Abstract
Significance: Cerebral ischemia is a major cause of death and disability throughout the world, yet therapeutic options remain limited. The interplay between the cellular redox state and the immune response plays a critical role in determining the extent of neural cell injury after ischemia and reperfusion. Excessive amounts of reactive oxygen species (ROS) generated by mitochondria and other sources act both as triggers and effectors of inflammation. This review will focus on the interplay between these two mechanisms. Recent Advances: MicroRNAs (miRNAs) are important post-transcriptional regulators that interact with multiple target messenger RNAs coordinately regulating target genes, including those involved in controlling mitochondrial function, redox state, and inflammatory pathways. This review will focus on the regulation of mitochondria, ROS, and inflammation by miRNAs in the chain of deleterious intra- and intercellular events that lead to brain cell death after cerebral ischemia. Critical Issues: Although pretreatment using miRNAs was effective in cerebral ischemia in rodents, testing treatment after the onset of ischemia is an essential next step in the development of acute stroke treatment. In addition, miRNA formulation and delivery into the CNS remain a challenge in the clinical translation of miRNA therapy. Future Directions: Future research should focus on post-treatment and potential clinical use of miRNAs. Antioxid. Redox Signal. 22, 187–202.
Introduction
Stroke is one of the leading causes of death worldwide and the most prominent cause of long-term disability (105). Although many clinical stroke trials have been completed, the only efficacious treatment identified to date is early (<4.5 h) thrombolysis (17). One reason for the widespread failure to translate promising findings in animal studies to successful human clinical trials likely resides in the complex interplay between signaling pathways and the potentially short therapeutic window for acute neuroprotection. Recent evidence increasingly supports a role for microRNAs (miRNAs) in the response to cerebral ischemia, as we have reviewed recently (131). The faster post-transcriptional effect of miRNAs, and their ability to simultaneously regulate many target genes, suggests that miRNAs may have a greater therapeutic potential as candidates for the treatment of stroke than therapies targeting a single gene by direct transcriptional control. Further increasing their potential for translation, several miRNAs are already in clinical trials, suggesting that formulation and administration will be straightforward in a new disease setting or for a new miRNA target.
In ischemic stroke, the damage is most rapid and severe in the center of the ischemic territory (ischemic core) where neurons are destined to become necrotic and die within hours after the onset of stroke (78). However, the fate of neurons in the adjacent peri-ischemic (penumbral) area is less certain; they may either maintain metabolic homeostasis, re-establish protein synthesis, and survive via induction of prosurvival and antiapoptotic signaling pathways, or will die at a later period of reperfusion via initiation of proapoptotic pathways (44). This vulnerable region of brain therefore represents a significant target for therapeutic strategies in the poststroke period, which seek to improve clinical outcome by ultimately minimizing the total volume of infarct. Oxidative stress and inflammation are two widely accepted mechanisms of penumbral cell death after cerebral ischemia, which have been reviewed recently (71, 78). The present review will focus on the interplay of these two mechanisms, emphasizing regulation by miRNAs and the central role of mitochondria in regulation of reactive oxygen species (ROS) and inflammation.
Redox in Stroke
Cellular redox homeostasis is crucial for many biological processes. Whereas ROS have key signaling roles in the cell, excess levels can damage essentially all the constituents of the cell. Oxidative stress is defined as an excess production of ROS relative to antioxidant defense. Oxidative stress disrupts essential cellular functions and is implicated in diseases, including stroke, head trauma, and chronic neurodegenerative diseases, as well as aging.
ROS and antioxidants
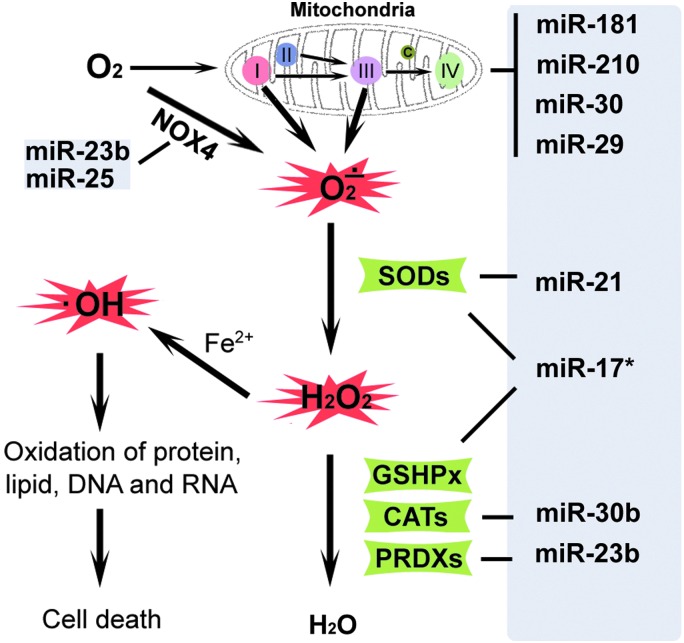
ROS are highly reactive and generally short-lived molecules that are O2-derived free radicals and reactive nonradical species, of which superoxide anion (O2•−), hydroxyl radical ( •OH), and the nonradical species hydrogen peroxide (H2O2), plus some of their reaction products, including peroxynitrite, are most common (Fig. 1). Mitochondria are thought to be the major intracellular source of ROS in most mammalian cells. The respiratory chain is localized in the inner membrane of the mitochondrion and is the main source of superoxide anion in many settings. Among components of the respiratory chain, complex I and III are the main sites of superoxide anion production (42, 90, 95, 117). During respiration, an estimated 1%–2% of the O2 consumed gains an extra electron and is reduced to O2•− (22), which can subsequently be converted to H2O2 (20). •OH, a highly ROS, is produced from H2O2 through the Fenton or Haber-Weiss reactions. In addition to mitochondrial sources, ROS can be produced by other pathways, including the endoplasmic reticulum (ER), where ROS are generated concomitant with oxidative protein folding (110), the NADPH oxidases (15), xanthine oxidase (60), and other oxidases. ROS cause cell injury through oxidation of lipids, protein, DNA, and RNA.
FIG. 1.
Reactive oxygen species (ROS) metabolism and regulation by miRNA. Excessive amounts of superoxide anion (O2•−) are produced in mitochondria, mainly through complex I and III, during ischemia–reperfusion. Superoxide dismutase (SOD) detoxifies O2•− to hydrogen peroxide (H2O2), which is converted to water (H2O) by catalase (CATs) or glutathione peroxidase (GSHPx). Hydroxyl radicals ( •OH), produced from H2O2 through the Fenton or Haber-Weiss reactions, cause cell injury through oxidized lipid, protein, DNA, and RNA. On the right side, miRNAs reported to target mitochondrial protective proteins and antioxidative enzymes are indicated. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Under physiologic conditions, production of ROS is not harmful to cells, as antioxidant systems exist intracellularly and extracellularly to detoxify ROS and protect cells from oxidative damage. Antioxidant defense systems include both enzymatic and nonenzymatic antioxidants. Enzymatic antioxidants include superoxide dismutases (SODs), glutathione peroxidase (GSHPx), catalases (CATs), and peroxiredoxins (PRDXs) (99). SODs comprise a family of metal-containing proteins that catalyze dismutation of O2•− to form H2O2 and O2 (5, 76, 99). Among the SOD family members, SOD1/CuZn-SOD is a copper- and zinc-containing homodimer, primarily localized in the cytoplasm; SOD2/MnSOD is a manganese-containing enzyme exclusively localized in mitochondria; and SOD3/ECSOD is a copper- and zinc-containing tetramer, present largely in the extracellular space (204). H2O2 produced by SODs is also harmful to cells, and is converted to the end product water mainly by GSHPx, CATs, or PRDXs (76, 99). GSHPx-1, present in the cytoplasm and mitochondria of most cells, inactivates peroxides using glutathione as a source of reducing equivalents. In addition to antioxidant enzymes, nonenzymatic antioxidants such as glutathione, NAD(P)H, vitamin C, vitamin E, uric acid, and bilirubin play important roles in the scavenging of ROS.
Immediate increase of ROS after stroke
During brain ischemia–reperfusion, multiple detrimental processes occur concurrently, including overproduction of oxidants, inactivation of detoxification systems, and consumption of antioxidants. These changes disrupt the brain's normal antioxidant defenses (27, 32). Although many ROS have very short half-lives and are difficult to measure directly in the intact brain, several useful techniques have been developed. Electron paramagnetic resonance spectroscopy and hydroxyl radical trapping with salicylate and subsequent analysis by high performance liquid chromatography and electrochemical detection have shown that a rapid increase in ROS occurs during and following ischemia. Hydroxyl radical production was detected in the brain during occlusion and following reperfusion (24, 141, 200). These researchers further demonstrated that mitochondria are an important source of these hydroxyl radicals (141). Oxidative stress has also been demonstrated in cerebral ischemia using hydroethidine (HEt), or by measuring the oxidized products of nucleic acid, lipids, or protein (32). Using HEt and live cell imaging, we found that ROS increased immediately after ischemia-like stress in cultured primary neural cells (126). ROS generation was readily detected using HEt after both focal and global cerebral ischemia (79, 115, 194).
In addition to increased production of ROS, cerebral ischemia also impairs scavenging. Endogenous MnSOD generation was shown to be reduced after ischemia, further aggravating oxidative brain damage (77). SODs have been demonstrated to protect against cerebral ischemic injury (26, 83, 89, 115). Overexpression of SOD1 was protective in in vivo cerebral ischemia models (28, 89, 199). Several laboratories, including ours, have found that overexpression of SOD2 also protects and reduces brain infarction volume after focal ischemia (80) and CA1 delayed neuronal death after global cerebral ischemia (194). Astrocytes have a central role in scavenging ROS because they contain high levels of antioxidants and can initially maintain adenosine triphosphate levels via glycolysis (4, 165). Astrocyte cultures from transgenic mice overexpressing SOD1 show increased resistance to xanthine oxidase/hypoxanthine and the superoxide generator, menadione (33). We found earlier that vulnerability to glucose deprivation injury correlates with glutathione levels (137) and vulnerability to oxidative injury increases with age in astrocytes (138).
Immune response to stroke
Cerebral ischemia engages both innate and adaptive immunity, the two main branches of the vertebrate immune system (1). The innate immune system responds quickly to specific molecular patterns and prepares the adaptive immune response by initiating the inflammatory process. The adaptive immune response leads to generation of antigen-specific lymphocytes, which respond to, and retain over time, long-lasting immunity against specific antigens. Cerebral ischemia induces a rapid, localized innate inflammatory response, which contributes to the early phase of irreversible infarction, and which also includes induction of early immunodepression in the days after a stroke, as well as some signs of long-term proinflammatory activation in the brain [for reviews, see Refs. (8, 14, 40, 166, 189)]. Given both the immediate/innate and sustained/adaptive nature of the immune response to stroke, modulation of different immune mediators may represent a therapeutic strategy targeting the early (necrotic) phase, the later (apoptotic) phase of neuronal cell death following cerebral ischemia–reperfusion, and the longer term recovery phase. However, much remains to be learned about the immune response to stroke.
The immune response is an important element contributing to the fate of the ischemic brain, both early and during long-term recovery. The absence of T cells has been shown by several groups to reduce ischemic injury, although the extent of reperfusion may be important to this effect (193). Paradoxically, poststroke immune suppression is known to render stroke patients vulnerable to infections, including pneumonia and sepsis, acting, in part, by activation of the α7 nicotinic acetylcholine receptor to inhibit neutrophil and macrophage accumulation and function (91). Increased sympathetic activation and impairment of the hypothalamic/pituitary/adrenal axis lead to a reduction in T and B lymphocytes, thereby increasing the risk of diminished neurological outcome and death (78). The immune response to stroke has been extensively reviewed (14, 71, 78), so only those factors directly related to oxidative stress, mitochondria, and miRNAs will be considered in this study. In response to cerebral ischemia, immune-responsive cells within the brain (microglia, endothelial cells, astrocytes, and neurons) act immediately to increase the levels of proinflammatory cytokines (8, 38, 45).
Innate immune response after stroke—cytokines and nuclear factor-kappa B
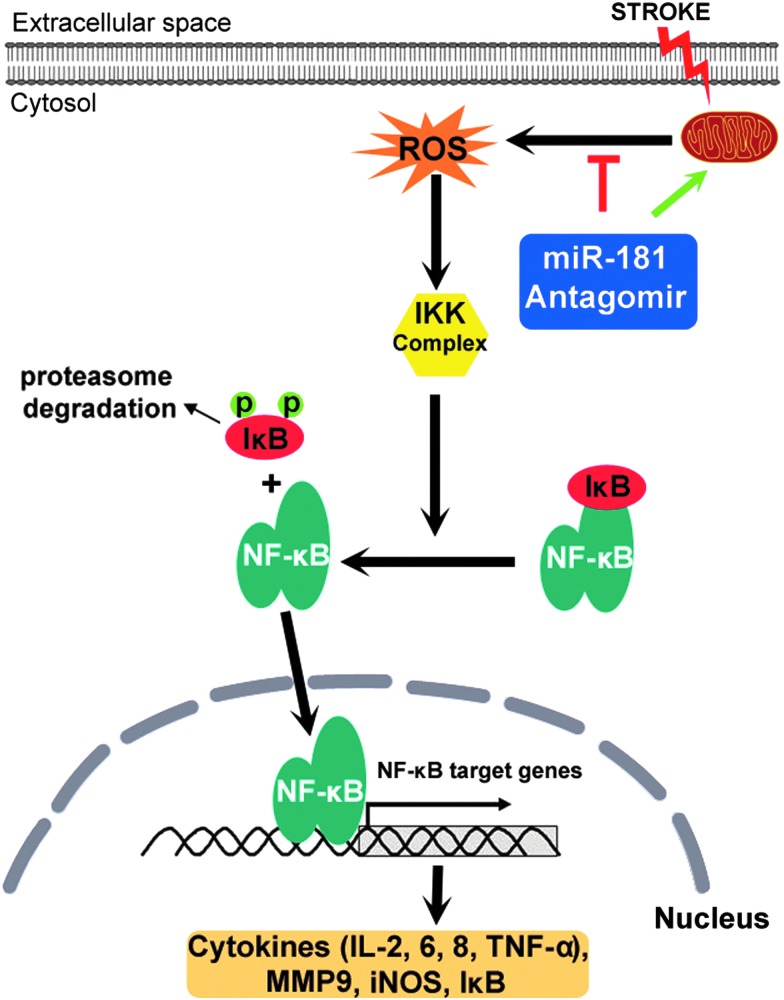
Most notably, tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and IL-6 appear to be produced by resident microglia and have been shown to be key inflammatory mediators contributing to tissue injury in both human and experimental models of stroke (92) (Fig. 2). TNF-α mediates many physiological and pathological cellular processes, including acute and chronic inflammation, infection, and apoptosis (171), whereas IL-6 has been shown to be a strong predictor of early neurological deterioration after stroke and final volume of infarct (25). These cytokines also inhibit mitochondrial function (16, 161), contributing to the impairment of mitochondrial function and increase in oxidative stress following ischemia. These inflammatory cytokines increase within the therapeutic window (<4.5 h) after the onset of experimental stroke (187), but remain elevated for up to 72 h, likely due to persistent activation of microglia (8). A key element of microglial-mediated inflammation is activation of the master inflammatory transcription factor nuclear factor-kappa B (NF-κB), which also plays a role in the control of apoptosis (59, 66) (Fig. 2).
FIG. 2.
Nuclear factor-kappa B (NF-κB) proinflammatory signaling pathway and regulation by miR-181. NF-κB, a dimer often consisting of p50/p65 subunits, is normally resident in the cytosol and is maintained in an inactive form by its inhibitor IκB. Stroke stimulates mitochondria to release ROS that activate the IκB kinase (IKK) complex. The activated IKK complex phosphorylates IκB and initiates its ubiquitination and degradation, freeing NF-κB to translocate to the nucleus, and binds the promoters of genes expressing proinflammatory cytokines, IκB, and other targets. miR-181 interacts with this pathway at multiple points. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
NF-κB is a family of dimeric transcription factors that regulate the transcription of hundreds of genes in a coordinated manner in response to an inducing signal. In resting cells, NF-κB is found primarily in the cytosol bound to its inhibitor—IκB proteins. Upon stimulation by cytokines or other inducers, IκB proteins are targeted for proteasomal degradation by the IκB kinase. Once IκB degrades, NF-κB translocates to the nucleus and binds DNA at κB sites in the regulatory region of proinflammatory genes and promotes their transcription (49, 61). Its target genes include its own inhibitors and other regulatory proteins that form a complex network that tightly regulates the dynamic response and determines which of the downstream genes are transcriptionally activated. An ordinary differential equation computational model of NF-κB activation specific for microglia has been developed recently to better understand the regulation of NF-κB at a systems level in this individual cell type (151).
Adaptive immune response after stroke and the role of cytokines
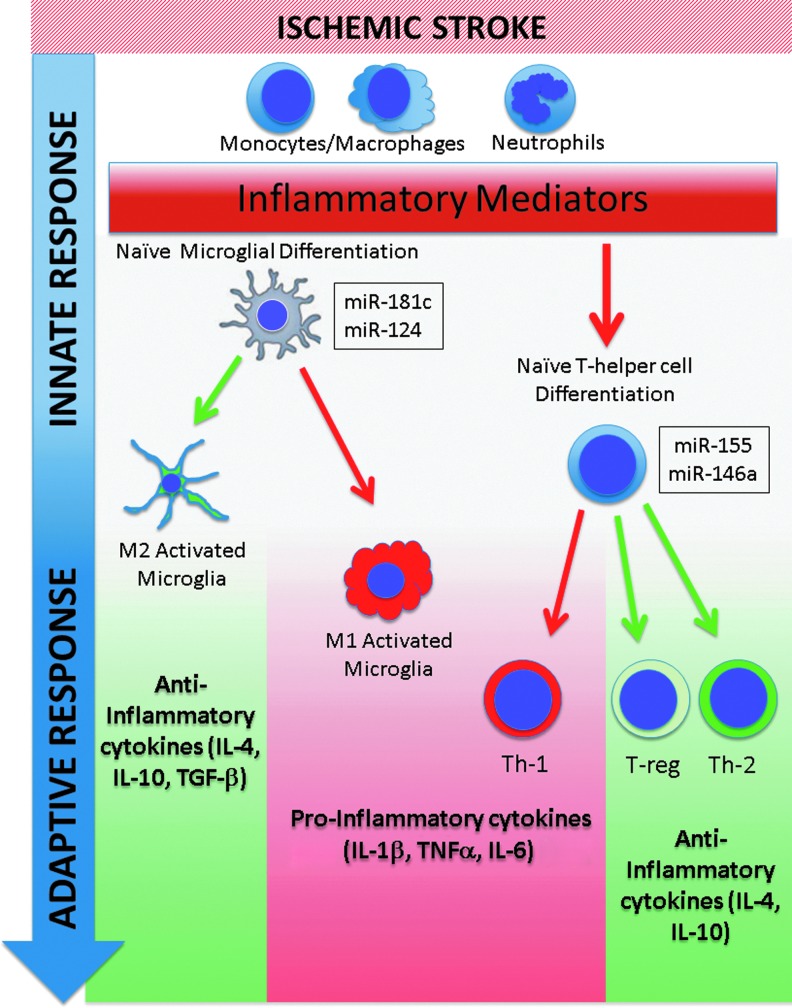
The adaptive or acquired immune response maintains a sustained response to specific antigens (Fig. 3). Support for involvement of adaptive immunity in stroke outcome is derived from studies investigating the role of lymphocytes in models of focal cerebral ischemia. Ischemia triggers infiltration of lymphocytes into the ischemic brain, which contribute to injury (48, 52). Lymphocyte-deficient mice are protected from ischemic damage (52, 70, 87). The protection has been attributed to the absence of T cells, as lymphocyte-deficient mice supplemented with reconstituted T cells, but not B cells, are no longer protected from injury. Undifferentiated T-helper lymphocytes have the capacity to differentiate into proinflammatory Th1 or anti-inflammatory Th2 and T-regulatory (T-reg) T-helper phenotypes, and increased Th2 induction appeared protective (64, 188). Recent work suggests that altering the ability to induce Th1 and Th2/T-reg lymphocyte phenotypes plays a large role in the outcome after stroke. The absence of T-regs increased delayed brain damage after stroke and worsened functional outcome (98). Gu et al. recently demonstrated that mice with impaired Th1 immunity were protected from experimental cerebral ischemia, while decreased Th2 polarization aggravated brain injury (52, 192). This effect appears to be due to alterations in the expression profile of an array of anti-inflammatory mediators associated with the Th2/T-reg response, including IL-4, IL-10, and transforming growth factor beta (TGF-β), a pleiotropic growth factor found in activated microglia and macrophages (63, 98, 100).
FIG. 3.
microRNAs modulate the immune response to stroke. Immediately following stroke, the innate immune system triggers a localized inflammatory response, which then activates the adaptive immune response. The adaptive immune response can promote a proinflammatory state, exacerbating outcome, or can inhibit proinflammatory activation, depending on microglial and T-helper cell subtype differentiation, and local environmental cues. To date, several miRNAs (miR-124, miR-146a, miR-155, and miR-181c) appear to modulate both the innate and adaptive immune responses to stroke and may provide a therapeutic avenue for improving clinical outcome. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In addition to altering T-cell polarization, cytokine production has recently been shown to contribute to polarization of macrophages and microglia in the evolution of the inflammatory response after stroke. When stimulated with lipopolysaccharides or interferon-γ, macrophages and microglia undergo transformation to an M1 phenotype characterized by production of proinflammatory cytokines, while stimulation with IL-4 or TGF-β leads to a neuroprotective M2 phenotype. When markers of macrophage/microglial activation were characterized in a model of permanent focal ischemia, expression was shown to vary with time after occlusion, consistent with early M2 polarization and subsequently followed by later M1 polarization and phagocytic morphology (140). A second study investigating polarization of macrophages and microglia after transient middle cerebral artery occlusion (MCAO) documented a similar evolution over time (67).
Anti-inflammatory cytokines appear to protect neurons both directly and indirectly by altering the response of immune cells in the brain and periphery. Male IL-4 knockout mice had a greater Th1/Th2 ratio, larger infarct volume, and worsened neurologic outcome following MCAO compared with wild-type mice (192). Intracerebroventricular administration of exogenous IL-4 reduced injury to that seen with wild-type mice and decreased activation of immune-responsive microglia and astrocytes adjacent to the infarct. A recent study by Engelbertsen et al. demonstrated that an increase in the Th2 population and circulating IL-4 levels in humans are independently associated with reduced levels of myocardial infarction and stroke (43). Furthermore, whereas IL-10 reduces the proinflammatory response after ischemic stroke by acting on glia, endothelium, and immune cells, it has also been shown to provide protection to primary cortical neurons in culture by activation of the AKT/PI3K prosurvival pathways (150).
TGF-β has been shown to be neuroprotective both in vitro and in vivo (65). The spatial and temporal expression of TGF-β in activated microglia and macrophages within the ischemic penumbra suggests that microglial-derived TGF-β functions both in inhibiting cell death and in promoting neuronal regeneration following focal cerebral ischemia (41, 182). These findings suggest that promoting a Th2/T-reg adaptive response may serve to inhibit a proinflammatory response and augment an anti-inflammatory response, thereby providing neuroprotection after stroke, a theory supported by findings using neuropeptides to induce anti-inflammatory activation in other neuroinflammatory and neurodegenerative models (184). However, the deleterious effect of peripheral immune suppression also needs to be taken into account in developing strategies that could also increase immune suppression following stroke. Despite this, lymphocyte-targeted neuroprotection is still an exciting possibility that might simultaneously target multiple immune-responsive bioactive molecules to improve outcome.
Important Roles of Mitochondria in Stroke
Mitochondria are centrally involved in both ischemic injury and recovery of brain tissue after cerebral ischemia due to their major roles in ROS production, regulation of inflammation and apoptosis, and role in neurogenesis (107, 175). This is reflected in observations that mitochondrial protection is associated with reduced brain injury and improved neurogenesis following cerebral ischemia.
Interplay between mitochondrial ROS and inflammation
Ischemia leads to significant mitochondrial dysfunction (156) (Fig. 4). Unsaturation of cytochrome c oxidase at the terminus of the electron transport chain disrupts mitochondrial respiratory function, including the synthesis of ATP, and leads to overproduction of ROS (128) by complex I and III (Fig. 1). ROS interact with NF-κB at various places within the signaling pathway, and can even have opposing effects on NF-κB activation in the cytosol versus the nucleus [For reviews, see Morgan and Liu (113) and Siomek (159)]. Conversely, NF-κB has both pro-oxidant inflammatory targets and antioxidant targets. ROS produced by mitochondria can trigger NF-κB activation (Fig. 2) and synthesis of target messenger RNAs (mRNAs). These include adhesion molecules that promote localization of circulating leukocytes (neutrophils, lymphocytes, and macrophages) to the ischemic region, induction of iNOS, MMPs, and proinflammatory cytokines, as well as maturation of undifferentiated T-helper lymphocytes into a proinflammatory Th1 subtype (Fig. 3), further contributing to the production of inflammatory cytokines (8).
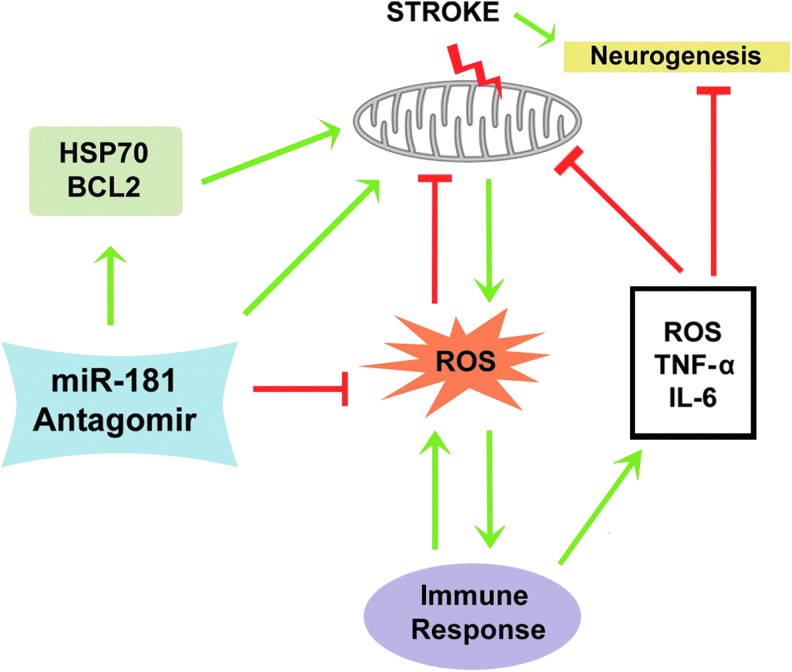
FIG. 4.
miR-181 influences immune response, mitochondria, and ROS. Stroke leads to increased proneurogenic signals, but also dysfunction of mitochondria, which inhibits neurogenesis and increases ROS. Overproduction of ROS triggers immune response and the release of inflammatory factors, such as tumor necrosis factor alpha (TNF-α) and IL-6 as well as further increasing ROS, causing additional mitochondrial damage. miR-181 antagomir can increase mitochondrial protective proteins (HSP70 family members and BCL2 family members) to reduce ROS production and inhibit inflammation, thereby efficiently regulating multiple ischemic cell death pathways and facilitating neurogenesis. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The inflammation triggered by ROS may lead to disruption of mitochondrial homeostasis (148). Activated microglia appear to induce neuronal death via mitochondrial dysfunction both in vitro (191) and in vivo (69), likely mediated by microglial-derived IL-6, TNF-α, and nitric oxide (57, 142, 147). IL-6 augments production of ROS (16), which impair oxidative phosphorylation via oxidation of mitochondrial lipids and respiratory enzymes (56, 177). TNF-α induces mitochondrial damage through suppression of mitochondrial complexes I and IV and pyruvate dehydrogenase (149, 161, 205), while nitric oxide inhibits complex IV (21, 51). All of this may explain the secondary mitochondrial failure with longer reperfusion after cerebral ischemia (157). Taken together, these results support the central role of mitochondria in the cascade of ROS and inflammation-induced neurotoxicity.
Mitochondria and neurogenesis
Following central nervous system development, the birth of neurons in the adult brain is restricted to two regions, the subventricular zone and the subgranular zone of the hippocampal dentate gyrus (47). Recent studies have suggested that this neurogenesis is important in the consolidation of memories (86), and that disruption of neurogenesis via irradiation is detrimental to memory and cognitive function (112, 145). After stroke, although neural progenitor cells proliferate, very few survive to replace the lost neurons (7). Furthermore, increasing neurogenesis using factors such as fibroblast growth factor 2 promotes poststroke functional recovery (55, 94).
Recent studies suggest that mitochondrial function may have a significant direct impact on neurogenesis. Neural progenitor cells from aged brains, which have impaired neuronal production, have decreased mitochondrial proteins and oxygen consumption (162). Notably, mitochondrial DNA damage results in attenuated neurogenesis. Neural progenitor cells lacking 8-oxoguanine glycosylase, a protein needed for repair of mitochondrial DNA damage, have decreased neurogenesis and increased astrogenesis (180), likely due to decreased mitochondrial metabolism (181). In addition, embryonic stem cells with mutations in mitochondrial DNA exhibit impaired neuronal differentiation (84). Multiple studies have found that alterations in Krebs cycle proteins are associated with dysfunctional neurogenesis. Mutations in the electron transfer flavoprotein lead to attenuated neurogenesis via the PPARG-ERK pathway (160), and mice lacking either dihydrolipoamide dehydrogenase (Dld) or dihydrolipoyl succinyltransferase (E2k), subunits of the essential mitochondrial enzyme oxoglutarate dehydrogenase complex, have decreased hippocampal neuroblasts compared with wild-type mice (23). Additionally, P19 cells deficient in the mitochondrial protein, frataxin, exhibit increased apoptosis and decreased neuronal differentiation following induction of neurogenesis (136).
ROS and inflammation are detrimental to neurogenesis (112, 145, 175). NeuroD6, a transcription factor that promotes survival of newborn neurons, acts by preserving mitochondrial function after exposure to ROS (172) and increasing mitochondrial mass (13). Several studies have also shown that proinflammatory factors produced by activated microglia, such as IL-6, TNF-α, and nitric oxide, lead to mitochondrial dysfunction in progenitor cells and subsequent inhibition of neurogenesis (175). Our laboratory has directly examined the influence of mitochondrial dysfunction on neurogenesis (176). Treatment of progenitor cells in vitro with antimycin-A, a mitochondrial inhibitor, significantly decreased expression of doublecortin (Dcx) - expressing immature neurons and MAP2- expressing mature neurons. Similarly, conditioned media from activated microglia decrease Dcx-positive cells, while overexpression of the mitochondrial-specific heat-shock protein (HSP) GRP75 partially rescues these cultures, suggesting a significant role of mitochondrial dysfunction in inflammation-induced neurogenesis impairment.
Protecting mitochondrial function
Two families of well-known cell protective proteins, the HSP70 family of protein chaperones and the antiapoptotic BCL2 protein family, are also related to mitochondrial function. Both have also been shown to protect the brain from ischemia when overexpressed. HSP72, the inducible cytosolic member of the HSP70 family, is known to protect from both necrotic and apoptotic cell death, and affects several different steps in the apoptosis cascade, including reduction of mitochondrion-dependent apoptotic signaling [see Fig. 1 in (50)]. Several studies have shown that overexpression of GRP75/HSP75/mortalin, the mitochondrial-specific member of the HSP70 family, reduces damage in both in vitro and in vivo models of ischemic stroke (174, 195). The mechanisms of GRP75 protection against ischemia include attenuated oxidative stress, preservation of mitochondrial function, inhibition of apoptosis, and enhanced neurogenesis. For more detailed information on the protective effect of GRP75 on ischemic brain injury and the mechanisms involved, the reader is referred to a recent book chapter (185). GRP78/HSP78/BIP, another member of the HSP70 family, is largely localized to the ER and is a master regulator of the unfolded protein response. Overexpressing GRP78 protects neural cells against ischemic injury, preserves the respiratory activity and mitochondrial membrane potential, and reduces ROS production after ischemia-like stress (132). Interestingly, GRP78 relocates from ER to mitochondria shortly after stress (134, 163). It is increasingly accepted that the HSP70 family members, together with other molecular chaperones, are organized in a chaperoning network serving as a basic regulatory mechanism in diverse cellular functions [for a recent review, see Ouyang and Giffard (134)].
The BCL2 protein family (2) is a principal regulator of apoptosis through mitochondrial membrane integrity, function, and apoptotic signaling (139). The BCL2 protein family consists of three subgroups: the prosurvival proteins (BCL2, BCLxL, BCLw, MCL1, and A1), the multidomain proapoptotic proteins (BAX and BAK), and the BH3 domain-only proapoptotic proteins (BIM, PUMA, BID, BAD, BIK, BMF, HRK, and NOXA). In response to stress, the decision whether or not to undergo apoptosis is determined by interactions between these three groups. BH3-only proteins are upregulated in response to apoptotic stimuli and transduce the damage signal. In addition, they inhibit antiapoptotic proteins and activate proapoptotic proteins resulting in mitochondrial outer membrane permeabilization, cytochrome c release, and activation of caspases to initiate apoptosis (202). Overexpressing prosurvival BCL2 family members protects against cerebral ischemia in vivo (85, 209) and in vitro (126). Together, these results show that maintaining mitochondrial function is integral for neuroprotection [for review, see Ouyang and Giffard (127)].
Interestingly, HSP72 and BCL2 share a close relationship. HSP72 overexpression increases the expression of BCL2 in vitro and in vivo (81). The HSP70 family members and the BCL2 family members coexist in the mitochondrion-associated ER membrane, and affect ER and mitochondrial calcium homeostasis after cerebral ischemia [(134) and Fig. 2 in (131)].
miRNAs in Stroke
Multitarget therapeutic strategies offer a potential solution to the translational barrier that has burdened research in the treatment of stroke. Given that a single miRNA can theoretically bind to and inhibit a large number of related targets, the potential for miRNA modulation in cerebral ischemia is promising. miRNAs are small (∼22 nucleotides) noncoding RNAs that participate in mRNA translational regulation. Despite their relatively recent discovery, it is already known that miRNAs play important roles in ischemic disease. Changes in miRNAs with ischemic brain injury have been identified using miRNA profiling techniques in a rat focal cerebral ischemia model (39, 74, 102), in forebrain ischemia (203), and in stroke patients (167). Recently, a few studies have evaluated the significance of individual miRNAs and their regional expression in ischemic brain damage [for a recent review, see Ouyang et al. (131)]. This section will focus on miRNA modulation of ROS, inflammation, and mitochondrial function.
miRNAs modulate cellular redox state
miRNAs have recently been found to be critical regulatory molecules in cellular redox regulation (Fig. 1). Mitochondria are the main site of ROS production and Shi and Gibson (155) reported that post-transcriptional upregulation of the mitochondrial enzyme malate dehydrogenase by oxidative stress in a neuronal cell line is mediated by miRNA-743a, providing insight into possible roles of miRNAs in oxidative stress. miR-338 regulates multiple mitochondrial mRNAs that encode subunits of the oxidative phosphorylation machinery (9). miR-145 protects cardiomyocytes against H2O2-induced apoptosis through targeting the mitochondrial apoptotic pathway (97). In addition to regulation of mitochondrial targets, there are already a few studies noting that miRNAs regulate other sources of ROS. Type 4 NADPH oxidase (NOX4) is a direct target of miR-23b in the spinal cord (72) and of miR-25 in the heart and kidney (46, 173). miRNAs also influence the ROS defense system. miR-21 inhibits the metabolism of superoxide to H2O2 by directing attenuating SOD3 or by indirectly reducing SOD2 levels (208). In an epithelial cell line, miR-30b regulates ROS levels by targeting CATs (58). miR-17* suppresses tumorigenicity of prostate cancer by inhibiting mitochondrial antioxidant enzymes such as MnSOD and GSHPx (197). In addition, miR-23b downregulates PRDX3 in human prostate cancer (62). miR-210, nicknamed “the hypoximir,” is strongly induced in response to hypoxia and represses mitochondrial respiration, in addition to several other important effects (30).
Oxidative stress can also alter the miRNA expression profile (196). This suggests they can be part of a feedback mechanism on overall ROS regulation. Possible feedback between miRNA and ROS has recently been reviewed (109). Nelson et al. reviewed accumulating evidence for the roles of miRNAs and discussed a possible involvement of miRNA oxidation in the pathogenesis of neurodegenerative disorders (120). It has been hypothesized that RNA oxidation causes aberrant expression of miRNAs and proteins, subsequently initiating inappropriate cell fate choices (124).
Direct roles for miRNAs in neurogenesis
Several recent articles have highlighted potential ways in which miRNA manipulation may influence neurogenesis. During normal development, a specific miRNA profile is expressed in the cerebral cortex, suggesting a role for miRNAs in brain maturation (121). The function of miRNAs in poststroke neurogenesis has also been examined. Following focal cerebral ischemia in rats, progenitor cells in the subventricular zone express decreased levels of miR-124a (104). The same study showed that, in vitro, overexpression of miR-124a in progenitor cells decreases expression of the Notch ligand JAG1 and subsequently decreases proliferation and increases neuronal differentiation. The miR-17-92 cluster is also overexpressed in adult mouse neural progenitor cells after stroke, which increases proliferation in vitro (103). Interestingly, the miR-17-92 cluster targets phosphatase and tensin homolog (PTEN), and is increased by the neural patterning protein Sonic hedgehog (103).
miRNAs modulate the immune response
Recent work has begun to reveal many ways in which miRNAs regulate the immune system, including regulating the development of immune cells as well as modulating the innate and adaptive immune responses (106) (Figs. 2 and 3). Two well-studied miRNAs appear to coordinate both the innate and adaptive arms of the immune response (Fig. 3). miR-155 is rapidly induced in mice treated with the strongly proinflammatory agent lipopolysaccharide, and appears to augment the expression of innate inflammatory cytokines via repression of the negative regulators of inflammation, suppressor of cytokine signaling 1 (SOCS1) (6). However, miRNA-155 also promotes an adaptive proinflammatory immune response by coordinating T-cell development (125). A second miRNA, miR-146a, appears necessary in suppressing inflammatory cytokine production, including IL-6, IL-1β, and TNF-α (18). The miR-146a promoter contains two consensus NF-κB sites that are essential for the transcriptional activation of the miR-146a gene in response to inflammatory stimuli [for a recent review, see Boldin and Baltimore (19)]. By repressing TNF receptor-associated factor 6 (TRAF6) and IL-1 receptor-associated kinase 1 (IRAK1) molecules, miR-146a acts as a negative regulator of the NF-κB pathway and an important regulator of toll-like receptor signaling (19). On the other hand, miR-146a-deficient mice display a spectrum of immunoproliferative and autoimmune diseases (18), indicating a regulatory role in lymphocyte proliferation and differentiation, contributions that serve to define the adaptive immune response.
T-helper lymphocytes serve to both activate and suppress inflammatory components of the immune system, and preliminary research suggests that miRNAs play an important role in the development, differentiation, and activation of specific subpopulations of T-helper lymphocytes [for review, see Contreras and Rao (36)]. Early observations from a global knockout of Dicer, a key regulatory enzyme in the processing of miRNAs, indicated that miRNAs were necessary in T-cell development and differentiation (35, 114). Subsequently, Liston et al. demonstrated that specific knockout of anti-inflammatory T-reg cells leads to a lethal and severe autoimmune disease in mice (101). Interestingly, both miR-155 and miR-146a appear to regulate T-reg function. For example, miR-155/T-reg-deficient mice have impaired survival compared with wild-type T-reg mice (108) and miR-146a knockout mice display a hyperinflammatory phenotype (18, 210). These studies highlight the relevance of miRNAs in differentiation of the T-helper lymphocyte lineage, and offer insight into potential neuroprotective therapeutic strategies whereby modulation of specific miRNAs effects systemic immune signaling and inflammation. Involved in redox and immune response, miRNAs and their targets are summarized in Table 1.
Table 1.
miRNA Involved in Redox and Immune Response and Their Targets
| miRNAs | Targets | References |
|---|---|---|
| miR-1 | HSP60 | (135) |
| miR-15b | Arl2, BCL2 | (122, 153) |
| miR-17* | SOD2, GPX2, TrxR2 | (197) |
| miR-17-92 | PTEN | (103) |
| miR-21 | SOD3, TNF-α | (208) |
| miR-23b | PRDX3, NOX4 | (62, 72) |
| miR-24-2 | BCL2 | (158) |
| miR-25 | MCU, NOX4 | (46, 111, 173) |
| miR-29a | PUMA | (133) |
| miR-29b | BCL-w, BIM, BMF, HRK, PUMA, BAK | (88, 152) |
| miR-30a | P53 | (96) |
| miR-30b | Catalase, p53 | (58, 96) |
| miR-30e | UCP2, BCL2 | (75, 82) |
| miR-34a | BCL2 | (82) |
| miR-92a | BIM | (123) |
| miR-124a | JAG1 | (104, 143) |
| miR-125b | BCL2, MCL1, BAK1 | (154, 206) |
| miR-128 | BAX | (3) |
| miR-141 | Slc25a3 | (12) |
| miR-145 | Bnip3 | (97) |
| miR-146a | TRAF6, IRAK1 | (18, 73, 210) |
| miR-155 | SOCS1 | (108, 125, 168) |
| miR-181a | BCL2, MCL1, GRP78, c-Fos, | (93, 129, 130, 190) |
| miR-181a-1* | BCL2 | (82) |
| miR-181b | Importin-α3 | (164) |
| miR-181c | Mt-COX1 | (207) |
| miR-181d | BCL2 | (183) |
| miR-195 | BCL2 | (158) |
| miR-210 | ISCU1/2, SDHD | (29, 30, 116, 144) |
| miR-320 | HSP20 | (146, 186) |
| miR-338 | COXIV, ATP5G1 | (9, 10) |
| miR-365-2 | BCL2 | (158) |
| miR-378* | HSP72 | (170) |
| miR-451 | BCL2 | (119) |
| miR-484 | Fis1 | (179) |
| miR-491-5p | BCL-xL | (53) |
| miR-497 | BCL2, BCL-w | (198, 201) |
| miR-499 | Calcineurin | (178) |
| miR-711 | HSP72 | (170) |
| miR-743 | Mdh2 | (155) |
| miR-885-3p | BCL2 | (68) |
In addition to systemic therapeutic strategies, several miRNAs appear to be selectively upregulated in the brain subsequent to inflammation [for review see Thounaojam et al. (169)]. The miR-181 family is one of the key regulators of immune response (31). In addition to its established role in T- and B-cell development, miR-181 also affects human natural killer cell development by regulating Notch signaling (34). miR-181a inhibited the secretion of IL-6 and TNF-α, and up-regulated IL-10, an important anti-inflammatory cytokine, targeting c-Fos (190). miR-181b has been identified as an essential regulator of downstream NF-κB signaling, endothelial cell activation, and vascular inflammation in vivo by directly targeting importin-α3, a protein critical for NF-κB nuclear translocation (164). miR-181 is also implicated in controlling viral infection (54) and plays an important role in systemic lupus erythematosus pathogenesis (93). The miR-181 family also targets the mitochondrial protective system as discussed below in the miRNAs Regulate Mitochondrial Function section.
miRNAs are thought to have distinct functions in different cell types. As described above, activated microglia play a role in disruption of mitochondrial homeostasis, contributing to neurotoxicity via production of proinflammatory cytokines such as TNF-α. Ponomarev et al. demonstrated that miR-124 was specifically expressed in quiescent microglia, but not in peripheral monocytes, and was subsequently downregulated in activated microglia (143). They identified a CCAAT enhancer-binding protein thought to play a role in microglial activation as a potential target. Recently, Zhang et al. reported TNF-α as a potential target for miR-181c in a model of microglial-mediated neurotoxicity, whereby inhibition of miR-181c decreased neuronal apoptosis induced by TNF-α (207).
Astrocytes contribute to neuroprotection by several mechanisms (11). Tarassishin et al. observed a decrease in miR-155 in astrocytes concurrent with suppression of proinflammatory cytokines, suggesting a proinflammatory role for miR-155 (168). More recently, Iyer et al. reported that the expression of miR-146a in human astrocytes was significantly upregulated by IL-1β (73). Interestingly, as noted above, miR-146a and miR-155 are also implicated in the differentiation and maturation of anti-inflammatory T-helper lymphocytes. Moreover, miRNAs are emerging as significant upstream modulators of neuropsychiatric disorders that contain a neuroinflammatory component, such as Alzheimer's disease, Parkinson's disease, Huntington's disease, multiple sclerosis, and traumatic brain injury (36). Exploration of miRNAs in these disorders may yield further insight into the role miRNAs play in the pathogenesis of immune-mediated inflammation in the CNS after stroke.
miRNAs regulate mitochondrial function
In recent years, multiple studies have shown that miRNAs target a variety of mitochondrial and mitochondrial-associated proteins and may be a useful tool for the manipulation of mitochondrial function (Fig. 4). miR-181c is encoded in the nucleus, assembled in the cytoplasm, translocated into the mitochondria, and targets cytochrome c oxidase subunit 1 (mt-COX1) mRNA (37). It causes electron transport chain complex IV remodeling and influences mitochondrial function. Some other miRNAs, such as the miR-30 family and miR-210, target multiple mitochondrial proteins and could impact mitochondrial functioning using several mechanisms. miR-30a and miR-30b decreases p53 and mitochondrial fission via dynamin-related protein-1 (96), while miR-30e targets mitochondrial uncoupling protein 2, contributing to kidney fibrosis (75).
miR-210 has multiple mitochondrial-related targets, such as the iron–sulfur cluster assembly enzyme (116) and succinate dehydrogenase complex subunit D, a component of the electron transport chain (144). miRNAs also target several genes required for ATP production, such as solute carrier family 25 member 3 [miR-141, (12)], COX-IV [miR-338, (10)], and ADP-ribosylation factor-like protein 2 [miR-15b, (122)]. Additional miRNAs decrease production of proteins that affect susceptibility to apoptosis, such as the mitochondrial calcium uniporter [miR-25, (111)], the calcineurin catalytic subunit [miR-499, (178)], and mitochondrial fission Fis1 protein [miR-484, (179)].
Thus, miRNAs can have a substantial impact on mitochondrial functioning. As mitochondria have multiple essential roles in the cell, these miRNAs may be used to alter cell functioning in both normal and injury/disease states, including brain ischemia. As described below, manipulation of mitochondrial proteins by miRNAs is a promising therapeutic tool to encourage neurogenesis, HSP-induced protection, and antiapoptotic mechanisms in the ischemic brain.
miRNAs and mitochondrial protective proteins
Recent research has shown that several miRNAs that target HSPs may be manipulated to improve the outcome after injury. miR-1, which exacerbates cardiac ischemia–reperfusion injury, decreases expression of HSP60 (135). Cardiac ischemia preconditioning, which protects against ischemic injury, decreases miR-378* and miR-711, both of which target the HSP70 family (170). Overexpression of miR-320 in cardiomyocytes enhances ischemia–reperfusion injury, in part, via downregulation of HSP20 (146). Finally, Gefitnib, a drug that decreases HSP72 expression via miRNA-mediated mechanisms, exacerbates interstitial lung disease (118).
Research has shown that BCL2 is targeted by multiple miRNAs, including miR-195, miR-24-2, and miR-365-2 (206), miR-125b (154), miR-885-3p (68), miR-181a-1*, miR-30e, and miR-34a (82), miR-451 (119), and miR-181d (183). Chronic exposure of neurons to alcohol increases the levels of miR-497, leading to apoptosis by targeting BCL2 (198). miR-15b, which is upregulated 72 h following MCAO, targets BCL2 as well (153). BCL-xL, another antiapoptotic member of the BCL2 family, is targeted by miR-491-5p (53). In addition, proapoptotic BAX is targeted by miR-128 (3) and BIM is decreased by miR-92a (153). We found that miR-29a targets BH3-only protein PUMA and reduces neuronal vulnerability to forebrain ischemia (133). In contrast, increasing miR-29b had the effect of promoting neuronal cell death in focal ischemia by inhibiting BCL-w, an antiapoptotic member of the BCL2 protein family (152).
A few miRNAs target multiple members of the BCL2 family. In addition to BCL2 (154), miR-125b decreases both proapoptotic BAK1 along with the antiapoptotic gene MCL1 (206). miR-497 also targets BCL-w in addition to BCL2 in Neuro-2A cells (201). Knockdown of miR-497, which targets both BCL2 and BCL-w (201), is protective against MCAO-induced neuronal death. Our laboratory has focused extensively on miR-181a, which downregulates both BCL2 and MCL1, antiapoptotic members of the BCL2 family. Indeed, overexpression of miR-181a in astrocytes subjected to glucose deprivation decreases mitochondrial membrane potential, increases ROS formation, and increases cell death (129). A group has reported that miR-29b is activated during neuronal maturation and targets several proapoptotic genes, BIM, BMF, HRK, PUMA, and BAK in the BCL2 family (88). In addition to targeting antiapoptotic members of the BCL2 family, miR-181 also targets the ER protein GRP78/HSP78. In our laboratory, we have found that knockdown of miR-181a, which increases in the ischemic core during reperfusion following transient MCAO, increases GRP78 levels in the brain and significantly decreases the infarct size following stroke (130). As miR-181a also targets BCL2 and MCL1 (129), it is likely that the protective phenotype of mice treated with the miR-181a antagomir results from enhanced protein levels of GRP78, BCL2, and MCL1. These data show that miRNAs targeting HSPs and/or BCL2 family members are promising candidates in the treatment of stroke by enhancing the mitochondrial function.
Future Directions
miRNAs may have greater therapeutic potential as candidates for the treatment of stroke than therapies targeting a single gene because of their faster post-transcriptional effect and their ability to simultaneously regulate many target genes. A single miRNA, such as miR-181, can simultaneously regulate target genes affecting ROS, mitochondrial metabolism, apoptosis, and inflammation regulatory pathways, all key players in the mechanisms of cerebral ischemia (Fig. 4). Another exciting possibility with miRNAs is targeting plasticity and recovery after the acute phase of stroke. miRNAs have been shown to be critical in neuronal development, and there is potential that plasticity could be increased following stroke by, for example, encouraging neurite outgrowth using miRNAs such as miR-320 (186). Whereas pretreatment using miR-181a (130) and miR-29a (133) was effective in focal and global cerebral ischemia in rodents, testing treatment after the onset of ischemia is an essential step for the development of acute stroke treatment. Several miRNAs are already in clinical trials in liver diseases, suggesting that formulation and administration will be possible in a new disease setting or for a new miRNA target. However, in this regard, delivery into the CNS is often challenging, and remains part of the challenge in the clinical translation of miRNA therapy.
Abbreviations Used
- Arl2
ADP-ribosylation factor-like 2
- ATP5G1
ATP synthase
- Bnip3
Bcl2/adenovirus E1B 19 kDa-interacting protein 3
- CATs
catalase
- COXIV
cytochrome c oxidase IV
- Dcx
doublecortin
- ER
endoplasmic reticulum
- Fis1
mitochondrial fission protein
- GPX2
glutathione peroxidase-2
- GSHPx
glutathione peroxidase
- H2O
water
- H2O2
hydrogen peroxide
- HEt
hydroethidine
- HSP70
heat shock protein 70
- IKK
IκB kinase
- IL
interleukin
- IRAK1
IL-1 receptor-associated kinase 1
- JAG1
Jagged-1, a ligand of Notch
- MCAO
middle cerebral artery occlusion
- MCU
mitochondrial calcium uniporter
- Mdh2
mitochondrial tricarboxylic acid cycle gene
- miRNAs
microRNAs
- MnSOD
manganese superoxide dismutase, SOD2,
- mRNA
messenger RNA
- mt-COX1
cytochrome c oxidase subunit 1
- NF-κB
nuclear factor-kappa B
- NOX4
NADPH oxidase 4
- PRDX3
peroxiredoxin III
- PTEN
phosphatase and tensin homolog
- ROS
reactive oxygen species
- SDHD
subunit D of succinate dehydrogenase complex
- Slc25a3
mitochondrial phosphate carrier
- SOCS1
suppressor of cytokine signaling 1
- TGF-β
transforming growth factor beta
- TRAF6
tumor necrosis factor (TNF) receptor-associated factor 6
- T-reg
T-regulatory
- TrxR2
thioredoxin reductase-2
- UCP2
mitochondrial uncoupling protein 2
Acknowledgments
Supported by NIH grants NS084396, NS053898, and NS080177 to R.G.G. and T32 GM089626 to R.E.W. and C.M.S. The authors would like to thank William Magruder for help preparing the article.
References
- 1.Abbas AK. and Lichtman AH. Basic Immunology Updated Edition: Functions and Disorders of the Immune System. Philadelphia: Saunders, 2010 [Google Scholar]
- 2.Adams JM. and Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol 19: 488–496, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adlakha Y. and Saini N. MicroRNA-128 downregulates Bax and induces apoptosis in human embryonic kidney cells. Cell Mol Life Sci 68: 1415–1428, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson M, Blomstrand F, Blomstrand C, Eriksson PS, and Nilsson M. Astrocytes and stroke: networking for survival? Neurochem Res 28: 293–305, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Andreyev AY, Kushnareva Y, and Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Moscow) 70: 200–214, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, Margioris A, Tsichlis P, and Tsatsanis C. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity 31: 220–231, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arvidsson A, Collin T, Kirik D, Kokaia Z, and Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8: 963–970, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Arvin B, Neville L, Barone F, and Feuerstein G. The role of inflammation and cytokines in brain injury. Neurosci Biobehav Rev 20: 445–452, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Aschrafi A, Kar A, Natera-Naranjo O, MacGibeny M, Gioio A, and Kaplan B. MicroRNA-338 regulates the axonal expression of multiple nuclear-encoded mitochondrial mRNAs encoding subunits of the oxidative phosphorylation machinery. Cell Mol Life Sci 69: 4017–4027, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aschrafi A, Schwechter AD, Mameza MG, Natera-Naranjo O, Gioio AE, and Kaplan BB. MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. J Neurosci 28: 12581–12590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barreto G, White R, Ouyang Y, Xu L, and Giffard R. Astrocytes: targets for neuroprotection in stroke. Cent Nerv Syst Agents Med Chem 11: 164–173, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baseler WA, Thapa D, Jagannathan R, Dabkowski ER, Croston TL, and Hollander J.M. miR-141 as a regulator of the mitochondrial phosphate carrier (Slc25a3) in the type 1 diabetic heart. Am J Physiol Cell Physiol 303: C1244–C1251, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baxter KK, Uittenbogaard M, Yoon J, and Chiaramello A. The neurogenic basic helix-loop-helix transcription factor NeuroD6 concomitantly increases mitochondrial mass and regulates cytoskeletal organization in the early stages of neuronal differentiation. ASN Neuro 1: pii , 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker KJ. Activation of immune responses to brain antigens after stroke. J Neurochem 123: 148–155, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedard K. and Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Behrens M, Ali S, and Dugan L. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci 28: 13957–13966, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blakeley JO. and Llinas RH. Thrombolytic therapy for acute ischemic stroke. J Neurol Sci 261: 55–62, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Boldin M, Taganov K, Rao D, Yang L, Zhao J, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J, Sun G, Tay J, Linsley P, and Baltimore D. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med 208: 1189–1201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boldin MP. and Baltimore D. MicroRNAs, new effectors and regulators of NF-κB. Immunol Rev 246: 205–220, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Boveris A, Oshino N, and Chance B. The cellular production of hydrogen peroxide. Biochem J 128: 617–630, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown G. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett 369: 136–139, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Cadenas E, Boveris A, Ragan CI, and Stoppani AOM. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys 180: 248–257, 1977 [DOI] [PubMed] [Google Scholar]
- 23.Calingasan NY, Ho DJ, Wille EJ, Campagna MV, Ruan J, Dumont M, Yang L, Shi Q, Gibson GE, and Beal MF. Influence of mitochondrial enzyme deficiency on adult neurogenesis in mouse models of neurodegenerative diseases. Neuroscience 153: 986–996, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao W, Carney JM, Duchon A, Floyd RA, and Chevion M. Oxygen free radical involvement in ischemia and reperfusion injury to brain. Neurosci Lett 88: 233–238, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Castillo J. and Rodriguez I. Biochemical changes and inflammatory response as markers for brain ischaemia: molecular markers of diagnostic utility and prognosis in human clinical practice. Cerebrovasc Dis 17Suppl 1: 7–18, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Chan PH. Role of oxidants in ischemic brain damage. Stroke 27: 1124–1129, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21: 2–14, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Chan PH, Kawase M, Murakami K, Chen SF, Li Y, Calagui B, Reola L, Carlson E, and Epstein CJ. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J Neurosci 18: 8292–8299, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan SY, Zhang Y-Y, Hemann C, Mahoney CE, Zweier JL, and Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab 10: 273–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan YC, Banerjee J, Choi SY, and Sen CK. miR-210: the master hypoxamir. Microcirculation 19: 215–223, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C-Z, Li L, Lodish HF, and Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science 303: 83–86, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Yoshioka H, Kim G, Jung J, Okami N, Sakata H, Maier C, Narasimhan P, Goeders C, and Chan P. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14: 1505–1517, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Chan PH, and Swanson RA. Astrocytes overexpressing Cu,Zn superoxide dismutase have increased resistance to oxidative injury. Glia 33: 343–347, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Cichocki F, Felices M, McCullar V, Presnell SR, Al-Attar A, Lutz CT, and Miller JS. Cutting edge: microRNA-181 promotes human NK cell development by regulating notch signaling. J Immunol 187: 6171–6175, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cobb B, Nesterova T, Thompson E, Hertweck A, O'Connor E, Godwin J, Wilson C, Brockdorff N, Fisher A, Smale S, and Merkenschlager M. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J Exp Med 201: 1367–1373, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Contreras J. and Rao D. MicroRNAs in inflammation and immune responses. Leukemia 26: 404–413, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Das S, Ferlito M, Kent OA, Fox-Talbot K, Wang R, Liu D, Raghavachari N, Yang Y, Wheelan SJ, Murphy E, and Steenbergen C. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ Res 110: 1596–1603, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.del Zoppo G, Ginis I, Hallenbeck J, Iadecola C, Wang X, and Feuerstein G. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol 10: 95–112, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dharap A, Bowen K, Place R, Li LC, and Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 29: 675–687, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dirnagl U, Iadecola C, and Moskowitz M. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22: 391–397, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Doyle K, Cekanaviciute E, Mamer L, and Buckwalter M. TGFβ signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation 7: 62–62, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dröse S. and Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J Biol Chem 283: 21649–21654, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Engelbertsen D, Andersson L, Ljungcrantz I, Wigren M, Hedblad B, Nilsson J, and Bjorkbacka H. T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler Thromb Vasc Biol 33: 637–644, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Ferrer I. and Planas A. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol 62: 329–339, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Feuerstein G, Wang X, and Barone F. The role of cytokines in the neuropathology of stroke and neurotrauma. Neuroimmunomodulation 5: 143–159, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Fu Y, Zhang Y, Wang Z, Wang L, Wei X, Zhang B, Wen Z, Fang H, Pang Q, and Yi F. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am J Nephrol 32: 581–589, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Gage FH. Mammalian neural stem cells. Science 287: 1433–1438, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe C-U, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, and Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40: 1849–1857, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Ghosh S. and Karin M. Missing pieces in the NF-κB puzzle. Cell 109: S81–S96, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Giffard RG, Han R-Q, Emery JF, Duan M, and Pittet JF. Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: the complex roles of heat shock protein 70. Anesthesiology 109: 339–348, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giuffre A, Sarti P, D'Itri E, Buse G, Soulimane T, and Brunori M. On the mechanism of inhibition of cytochrome c oxidase by nitric oxide. J Biol Chem 271: 33404–33408, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Gu L, Xiong X, Zhang H, Xu B, Steinberg G, and Zhao H. Distinctive effects of T cell subsets in neuronal injury induced by cocultured splenocytes in vitro and by in vivo stroke in mice. Stroke 43: 1941–1946, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo R, Wang Y, Shi W-Y, Liu B, Hou S-Q, and Liu L. MicroRNA miR-491-5p targeting both TP53 and Bcl-XL induces cell apoptosis in SW1990 pancreatic cancer cells through mitochondria mediated pathway. Molecules 17: 14733–14747, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo X-K, Zhang Q, Gao L, Li N, Chen X-X, and Feng W-H. Increasing expression of microRNA 181 inhibits porcine reproductive and respiratory syndrome virus replication and has implications for controlling virus infection. J Virol 87: 1159–1171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gutiérrez-Fernández M, Fuentes B, Rodríguez-Frutos B, Ramos-Cejudo J, Vallejo-Cremades MT, and Díez-Tejedor E. Trophic factors and cell therapy to stimulate brain repair after ischaemic stroke. J Cell Mol Med 16: 2280–2290, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem 97: 1634–1658, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Hanisch U. Microglia as a source and target of cytokines. Glia 40: 140–155, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Haque R, Chun E, Howell JC, Sengupta T, Chen D, and Kim H. MicroRNA-30b-mediated regulation of catalase expression in human ARPE-19 cells. PLoS One 7: e42542, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harari OA. and Liao JK. NF-κB and innate immunity in ischemic stroke. Ann N Y Acad Sci 1207: 32–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrison R. Physiological roles of xanthine oxidoreductase. Drug Metab Rev 36: 363–375, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Hayden MS. and Ghosh S. Shared principles in NF-κB signaling. Cell 132: 344–362, 2008 [DOI] [PubMed] [Google Scholar]
- 62.He H-C, Zhu J-G, Chen X-B, Chen S-M, Han Z-D, Dai Q-S, Ling X-H, Fu X, Lin Z-Y, Deng Y-H, Qin G-Q, Cai C, Chen J-H, and Zhong W-D. MicroRNA-23b downregulates peroxiredoxin III in human prostate cancer. FEBS Lett 586: 2451–2458, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Hedrick S. T cell development: bottoms-up. Immunity 16: 619–622, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Hendrix S. and Nitsch R. The role of T helper cells in neuroprotection and regeneration. J Neuroimmunol 184: 100–112, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Henrich-Noack P, Prehn J, and Krieglstein J. TGF-beta 1 protects hippocampal neurons against degeneration caused by transient global ischemia. Dose-response relationship and potential neuroprotective mechanisms. Stroke 27: 1609–1614; discussion 1615, 1996 [DOI] [PubMed] [Google Scholar]
- 66.Hoffmann A. and Baltimore D. Circuitry of nuclear factor κB signaling. Immunol Rev 210: 171–186, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, and Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43: 3063–3070, 2012 [DOI] [PubMed] [Google Scholar]
- 68.Huang Y, Chuang AY, and Ratovitski EA. Phospho-ΔNp63α/miR-885-3p axis in tumor cell life and cell death upon cisplatin exposure. Cell Cycle 10: 3938–3947, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hunter R, Dragicevic N, Seifert K, Choi D, Liu M, Kim H, Cass W, Sullivan P, and Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem 100: 1375–1386, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, and Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab 27: 1798–1805, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iadecola C. and Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 17: 796–808, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Im Y, Jee M, Jung J, Choi J, Jang J, and Kang S. miR23b ameliorates neuropathic pain in spinal cord by silencing NADPH oxidase 4. Antioxid Redox Signal 16: 1046–1060, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Iyer A, Zurolo E, Prabowo A, Fluiter K, Spliet W, van Rijen P, Gorter J, and Aronica E. MicroRNA-146a: a key regulator of astrocyte-mediated inflammatory response. PLoS One 7: e44789, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeyaseelan K, Lim KY, and Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 39: 959–966, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Jiang L, Qiu W, Zhou Y, Wen P, Fang L, Cao H, Zen K, He W, Zhang C, Dai C, and Yang J. A microRNA-30e/mitochondrial uncoupling protein 2 axis mediates TGF-[beta]1-induced tubular epithelial cell extracellular matrix production and kidney fibrosis. Kidney Int 84: 285–296, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson F. and Giulivi C. Superoxide dismutases and their impact upon human health. Mol Aspects Med 26: 340–352, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Jung JE, Kim GS, Narasimhan P, Song YS, and Chan PH. Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci 29: 7003–7014, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamel H. and Iadecola C. Brain-immune interactions and ischemic stroke: clinical implications. Arch Neurol 69: 576–581, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kawase M, Murakami K, Fujimura M, Morita-Fujimura Y, Gasche Y, Kondo T, Scott RW, and Chan PH. Exacerbation of delayed cell injury after transient global ischemia in mutant mice with CuZn superoxide dismutase deficiency. Stroke 30: 1962–1968, 1999 [DOI] [PubMed] [Google Scholar]
- 80.Keller JN, Kindy MS, Holtsberg FW, St. Clair DK, Yen H-C, Germeyer A, Steiner SM, Bruce-Keller AJ, Hutchins JB, and Mattson MP. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci 18: 687–697, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelly S, Zhang ZJ, Zhao H, Xu L, Giffard RG, Sapolsky RM, Yenari MA, and Steinberg GK. Gene transfer of HSP72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: influence of Bcl-2. Ann Neurol 52: 160–167, 2002 [DOI] [PubMed] [Google Scholar]
- 82.Khanna A, Muthusamy S, Liang R, Sarojini H, and Wang E. Gain of survival signaling by down-regulation of three key miRNAs in brain of calorie-restricted mice. Aging 3: 223–236, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, and Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci U S A 88: 11158–11162, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirby DM, Rennie KJ, Smulders-Srinivasan TK, Acin-Perez R, Whittington M, Enriquez JA, Trevelyan AJ, Turnbull DM, and Lightowlers RN. Transmitochondrial embryonic stem cells containing pathogenic mtDNA mutations are compromised in neuronal differentiation. Cell Prolif 42: 413–424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kitagawa K, Matsumoto M, Tsujimoto Y, Ohtsuki T, Kuwabara K, Matsushita K, Yang G, Tanabe H, Martinou J-C, Hori M, and Yanagihara T. Amelioration of hippocampal neuronal damage after global ischemia by neuronal overexpression of BCL-2 in transgenic mice. Stroke 29: 2616–2621, 1998 [DOI] [PubMed] [Google Scholar]
- 86.Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, and Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell 139: 814–827, 2009 [DOI] [PubMed] [Google Scholar]
- 87.Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, Austinat M, Nieswandt B, Wiendl H, and Stoll G. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood 115: 3835–3842, 2010 [DOI] [PubMed] [Google Scholar]
- 88.Kole AJ, Swahari V, Hammond SM, and Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev 25: 125–130, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kondo T, Reaume AG, Huang T-T, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, and Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci 17: 4180–4189, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, and Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med 47: 333–343, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Lafargue M, Xu L, Carlès M, Serve E, Anjum N, Iles KE, Xiong X, Giffard R, and Pittet J-F. Stroke-induced activation of the α7 nicotinic receptor increases Pseudomonas aeruginosa lung injury. FASEB J 26: 2919–2929, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lambertsen K, Biber K, and Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 32: 1677–1698, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lashine YA, Seoudi AM, Salah S, and Abdelaziz AI. Expression signature of microRNA-181-a reveals its crucial role in the pathogenesis of paediatric systemic lupus erythematosus. Clin Exp Rheumatol 29: 351–357, 2011 [PubMed] [Google Scholar]
- 94.Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-Theotokis A, and McKay RDG. Long-lasting regeneration after ischemia in the cerebral cortex. Stroke 38: 153–161, 2007 [DOI] [PubMed] [Google Scholar]
- 95.Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life 52: 159–164, 2001 [DOI] [PubMed] [Google Scholar]
- 96.Li J, Donath S, Li Y, Qin D, Prabhakar BS, and Li P. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet 6: e1000795, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li R, Yan G, Li Q, Sun H, Hu Y, Sun J, and Xu B. MicroRNA-145 protects cardiomyocytes against hydrogen peroxide H2O2-induced apoptosis through targeting the mitochondria apoptotic pathway. PLoS One 7: e44907, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, and Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 15: 192–199, 2009 [DOI] [PubMed] [Google Scholar]
- 99.Limón-Pacheco J. and Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res 674: 137–147, 2009 [DOI] [PubMed] [Google Scholar]
- 100.Lisak R, Benjamins J, Bealmear B, Nedelkoska L, Yao B, Land S, and Studzinski D. Differential effects of Th1, monocyte/macrophage and Th2 cytokine mixtures on early gene expression for glial and neural-related molecules in central nervous system mixed glial cell cultures: neurotrophins, growth factors and structural proteins. J Neuroinflammation 4: 30, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liston A, Lu L, O'Carroll D, Tarakhovsky A, and Rudensky A. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med 205: 1993–2004, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G, and Sharp FR. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab 30: 92–101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu XS, Chopp M, Wang XL, Zhang L, Hozeska-Solgot A, Tang T, Kassis H, Zhang RL, Chen C, Xu J, and Zhang ZG. MicroRNA-17–92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J Biol Chem 288: 12478–12488, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, Hozeska-Solgot A, Zhang L, Chen C, and Zhang ZG. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through notch signaling pathway. PLoS One 6: e23461, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J, Committee obotAHAS, and Subcommittee SS. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 106.Lodish HF, Zhou B, Liu G, and Chen C-Z. Micromanagement of the immune system by microRNAs. Nat Rev Immunol 8: 120–130, 2008 [DOI] [PubMed] [Google Scholar]
- 107.López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, and Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 13: 106–118, 2013 [DOI] [PubMed] [Google Scholar]
- 108.Lu L, Thai T, Calado D, Chaudhry A, Kubo M, Tanaka K, Loeb G, Lee H, Yoshimura A, Rajewsky K, and Rudensky A. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity 30: 80–91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Magenta A, Greco S, Gaetano C, and Martelli F. Oxidative stress and microRNAs in vascular diseases. Int J Mol Sci 14: 17319–17346, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Malhotra J. and Kaufman R. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9: 2277–2293, 2007 [DOI] [PubMed] [Google Scholar]
- 111.Marchi S, Lupini L, Patergnani S, Rimessi A, Missiroli S, Bonora M, Bononi A, Corrà F, Giorgi C, De Marchi E, Poletti F, Gafà R, Lanza G, Negrini M, Rizzuto R, and Pinton P. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr Biol 23: 58–63, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Monje ML, Toda H, and Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science 302: 1760–1765, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Morgan MJ. and Liu Z-g. Crosstalk of reactive oxygen species and NF-[kappa]B signaling. Cell Res 21: 103–115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Muljo S, Ansel K, Kanellopoulou C, Livingston D, Rao A, and Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med 202: 261–269, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, and Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci 18: 205–213, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, and Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta 33: 816–823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Namba T, Tanaka K-I, Hoshino T, Azuma A, and Mizushima T. Suppression of expression of heat shock protein 70 by gefitinib and its contribution to pulmonary fibrosis. PLoS One 6: e27296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nan Y, Han L, Zhang A, Wang G, Jia Z, Yang Y, Yue X, Pu P, Zhong Y, and Kang C. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Res 1359: 14–21, 2010 [DOI] [PubMed] [Google Scholar]
- 120.Nelson PT, Wang WX, and Rajeev BW. MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol 18: 130–138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nielsen J, Lau P, Maric D, Barker J, and Hudson L. Integrating microRNA and mRNA expression profiles of neuronal progenitors to identify regulatory networks underlying the onset of cortical neurogenesis. BMC Neurosci 10: 98–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nishi H, Ono K, Iwanaga Y, Horie T, Nagao K, Takemura G, Kinoshita M, Kuwabara Y, Mori RT, Hasegawa K, Kita T, and Kimura T. MicroRNA-15b modulates cellular ATP levels and degenerates mitochondria via Arl2 in neonatal rat cardiac myocytes. J Biol Chem 285: 4920–4930, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Niu H, Wang K, Zhang A, Yang S, Song Z, Wang W, Qian C, Li X, Zhu Y, and Wang Y. miR-92a is a critical regulator of the apoptosis pathway in glioblastoma with inverse expression of BCL2L11. Oncol Rep 28: 1771–1777, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Nunomura A, Moreira P, Castellani R, Lee H-g, Zhu X, Smith M, and Perry G. Oxidative damage to RNA in aging and neurodegenerative disorders. Neurotox Res 22: 231–248, 2012 [DOI] [PubMed] [Google Scholar]
- 125.O'Connell R, Kahn D, Gibson W, Round J, Scholz R, Chaudhuri A, Kahn M, Rao D, and Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 33: 607–619, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ouyang Y-B, Carriedo SG, and Giffard RG. Effect of Bcl-XL overexpression on reactive oxygen species, intracellular calcium, and mitochondrial membrane potential following injury in astrocytes. Free Radic Biol Med 33: 544–551, 2002 [DOI] [PubMed] [Google Scholar]
- 127.Ouyang Y-B. and Giffard RG. Cellular neuroprotective mechanisms in cerebral ischemia: Bcl-2 family proteins and protection of mitochondrial function. Cell Calcium 36: 303–311, 2004 [DOI] [PubMed] [Google Scholar]
- 128.Ouyang Y-B, Kristian T, Li P-A, and Siesjö B. Mitochondria, free radicals, and ischemic brain damage. Pharmacol Cereb Ischemia 87–96, 1998 [Google Scholar]
- 129.Ouyang Y-B, Lu Y, Yue S, and Giffard RG. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion 12: 213–219, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ouyang Y-B, Lu Y, Yue S, Xu L-J, Xiong X-X, White RE, Sun X, and Giffard RG. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol Dis 45: 555–563, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ouyang Y-B, Stary C, Yang G-Y, and Giffard R. microRNAs: innovative targets for cerebral ischemia and stroke. Curr Drug Targets 14: 90–101, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ouyang Y-B, Xu L-J, Emery JF, Lee AS, and Giffard RG. Overexpressing GRP78 influences Ca2+ handling and function of mitochondria in astrocytes after ischemia-like stress. Mitochondrion 11: 279–286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ouyang Y-B, Xu L, Lu Y, Sun X, Yue S, Xiong X, and Giffard RG. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia 61: 1784–1794, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ouyang YB. and Giffard RG. ER-mitochondria crosstalk during cerebral ischemia: molecular chaperones and ER-mitochondrial calcium transfer. Int J Cell Biol 2012: 1–8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pan Z, Sun X, Ren J, Li X, Gao X, Lu C, Zhang Y, Sun H, Wang Y, Wang H, Wang J, Xie L, Lu Y, and Yang B. miR-1 exacerbates cardiac ischemia-reperfusion injury in mouse models. PLoS One 7: e50515, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pandolfo M. Frataxin deficiency and mitochondrial dysfunction. Mitochondrion 2: 87–93, 2002 [DOI] [PubMed] [Google Scholar]
- 137.Papadopoulos MC, Koumenis IL, Dugan LL, and Giffard RG. Vulnerability to glucose deprivation injury correlates with glutathione levels in astrocytes. Brain Res 748: 151–156, 1997 [DOI] [PubMed] [Google Scholar]
- 138.Papadopoulos MC, Koumenis IL, Yuan TY, and Giffard RG. Increasing vulnerability of astrocytes to oxidative injury with age despite constant antioxidant defenses. Neuroscience 82: 915–925, 1997 [DOI] [PubMed] [Google Scholar]
- 139.Parsons M. and Green D. Mitochondria in cell death. Essays Biochem 47: 99–114, 2010 [DOI] [PubMed] [Google Scholar]
- 140.Perego C, Fumagalli S, and De Simoni M-G. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation 8: 174–174, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Piantadosi CA. and Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke 27: 327–332, 1996 [DOI] [PubMed] [Google Scholar]
- 142.Pocock J. and Liddle A. Microglial signalling cascades in neurodegenerative disease. Prog Brain Res 132: 555–565, 2001 [DOI] [PubMed] [Google Scholar]
- 143.Ponomarev E, Veremeyko T, Barteneva N, Krichevsky A, and Weiner H. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat Med 17: 64–70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Puissegur MP, Mazure NM, Bertero T, Pradelli L, Grosso S, Robbe Sermesant K, Maurin T, Lebrigand K, Cardinaud B, Hofman V, Fourre S, Magnone V, Ricci JE, Pouyssegur J, Gounon P, Hofman P, Barbry P, and Mari B. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ 18: 465–478, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg S, and Fike J. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res 162: 39–47, 2004 [DOI] [PubMed] [Google Scholar]
- 146.Ren X-P, Wu J, Wang X, Sartor MA, Qian J, Jones K, Nicolaou P, Pritchard TJ, and Fan G-C. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation 119: 2357–2366, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rock R, Gekker G, Hu S, Sheng W, Cheeran M, Lokensgard J, and Peterson P. Role of microglia in central nervous system infections. Clin Microbiol Rev 17: 942–964, table of contents, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rupalla K, Allegrini P, Sauer D, and Wiessner C. Time course of microglia activation and apoptosis in various brain regions after permanent focal cerebral ischemia in mice. Acta Neuropathol 96: 172–178, 1998 [DOI] [PubMed] [Google Scholar]
- 149.Samavati L, Lee I, Mathes I, Lottspeich F, and Huttemann M. Tumor necrosis factor alpha inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J Biol Chem 283: 21134–21144, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sharma S, Yang B, Xi X, Grotta J, Aronowski J, and Savitz S. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res 1373: 189–194, 2011 [DOI] [PubMed] [Google Scholar]
- 151.Sheppard P, Sun X, Emery J, Giffard R, and Khammash M. Quantitative characterization and analysis of the dynamic NF-κB response in microglia. BMC Bioinform 12: 276, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shi G, Liu Y, Liu T, Yan W, Liu X, Wang Y, Shi J, and Jia L. Upregulated miR-29b promotes neuronal cell death by inhibiting Bcl2L2 after ischemic brain injury. Exp Brain Res 216: 225–230, 2012 [DOI] [PubMed] [Google Scholar]
- 153.Shi H, Sun BL, Zhang J, Lu S, Zhang P, Wang H, Yu Q, Stetler RA, Vosler PS, Chen J, and Gao Y. miR-15b suppression of Bcl-2 contributes to cerebral ischemic injury and is reversed by sevoflurane preconditioning. CNS Neurol Disord Drug Targets 12: 381–391, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shi L, Zhang S, Feng K, Wu F, Wan Y, Wang Z, Zhang J, Wang Y, Yan W, Fu Z, and You Y. MicroRNA-125b-2 confers human glioblastoma stem cells resistance to temozolomide through the mitochondrial pathway of apoptosis. Int J Oncol 40: 119–129, 2012 [DOI] [PubMed] [Google Scholar]
- 155.Shi Q. and Gibson GE. Up-regulation of the mitochondrial malate dehydrogenase by oxidative stress is mediated by miR-743a. J Neurochem 118: 440–448, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Siesjo B-K.Ouyang Y, Kristian T, Elmer E, Li P-A, and Uchino H. Role of mitochondria in immediate and delayed reperfusion damage. In: Maturation Phenomenon in Cerebral Ischemia III, edited by Ito U, Fieschi C, Orzi F, Kuroiwa T, and Klatzo I. Berlin: Springer-Verlag, 1999, pp. 217–225 [Google Scholar]
- 157.Siesjo BK, Elmer E, Janelidze S, Keep M, Kristian T, Ouyang YB, and Uchino H. Role and mechanisms of secondary mitochondrial failure. Acta Neurochir Suppl 73: 7–13, 1999 [DOI] [PubMed] [Google Scholar]
- 158.Singh R. and Saini N. Downregulation of BCL2 by miRNAs augments drug-induced apoptosis—a combined computational and experimental approach. J Cell Sci 125: 1568–1578, 2012 [DOI] [PubMed] [Google Scholar]
- 159.Siomek A. NF-kB signaling pathway and free radical impact. Acta Biochim Pol 59: 323–331, 2012 [PubMed] [Google Scholar]
- 160.Song Y, Selak MA, Watson CT, Coutts C, Scherer PC, Panzer JA, Gibbs S, Scott MO, Willer G, Gregg RG, Ali DW, Bennett MJ, and Balice-Gordon RJ. Mechanisms underlying metabolic and neural defects in zebrafish and human multiple acyl-CoA dehydrogenase deficiency (MADD). PLoS One 4: e8329, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stadler J, Bentz B, Harbrecht B, Di Silvio M, Curran R, Billiar T, Hoffman R, and Simmons R. Tumor necrosis factor alpha inhibits hepatocyte mitochondrial respiration. Ann Surg 216: 539–546, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stoll EA, Cheung W, Mikheev AM, Sweet IR, Bielas JH, Zhang J, Rostomily RC, and Horner PJ. Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism. J Biol Chem 286: 38592–38601, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun F-C, Wei S, Li C-W, Chang Y-S, Chao C-C, and Lai Y-K. Localization of GRP78 to mitochondria under the unfolded protein response. Biochem J 396: 31–39, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, Blackwell TS, Baron RM, and Feinberg MW. MicroRNA-181b regulates NF-κB–mediated vascular inflammation. J Clin Invest 122: 1973–1990, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Swanson R, Ying W, and Kauppinen T. Astrocyte influences on ischemic neuronal death. Curr Mol Med 4: 193–205, 2004 [DOI] [PubMed] [Google Scholar]
- 166.Tan K, Lip G, and Blann A. Post-stroke inflammatory response: effects of stroke evolution and outcome. Curr Atheroscler Rep 5: 245–251, 2003 [DOI] [PubMed] [Google Scholar]
- 167.Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, and Jeyaseelan K. Expression profile of MicroRNAs in young stroke patients. PLoS One 4: e7689, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tarassishin L, Loudig O, Bauman A, Shafit-Zagardo B, Suh H, and Lee S. Interferon regulatory factor 3 inhibits astrocyte inflammatory gene expression through suppression of the proinflammatory miR-155 and miR-155*. Glia 59: 1911–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Thounaojam M, Kaushik D, and Basu A. MicroRNAs in the brain: it's regulatory role in neuroinflammation. Mol Neurobiol 47: 1034–1044, 2013 [DOI] [PubMed] [Google Scholar]
- 170.Tranter M, Helsley RN, Paulding WR, McGuinness M, Brokamp C, Haar L, Liu Y, Ren X, and Jones WK. Coordinated post-transcriptional regulation of Hsp70.3 gene expression by microRNA and alternative polyadenylation. J Biol Chem 286: 29828–29837, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tuttolomondo A, Di Sciacca R, Di Raimondo D, Renda C, Pinto A, and Licata G. Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr Top Med Chem 9: 1240–1260, 2009 [DOI] [PubMed] [Google Scholar]
- 172.Uittenbogaard M, Baxter K, and Chiaramello A. The neurogenic basic helix-loop-helix transcription factor NeuroD6 confers tolerance to oxidative stress by triggering an antioxidant response and sustaining the mitochondrial biomass. ASN Neuro 2: e00034–e00034, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Varga ZV, Kupai K, Szűcs G, Gáspár R, Pálóczi J, Faragó N, Zvara Á, Puskás LG, Rázga Z, Tiszlavicz L, Bencsik P, Görbe A, Csonka C, Ferdinandy P, and Csont T. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J Mol Cell Cardiol 62: 111–121, 2013 [DOI] [PubMed] [Google Scholar]
- 174.Voloboueva LA, Duan M, Ouyang Y, Emery JF, Stary C, and Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 protects astrocytes against ischemic injury in vitro. J Cereb Blood Flow Metab 28: 1009–1016, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Voloboueva LA. and Giffard RG. Inflammation, mitochondria, and the inhibition of adult neurogenesis. J Neurosci Res 89: 1989–1996, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Voloboueva LA, Lee SW, Emery JF, Palmer TD, and Giffard RG. Mitochondrial protection attenuates inflammation-induced impairment of neurogenesis in vitro and in vivo. J Neurosci 30: 12242–12251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wagner K, Kleinholz M, and Myers R. Delayed decreases in specific brain mitochondrial electron transfer complex activities and cytochrome concentrations following anoxia/ischemia. J Neurol Sci 100: 142–151, 1990 [DOI] [PubMed] [Google Scholar]
- 178.Wang J-X, Jiao J-Q, Li Q, Long B, Wang K, Liu J-P, Li Y-R, and Li P-F. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med 17: 71–78, 2011 [DOI] [PubMed] [Google Scholar]
- 179.Wang K, Long B, Jiao J-Q, Wang J-X, Liu J-P, Li Q, and Li P-F. miR-484 regulates mitochondrial network through targeting Fis1. Nat Commun 3: 781, 2012 [DOI] [PubMed] [Google Scholar]
- 180.Wang W, Esbensen Y, Kunke D, Suganthan R, Rachek L, Bjørås M, and Eide L. Mitochondrial DNA damage level determines neural stem cell differentiation fate. J Neurosci 31: 9746–9751, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang W, Osenbroch P, Skinnes R, Esbensen Y, Bjørås M, and Eide L. Mitochondrial DNA integrity is essential for mitochondrial maturation during differentiation of neural stem cells. Stem Cells 28: 2195–2204, 2010 [DOI] [PubMed] [Google Scholar]
- 182.Wang X, Yue T, White R, Barone F, and Feuerstein G. Transforming growth factor-beta 1 exhibits delayed gene expression following focal cerebral ischemia. Brain Res Bull 36: 607–609, 1995 [DOI] [PubMed] [Google Scholar]
- 183.Wang X-F, Shi Z-M, Wang X-R, Cao L, Wang Y-Y, Zhang J-X, Yin Y, Luo H, Kang C-S, Liu N, Jiang T, and You Y-P. MiR-181d acts as a tumor suppressor in glioma by targeting K-ras and Bcl-2. J Cancer Res Clin Oncol 138: 573–584, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Waschek J. VIP and PACAP: neuropeptide modulators of CNS inflammation, injury, and repair. Br J Pharmacol 169: 512–523, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.White R, Ouyang Y, and Giffard R. Hsp75/mortalin and protection from ischemic brain injury. In: Mortalin Biology: Life, Stress and Death, edited by Kaul S. and Wadhwa R. Berlin: Springer, 179–190, 2012 [Google Scholar]
- 186.White RE. and Giffard RG. MicroRNA-320 induces neurite outgrowth by targeting ARPP-1. NeuroReport 23: 590–595, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Williams A, Dave J, and Tortella F. Neuroprotection with the proteasome inhibitor MLN519 in focal ischemic brain injury: relation to nuclear factor kappaB (NF-kappaB), inflammatory gene expression, and leukocyte infiltration. Neurochem Int 49: 106–112, 2006 [DOI] [PubMed] [Google Scholar]
- 188.Wolf S, Fisher J, Bechmann I, Steiner B, Kwidzinski E, and Nitsch R. Neuroprotection by T-cells depends on their subtype and activation state. J Neuroimmunol 133: 72–80, 2002 [DOI] [PubMed] [Google Scholar]
- 189.Wong C. and Crack P. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem 15: 1–14, 2008 [DOI] [PubMed] [Google Scholar]
- 190.Wu C, Gong Y, Yuan J, Zhang W, Zhao G, Li H, Sun A, KaiHu , Zou Y, and Ge J. microRNA-181a represses ox-LDL-stimulated inflammatory response in dendritic cell by targeting c-Fos. J Lipid Res 53: 2355–2363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xie Z, Smith C, and Van Eldik L. Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia 45: 170–179, 2004 [DOI] [PubMed] [Google Scholar]
- 192.Xiong X, Barreto G, Xu L, Ouyang Y, Xie X, and Giffard R. Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke 42: 2026–2032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xiong X, Gu L, Zhang H, Xu B, Zhu S, and Zhao H. The protective effects of T cell deficiency against brain injury are ischemic model-dependent in rats. Neurochem Int 62: 265–270, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu L, Emery J, Ouyang Y, Voloboueva L, and Giffard R. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia 58: 1042–1049, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xu L, Voloboueva LA, Ouyang Y, Emery JF, and Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J Cereb Blood Flow Metab 29: 365–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xu S, Zhang R, Niu J, Cui D, Xie B, Zhang B, Lu K, Yu W, Wang X, and Zhang Q. Oxidative stress mediated-alterations of the microRNA expression profile in mouse hippocampal neurons. Int J Mol Sci 13: 16945–16960, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xu Y, Fang F, Zhang J, Josson S, St. Clair WH, and St. Clair DK. miR-17* suppresses tumorigenicity of prostate cancer by inhibiting mitochondrial antioxidant enzymes. PLoS One 5: e14356, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yadav S, Pandey A, Shukla A, Talwelkar SS, Kumar A, Pant AB, and Parmar D. miR-497 and miR-302b regulate ethanol-induced neuronal cell death through BCL2 protein and cyclin D2. J Biol Chem 286: 37347–37357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang G, Chan PH, Chen J, Carlson E, Chen SF, Weinstein P, Epstein CJ, and Kamii H. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke 25: 165–170, 1994 [DOI] [PubMed] [Google Scholar]
- 200.Yang Z-j, Xie Y, Bosco G, Chen C, and Camporesi E. Hyperbaric oxygenation alleviates MCAO-induced brain injury and reduces hydroxyl radical formation and glutamate release. Eur J Appl Physiol 108: 513–522, 2010 [DOI] [PubMed] [Google Scholar]
- 201.Yin K-J, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, and Chen YE. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis 38: 17–26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Youle RJ. and Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59, 2008 [DOI] [PubMed] [Google Scholar]
- 203.Yuan Y, Wang JY, Xu LY, Cai R, Chen Z, and Luo BY. MicroRNA expression changes in the hippocampi of rats subjected to global ischemia. J Clin Neurosci 17: 774–778, 2010 [DOI] [PubMed] [Google Scholar]
- 204.Zelko IN, Mariani TJ, and Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 33: 337–349, 2002 [DOI] [PubMed] [Google Scholar]
- 205.Zell R, Geck P, Werdan K, and Boekstegers P. TNF-alpha and IL-1 alpha inhibit both pyruvate dehydrogenase activity and mitochondrial function in cardiomyocytes: evidence for primary impairment of mitochondrial function. Mol Cell Biochem 177: 61–67, 1997 [DOI] [PubMed] [Google Scholar]
- 206.Zeng C-W, Zhang X-J, Lin K-Y, Ye H, Feng S-Y, Zhang H, and Chen Y-Q. Camptothecin induces apoptosis in cancer cells via microRNA-125b-mediated mitochondrial pathways. Mol Pharmacol 81: 578–586, 2012 [DOI] [PubMed] [Google Scholar]
- 207.Zhang L, Dong L, Li Y, Hong Z, and Wei W. The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor. J Neuroinflammation 6: 211–23, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang X, Ng W-L, Wang P, Tian L, Werner E, Wang H, Doetsch P, and Wang Y. MicroRNA-21 modulates the levels of reactive oxygen species by targeting SOD3 and TNFα. Cancer Res 72: 4707–4713, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 209.Zhao H, Yenari MA, Cheng D, Sapolsky RM, and Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem 85: 1026–1036, 2003 [DOI] [PubMed] [Google Scholar]
- 210.Zhao J, Rao D, Boldin M, Taganov K, O'Connell R, and Baltimore D. NF-kappaB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc Natl Acad Sci U S A 108: 9184–9189, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]