief Model provided the theoretical framework that guided data collection and analysis (Fig. 1).34,35 The PEN-3, originally developed for use in African countries and subsequently used among African Americans and Latinos in the US, is a conceptual model that is used to develop culturally relevant health education programs.36–41 It consists of three interrelated and interdependent dimensions of health (Cultural Empowerment, Relationships and Expectations, and Cultural Identity). Each dimension has three components that form the PEN acronym. The first dimension, Cultural Empowerment, assists in defining the target audience (person, extended family, and neighborhood). The second dimension, Relationships and Expectations, focuses on determining the factors that influence the person, family, and/or community actions (perceptions, enablers, and nurturers). Perceptions include the knowledge, attitudes, and beliefs that may contribute to or hinder engagement in a particular health behavior. Enablers include community or structural factors such as availability of resources, accessibility, and referrals. Nurturers refer to reinforcing factors that the target audience receives from their social networks. The third, most important and unique, dimension of the model is the Cultural Identity, which is crucial in the development of culturally relevant interventions among racial/ethnic minorities. Its components are positive, existential, and negative. The “positive” component refers to perceptions, enablers, and nurturers who lead the target audience to engage in healthy behaviors or deter them from engaging in harmful behaviors. The “existential” component refers to cultural perceptions and practices that may be at odds with medical orthodoxy but have no harmful health consequences and should not be changed but incorporated in interventions and assessments. The “negative” component refers to perceptions, enablers, and nurturers who lead the target audience not to engage in healthy behaviors or to engage in harmful behaviors.38,39
FIG. 1.
Conceptual framework based on the PEN-3 and Health Belief Models.
Although the PEN-3 model takes into account cultural sensitivity and appropriateness in the data collection and analysis, we believe that there are other components that may be relevant when examining secondary prevention of cervical cancer among racial/ethnic minority populations such as African Americans. Several of the most relevant of these components are integral parts of the Health Belief Model (HBM). Under the HBM, individuals will change their behavior(s) to prevent a particular disease if: (a) they consider themselves to be susceptible to the disease or condition (e.g., as HIV-infected women they are likely to have been exposed to HPV and HIV-infection increases their risk for poor HPV associated outcomes); (b) if they perceive that such disease or condition can have serious consequences (e.g., HPV infection can lead to cervical cancer that can be fatal even in women on highly active antiretroviral therapy); (c) they are threatened by the disease or condition (e.g., the disease or condition is likely to have a negative effect on something of value to them personally); (d) they perceive that engagement in a particular behavior (e.g., getting screened) will be beneficial in reducing the susceptibility to and/or the severity of the disease; and (e) they believe that the benefits outweigh the barriers or costs. The final model for this study incorporates the components of the PEN-3 and the HBM (see Fig. 1).
The research was approved by the Institutional Review Board of the authors' university. Participants were recruited through flyers that were distributed at two community-based HIV/AIDS service organizations that serve African American women in rural and urban areas of Alabama and by word of mouth. A total of 20 participants were recruited who met the following inclusion criteria: (1) female; (2) African American; (3) HIV positive; (4) age 19 and older; (5) English speaking; and (6) absence of mental or physical limitations that would preclude participation in focused discussions. Additionally, the study sample was stratified into two groups: women who reported having had a Pap test in the previous 12 months (Pap Test), and women who reported not having had a Pap test in the previous 12 months (No Pap Test).
Staff at collaborating clinics served as intermediaries for the investigators in recruitment of participants through distribution of a brochure that described the research and the nature of participation. Women who gave their permission were contacted by the investigators and screened according to inclusion/exclusion criteria. Women who met study criteria were provided a date and time for the interview. Written informed consent was obtained from each participant prior to study enrollment. Participants also completed a brief sociodemographic questionnaire that assessed age, education, HIV clinical history, and date of last Pap test. At the completion of the interview, each participant was given $30 cash as honorarium to compensate them for their time.
Interviews were conducted using a detailed interview protocol that included open-ended questions considering the major constructs within the theoretical framework (PEN-3 and HBM). Examples of interview questions used to explore participants' social construction of cervical cancer included: (1) What are some of the serious health problems that women with HIV have to be concerned about?; (2) What do you know about cervical cancer?; and (3) What do you think your chances are of having cervical cancer? Similar questions were used to explore other constructs of interest. Probes were used (e.g., Tell me more about that.) as needed to encourage in-depth descriptions of experiences and perceptions. The interviews were audio-recorded and transcribed verbatim. The transcripts, interviewer notes, and sociodemographic questionnaire provided the data for analysis.
Three members of the research team conducted the data analysis. During the first round of coding, each transcript was independently analyzed by two members of the research team. To ensure that the data was coded consistently, the research team met to compare the coded data. Inconsistencies in the coded data were discussed and the research team arrived at a consensus on how the data should be coded. Data analysis was conducted using a content analysis approach, with categories defined by the theoretical framework (PEN-3 and HBM). Two dimensions of the PEN-3 model were used to organize the coded data into the following categories: (1) Relationships and Expectations: perceptions, enablers, and nurturers; and (2) Cultural Empowerment: positive, existential, and negative. In order to facilitate coding, the HBM constructs were included along with the PEN-3 constructs since they are complementary and helped to better explain engagement in cervical cancer screening among the participants in this study. Given the overlap between perceived barriers and benefits (HBM) as negative/positive perceptions, enablers, and nurtures, they were grouped within these categories.
Results
The study sample included 20 African American women, 11 who reported having had a Pap test within the past year, and 9 who reported not having a Pap test within the past year (Table 1). The participants ranged in age from 28 to 62 years old, with a mean age of 49 years. Approximately 50% of the participants had less than a high school education. Most (80%) were neither married nor living with a partner, and 92% had at least one child. The length of time since HIV diagnosis ranged from 2 to 25 years, with a mean of 12.5 years. The two groups were similar with regard to their demographic profile.
Table 1.
Demographic and Health Characteristics of Alabama Cervical Health Project Participants
| Group | ||
|---|---|---|
| Variable | Pap w/in past year (n=11) | No Pap w/in past year (n=9) |
| Age, M (SD) | 49.8 (11.0) | 49.0 (5.3) |
| Years since HIV diagnosis, M (SD) | 13.8 (5.8) | 12.4 (6.8) |
| Ever been told you had cervical cancer | ||
| Yes | 0 | 1 |
| No | 9 | 8 |
| Marital status | ||
| Single | 6 (54.5%) | 4 (44.4%) |
| Divorced | 2 (18.2%) | 0 |
| Married | 2 (18.2%) | 1 (11.1%) |
| Living together, but not married | 0 | 1 (11.1%) |
| Separated | 1 (9.1%) | 1 (11.1%) |
| Widow | 0 | 2 (22.2%) |
| Years of school completed | ||
| Less than high school | 6 (54.5%) | 4 (44.4%) |
| High school | 1 (9.09%) | 3 (33.3%) |
| Some college | 4 (36.4%) | 0 |
| Associates degree or higher | 0 | 2 (22.2%) |
| Have children | ||
| Yes | 9 (81.8%) | 9 (100%) |
| No | 2 (18.2%) | 0 |
The qualitative data was arranged into nine categories that resulted when the elements of the Cultural Empowerment domain and the Relationships and Expectations domain are crossed to produce the following constructs: positive perceptions, existential perceptions, negative perceptions, positive enablers, existential enablers, negative enablers, positive nurturers, existential nurturers, and negative nurturers. The data analysis did not reveal substantial evidence for existential perceptions, enablers, and nurturers, therefore, the findings discussed below focus on the six remaining constructs of the PEN-3 model and two constructs of the HBM (perceived susceptibility and perceived severity).
Social construction of cervical cancer
Perceptions (positive)
In order to understand how participants conceptualized their risk for cervical cancer, women were asked how they conceptualized health. When asked what they do to stay healthy, all participants talked about things that can be done to maintain good health in general, such as “eating right” and “exercising.” None of the participants identified “getting a Pap test” as something they needed to do to stay healthy. However, when asked specifically about things they could to reduce their risks for cervical cancer, several participants stated that Pap tests were important for reducing their risk for cervical cancer. For example, I guess having a mammogram, you know, and Pap smears on a regular basis. (Age 57, Pap Test)
The most frequent positive perception among women who reported having a Pap test within the past 12 months and the ones who did not was an intrinsic motivation to know they are well. For example: Just to make sure that everything is what it should be. Surely I don't need anything else, HIV is enough. (Age 40, No Pap Test)
Both groups of participants were aware of the importance of Pap tests. However, women who reported having a Pap test within the past 12 months articulated more clearly that Pap tests are important for the early detection of cervical cancer than women who reported not having a Pap test within the past 12 months. For example a participant who had a Pap test within the last 12 months stated: You need to be trying to find out earlier, you know, in your stages, if you have cancer or not. (Age 62, Pap Test) A participant who had not had a Pap test within the last 12 months stated: Well, mainly because you have the virus and you just want to keep a check on other areas, other things of your body. (Age 45, No Pap Test).
When asked about the importance of Pap tests for HIV-infected women, several participants stated that its was important for women living with HIV disease to get regular Pap tests, because they must be “extra concerned” about their health. For example: …as a woman with HIV, we should just be more diligent and own our health in any form—Pap, dental, mental, breast—whatever it takes, and we just need to be extra concerned about our health. (Age 61, Pap Test)
Despite not having had a Pap test in the last 12 months, more women who reported not having had a Pap test in the past 12 months indicated that they did not have any barriers to getting a Pap test. For example: I might be uncomfortable true enough, but still I'm gonna do it. (Age 60, No Pap Test)
Perceptions (negative)
An equal number of women in both groups revealed that they did not have much knowledge about cervical cancer or risk factors associated with cervical cancer. Even women who had a history of abnormal Pap tests and possible cervical cancer diagnosis had misconceptions about the signs and symptoms of cervical cancer. For example: I don't even really know that much about cervix cancer. All I know I don't want it. (Age 46, Pap Test)
Almost half of the participants who reported not having a Pap test within the past 12 months were not aware of the recommended cervical cancer screening intervals for women in general nor for HIV-infected women specifically. One participant said that she had been told that she needed a Pap test every 10 years: Well, they're telling me, they tell every 10 years. (Age 52, Pap Test)
Most participants were not aware of the age at which cervical cancer screening should be initiated. Several participants believed that girls should begin getting Pap test as early as 12 years old. For example: …Now in these days, up from 12 on up to age, you know what I'm saying, need to start getting a Pap smear. (Age 48, No Pap Test)
A frequently reported barrier to getting Pap tests was the fear that the test would reveal an additional health problem. Several participants indicated that living with HIV disease was enough and that an additional health problem would be a major burden: [I] was scared to have a Pap smear because I'm thinking they're going to see something bad—cancer or something like that. …because there'd be something else that I'd have to be very concerned about. (Age 62, Pap Test)
Several participants stated that they “hated Pap smears” and the term Pap smear caused some participants to “cringe.” For example: Pap smears, I hate them…I know it hurts because I done been there and done that. (Age 50, Pap Test) The pain and discomfort that occurred during a Pap smear was a challenge for some participants. One participant described it this way: Oh, the pain! [Laughing]. You know it's gonna be uncomfortable. (Age 62, Pap Test)
Enablers (positive)
Several participants indicated that the gender of the healthcare provider performing a Pap test was not a barrier to them. For example: Anything that's going to help me, anybody that's going to help me, I don't have a problem with who do. (Age 46, Pap Test)
Some participants pointed out that in the past they did not want a male to perform the Pap test, but that the gender of the healthcare provider was no longer a factor. For example, a participant stated: I went to the health department the other day…they said that we got a male doctor…it don't bother me no more, because, I don't know why it don't bother me no more, but it used to be like, I don't want no man looking at me. (Age 47, No Pap Test)
Enablers (negative)
Although some participants did not have an issue with the gender of the healthcare provider performing the Pap test, other participants did, in particular, some participants from the group that had not had a Pap test in the previous 12 months expressed concerns regarding embarrassment and partner jealously. One such participant noted that some women who find the Pap test embarrassing or who fear partner jealously are…women who their religion or their moral values would not allow another man to look at them, other than their husbands (Age 47, No Pap). This was expressed in the context of themselves as well as HIV-infected women in general.
Nurturers (Positive)
When asked what motivated them to get Pap tests, several participants in both groups mentioned that they received encouragement from family, friends, and spouses. For example, one participant stated: He's [husband] all for me going and getting Pap tests. (Age 42, No Pap Test)
Several participants also indicated that they were motivated to get Pap tests because they wanted to live and be able to take care of their children and their grandchildren. For example, one participant stated, I'll be having two of my very own grandchildren. That's motivation. (Age 48, No Pap Test)
Nurturers (negative)
Women in both groups stated that their family and friends do not talk about Pap tests, however this was reported more frequently by women who reported not having had a Pap test in the past 12 months. For example, one participant stated: my family, they haven't said nothing to me about it. (Age 60, No Pap Test)
Another frequent negative nurturer reported by women in both groups was the fear of stigma and discrimination, and the lack of acceptance by others regarding their HIV-positive status. This included stigma and discrimination from healthcare providers, hospital staff, friends, and family. When discussing a recent experience during a visit to the hospital, one participant stated: There's a lot of stigma and, you know, people, they will treat you differently. (Age 48, No Pap Test)
Perceived susceptibility (negative)
There was a clear distinction between how the participants' perceived their susceptibility to cervical cancer and the cervical cancer susceptibility of HIV-infected women in general. Several participants stated that women living with HIV disease have a higher chance of getting cervical cancer due to having “weaker bodies.” For example, one participant stated, …cause we are more high risk by already having the infection, and we can catch things easier than other women. (Age 62, Pap Test) However, nearly half of the participants (regardless if they had a Pap test within the past 12 months) did not believe that they were more susceptible to cervical cancer than HIV-uninfected women, as exemplified in the following statement by one participant: I don't believe because I'm positive I'll get cervical cancer. (Age 47, No Pap Test)
Several participants believed that they were not susceptible to cervical cancer because they did not have a family history of the disease. For example, one participant stated: Well, I know a lot of people that's HIV positive that's got some form of cancer, but the research that I'm going by is I don't have any family members with cancer, but I always keep my mammogram and my Pap smears done so I won't catch it. (Age 54, Pap Test)
Participants with a history of abnormal Pap tests more frequently indicated they were afraid that that they may get cervical cancer. For example, one participant described it this way: …when I found out that I had an abnormal Pap smear, it kind of scared me, and when they took them cells, you know, I thought maybe it would turn into cancer. (Age 60, No Pap Test)
A few participants thought that they had a higher risk for cervical cancer because of factors other than HIV infection, including a family history of cancer. For example: I might be a candidate for it by my mother and father, died from cancer. (Age 62, Pap Test)
When asked why some HIV-infected women do not get Pap tests, some participants stated that HIV-infected women stop having sex after they receive their HIV diagnosis and therefore feel that they do not need to get a Pap test. One woman stated:…maybe she's thinking that once she was diagnosed, some women stop having sex or whatever and they said, well I don't need a Pap smear. (Age 60, Pap Test)
Perceived severity
When asked what health problems HIV-infected women should be concerned about, participants did not identify cervical cancer as one of them. Even when asked specifically about cancer, most of them did not see its relevance. For example: I don't know. I think it is, but I think with HIV and all the other stuff going on, cancer probably at the back of your head. It's not really in the forefront of your thinking. I mean, just dealing with HIV everyday. You don't really think about cancer. (Age 53, Pap Test)
Discussion
While results from this study confirm previous findings related to knowledge of cervical cancer risk among African Americans in general, some results appear to be specific to the context of the lives of women with HIV disease. Also, there were no major differences between participants who reported having had a Pap test within the past 12 months and the ones who reported not having had a Pap test within the past 12 months.
The PEN-3 Model and the Health Belief Model were used as the theoretical frameworks to identify the perceptions, enablers, and nurturers, perceived susceptibility, perceived severity, and perceived benefits related to cervical cancer and screening among the participants. Lack of knowledge was a major theme of the perceptions of both women who were participating in cervical cancer screening and those who were not. This knowledge deficit was most obvious in that none of the women identified cervical cancer screening as a preventive practice that was needed in order to stay healthy. Most concerning, however, was the finding that the HIV-infected African American women who participated in the interviews lacked knowledge of their increased risk for cervical cancer and were not aware of the recommendations regarding cervical cancer screening for women with HIV disease. These findings are consistent with recent research revealing wide spread confusion about cervical cancer risk and Pap tests among a population of high risk women.42 Such findings suggest that HIV care providers may not be effectively communicating cervical cancer prevention and early detection messages to their clients as all participants had a regular source for health care, and most reported being adherent to their medical appointments. What is not known from these findings is whether the health care clinics where the women received care have specific protocols that address cervical cancer risk among HIV-infected women and their needs for information and education. Cervical cancer education may be offered, but may not be offered in the type or format that would be most relevant to this population. Clearly, further research is needed to determine the extent and type of education HIV-infected women receive regarding cervical cancer risk as well as determine the relevance of such education to the target population including the meaning these women make of such information.
The effect of social support and encouragement of family and significant others as a motivation to perform prevention activities such as cervical cancer screening was another important finding of this study. This finding is consistent with a significant body of evidence emphasizing the role that social networks play in prevention across various risk groups and populations.43–46 Support through social networks may mediate preventive behaviors, such as participation in cervical cancer screening by diminishing perceived barriers.44 On the other hand, family and friends can also present a barrier to cervical cancer screening depending on the context as some participants mention that family members and friends “do not want to talk about it.”
Some findings are likely to be specific to the context of HIV/AIDS. The role that perceived HIV-related stigma and discrimination can play as a barrier to care was an important theme in this study that has been reported in other studies of HIV-infected African American women in the Deep South; in particular, unease about seeking health care from non-HIV providers who may respond negatively when the client discloses his/her HIV serostatus.14,47–49
Of particular concern were two perceptions revealed in this study: (1) not participating in cervical cancer screening is a way to avoid facing another potential major health problems when already feeling burdened and overwhelmed by living with HIV disease; and (2) choosing not to have sex after being diagnosed with HIV eliminates the risk of cervical cancer. Such beliefs must be addressed in any cervical cancer prevention intervention designed for this target population.
Limitations of this study include a small sample size and that all of the participants were recruited from one city in the urban South, with the majority reporting low educational attainment. Therefore, these results may not be generalizable to HIV-infected women who live in rural areas or have a higher educational attainment. In addition, participants were recruited via word of mouth and through clinics serving HIV-infected patients. The participants that self-selected to participate in the study may not be representative of other HIV-infected women in the area. In addition, since the data was collected during in-depth interviews, there is the possibility that the information provided by the participants was effects by social desirability bias and/or recall bias.
To our knowledge, this is the first study to assess the sociocultural and structural factors that effect HIV-infected African American women's uptake of cervical cancer screening. Since the prevalence of HIV infection and cervical cancer among African American women is disproportionately high in the Southern US, the results of this study make a significant contribution to the literature. The results of this study will be critical in the development, implementation, and evaluation of health education interventions that target HIV-infected African American women.
Acknowledgments
This study was supported by a grant from the University of Alabama at Birmingham Cancer Center Core Support Grant (UAB CCSG P30 CA013148) and the University of Alabama at Birmingham Center For AIDS Research, an NIH funded program (P30 AI027767) that was made possible by the following institutes: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH,NIA, NIDDK, NIGMS, FIC, and OAR. The authors would like to thank the participants for their invaluable contributions to the study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Heard I. Prevention of cervical cancer in women with HIV. Curr Opin HIV AIDS 2009. 2009;4:68–73 [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst 2009;101:1120–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mogtomo MLK, Malieugoue LCG, Djiepgang C, Wankam M, Moune A, Ngane AN. Incidence of cervical disease associated to HPV in human immunodeficiency infected women under highly active antiretroviral therapy. Infect Agent Cancer 2009;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stier E. Human papillomavirus related diseases in HIV-infected individuals. Curr Opin Oncol 2008;20:541–-546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keller MJ, Burk RD, Xie X, et al. Risk of cervical precancer and cancer among HIV-infected women with normal cervical cytology and no evidence of oncogenic HPV infection. JAMA 2012;308:362–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris TG, Burk RD, Palefsky JM, et al. Incidence of cervical squamous intraepithelial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results. JAMA 2005;293:1471–1476 [DOI] [PubMed] [Google Scholar]
- 7.Tebeu PM, Major AL, Mhawech P, Rapiti E. The recurrence of cervical intraepithelial neoplasia in HIV-positive women: A review of the literature. Int J STD AIDS 2006;17:507–511 [DOI] [PubMed] [Google Scholar]
- 8.Pantanowitz L, Michelow P. Review of human immunodeficiency virus (HIV) and squamous lesions of the uterine cervix. Diagn Cytopathol 2011;39:65–72 [DOI] [PubMed] [Google Scholar]
- 9.Palefsky J. Cervical human papillomavirus infection and cervical intraepithelial neoplasia in women positive for human immunodeficiency virus in the era of highly active antiretroviral therapy. Curr Opin Oncol 2003;15:382–388 [DOI] [PubMed] [Google Scholar]
- 10.Denny LA, Franceschi S, de Sanjosá S, Heard I, Moscicki AB, Palefsky J. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine 2012;30:F168–F174 [DOI] [PubMed] [Google Scholar]
- 11.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst 2000;92:1500–-1510 [DOI] [PubMed] [Google Scholar]
- 12.Massad LS, Evans CT, Minkoff H, et al. Natural history of grade 1 cervical intraepithelial neoplasia in women with human immunodeficiency virus. Obstet Gynecol 2004;104:1077–1085 [DOI] [PubMed] [Google Scholar]
- 13.Kempf MC, McLeod J, Boehme AK, et al. A qualitative study of the barriers and facilitators to retention-in-care among HIV-positive women in the rural southeastern United States: Implications for targeted interventions. Aids Patient Care STDS 2010;24:515–520 [DOI] [PubMed] [Google Scholar]
- 14.Boehme AK, Moneyham L, McLeod J, et al. HIV-infected women's relationships with their health care providers in the rural deep south: An exploratory study. Heal Care Women Int 2012;33:403–419 [DOI] [PubMed] [Google Scholar]
- 15.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30 [DOI] [PubMed] [Google Scholar]
- 16.Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer 2008;113:2855–2864 [DOI] [PubMed] [Google Scholar]
- 17.Barnholtz-Sloan J, Patel N, Rollison D, Kortepeter K, MacKinnon J, Giuliano A. Incidence trends of invasive cervical cancer in the United States by combined race and ethnicity. Cancer Causes Control CCC 2009;20:1129–1138 [DOI] [PubMed] [Google Scholar]
- 18.Patel NR, Rollison DE, Barnholtz-Sloan J, MacKinnon J, Green L, Giuliano AR. Racial and ethnic disparities in the incidence of invasive cervical cancer in Florida. Cancer 2009;115:3991–4000 [DOI] [PubMed] [Google Scholar]
- 19.Alabama Department of Public Health. HIV Incidence Estimates 2006–2009 - Alabama. Montgomery, AL: Alabama Department of Public Health, 2012 [Google Scholar]
- 20.Massad LS, Seaberg EC, Watts DH, et al. Long-term incidence of cervical cancer in women with HIV. Cancer 2009;115:524–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;156:880–891, W312 [DOI] [PubMed] [Google Scholar]
- 22.ACOG Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 117: Gynecologic care for women with human immunodeficiency virus. Obstet Gynecol 2010;116:1492–1509 [DOI] [PubMed] [Google Scholar]
- 23.Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012;30:F123–F138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firnhaber C, Wilkin T. Human papillomavirus vaccines: Where do they fit in HIV-infected individuals? Curr HIV/AIDS Rep 2012;9:278–286 [DOI] [PubMed] [Google Scholar]
- 25.Andrasik MP, Rose R, Pereira D, Antoni M. Barriers to cervical cancer screening among low-income HIV-positive African American women. J Health Care Poor Underserved 2008;19:912–925 [DOI] [PubMed] [Google Scholar]
- 26.Fletcher FE, Vidrine DJ, Tami-Maury I, et al. Cervical cancer screening adherence among HIV-positive female smokers from a comprehensive HIV clinic. AIDS Behav 2014;18:544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logan JL, Khambaty MQ, D'Souza KM, Menezes LJ. Cervical cancer screening among HIV-infected women in a health department setting. Aids Patient Care STDS 2010;24:471–475 [DOI] [PubMed] [Google Scholar]
- 28.Rahangdale L, Sarnquist C, Yavari A, Blumenthal P, Israelski D. Frequency of cervical cancer and breast cancer screening in HIV-infected women in a county-based HIV clinic in the Western United States. J Womens Health 2010;19:709–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bazargan M, Bazargan SH, Farooq M, Baker RS. Correlates of cervical cancer screening among underserved Hispanic and African-American women. Prev Med 2004;39:465–473 [DOI] [PubMed] [Google Scholar]
- 30.Datta GD, Colditz GA, Kawachi I, Subramanian S, Palmer JR, Rosenberg L. Individual-, neighborhood-, and state-level socioeconomic predictors of cervical carcinoma screening among U.S. black women. Cancer 2006;106:664–669 [DOI] [PubMed] [Google Scholar]
- 31.Hewitt M, Devesa SS, Breen N. Cervical cancer screening among U.S. women: Analyses of the 2000 National Health Interview Survey. Prev Med 2004;39:270–278 [DOI] [PubMed] [Google Scholar]
- 32.Airhihenbuwa CO, Makinwa B, Obregon R. Toward a new communications framework for HIV/AIDS. J Heal Commun 2000;5:101–111 [DOI] [PubMed] [Google Scholar]
- 33.Blitz S, Baxter J, Raboud J, et al. Evaluation of HIV and highly active antiretroviral therapy on the natural history of human papillomavirus infection and cervical cytopathologic findings in HIV-positive and high-risk HIV-negative women. J Infect Dis 2013;208:454–462 [DOI] [PubMed] [Google Scholar]
- 34.Airhihenbuwa CO. A conceptual model for culturally appropriate health education programs in developing countries. Int Q Community Heal Educ 1990;11:53–62 [DOI] [PubMed] [Google Scholar]
- 35.Rosenstock IM. Why people use health services. Milbank Mem Fund Q 1966;44:94–127 [PubMed] [Google Scholar]
- 36.Airhihenbuwa C. Health promotion and disease prevention strategies for African Americans: A conceptual model. In: Health issues in the Black Community. San Francisco, CA: Jossey-Bass Publishers; 1992. p. 267–280 [Google Scholar]
- 37.Beech BM, Scarinci IC. Smoking attitudes and practices among low-income African-Americans: Qualitative assessment of contributing factors. Am J Health Promot 2003;17:240–248 [DOI] [PubMed] [Google Scholar]
- 38.Garces IC, Scarinci IC, Harrison L. An examination of sociocultural factors associated with health and health care seeking among Latina immigrants. J Immigr Minor Health 2006;8:377–385 [DOI] [PubMed] [Google Scholar]
- 39.Scarinci IC, Beech BM, Kovach KW, Bailey TL. An examination of sociocultural factors associated with cervical cancer screening among low-income Latina immigrants of reproductive age. J Immigr Heal 2003;5:119–128 [DOI] [PubMed] [Google Scholar]
- 40.Scarinci IC, Bandura L, Hidalgo B, Cherrington A. Development of a theory-based (PEN-3 and Health Belief Model), culturally relevant intervention on cervical cancer prevention among Latina immigrants using intervention mapping. Health Promot Pract 2012;13:29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White K, Garces IC, Bandura L, McGuire AA, Scarinci IC. Design and evaluation of a theory-based, culturally relevant outreach model for breast and cervical cancer screening for Latina immigrants. Ethn Dis 2012;22:274–280 [PMC free article] [PubMed] [Google Scholar]
- 42.Daley E, Perrin K, Vamos C, et al. Confusion about Pap smears: Lack of knowledge among high-risk women. J Womens Health 2013;22:67–74 [DOI] [PubMed] [Google Scholar]
- 43.Brown DR, Wilson RM, Boothe MA, Harris CE. Cervical cancer screening among ethnically diverse black women: Knowledge, attitudes, beliefs, and practices. J Natl Med Assoc 2011;103:719–728 [DOI] [PubMed] [Google Scholar]
- 44.Luszczynska A, Durawa AB, Scholz U, Knoll N. Empowerment beliefs and intention to uptake cervical cancer screening: Three psychosocial mediating mechanisms. Women Health 2012;52:162–181 [DOI] [PubMed] [Google Scholar]
- 45.Bennett KK, Buchanan JA, Adams AD. Social-cognitive predictors of intention to vaccinate against the human papillomavirus in college-age women. J Soc Psychol 2012;152:480–492 [DOI] [PubMed] [Google Scholar]
- 46.Torres E, Erwin DO, Treviño M, Jandorf L. Understanding factors influencing Latina women's screening behavior: A qualitative approach. Health Educ Res 2013;28:772–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vyavaharkar MV, Moneyham L, Corwin S. Health care utilization: The experiences of rural HIV-positive African American women. J Health Care Poor Underserved 2008;19:294–306 [DOI] [PubMed] [Google Scholar]
- 48.Vyavaharkar M, Moneyham L, Corwin S, Saunders R, Annang L, Tavakoli A. Relationships between stigma, social support, and depression in HIV-infected African American women living in the rural Southeastern United States. J Assoc Nurses AIDS Care JANAC 2010;21:144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moneyham L, McLeod J, Boehme A, et al. Perceived barriers to HIV care among HIV-infected women in the Deep South. J Assoc Nurses AIDS Care JANAC 2010;21:467–477 [DOI] [PubMed] [Google Scholar]