Abstract
Objective
The aim of the study was to characterize the expression, regulation and pathogenic role of TLR7 and TLR8 in rheumatoid arthritis (RA).
Methods
Expression of TLR7 and TLR8 was demonstrated in RA, osteoarthritis (OA) and normal (NL) synovial tissues (ST) employing immunohistochemistry. We next examined the mechanism by which TLR7 and TLR8 ligation mediates proinflammatory response by Western blot analysis and ELISA. Expression of TLR7 and TLR8 in RA monocytes was correlated to disease activity score (DAS28) and TNF-α levels. Further the effect of TLR7 ligation in RA monocytes was determined on synovial fluid (SF) mediated TNF-α transcription.
Results
TLR7/TLR8 are predominately expressed in RA ST lining and sublining macrophages. We show that NF-κB and/or PI3K pathways are essential for TLR7/TLR8 induction of proinflammatory factors in RA peripheral blood (PB) differentiated macrophages. Expression of TLR7 in RA monocytes shows a strong correlation with DAS28 and TNF-α levels. In contrast, expression of TLR8 in these cells does not correlate with DAS28, TLR7 or TNF-α levels. We further demonstrate that RNA from RA SF but not RA or NL plasma could modulate TNF-α transcription from RA monocytes that can be downregulated by antagonizing TLR7 ligation or degradation of single stand (ss) RNA. Thus, ssRNA present in RA SF may function as a potential endogenous ligand for TLR7.
Conclusions
These results suggest that expression of TLR7 but not TLR8 may be a predictor for RA disease activity and anti-TNF-α responsiveness, and targeting TLR7 may suppress chronic progression of RA.
Keywords: RA synovial tissue, RA monocytes/macrophages, TLR7, TLR8 and ssRNA
RA is a chronic autoimmune disorder in which the innate immune system plays an important role1, 2. Although monokines such as TNF-α, IL-6 and CCL2 play an integral role in the pathogenesis of RA, it is unclear which stimuli are involved in driving their chronic production. Potential candidates for the induction of these inflammatory monokines may include Toll-like receptor (TLR) activation.
TLRs are pattern recognition receptors that are classified into 2 groups based on their cellular distribution and receptor selection. TLRs 1, 2, 4, 5, and 6, expressed on the cell surface, recognize microbial components, known as pathogen associated molecular patterns (PAMPs)3, whereas endosomal TLRs 3, 7, 8 and 9 mainly detect nucleic acids4–6. Our understanding of the role of TLRs in RA pathogenesis originates from findings showing endogenous TLR ligands called damage associated molecular patterns (DAMPs) in RA synovial tissue (ST) and fluid (SF). Endogenously expressed DAMPs include, fibrinogen, HSP60, 70 and 96 as well as EDA fibronectin, all of which bind to TLR2 and/or TLR47, 8. Previous studies demonstrate that expression of TLR2 and TLR4 are elevated in RA peripheral blood (PB) monocytes as well as in RA SF and ST macrophages9–12. Further, the data obtained from experimental arthritis models strongly support the role of TLR4 in the pathogenesis of arthritis seen in collagen induced arthritis (CIA)13, as well as in IL-1RA−/− model13, 14. However, little is known about the endosomal TLRs in RA or experimental arthritis models.
Expression levels of endosomal TLR3 and TLR7 were demonstrated in RA synovial tissues fibroblasts15 and dendritic cells16. Further it was shown that in RA dendritic cells, ligands to TLR3 or TLR7 were unable to induce production of proinflammatory cytokines without synergistic activation from TLR4 ligands suggesting that the effect of TLR3 or TLR7 ligation was less potent than the combinational effect with LPS stimulation in this cell type16.
In this study, we document that TLR7/ TLR8 are elevated in RA ST lining and sublining macrophages compared to normal ST. Consistently, our data demonstrates that TLR7/TLR8 are greatly elevated in RA SF macrophages and PB monocytes compared to their normal counterparts. Most importantly we show that patients with higher DAS28 express elevated levels TLR7 but not TLR8 in RA monocytes. Levels of TLR7 correlate with TNF-α concentration and inhibition of TLR7 ligation or degradation of ssRNA reduces RA SF induced TNF-α transcription in RA monocytes. Interestingly, we show for the first time that only RNA from RA SF and not RA or NL plasma could modulate TNF-α levels through TLR7 ligation in RA monocytes suggesting that joint ssRNA may function as a TLR7 endogenous ligand.
MATERIALS AND METHODS
Antibodies and immunohistochemistry
The studies were approved by the Institutional Review Board and all donors gave informed written consent. RA, OA, and NL ST were formalin fixed, paraffin embedded and sectioned. STs were immunoperoxidase-stained using Vector Elite ABC Kits (Vector Laboratories, Burlingame, CA), with diaminobenzidine (Vector Laboratories) as a chromogen. Briefly, slides were deparaffinized in xylene, and antigens were unmasked by microwave exposure and incubation in citrate buffer. STs were incubated with antibodies to human TLR7 and TLR8 (1:50, Santa Cruz Biotechnology, Santa Cruz, CA) or an IgG control antibody. Each slide was evaluated by two blinded observers (A.M.M. and M.M.V.) on a 0–5 scale17, 18. For experiments performed in Figs. 1E and F, RA ST serial sections were stained with anti-TLR7, anti-TLR8 and anti-CD68 (Vector Laboratories; 1:100) antibodies or anti-TLR7 and anti-vimentin antibodies (Lab Vision, Kalamazoo, MI; 1:2000).
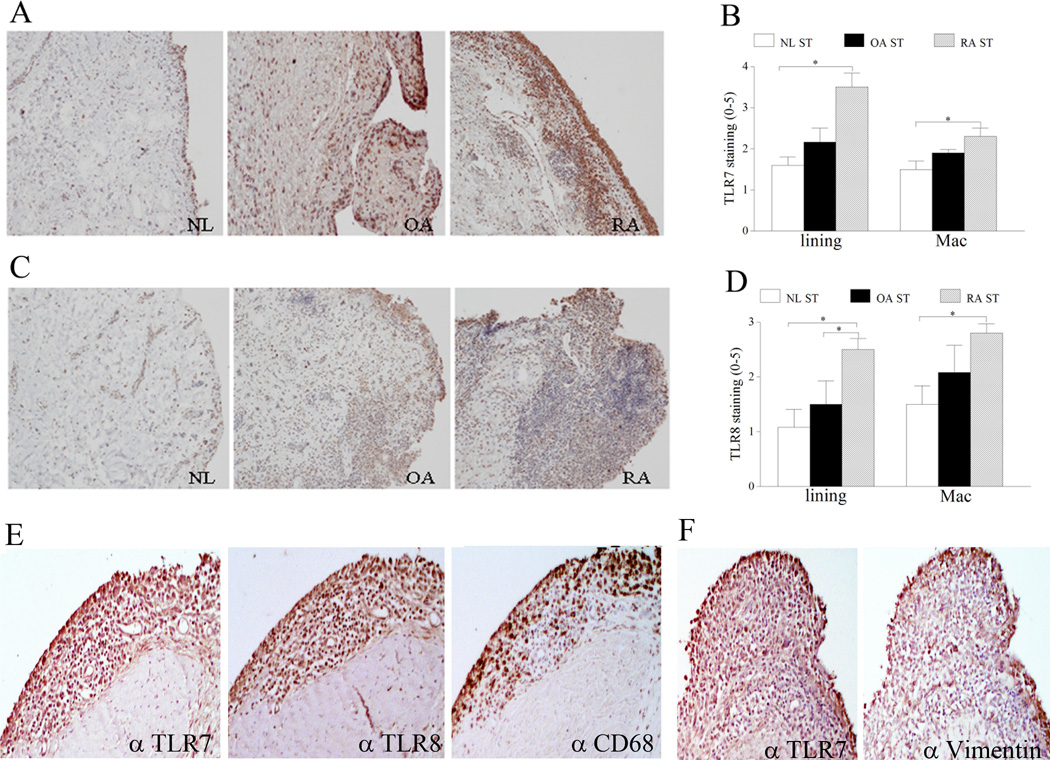
Figure 1. TLR7 and TLR8 expression is increased in RA synovial tissue (ST) lining and sublining macrophages compared to normal (NL) ST.
NL, OA and RA ST were stained with anti-human TLR7 (A) or anti-human TLR8 (C) (original magnification × 200) and positive immunostaining was scored on a 0–5 scale (B and D). ST lining and sublining macrophage immunostaining are shown as mean ±SEM, (n=5–7). E. RA serial sections were stained with anti-TLR7, anti-TLR8 and anti-CD68 antibodies in order to distinguish TLR7 and TLR8 staining on RA ST lining and sublining macrophages (original magnification × 400). F. RA serial sections were stained with anti-TLR7 and anti-vimentin antibodies in order to co-localize TLR7 staining in RA ST fibroblasts (original magnification × 400).
RA patient population
RA blood19 was obtained from 30 women and 5 men (mean age 51.6 ± 2.7 years). Patients were either receiving no treatment (n=3), taking disease-modifying anti-rheumatic drug (DMARD)s (methotrexate, leflunomide, sulfasalizine, azathioprine) alone (n=5), taking DMARDS plus hydroxychloroquine (n=8), taking DMARDs plus prednisone (n=4), taking DMARDs plus rituximab (n=1), taking DMARDs plus hydroxychloroquine plus minocycline (n=1), taking DMARDs plus hydroxycholoquine and predisone (n=1), or taking an anti-TNF-α either alone (n=5), with a DMARD (n=5), with a DMARD plus hydroxychloroquine (n=1), or with a DMARD plus hydroxychloroquine and prednisone (n=1). These studies were approved by the University of Illinois at Chicago Institutional Ethics Review Board and all donors gave informed written consent.
Cell isolation, culture and procedures
NL and RA PB and RA SF mononuclear cells were isolated by Histopaque gradient centrifugation (Sigma-aldrich, St. Louis, MO, USA)20, 21. Monocytes from NL and RA PB or macrophages from RA SF were isolated employing a negative selection kit (StemCell Technologies, Vancouver, Canada) according to the manufacturers' instructions17, 18. PB monocytes were differentiated to macrophages by culturing in RPMI containing 20% FBS for 7 days.
Quantification of chemokines and cytokines
Human TNF-α, IL-6 and CCL2 ELISA kits (R&D Systems, Minneapolis, MN, USA) were used according to the manufacturers' instructions.
Isolation of RA ST fibroblasts
RA fibroblasts were isolated from fresh STs by mincing and digestion in a solution of dispase, collagenase, and DNase21. Cells were used between passages 3 and 9 and cultured in DMEM containing 10% FBS.
Cell treatment
RA PB monocytes and in vitro differentiated macrophages or RA fibroblasts were treated with LPS (Sigma, 10 ng/ml), IL-1β (R&D Systems, 10 ng/ml), TNF-α (R&D Systems, 10 ng/ml), IL-17 (R&D Systems, 50 ng/ml), IL-6 (R&D Systems, 10 ng/ml), IL-8 (R&D Systems, 10 ng/ml), or RA SF (10%). Cells were harvested after 6 h and the TLR7 and TLR8 mRNA levels were quantified by real-time RT-PCR. RA fibroblasts, RA PB monocytes, NL and RA differentiated macrophages were treated with R848 (100 ng/ml and 1 µg/ml) (InvivoGen, San Diego, CA) and cells (6h) or conditioned media (24h) were harvested to determine TNF-α, IL-6 and CCL2 expression levels. In a different experiment, RA monocytes from 4 different patients were untreated or treated with PBS, Chloroquine (50 µM; InvivoGen) or A151 (1µM; InvivoGen)22 for 1h prior to being treated with R848 (100 ng/ml) or RA SF (10%) for 6h. To demonstrate the role of ssRNA and/or TLR7 ligation, RA monocytes were untreated or preincubated with PBS, RNAse A/T1 (RNAse A, 40 µg/ml; T1, 100 U/ml) (Fermentas, Glen Burnie, Maryland), A151 (1µM) or RNAse A/T1 plus A151 for 1h. Following incubation, RA SFs were added to the pretreated RA monocytes for 6h while untreated RA monocytes were employed as baseline control. To examine whether TLR7 ligand was present in RA SF and plasma compared to NL plasma, mRNA extracted from these samples was used to stimulate RA monocytes. For this purpose, RA monocytes were either untreated or treated with 5µg of RNA extracted from RA SF, RA and NL plasma [only RNA that had purity >1.8 (260/280 ratio) was used] or RA SF RNA plus A151 (1µM) for 6h. To validate that RNA extracted from RA SF specifically ligates to TLR7, NL monocytes and 293T cells were either untransfected or transfected with pCDNA3-TLR7-YFP (gift from Dr. Golenbock; Addgene plasmid #13022) (0.5 and/or 0.2µg). Cells were then stimulated with RA SF (10%) or RA SF mRNA (5µg) for 6h. Response to various treatments was determined by quantifying TNF-α transcription by real-time RT-PCR.
Real-time RT-PCR
Total cellular RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA). Subsequently, reverse transcription and real-time RT-PCR were performed to determine TLR7, TLR8, TNF-α, IL-6 and CCL2 expression levels as described previously17, 18. Relative gene expression was determined by the ΔΔCt method, and results were expressed as fold increase above conditions as indicated in the figure legends.
Flow cytometry
In order to determine TLR7+ or TLR8+ cells, normal and RA monocytes and macrophages were washed and blocked with 50% human serum in 0.5% BSA. Cells were then fixed and permeabilized using IC-Flow kit according to manufacturers' instructions (Imgenex, San Diego, CA). Next, cells were stained with PE conjugated anti-TLR7, Alexa-647 conjugated anti-TLR8 (Imgenex), and FITC labeled anti-CD14 (Becton Dickinson, Franklin Lakes, NJ) or isotype control antibodies and the percentage of CD14+TLR7+ or CD14+TLR8+ cells were determined. In a different experiment TLR7 transfection was determined by analyzing YFP expression in NL monocytes and 293T cells.
R848 signaling pathways in RA macrophages
RA differentiated macrophages (2x106/ml) were untreated or treated with R848 (1 µg/ml) for 0 to 65 min. Cell lysates were examined by Western blot analysis as previously described21. Blots were probed with phospho (p)-ERK, p-p38 MAPK, p-AKT1 (Cell Signaling; 1:1000) or degradation of IκB (Santa Cruz; 1:1000) overnight or probed with ERK, p38 and AKT or actin (Cell Signaling or Sigma; 1:3000).
Inhibition of the signaling pathways in RA PB macrophages
To define which signaling pathways mediate R848-induced CCL2 secretion, RA differentiated macrophages were incubated with DMSO or 10 µM inhibitors to p38 (SB203580), ERK (PD98059), PI3K (LY294002) or NF-κB (MG-132) for 1 h. Cells were subsequently activated with R848 for 24h and the media was collected to quantify CCL2 levels by ELISA.
Statistical analysis
The data was analyzed employing 1-way ANOVA followed by post hoc two-tailed Student’s t-tests for paired and unpaired samples. Expression levels of TLR7 and TLR8 (n=35) were correlated with each other, DAS28 score and concentration of TNF-α in RA monocytes by linear regression analysis. Values of p < 0.05 were considered significant.
RESULTS
TLR7 and 8 immunostaining is elevated in RA ST lining and sublining macrophages
To characterize the expression pattern of TLR7 and TLR8 in RA, OA, and NL joints, STs were stained with antibodies specific for these receptors. We found that in RA, TLR7 and TLR8 immunostaining was markedly higher in ST lining and sublining macrophages compared to normal ST (Figs. 1A–D). Confirming these findings TLR7/8 staining was co-localized to lining and sublining CD68+ cells while lining vimentin expressing cells were co-localized to TLR7+ cells (Figs. 1E–F) using serial section analysis. These results suggest that cells in the lining and macrophages in the sublining may be effector cells for TLR7 and TLR8 ligation.
TLR7 is present in RA ST fibroblasts, RA PB monocytes and macrophages
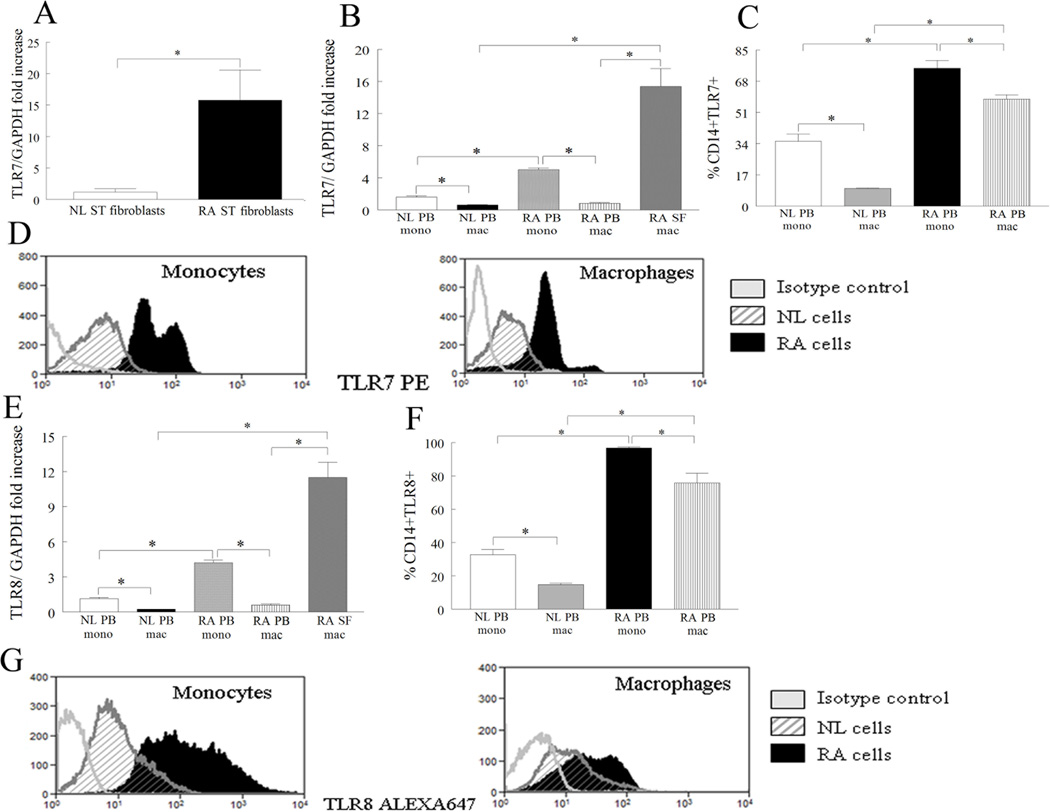
Since expression levels of TLR7 were elevated in RA ST lining and sublining macrophages, we asked if TLR7 was differentially expressed in RA fibroblasts, monocytes and macrophages compared to control cells. We found that TLR7 expression was 13-fold higher in RA compared to NL fibroblasts (Fig. 2A), however its expression was not inducible when RA fibroblasts were stimulated with LPS, TNF-α, IL-1β, IL-17, IL-6, IL-8 or RA synovial fluid (Fig. S1). Further, expression of TLR7 was elevated 18 and 24 fold in RA SF macrophages compared to RA and NL PB differentiated macrophages respectively by real-time RT-PCR (Fig. 2B). Levels of TLR7 were significantly elevated in RA monocytes compared to RA differentiated macrophages and NL monocytes (Fig. 2B). Interestingly, mRNA and endosomal expression of TLR7 is reduced when RA PB monocytes differentiate to macrophages (Figs. 2B–D). Our results suggest that RA fibroblasts, monocytes and macrophages may be an important source for TLR7 ligation.
Figure 2. TLR7 and TLR8 are upregulated in RA synovial fluid (SF) compared to RA and NL PB macrophages.
A. TLR7 mRNA levels were determined in NL (n=4) and RA ST fibroblasts (n=14) employing real-time RT-PCR. The data are shown as fold increase above NL ST fibroblasts and are normalized to GAPDH. B and E. TLR7 or TLR8 mRNA levels were determined in NL (n for monocytes or macrophages=11 or 18) and RA PB monocytes (n=11) and differentiated macrophages (n=15) as well as in RA SF macrophages (n=10) by employing real-time RT-PCR. The data are shown as fold increase above NL PB monocytes and are normalized to GAPDH. NL and RA PB monocyte and differentiated macrophages were immunostained with FITC labeled anti-CD14 antibody and PE-conjugated anti-TLR7 (C–D) or FITC labeled anti-CD14 antibody and Alexa 647-conjugated anti-TLR8 (F–G) in order to determine the percentage of CD14+ and TLR7/8 positive cells. The values are presented as mean ± SEM of % CD14+TLR7+ (n=5–8) (C) or %CD14+TLR8+ (n=5–9) (F) in each cell population. D and G are representative flow cytometry histograms of Figs. C and F. Values demonstrate mean ± SEM, * represents p<0.05.
TLR8 is not expressed in RA fibroblasts but has a similar expression pattern to TLR7 in RA SF and PB differentiated macrophages
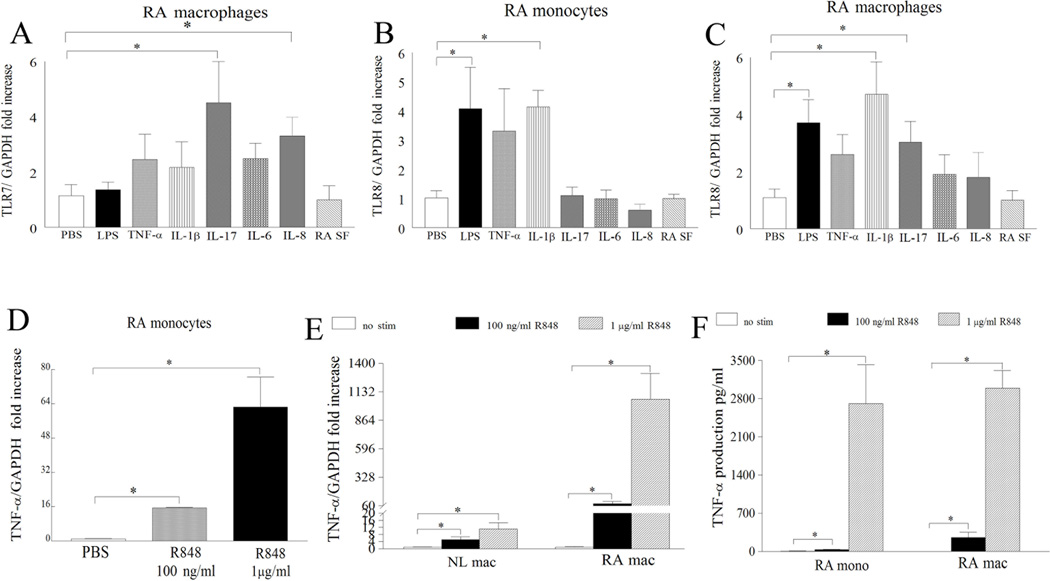
To determine the cell type that expresses TLR8 in RA joints, RA fibroblasts and macrophages were examined for TLR8 expression. Unlike TLR7 (Fig. 2A), TLR8 was undetectable in RA and NL fibroblasts (CT>40 cycles), however similar to TLR7 (Fig. 2B) expression of TLR8 was highly elevated in RA SF macrophages compared to RA and NL macrophages (Fig. 2E). Expression of TLR8 was higher in NL and RA monocytes compared to counterpart macrophages (Figs. 2E–G). Confirming the histological and mRNA studies, the percentages of TLR8 positive RA monocytes and macrophages (95% and 75%) were comparable to TLR7 positive cells (75% and 55%) (Figs. 2C–D and 2F–G). Next, TLR7/8 modulating factors were determined in RA PB monocytes and differentiated macrophages. We found that TLR8 expression is enhanced by LPS and IL-1β stimulation in both cell types (Figs. 3B–C). We also show that in RA differentiated macrophages IL-17 is the common factor that drives TLR7 and TLR8 expression levels (Figs. 3A, C) while TLR7 expression was unresponsive to stimulation in RA monocytes (Fig. S2). These results suggest that TLR7 and TLR8 have similar expression patterns in RA blood and ST and that both receptors are modulated by IL-17 in RA differentiated macrophages.
Figure 3. Proinflammatory factors induce the expression of TLR7/8 in RA PB differentiated macrophages and ligation of TLR7/8 induces expression and production of TNF-α in RA monocytes and differentiated macrophages.
RA differentiated macrophages (A–C) or monocytes (B) were untreated (PBS) or treated with LPS (10 ng/ml), TNF-α (10 ng/ml), IL-1β (10 ng/ml), IL-17 (50 ng/ml), IL-6 (10 ng/ml), IL-8 (10 ng/ml), or RA SF (10%) for 6h and expression of TLR7 was determined in RA macrophages (A) as well as expression levels of TLR8 in RA monocytes (B) and differentiated macrophages (C) were measured by real-time RT-PCR (n=5–10). The data are shown as fold increase above untreated RA PB monocytes or differentiated macrophages and are normalized to GAPDH. RA monocytes (mono) (n=7–10) (D and F) or NL (E) and RA differentiated macrophages (mac) (n=4–5) (E and F) were either untreated (PBS) or treated with R848 100 ng/ml or 1 µg/ml for 6h and expression levels of TNF-α (D and E) were quantified by real-time RT-PCR. The data are shown as fold increase above untreated cells and were normalized to GAPDH. Supernatants were harvested from RA monocytes (n=5–6) or differentiated macrophages (n=7–8) untreated (PBS) or treated with R848 at 100 ng/ml and 1 µg/ml for 24h and TNF-α (F) levels were determined by ELISA. Values demonstrate mean ± SEM, * represents p<0.05.
RA monocytes and macrophages but not fibroblasts are responsive to TLR7/8 ligation
While ss viral RNA is a natural ligand of TLR7/TLR8, imidazoquinoline resiquimod (R848) is recognized as a potent synthetic agonist of TLR7/TLR823. To determine whether RA PB monocytes, macrophages and RA fibroblasts respond to ligation of R848, cells were exposed to different doses of R848 and screened for transcription and production of TNF-α, IL-6 and CCL2. Although TLR7 is expressed in RA fibroblasts, exposure of these cells to R848 did not result in production of CCL2 or IL-8 (Figs. S3–4). In contrast, transcription and secretion of TNF-α (Figs. 3D–F) and IL-6 (Fig. S5–7) were dose dependently increased in R848 stimulated RA monocytes and macrophages. Although TLR7 and TLR8 expression was greatly elevated in RA monocytes compared to RA macrophages, ligation of these receptors with a higher dose of R848 (1 µg/ml) resulted in similar production levels of TNF-α and CCL2 in both cell types (Figs 3F, 4C). However our results demonstrate that when NL and RA macrophages are activated with same concentrations of R848 significantly higher levels of TNF-α are expressed in RA compared to NL cells perhaps due to higher levels of TLR7/8 expression (Fig 3E). These results suggest that while RA monocytes and macrophages respond to TLR7/8 ligation, RA fibroblasts do not.
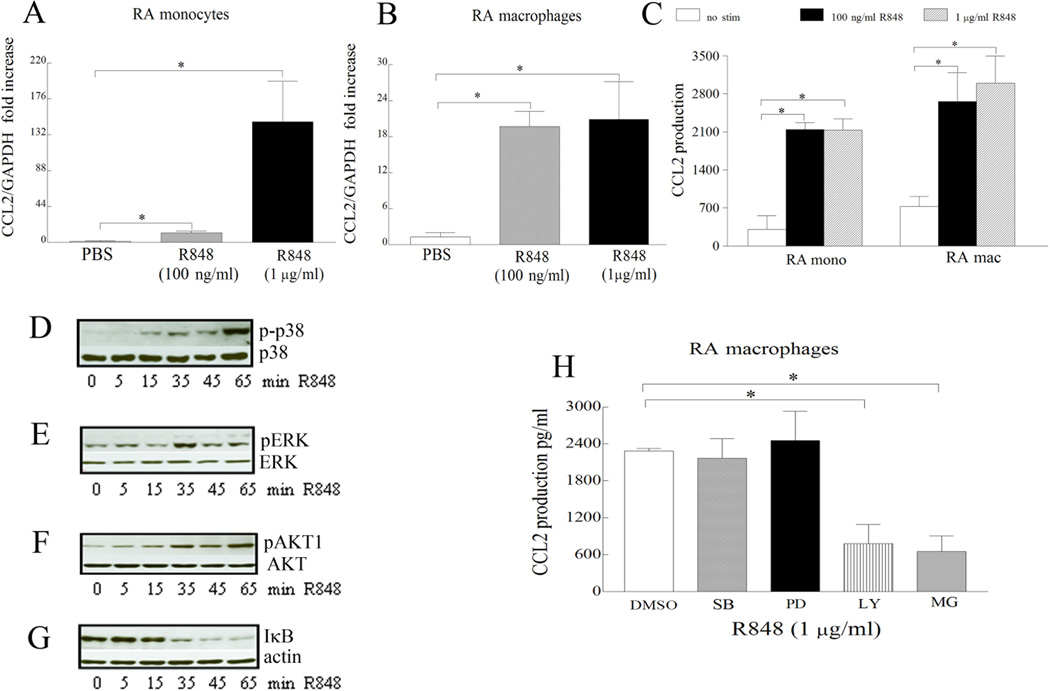
Figure 4. CCL2 levels are increased following TLR7 and TLR8 ligation in RA monocytes and differentiated macrophages and R848-induced CCL2 production is modulated by PI3K, NF-κB pathways in RA differentiated macrophages.
RA monocytes (mono) (n=7–10) (A) and differentiated macrophages (mac) (n=4–5) (B) were either untreated (PBS) or treated with R848 100 ng/ml or 1 µg/ml for 6h and expression levels of CCL2 were quantified by real-time RT-PCR. The data are shown as fold increase above untreated cells and were normalized to GAPDH. Supernatants were harvested from RA monocytes (n=5–6) or differentiated macrophages (n=7–8) untreated (PBS) or treated with R848 100 ng/ml and 1 µg/ml for 24h and CCL2 (C) levels were determined by ELISA. In order to determine the mechanism of TLR7/8 activation in RA differentiated macrophages, cells were stimulated with R848 (1 µg/ml) for 0–65 minutes, and the cell lysates were probed with antibodies specific for p-p38 (D), p-ERK (E), p-AKT (F), or IκB (G). To examine which of the signaling pathways were associated with TLR7 and TLR8-induced CCL2 production, in RA macrophages, cells were untreated (DMSO) or treated with 10 µM inhibitors to p38 (SB203580), ERK (PD98059), PI3K (LY294002) or NF-κB (MG-132) for 1h. Cells were subsequently activated with R848 (1 µg/ml) for 24h and the conditioned media was collected in order to quantify the levels of CCL2 employing ELISA (H). Values are the mean ± SEM, n=5–8. * represents p <0.05.
R848-induced CCL2 is regulated by NF-κB and PI3K pathways in RA macrophages
In RA macrophages, R848 activated pathways were inhibited to determine signaling cascades that contribute to TLR7/8 inflammatory response. We found that although p38, ERK, AKT1 and NF-κB pathways were activated by R848 (Fig. 4D–G) only inhibition of NF-κB and PI3K suppressed R848 induced CCL2 secretion by 3 fold (Fig. 4H). Our results suggest that activation of NF-κB and PI3K by R848 modulates CCL2 production in RA macrophages.
In RA monocytes TLR7 but not TLR8 correlates with DAS28 score and TNF-α levels
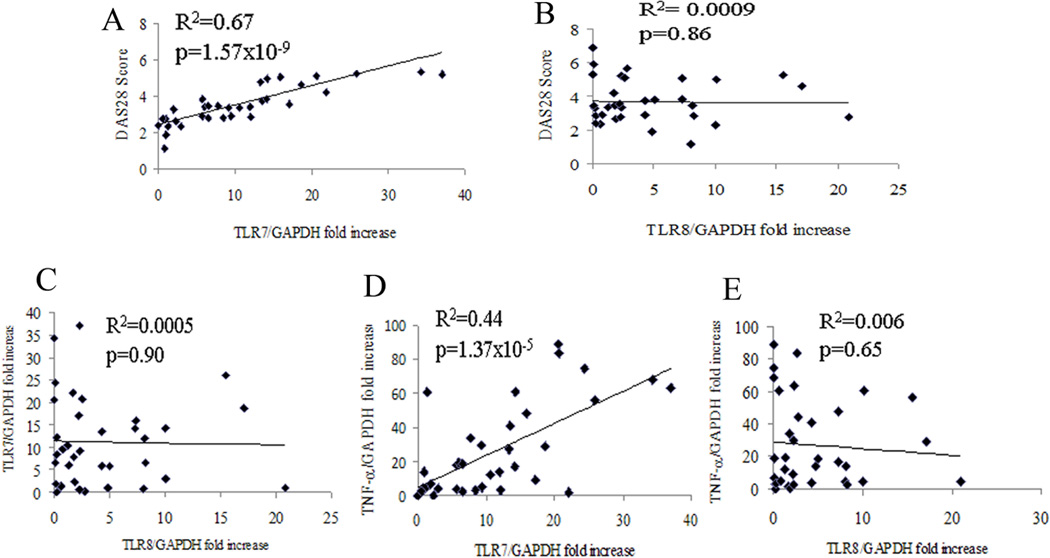
Since expression of TLR7 and TLR8 are significantly higher in RA monocytes compared to macrophages and ligation of a TLR7/8 agonist induces TNF-α production, we next asked whether mRNA levels of TLR7 and TLR8 in RA monocytes correlate with DAS28, TNF-α levels or each other. Regression analysis demonstrated that patients with greater concentrations of TLR7 had increased DAS28 and TNF-α levels in RA monocytes (Fig. 5A and D). In contrast, there was no correlation found between TLR8 expression in RA monocytes with DAS28, TLR7 or TNF-α levels (Fig. 5B, C, E). Interestingly, mRNA levels of TLR7 and TLR8 in RA macrophages (R2=0.04, p=0.5) were also uncorrelated. These results suggest that elevated levels of TLR7 but not TLR8 may predict higher RA disease activity and anti-TNF-α responsiveness.
Figure 5. TLR7 but not TLR8 expression correlates with DAS28 and TNF-α levels in RA monocytes.
Linear regression analysis was used to compare expression of TLR7 (n=35) with DAS28 (A), TLR8 (C), and TNF-α (D) mRNA levels in RA monocytes. Correlation was also calculated for TLR8 expression (n=35) and levels of DAS28 (B) and TNF-α (E) in RA monocytes. The mRNA expression levels in RA monocytes are shown as fold increase above NL PB monocytes and are normalized to GAPDH. Values of p < 0.05 were considered significant.
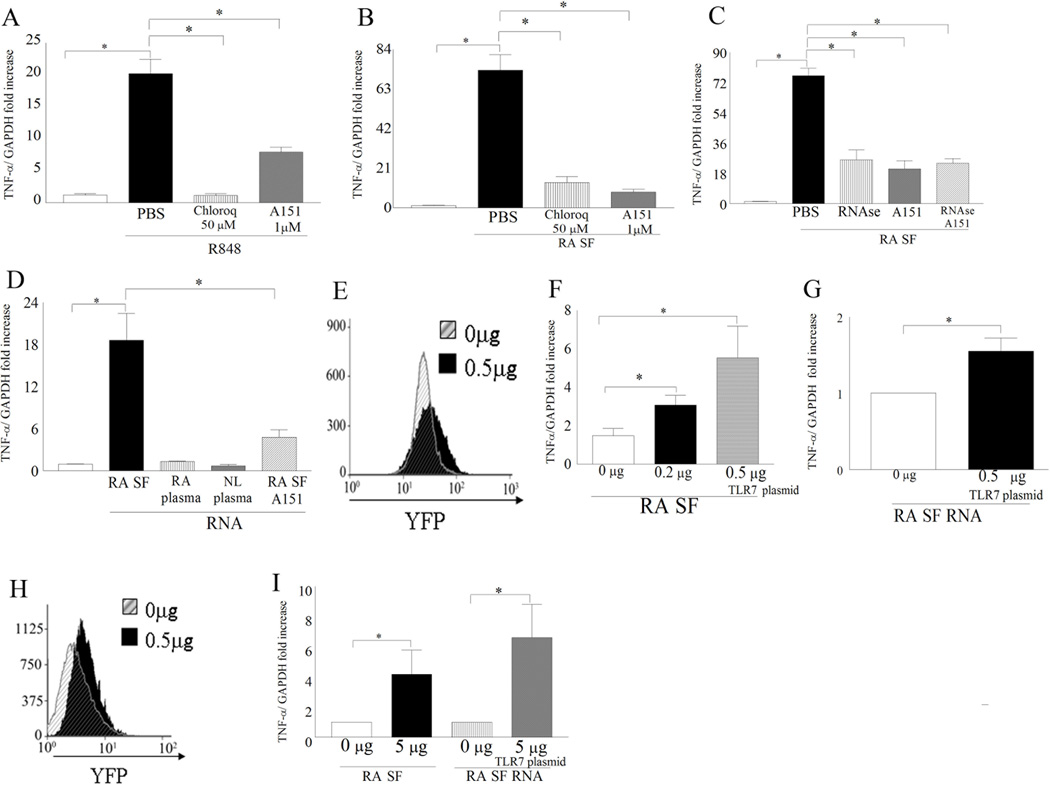
Inhibition of TLR7 ligation to RA SF RNA can significantly reduce SF induced TNF-α transcription in RA monocytes
Since TLR7 expression demonstrated a strong correlation with DAS28 and TNF-α transcription in RA monocytes we asked whether inhibition of TLR7 ligation can affect TNF-α modulation in these cells. Our results show that when an inhibitor for endosomal acidification (Chloroquine) was employed, the R848 ligation to TLR7/8 was completely abrogated whereas the TLR7 antagonist A15122 partially reduces the R848 induced TNF-α transcription in RA monocytes confirming its specificity for TLR7 ligation (Fig. 6A). We next demonstrate that TLR7 endogenous ligand(s) are present in RA synovial fluid since blockade of TLR7 ligation in RA monocytes greatly downregulates (8.5 fold decrease) SF mediated TNF-α transcription (Fig. 6B). Given that ssRNA is an agonist for TLR7 ligation24 we asked whether degradation of SF ssRNA or blockade of TLR7 ligation on RA monocytes would similarly affect RA SF mediated TNF-α transcription. We document that suppression of TLR7 ligation or degradation of SF ssRNA is equally effective (3 fold) in reducing synovial fluid induced TNF-α transcription in RA monocytes and that the combined therapy does not have an enhanced effect (Fig. 6C). To demonstrate that RA SF RNA is a potential TLR7 endogenous ligand, RA monocytes were stimulated with RNA extracted from RA SF as well as RA and NL plasma. We found that while RNA extracted from RA SF increased monocyte TNF-α transcription by 18 fold through TLR7 ligation, RNA obtained from RA or NL plasma was incapable of this process (Fig. 6D). To confirm that RA SF and or SF mRNA specifically ligates to TLR7 we show that in NL monocytes, RA SF mediated TNF-α levels were dose dependently increased with higher TLR7 transfection levels however higher transfection levels of TLR7 were required to induce response by SF RNA (Figs 6F–G). Interestingly in 293T cells only cells transfected with 0.5 µg of TLR7 plasmid responded to RA SF and SF mRNA activation (Fig I). Taken together our data indicates that ssRNA present in RA SF is a potential TLR7 endogenous ligand that can perpetuate RA inflammation by increasing TNF-α production.
Figure 6. Inhibition of TLR7 or degradation of ssRNA in RA synovial fluids (SF)s similarly reduces RA monocyte induced TNF-α transcription. Ligation of RNA extracted from RA SF but not RA or NL plasma to TLR7 in monocytes activates transcription of TNF-α.
RA monocytes were untreated or treated with PBS, Chloroquine (50 µM) or A151 (1µM) for 1h prior to being treated with R848 (100 ng/ml; n=4) (A) or RA synovial fluid (10%; n=4) (B) for 6h. C. RA monocytes (different patients from Figs. A and B) were untreated or pre-incubated with PBS, RNAse A/T1 (RNAse A, 40 µg/ml; T1, 100 U/ml), A151 (1µM) or RNAse A/T1 plus A151 for 1h. Subsequently RA synovial fluids (n=4) were added to the pretreated RA monocytes for 6h. D. RA monocytes were either untreated or treated with 5µg of RNA extracted from RA synovial fluid, RA plasma, NL plasma or RA synovial fluid RNA plus A151 (1µM) for 6h. NL monocytes (F and G) or 293T cells (I) are either untransfected or transfected with 0.5 µg and/or 0.2 µg of pCDNA3-TLR7-YFP and cells were treated with 10% RA SF (F and I) (n=4) or 5 µg RA SF mRNA (G and I) (n=4). TLR7 transfection was determined by analyzing YFP expression in NL monocytes (E) and 293T cells (H). In figures A–D and F, G and I and TNF-α mRNA levels were determined by real-time RT-PCR and normalized to untreated RA monocytes. Values are the mean ± SEM, * represents p <0.05.
DISCUSSION
In the current study, we found that expression levels of TLR7 and TLR8 were elevated in RA SF macrophages and RA monocytes compared to RA and normal macrophages. Next, we demonstrated that in RA monocytes expression levels of TLR7 but not TLR8 have a linear correlation with DAS28 and TNF-α levels. Further, inhibition of TLR7 ligation or degradation of ssRNA in RA SF reduces joint TNF-α transcription to similar levels in RA monocytes. Finally, we show that RNA extracted from RA SF is a unique source of TLR7 endogenous ligand. These novel results suggest that RA SF ssRNA, possibly released from necrotic cells, function as TLR7 endogenous ligands and potentially activate RA monocytes and contribute to the production of joint TNF-α and disease perpetuation.
Previous studies have shown that TLR7 is expressed in RA monocyte derived dendritic cells16 and RA fibroblasts15. Like TLR7, expression of TLR8 has been detected in dendritic cells25 as well as in monocytes26. Although, mRNA and endosomal expression of TLR7 and TLR8 were detected in RA synovial tissues27, their cellular distribution, modulation and role in RA pathogenesis are undefined. Elevated levels of TLR7 and TLR8 were found in RA lining and sublining macrophages. Our results are consistent with others demonstrating that TLR7 is present in RA fibroblasts, however previous studies show that TLR7 expression is lower in RA compared to OA fibroblasts15. Nevertheless, since ligation of TLR7 in RA fibroblasts did not result in production of proinflammatory factors and its expression was not enhanced by stimulation we conclude that RA fibroblasts may not be important effector cells for TLR7 function. While other studies demonstrated that TLR7 agonist imiquimod could increase TLR7 expression in RA fibroblasts15. Unlike TLR7, TLR8 was undetectable in RA and NL fibroblasts.
Like TLR7 and TLR8 expression, NL PB monocyte differentiation to macrophages reduced the frequency of TLR2 positive cells, while the percentage of TLR4 positive cells was unchanged in these cell types12. Despite reduced abundance of TLR7 and TLR8 in RA PB macrophages compared to PB monocytes, RA SF macrophages express the highest levels of these receptors. In light of these findings, we looked for endogenous ligand(s) for TLR7 or TLR8 in RA joint SF.
Expression of TLR8 but not TLR7 was induced by LPS and IL-1β stimulation in RA monocytes and macrophages. In contrast, ligation of TLR4 was capable of increasing TLR7 expression in dendritic cells28 suggesting that expression of TLR7 is differentially regulated in dendritic cells compared to RA differentiated macrophages. Earlier studies demonstrate that topical application of imiquimod, a TLR7 ligand, can induce and exacerbate psoriasis which is dependent on expression of IL-23, IL-17 and elevated splenic TH-17 cells29 suggesting that activation of TLR7 may play a role in autoimmune disease by triggering TH-17 polarization. Our results show that in RA macrophages expression of TLR7 and TLR8 is modulated by IL-17 and therefore there may be a positive feed back loop between ligation of TLR7 and TLR8 and polarization of TH-17 cells. Perhaps elevated TH-17 cells in SF30 can induce the expression of TLR7 and 8 in RA SF macrophages which can then be activated by binding to ssRNA released from necrotic synovial fluid cells.
When RA monocytes and macrophages were stimulated with a higher dose of R848, similar levels of TNF-α, IL-6 and CCL2 were produced despite RA macrophages having lower TLR7 or TLR8 expression compared to RA monocytes. This may be due to monocytes being in circulation while macrophages are immobilized in the inflammatory milieu of RA ST with cell to cell contact with other macrophages or RA fibroblasts therefore amplifying the activation response. However in RA myeloid cells, TLR7 expression levels play an important role in the R848 inflammatory response as TNF-α transcription is significantly lower in NL compared to RA macrophages. Confirming these finding we show that while RA SF or SF RNA can readily activate RA monocytes yet in NL monocytes and 293T cells transfection of TLR7 was required to achieve response. TLR7 and 8 signaling through MyD88 results in NF-κB, MAPK and PI3K activation which leads to production of a number of proinflammatory factors31. We show that in RA macrophages, NF-κB and/or PI3K pathways are essential for TLR7/8 induction of proinflammatory factors which corroborates with production of IFN through TLR7/8 ligation in dendritic cell via the same signaling pathways32.
Despite TLR7 and TLR8 having similar pattern of expression in RA ST and PB cells, mRNA expression of these receptors does not correlate in RA monocytes or macrophages and as a result only TLR7 expression correlates with higher disease activity and TNF-α transcription in the RA joint. The pathogenic role of TLR7 in RA was documented when an antagonist to TLR7 ligation, degradation of SF ssRNA or combination of both treatments comparably reduced RA SF mediated TNF-α transcription. This finding suggests that ligation of joint ssRNA to TLR7 modulates TNF-α levels through an overlapping pathway and it further points out that although ssRNA is a potential ligand for both TLR7 and TLR824, 33, TLR7 is the main sensor for ssRNA endogenous ligands in RA SF. Confirming these observations treatment with endosomal inhibitor and/or RNase/DNase ameliorate rat pristine induced arthritis through blocking TLR7 and 9 ligation34. Interestingly, we found that TLR7 endogenous ligands were undetectable in RA plasma and were only expressed in RA SF. This may be due to ssRNA released from the necrotic SF cells remains stagnated until removed by arthrocentesis while in RA plasma ssRNA from necrotic cells is removed in the blood filtration process hence making it more difficult to maintain ssRNA levels.
In conclusion, our data demonstrate that macrophages but not fibroblasts in RA synovial tissue and/or fluid are effector cells for TLR7 and TLR8 ligation. We document modulating factors and pathways contributing to the TLR7/8 inflammatory response. Notably, our study documents for the first time, the presence of ssRNA as a potential TLR7 endogenous ligand in RA synovial fluid and further highlights the correlation between TLR7 expression and DAS28 as well as TNF-α levels in RA monocytes. Collectively, these results suggest that TLR7 may be a TNF-α responsive gene that is linked to chronic progression of RA.
Supplementary Material
Acknowledgments
FUNDING:
This work was supported in part by awards from the National Institutes of Health (AR056099, AR055240), Arthritis National Research Foundation, grants from Within Our Reach from The American College of Rheumatology and funding provided by Department of Defense PR093477.
Footnotes
COMPETING INTERESTS:
None declared.
ETHICS APPPROVAL:
Approved by local ethical committees.
AUTHORS CONTRIBUTION:
Designed the research: N.D.C., S.S.
Performed the research: N.D.C., SJ.K., MV.V.
Analyzed the data: N.D.C., SJ.K., S.S., A.A.M., M.V.V.
Provided essential reagents: O.M.V., S.V., R.M.P., S.A.
Writing the paper: All the authors contributed to writing the paper.
REFERENCES
- 1.Brentano F, Kyburz D, Schorr O, et al. The role of Toll-like receptor signalling in the pathogenesis of arthritis. Cell Immunol. 2005;233:90–96. doi: 10.1016/j.cellimm.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Drexler SK, Sacre SM, Foxwell BM. Toll-like receptors: a new target in rheumatoid arthritis? Expert Rev Clin Immunol. 2006;2:585–599. doi: 10.1586/1744666X.2.4.585. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulou L, Holt AC, Medzhitov R, et al. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 5.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 6.Hoshino K, Sugiyama T, Matsumoto M, et al. IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature. 2006;440:949–953. doi: 10.1038/nature04641. [DOI] [PubMed] [Google Scholar]
- 7.Huang QQ, Pope RM. The role of toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:357–364. doi: 10.1007/s11926-009-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foell D, Wittkowski H, Roth J. Mechanisms of disease: a 'DAMP' view of inflammatory arthritis. Nat Clin Pract Rheumatol. 2007;3:382–390. doi: 10.1038/ncprheum0531. [DOI] [PubMed] [Google Scholar]
- 9.Iwahashi M, Yamamura M, Aita T, et al. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2004;50:1457–1467. doi: 10.1002/art.20219. [DOI] [PubMed] [Google Scholar]
- 10.Radstake TR, Roelofs MF, Jenniskens YM, et al. Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum. 2004;50:3856–3865. doi: 10.1002/art.20678. [DOI] [PubMed] [Google Scholar]
- 11.Sorensen LK, Havemose-Poulsen A, Sonder SU, et al. Blood cell gene expression profiling in subjects with aggressive periodontitis and chronic arthritis. J Periodontol. 2008;79:477–485. doi: 10.1902/jop.2008.070309. [DOI] [PubMed] [Google Scholar]
- 12.Huang Q, Ma Y, Adebayo A, et al. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56:2192–2201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- 13.Abdollahi-Roodsaz S, Joosten LA, Roelofs MF, et al. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 2007;56:2957–2967. doi: 10.1002/art.22848. [DOI] [PubMed] [Google Scholar]
- 14.Abdollahi-Roodsaz S, Joosten LA, Koenders MI, et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118:205–216. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrion M, Juarranz Y, Perez-Garcia S, et al. RNA sensors in human osteoarthritis and rheumatoid arthritis synovial fibroblasts: immune regulation by vasoactive intestinal peptide. Arthritis Rheum. 2011;63:1626–1636. doi: 10.1002/art.30294. [DOI] [PubMed] [Google Scholar]
- 16.Roelofs MF, Joosten LA, Abdollahi-Roodsaz S, et al. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005;52:2313–2322. doi: 10.1002/art.21278. [DOI] [PubMed] [Google Scholar]
- 17.Pickens SR, Chamberlain ND, Volin MV, et al. Characterization of CCL19 and CCL21 in rheumatoid arthritis. Arthritis Rheum. 2011;63:914–922. doi: 10.1002/art.30232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickens SR, Chamberlain ND, Volin MV, et al. Characterization of interleukin-7 and interleukin-7 receptor in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2011;63:2884–2893. doi: 10.1002/art.30493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 20.Shahrara S, Pickens SR, Dorfleutner A, et al. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182:3884–3891. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahrara S, Pickens SR, Mandelin AM, 2nd, et al. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J Immunol. 2010;184:4479–4487. doi: 10.4049/jimmunol.0901942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colisson R, Barblu L, Gras C, et al. Free HTLV-1 induces TLR7-dependent innate immune response and TRAIL relocalization in killer plasmacytoid dendritic cells. Blood. 2010;115:2177–2185. doi: 10.1182/blood-2009-06-224741. [DOI] [PubMed] [Google Scholar]
- 23.Schon MP, Schon M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190–199. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 24.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lombardi V, Van Overtvelt L, Horiot S, et al. Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of Il-10, IFN-gamma, and IL-17A by naive CD4+ T cells. J Immunol. 2009;182:3372–3379. doi: 10.4049/jimmunol.0801969. [DOI] [PubMed] [Google Scholar]
- 26.Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sacre SM, Lo A, Gregory B, et al. Inhibitors of TLR8 reduce TNF production from human rheumatoid synovial membrane cultures. J Immunol. 2008;181:8002–8009. doi: 10.4049/jimmunol.181.11.8002. [DOI] [PubMed] [Google Scholar]
- 28.Severa M, Remoli ME, Giacomini E, et al. Sensitization to TLR7 agonist in IFN-beta-preactivated dendritic cells. J Immunol. 2007;178:6208–6216. doi: 10.4049/jimmunol.178.10.6208. [DOI] [PubMed] [Google Scholar]
- 29.van der Fits L, Mourits S, Voerman JS, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 30.Shahrara S, Huang Q, Mandelin AM, 2nd, et al. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R93. doi: 10.1186/ar2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 32.Makela SM, Osterlund P, Julkunen I. TLR ligands induce synergistic interferon-beta and interferon-lambda1 gene expression in human monocyte-derived dendritic cells. Mol Immunol. 2011;48:505–515. doi: 10.1016/j.molimm.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann MH, Skriner K, Herman S, et al. Nucleic acid-stimulated antigen-presenting cells trigger T cells to induce disease in a rat transfer model of inflammatory arthritis. J Autoimmun. 2011;36:288–300. doi: 10.1016/j.jaut.2011.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.