Abstract
Background & Aims
Guidelines recommend a 10 year interval between screening colonoscopies with negative results for average-risk individuals. However, many patients are examined at shorter intervals. We investigated outcomes of individuals with no polyps who had repeat colonoscopy in less than 10 years.
Methods
Data were collected using the National Endoscopic Database, from 69 gastroenterology centers, on 264,184 asymptomatic subjects who underwent screening colonoscopies from 2000 through 2006, were found to have no polyps, and received another colonoscopy examination within less than 10 years.
Results
No polyps were found in 147,375 patients during a baseline colonoscopy; 17,525 patients (11.9%) had a follow-up colonoscopy within less than 10 years, including 1806 (10.3%) who received the follow-up colonoscopy within less than 1 year. The most common reason for repeating the examination within 1 year was that the first was compromised by inadequate bowel preparation or incomplete examination. Of these patients, 6.5% (95% confidence interval [CI], 5.3–7.6) had large polyp(s) >9 mm—a proportion similar to the prevalence in the average-risk screening population. Reasons that examinations were repeated within 1–5 years included average-risk screening (15.7%), family history of colon polyps or cancer (30.1%), bleeding (31.2%), gastrointestinal symptoms (11.8%), or a positive result from a fecal blood test (5.5%). If the baseline exam was adequate, the incidence of large polyps within 1–5 years after baseline colonoscopy was 3.1% (95% CI, 2.7–3.5) and within years 5–10 years was 3.7% (95% CI, 3.3–4.1).
Conclusions
Repeat colonoscopies within 10 years are of no benefit to patients who had adequate examinations and were found to have no polyps. Repeat colonoscopies are beneficial to patients when the baseline examination was compromised.
Keywords: neoplasm, colorectal, prevention, early detection, endoscopy
Introduction
Colorectal cancer (CRC) screening guidelines recommend a 10 year interval if there was no neoplasia detected at a high quality baseline screening colonoscopy.1–3 This recommendation is based on the natural history of colorectal neoplasia, randomized controlled trials of endoscopic screening with sigmoidoscopy,4–6 and case-control and cohort studies of colonoscopy.7–14 All of these data suggest that endoscopic screening has a durable reduction in CRC incidence and mortality of at least 10 years. Individuals with first degree relatives who had CRC before age 60 years, are advised to have follow-up at 5 years after a negative baseline exam.
Many patients have follow-up exams earlier than 10 years15,16 despite a negative baseline colonoscopy. In a study of utilization of colonoscopy in Medicare beneficiaries, 30% of individuals had a second colonoscopy within 5 years after a negative baseline exam.16 In the PLCO study, more than 25% of individuals with no neoplasia at baseline colonoscopy had repeat colonoscopy within 5 years.15 The reasons for most early exams are unknown. In some cases the baseline exam may have been incomplete or compromised by an inadequate bowel prep, in other cases new symptoms may have resulted in a colonoscopy. Another factor driving early colonoscopy may be concerns about development of interval cancer before 10 years.
The outcomes of patients who have no neoplasia found at screening colonoscopy are uncertain. There are several prospective studies which have followed cohorts for 5 years after negative screening colonoscopy to determine rates of advanced neoplasia (defined as tubular adenoma >10mm, or adenoma with villous histology, HGD or cancer). The rates of advanced neoplasia at 5 years ranges from 1.4–4.4%,17–22 which are lower than rates found in baseline average-risk screening.23
The purpose of this study was to determine why patients with negative colonoscopy have early follow-up exams at intervals less than 10 years, and their endoscopic outcomes in diverse practice settings. The study cohort was obtained from endoscopy practices which participate in the Clinical Outcomes Research Initiative (CORI), which was established in 1995 to study endoscopy in practice settings throughout the United States. Participating endoscopists use a computerized report generator to produce their reports, and data files are electronically transmitted to a central data repository. Subjects who received screening exams from 2000–2006 (average-risk, family history of CRC, positive fecal occult blood test (FOBT), or positive sigmoidoscopy) were identified. Those who had no polyps or tumors and had a follow-up exam within 10 years were included in this analysis. Our primary aims were to determine the demographic characteristics of these patients, completeness of the baseline exam, indication for the follow-up exam and a key endoscopic outcome (rate of polyp(s) >9mm) of the follow-up exam. Our hypotheses, based on patients with follow-up in our consortium, were: 1) that patients who have follow-up in less than 10 years after an adequate index exam will have a low rate of polyp(s) >9mm, relative to average-risk screening; and 2) patients who have exams within the first year are more likely to have an incomplete baseline exam, due to bowel prep or other factors.
Methods
Clinical Outcomes Research Initiative
This project was developed in 1995 with the goal of creating a consortium of clinical practice settings to determine utilization and outcomes of endoscopic procedures. Endoscopists use a structured computerized endoscopic report generator to produce endoscopic reports. The data that is transmitted from the local site to the National Endoscopic Database does not contain most patient or provider identifiers and qualifies as a Limited Data Set under 45 C.F.R. Section 164.514(e)(2). After completion of quality control checks, data from all sites are merged in the data repository for analysis. Procedure counts are monitored on a weekly basis for atypical activity. The repository is checked for anomalies on a daily basis. Any unusual activity prompts follow-up contact by CORI staff. During the study period, the practice sites contributing colonoscopy reports include private practices and endoscopy centers (82.7 %), academic centers (8.2%) and Veterans Affairs (VA)/Military medical centers (9.1 %). CORI was given approval by the IRB of the Oregon Health & Science University (eIRB #7331) in October 2011. This specific study utilized a limited dataset and was therefore exempted from further IRB review.
Patients
We included all complete colonoscopy reports from 2000 to 2006 in patients undergoing screening exams without any other indication for colonoscopy. We excluded reports in patients less than 18 years old. Screening exams were defined as average-risk, family history of CRC, positive fecal occult blood test (FOBT) or positive sigmoidoscopy in the absence of any gastrointestinal symptoms. Patients who had no polyps or tumors of any kind represent the study cohort. Among these patients, we followed the outcomes of patients who had one or more colonoscopy exams documented in CORI during the follow-up period until 2012. Patients are stratified by interval for the first follow-up exam. Characteristics of the baseline exam, patient demographics, practice site and indication for the follow-up exam were analyzed.
Outcome measurement
There are many possible important outcomes of colonoscopy. For this analysis, we have focused on neoplasia, which would be an important outcome of screening examinations. In non-screening procedures, other outcomes may be even more important. In this structured database, endoscopists are asked to provide detailed descriptors of every polyp, including size, location, morphology (pudunculated, sessile or flat) and method of removal. The determination of neoplasia in a polyp requires the addition of histopathology results, which arrive days after the endoscopy. We receive pathology results in 20% of the endoscopy reports, which are a representative sample of the entire cohort in terms of patient demographics and procedure indications.
Our key endpoint was the finding of one or more polyps sized more than 9mm or described as a suspected malignant tumor, hereafter termed large polyp. This endpoint is a surrogate for advanced neoplasia (defined as tubular adenoma >10mm adenoma with villous histology or high-grade dysplasia and cancer). Our previous analysis of 13,992 screening examinations with histopathology, demonstrated that this surrogate was robust (24). In this analysis, 4.8% of large polyps (sized more than 9mm) did not have advanced histology, and 2.9% of small polyps (1–9mm) did have advanced histology, and would be misclassified with our surrogate endpoint. Most the large polyps without advanced histology were classified as “hyperplastic.” Today we would likely refer to these polyps as serrated lesions, with follow-up management similar to high-risk adenomas.3 Thus, the lesions which are most likely misclassified as non-advanced in the current analysis are 2.9% of small polyps with advanced histologic features, most of which are 6–9mm in size. The incidence of these lesions are included in this report.
Analysis
Categorical data is presented as proportions and 95% confidence intervals. Comparisons of categorical data were performed using Pearson’s chi-square tests while continuous variables were compared using analysis of variance. All tests were two-sided and a p value of < 0.05 was considered statistically significant. All analyses were performed using SAS software v 9.2 (SAS Institute, Inc., Cary, NC).
Results
From 2000 to 2006, 264,184 asymptomatic patients had screening colonoscopy. Indications for screening included average-risk (n= 159,885, 60.5%), family history of CRC or polyps (n = 59,393 22.5%), positive FOBT (n=35,110 13.3%) or positive sigmoidoscopy (n =9,796 3.7%).
147,375 (55.8%) had no polyps or tumors (Figure 1). Among these patients, 17,525 patients (11.9%) had at least one follow-up colonoscopy documented in CORI within 10 years. These individuals are the subjects of this analysis. The indications for the baseline exam was average-risk (n=7,617, 43.5%), family history of CRC or polyps (n=7,420, 42.3%), positive FOBT (n=2,382, 13.6%) and positive sigmoidoscopy (n=301, 1.7%). The demographic characteristics of the cohort are described in Table 1. The cohort receiving repeat exams in the first 5 years was somewhat older than those receiving follow-up in the second 5 years. There was a female predominance after the first year. The baseline exam was compromised by either poor prep or incomplete exam in 15.5% of patients who had a follow-up exam in less than 10 years. For comparison, the rate of compromised exams in the entire cohort with no polyps at baseline (n=147,375), was 7.3%; the rate of compromised exams among all patients receiving screening colonoscopy (n=264,184) was 6.3%.
Figure 1.
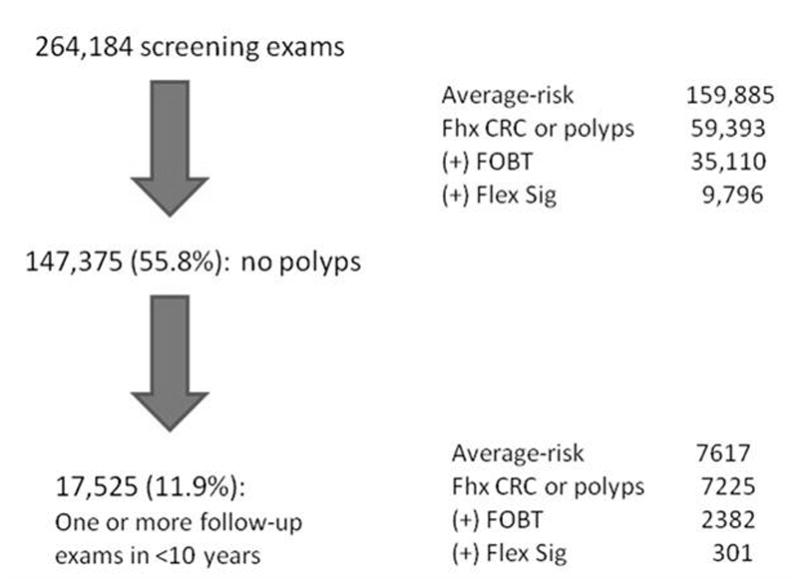
Consort diagram describing patient selection process from 264,184 patients undergoing screening colonoscopy from 2000–2006.
Table 1.
Patients with negative baseline colonoscopy and follow-up colonoscopy within 10 years
| Year of follow-up after baseline colonoscopy | ||||||
|---|---|---|---|---|---|---|
| <1 | 1–<2 | 2–<5 | 5–<7 | 7–<10 | Entire screen cohort | |
| Total N = 17,525 | 1806 (10.3) | 1058 (6.0) | 6314 (36.0) | 6681 (38.1) | 1666 (9.5) | N=264,184 |
| Baseline exam demographics | ||||||
| Mean age first exam (years) | 60.7 | 61.9 | 60.1 | 58.3 | 58.0 | 60.6 |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Male Gender | 1010 (55.9) | 501 (47.4) | 2829 (44.8) | 2957 (44.3) | 802 (48.1) | 140937 (53.4) |
| Race/Ethnicity1 | ||||||
| White | 1,287 (71.3) | 807 (76.3) | 5,233 (82.9) | 5,770 (86.4) | 1,390 (83.4) | 225,097 (85.2) |
| Black | 183 (10.1) | 78 (7.4) | 329 (5.2) | 324 (4.9) | 89 (5.3) | 15,621 (5.9) |
| Asian/Pacific Islander | 65 (3.6) | 27 (2.6) | 127 (2.0) | 113 (1.7) | 43 (2.6) | 4,371 (1.7) |
| Native American | 15 (0.8) | 4 (0.4) | 36 (0.6) | 27 (0.4) | 8 (0.5) | 1,135 (0.4) |
| Multi-racial | 2 (0.1) | 1 (0.1) | 15 (0.2) | 16 (0.2) | 1 (0.1) | 412 (0.2) |
| Hispanic | 238 (13.2) | 135 (12.8) | 559 (8.9) | 416 (6.2) | 126 (7.6) | 14,451 (5.5) |
| unknown | 16 (0.9) | 6 (0.6) | 15 (0.2) | 15 (0.2) | 9 (0.5) | 3,097 (1.2) |
| Practice Setting | ||||||
| Private % of all exams = 81.3 |
1080 (59.8) | 803 (75.9) | 5221 (82.7) | 5841 (87.4) | 1302 (78.2) | 205445 (77.8) |
| Academic % of all exams = 6.9 |
299 (16.6) | 127 (12.0) | 434 (6.9) | 218 (3.3) | 134 (8.0) | 26893 (10.2) |
| VA2/Military % of all exams = 11.8 |
427 (23.6) | 128 (12.1) | 659 (10.4) | 622 (9.3) | 230 (13.8) | 31846 (12.1) |
| Baseline exam procedure information | ||||||
| Poor/compromised prep | 1046 (57.9) | 184 (17.4) | 351 (5.6) | 234 (3.5) | 55 (3.3) | 9910 (3.8) |
| Cecum not reached | 1032 (57.1) | 128 (12.1) | 302 (4.8) | 208 (3.1) | 66 (4.0) | 8775 (3.3) |
| Poor/compromised prep and/or cecum not reached | 1325 (73.4) | 266 (25.1) | 596 (9.4) | 411 (6.2) | 112 (6.7) | 16566 (6.3) |
All race categories are non-Hispanic; Hispanic ethnicity is regardless of race
Veteran Affairs
The indications for the repeat procedures, stratified by interval are shown in Table 2. The most common reason for early exam was a family history of CRC or polyps (n=6,535; 37.3%); 93.1% had repeat exams in 7 years or less. Positive FOBT or sigmoidoscopy was an indication in 5.3%; 53.4% had a baseline positive FOBT; 46.6% had a new positive FOBT during follow-up. New symptoms occurred in 32.4% of the cohort. Bleeding was an indication in 23.1% of the repeat exams and other gastrointestinal (GI) symptoms accounted for 9.3%. Among individuals receiving a second exam in less than 10 years, 20.2% were average-risk without GI symptoms.
Table 2.
Indication for second exam, stratified by follow-up interval
| Total N | Overall | <1 | 1–<2 | 2–<5 | 5–<7 | 7–<10 |
|---|---|---|---|---|---|---|
| 17,525 | 1806 | 1058 | 6314 | 6681 | 1666 | |
| 2nd Exam Indication: | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) |
| Average-risk Screening only | 3,550 (20.2) | 679 (37.6) | 162 (15.3) | 999 (15.8) | 1,157 (17.3) | 547 (32.8) |
| Family History (may have other indications) | 6,535 (37.3) | 235 (13.0) | 137 (12.9) | 2,083 (33.0) | 3,629 (54.3) | 451 (27.1) |
| Family History as the only indication | 5,350 (30.5) | 193 (10.7) | 68 (6.4) | 1,486 (23.5) | 3,229 (48.3) | 374 (22.5) |
| +FOBT or polyps found on sigmoidoscopy1 | 921 (5.3) | 274 (15.2) | 67 (6.3) | 348 (5.5) | 164 (2.5) | 68 (4.1) |
| Bleeding/anemia2 | 4,049 (23.1) | 461 (25.5) | 423 (40.0) | 1,879 (29.8) | 937 (14.0) | 349 (21.0) |
| Other symptoms3 | 1,621 (9.3) | 94 (5.2) | 127 (12.0) | 741 (11.7) | 469 (7.0) | 190 (11.4) |
+FOBT and/or polyps found on sigmoidoscopy; may have average-risk screening or family history of CRC/polyps but no other indications
melena, hematochezia, iron deficiency without anemia, anemia, +FOBT; may have other indications
change in bowel habits, constipation, diarrhea, abdominal pain/bloating
1,806 patients (10.3%) had follow-up in less than 1 year after the baseline colonoscopy. In 73.4% (n = 1,325) of cases, the baseline exam was either incomplete or had poor/compromised bowel prep. They were more likely to be male, and had a mean age of 60.7 years. We compared the demographics of the baseline colonoscopy cohort (n= 264,184) and the 1,806 individuals receiving follow-up exam in less than one year (Table 1). Blacks accounted for 10.1% of the early exams (compared to 5.9% in the baseline cohort); Hispanics accounted for13.2% of the early exams (compared to 5.5% in the baseline cohort).
Among the 1,806 patients with negative baseline colonoscopy who had repeat exam in less than 1 year, 6.5% had large polyp(s) (Table 3). This rate is similar to the prevalence of large polyps among all patients receiving average-risk screening colonoscopy (6.4% of 158,844 subjects not in our study; P = 0.89).
Table 3.
Rate of polyp(s) >9mm stratified by follow-up interval
| Total N | Overall | Follow-up Interval (years) | ||||
|---|---|---|---|---|---|---|
| <1 | 1–<2 | 2–<5 | 5–<7 | 7–<10 | ||
| 17,525 | 1806 | 1058 | 6314 | 6681 | 1666 | |
|
| ||||||
| Polyp >9 mm (%; 95% Confidence Interval) | 654 (3.7; 3.5 –4.0) | 117 (6.5; 5.3 –7.6) | 33 (3.1; 2.1 –4.2) | 197 (3.1; 2.7 –3.5) | 230 (3.4; 3.0–3.9) | 77 4.6% (3.6 – 5.6) |
| N with proximal location (% of polyp >9; 95% CI) | 411 (62.8; 59.1–66.5) | 64 (54.7; 45.7–63.7) | 18 (54.5; 37.6–71.5) | 119 (60.4; 53.6–67.2) | 158 (68.7; 62.7–74.7) | 52 (67.5; 57.0–78.0) |
| Most advanced polyp finding by interval
| ||||||
|---|---|---|---|---|---|---|
| Total N | Overall | Follow-up Interval (years) | ||||
| <1 | 1–<2 | 2–<5 | 5–<7 | 7–<10 | ||
| 17,525 | 1,806 | 1,058 | 6,314 | 6,681 | 1,666 | |
|
| ||||||
| N (%;95% CI) | N (%;95% CI) | N (%;95% CI) | N (%;95% CI) | N (%;95% CI) | N (%;95% CI) | |
| No polyps or tumors | 12,417 (70.9; 70.2–71.5) | 1,242 (68.8; 66.6–70.9) | 815 (77.0; 74.5–79.6) | 4,670 (74.0; 72.9–75.0) | 4,594 (68.8; 67.7–69.9) | 1,096 (65.8; 63.5–68.1) |
| Polyp unknown size | 112 (0.6; 0.52–0.8) | 32 (1.8; 1.2–2.4) | 9 (0.9; 0.3–1.4) | 42 (0.7; 0.5–0.9) | 24 (0.4; 0.2–0.5) | 5 (0.3; 0.0–0.6) |
| Polyp < 6mm | 3,360 (19.2; 18.6–19.8) | 303 (16.8; 15.1–18.5) | 149 (14.1; 12.0–16.2) | 1,095 (17.3; 16.4–18.3) | 1,434 (21.5; 20.5–22.4) | 379 (22.7; 20.7–24.8) |
| Polyp 6 – 9 mm | 951 (5.4; 5.1–5.8) | 106 (5.9; 4.8–7.0) | 49 (4.6; 3.4–5.9) | 300 (4.8; 4.2–5.3) | 392 (5.9; 5.3–6.4) | 104 (6.2; 5.1–7.4) |
| Polyp >9 mm | 607 (3.5; 3.2–3.7) | 106 (5.9; 4.8–7.0) | 31 (2.9; 1.9–3.9) | 176 (2.8; 2.4–3.2) | 221 (3.3; 2.9–3.7) | 73 (4.4; 3.4–5.4) |
| Tumor (any) | 78 (0.4; 0.3–0.5) | 17 (0.9; 0.5–1.3) | 5 (0.5; 0.1–0.9) | 31 (0.5; 0.3–0.7) | 16 (0.2; 0.1–0.4) | 9 (0.5; 0.2–0.9) |
| Suspected malignant | 43 (0.2; 0.2–0.3) | 10 (0.6; 0.2–0.9) | 2 (0.2; 0.0–0.5) | 19 (0.3; 0.2–0.4) | 8 (0.1; 0.0–0.2) | 4 (0.2; 0.0–0.5) |
| Established malignant | 3 (0.02; 0.00–0.04) | 2 (0.11; 0.00–0.26) | 0 (0) | 1 (0.02; 0.00–0.05) | 0 (0) | 0 (0) |
15,719 had follow-up colonoscopy between 1 and 10 years after a negative baseline colonoscopy; the incidence of large polyp(s) was 3.1% (95% CI: 2.7–3.5) in years 1 to less than 5 and 3.7% (95% CI 3.3–4.1) in years 5 up to 10, which was significantly lower than the 1 year cohort (p < 0.0001) (Table 3). The incidence of newly discovered proximal large polyps increased with longer duration of follow-up (Table 3) from 55% in the first two years to 68% in the last 5 years. The proportion of exams where the most advanced polyp finding was a small polyp (less than 6 mm) was higher in years 5–10 compared to year 1 to less than 5 years (21.7% vs 16.9%; p<.0001).
The impact of a compromised baseline exam was assessed over the entire study period (within 10 years of baseline colonoscopy), by comparing all individuals who had compromised baseline exam with those who had a complete exam (Table 4). Patients with compromised exams were slightly older, and more likely to be male. Data were classified based on size of largest polyp. Incidence of largest polyp(s) >9mm (5.4% vs 3.4%; P <0.0001) and largest polyp(s) 6–9mm in diameter (6.6% vs 5.5%; P = 0.030) was higher in patients with compromised baseline exams. The incidence of polyps over time (stratified by size of largest polyp) is shown in Figure 2.
Table 4.
Comparison of patients with compromised baseline exam with individuals who had complete baseline exam
| Compromised baseline exam | Complete baseline exam | P | |
|---|---|---|---|
| N=2,710 (15.5%) | N=14,815 (84.5%) | ||
| Demographics | |||
| Mean age (SD*) | 60.6 (9.8) | 59.2 (10.0) | <0.0001 |
| Age category (years) | N (%) | N (%) | |
| <50 | 244 (9.0) | 2,214 (14.9) | <0.0001 |
| 50–59 | 1,164 (43.0) | 6,113 (41.3) | |
| 60–69 | 801 (29.6) | 4,178 (28.2) | |
| 70–79 | 428(15.8) | 2,055 (13.9) | |
| ≥80 | 73 (2.7) | 255 (1.7) | |
| Male gender | 1,436 (53.0) | 6,663 (45.0) | <0.0001 |
| Outcomes | |||
| Polyp >9mm/tumor | 145 (5.4) | 509 (3.4) | <.0001 |
| Largest polyp 6–9 mm (excluding polyps >9mm) N=16,871 |
168 (6.6) | 783 (5.5) | .030 |
Standard deviation
Figure 2.
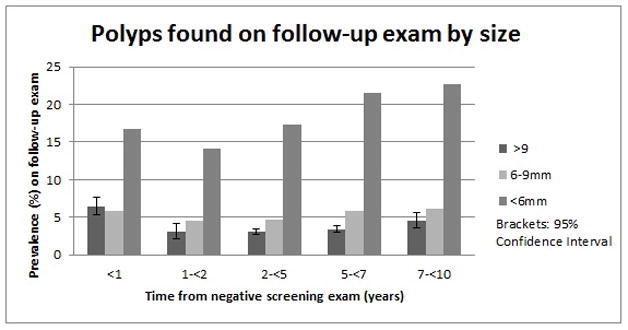
Incidence of polyps found at follow-up colonoscopy exam, stratified by size of largest polyp and time-interval from baseline negative colonoscopy.
Table 5 summarizes the outcomes of follow-up exam based on the procedure indication for the follow-up exam, stratified by interval from baseline colonoscopy. These data show that across each procedure indication, the highest yield of large polyps is in the period less than one year, when inadequate exams accounted for nearly 75% of patients. The rate of polyp(s) >9mm after a positive FOBT was only 2.2% in years 1-<5, and 4.0% in year 5–<10 after a baseline negative colonoscopy.
Table 5.
Analysis of outcome of follow-up exam by indication and interval
| Indication for follow-up exam | Interval from baseline colonoscopy | ||
|---|---|---|---|
| <1 year | 1–<5 years | 5–<10 years | |
| N (%) | N (%) | N (%) | |
| Average-risk1 | 679 | 1,161 | 1,704 |
| Polyp(s)>9mm | 33 (4.9) | 22 (1.9) | 57 (3.3) |
|
| |||
| Family History of CRC or polyps2 | 235 | 2,220 | 4,080 |
| Polyp(s)>9mm | 10 (4.3) | 55 (2.5) | 125 (3.1) |
|
| |||
| FOBT (+)3 | |||
| On follow-up exam only | 15 | 267 | 126 |
| Polyp(s)>9mm | 0 (0) | 7 (2.6) | 5 (4.0) |
| On follow-up and baseline exams | 228 | 138 | 101 |
| Polyp(s) >9mm | 19 (8.3) | 2 (1.4) | 4 (4.0) |
|
| |||
| Bleeding4 | 461 | 2,302 | 1,286 |
| Polyp(s)>9mm | 31 (6.7%) | 80 (3.5%) | 69(5.4%) |
|
| |||
| Other Symptoms5 | 94 | 868 | 659 |
| Polyp(s)>9mm | 0 (0%) | 29 (3.3%) | 29 (4.4%) |
Average-risk screening as the only indication
Family history of CRC or polyps; may have other indications
+FOBT, may have average-risk screening or family history of polyps/CRC but no other indications
Any of the following bleeding indications: melena, hematochezia, iron deficiency without anemia, anemia, +FOBT; may have other indications
Other symptoms include change in bowel habits, constipation, diarrhea, abdominal pain/bloating and no other indications
Discussion
We studied a unique cohort which had a negative baseline colonoscopy and had follow-up colonoscopy documented in the CORI database. The ideal study would have followed all patients prospectively to determine actual rates of interval colonoscopy. Therefore, this analysis provides only a snapshot of those patients who had follow-up colonoscopy within the CORI network, and does not include the rates of interval colonoscopy. To determine if our cohort was representative of individuals receiving colonoscopy screening exams, we examined the endoscopic outcomes of the entire cohort of individuals who received average-risk screening at baseline. Among 158,884 individuals undergoing screening that were not included in our study, 10,165 (6.4%) had one or more polyps >9mm. This finding is consistent with rates of advanced neoplasia from other large screening trials.23
17,525 patients with a negative baseline exam (11.9%) had repeat colonoscopy in less than 10 years within the CORI network. Among these subjects, 10.3% had the exam in less than 1 year. We found that 73.4% of these patients with one year exams had compromised baseline examinations, either due to poor prep (57.9%) and/or the cecum was not reached (57.1%). The prevalence of large polyp(s) was 6.5% in this group, which is similar to our baseline screening population and other studies.23 These data highlight several key points. Poor quality bowel preps which obscure visualization of the colon, may be associated with missed lesions at the baseline colonoscopy.25,26 Current quality indicators for colonoscopy call for monitoring bowel prep quality,27,28 with the goal of achieving preps adequate for detection of lesions greater than 5mm in 95% of exams. There is now substantial evidence29 that splitting the dose of bowel prep results in better quality and this practice is strongly encouraged by expert panels.3 The current data provide additional evidence of the importance of the quality of the baseline colonoscopy, and benefits of early re-examination if the baseline exam is compromised.
Among patients with exams at 1–5 years after baseline, the most common reasons for early repeat exams were family history (30.1%) and GI bleeding or anemia (31.2%) and other symptoms (11.8%). Most guidelines recommend a 5 year interval for screening of individuals with a family history of CRC if the index family member was less than 60 years,3 unless they have a hereditary syndrome associated with colorectal cancer such as Lynch syndrome or familial adenomatous polyposis. The evaluation of symptoms such as bleeding or interval fecal blood test or changes in bowel habits after a negative colonoscopy has never been carefully studied.
The incidence of large polyps in years 1 to 5 after a negative baseline colonoscopy is 3.1%, less than half the rate found at average-risk baseline screening (6.4%). There may be other benefits of repeating colonoscopy for evaluation of symptoms that were not measured. Given the low rate of large polyps, further study is needed to determine if there is any significant benefit to repeating colonoscopy early after a high-quality negative baseline exam. We did not find that family history of CRC was a predictor of increased risk in years 1 up to 5 or 5 to 10 after baseline negative colonoscopy. We do not know what proportion of patients had a first degree relative less than 60 years old, for whom screening intervals are recommended at 5 years. Further study is needed to clarify if this group would benefit from early colonoscopy.
These data demonstrate the lack of effectiveness of repeating FOBT in less than 5 years after a negative colonoscopy. At baseline colonoscopy, individuals with a positive FOBT have a two-fold increased prevalence of polyps >9mm compared to individuals undergoing average risk screening colonoscopy. We find that after a negative colonoscopy, the incidence of large polyps associated with a positive FOBT in years 1 to 4.9 years is only 2.2%. The role of FOBT in years 5 to 10 after a negative colonoscopy is also questionable. We found that 4.0% of such patients had incident large polyps, which is lower than the prevalence of large polyps found at baseline screening colonoscopy. These data reinforce the recommendation to avoid using FOBT after patients have had a negative baseline screening colonoscopy.
Current guidelines recommend a 10 year interval before repeating exams after a negative baseline colonoscopy in average-risk individuals. Prior cohort and case-control studies have found that colonoscopy screening may have a protective effect of 10 years or more.9,11,13,14 Two of these studies suggest that the protective effect increases after the first two years of follow-up.11,13 We suspect that significant neoplasia discovered in the first years after baseline colonoscopy likely represent lesions missed at the baseline exam. After the first year, we and others find low rates of large polyps after a negative baseline, confirming the likelihood that negative colonoscopy, performed with high quality, is associated with a low risk of developing advanced neoplasia over the next 10 years. We observed a trend in the relationship of incidence of proximal large polyps and timing of the interval exam. In the first two years after the baseline colonoscopy, 55% of large polyps were found in the proximal colon, and in the later years (5–10 years), 68% of the large polyps were proximal. These data support the hypothesis that proximal lesions are more likely to be missed at baseline colonoscopy, a finding consistent with other studies which raise questions about the protective effect of colonoscopy in the proximal colon.8–13
Strengths and Limitations
An important strength of our study is the inclusion of diverse practice sites throughout the United States, which are representative of endoscopic practice in this country.30 However, endoscopists who are comfortable sharing data from their practice might differ in important ways from those who will not share data or do not use electronic records to monitor quality in their practice. This potential bias could influence the frequency of repeat exams or reasons for repeat exams. We report on 10% of patients with negative baseline colonoscopy who had follow-up documented in CORI. It is likely that other patients had follow-up colonoscopies outside of CORI sites, and were not captured. Therefore, our follow-up is incomplete. Despite this limitation, this is the largest cohort with documented colonoscopy follow-up after negative baseline exams. Our follow-up was less than 10 years in patients who had colonoscopy after 2002. Nevertheless, our cutoff date captured follow-up exams within 5 years of the baseline colonoscopy for all subjects. The surrogate endpoint of polyp(s) >9mm has been shown to correlate well with rates of advanced neoplasia, but some individuals would be misclassified with this endpoint.
Conclusion
Our analysis focused on a cohort of community-based patients who had negative index screening colonoscopy and had follow-up colonoscopy in less than 10 years. There are several key findings. 10% of these patients had follow-up at less than 1 year after the baseline exam, most often because of a compromised baseline exam. The yield of large polyps in patients with compromised exams performed at less than one year was similar to the prevalence at index screening exams. These data support the need for repeat examination after a compromised index colonoscopy.3,25 Overall, the index screening colon exam was compromised in 16,650 of 264,284 patients receiving screening exams (6.3%), primarily due to poor bowel prep. If most of these patients have a follow-up exam within 6–12 months, they represent a significant cost burden. To reduce this burden, bowel prep quality should be monitored as a quality indicator,27,28 and split dose preps should be universally used.29
We find that if the baseline exam was not compromised, the incidence of significant findings at a 1 to 5 year interval is low, regardless of indication. In particular, our results suggest little benefit of screening with FOBT in the first five years after a baseline negative colonoscopy. In other cases, there may be other unmeasured benefits of colonoscopy that should be further evaluated. Finally, these data provide support for the durable protective effect of a negative colonoscopy, provided the baseline exam was adequate.
Acknowledgments
Grant Support: This project was supported with funding from NIDDK U01DK57132 and R33-DK61778-01. In addition, the practice network (Clinical Outcomes Research Initiative) has received support from the following entities to support the infrastructure of the practice-based network: AstraZeneca, Novartis, Bard International, Pentax USA, ProVation, Endosoft, GIVEN Imaging, and Ethicon. The commercial entities had no involvement in this research.
Abbreviations used in this paper
- CI
Confidence interval
- CORI
Clinical Outcomes Research Initiative
- CRC
colorectal cancer
- FOBT
fecal occult blood test
- GI
gastrointestinal
- PLCO
Prostate
- Lung
colorectal, and Ovarian Cancer screening trial
- SD
standard deviation
- VA
Veterans Affairs
Footnotes
Author Contributions:
Lieberman: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; study supervision
Holub: acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis; administrative, technical, or material support
Morris: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Logan: critical revision of the manuscript for important intellectual content; administrative, technical, or material support
Williams: statistical analysis; administrative, technical, or material support
Carney: critical revision of the manuscript for important intellectual content
Disclosures: Dr. Lieberman is the executive director of CORI, a non-profit organization that receives funding from federal and industry sources. This potential conflict of interest has been reviewed and managed by the OHSU and Portland VA Conflict of Interest in Research Committees. All other authors have no potential conflicts of interests to disclose.
References
Author names in bold designate shared co-first authorship.
- 1.U S. Preventive Services Task Force. Screening for colorectal cancer: U.S Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for early detection of colorectal cancer and adenomatous polyps, 2008:A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Atkin WS, Edwards R, Kralj-Hans I, et al. UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010 May 8;375(9726):1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 5.Segnan N, Armaroli P, Bonelli L, et al. SCORE Working Group. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011 Sep 7;103(17):1310–22. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- 6.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–57. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter NN, Sutradhar R, Forbes SS, et al. Analysis of administrative data finds endoscopist quality measures associate with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65–72. doi: 10.1053/j.gastro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Singh H, Nugent Z, Demers AA, et al. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: A population-based study. Am J Gastroenterol. 2010;105:2588–1596. doi: 10.1038/ajg.2010.390. [DOI] [PubMed] [Google Scholar]
- 9.Singh H, Turner D, Xue L, et al. Risk of developing colorectal cancer following a negative colonoscopy examination. JAMA. 2006;295:2366–73. doi: 10.1001/jama.295.20.2366. [DOI] [PubMed] [Google Scholar]
- 10.Bressler B, Paszat LF, Chen Z, et al. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: A population-based analysis. Gastroenterology. 2007;132:96–102. doi: 10.1053/j.gastro.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Lakoff J, Paszat LF, Saskin R, et al. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: A population-based study. Clin Gastroenterol Hep. 2008;6:1117–21. doi: 10.1016/j.cgh.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer: A population-based, case-control study. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 13.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 14.Nishihara R, Wu K, Lochhead P, Morikawa T, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoen RE, Pinsky PF, Weissfeld JL, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138:73–81. doi: 10.1053/j.gastro.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin JS, Singh A, Reddy N, et al. Overuse of screening colonoscopy in the Medicare population. Arch Int Med. 2011;171:1335–43. doi: 10.1001/archinternmed.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieberman DA, Weiss DG, Harford WV, et al. Five year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–85. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Imperiale TF, Glowinski EA, Lin-Cooper C, et al. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med. 2008;359:1218–24. doi: 10.1056/NEJMoa0803597. [DOI] [PubMed] [Google Scholar]
- 19.Leung WK, Lau JYW, Suen BY, et al. Repeat screeing colonoscopy 5 years after normal baseline screening colonoscopy in average-risk Chinese: A prospective study. Am J Gastroenterol. 2009;104:2028–34. doi: 10.1038/ajg.2009.202. [DOI] [PubMed] [Google Scholar]
- 20.Brenner H, Haug U, Arndt V, et al. Low risk of colorectal cancer and advanced adenomas more than 10 years after negative colonoscopy. Gastroenterology. 2010;138:870–6. doi: 10.1053/j.gastro.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 21.Miller H, Mukherjee R, Tian J, et al. Colonoscopy surveillance after polypectomy may be extended beyond five years. J Clin Gastroenterol. 2010;44:e162–e166. doi: 10.1097/MCG.0b013e3181e5cd22. [DOI] [PubMed] [Google Scholar]
- 22.Chung SJ, Kim YS, Yang SY, et al. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut. 2011;60:1537–43. doi: 10.1136/gut.2010.232876. [DOI] [PubMed] [Google Scholar]
- 23.Heitman SJ, Ronksley PE, Hisden RJ, et al. Prevalence of adenomas and colorectal cancer in average risk individuals: A systematic review and meta-analysis. Clin Gastroenterol Hep. 2009;7:1272–78. doi: 10.1016/j.cgh.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman DA, Moravec M, Holub J, et al. Polyp size and advanced histology in patients undergoing colonoscopy screening: Implications for CT Colonography. Gastroenterology. 2008;135:1100–5. doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebwohl B, Kastrinos F, Glick M, et al. The impact of suboptimal bowel preparation on adenoma miss rates and thef actors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73:1207–14. doi: 10.1016/j.gie.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neerincx M, Terhaarsive Droste JS, Mulder CJ, et al. Colonic work-up after incomplete colonoscopy: significant new findings during follow-up. Endoscopy. 2010 Sep;42(9):730–5. doi: 10.1055/s-0030-1255523. [DOI] [PubMed] [Google Scholar]
- 27.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006 Apr;101(4):873–85. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 28.Lieberman D, Nadel M, Smith R, et al. Standardized colonoscopy reporting and data system (CO-RADS): Report of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable. Gastrointest Endosc. 2007;65:757–66. doi: 10.1016/j.gie.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 29.Kilgore TW, Abdinoor AA, Szary NM, et al. Bowel preparation with split-dose polyethylene glycol before colonoscopy: a meta-analysis of randomized controlled trials. Gastrointest Endosc. 2011 Jun;73(6):1240–5. doi: 10.1016/j.gie.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Sonnenberg A, Amorosi SL, Lacey MJ, Lieberman DA. Patterns of endoscopy in the United States: analysis of data from the Centers for Medicare and Medicaid Services and the National Endoscopic Database. Gastrointest Endosc. 2008;67:489–96. doi: 10.1016/j.gie.2007.08.041. [DOI] [PubMed] [Google Scholar]


