Abstract
Purpose:
This study aims to validate the effect of autoclaved autogenous bone (AAB), incorporating Escherichia coli-derived recombinant human bone morphogenetic protein-2 (ErhBMP-2), on critical-sized, segmental radius defects in rabbits. Delivery systems using absorbable collagen sponge (ACS) and fibrin glue (FG) were also evaluated.
Methods:
Radius defects were made in 12 New Zealand white rabbits. After autoclaving, the resected bone was reinserted and fixed. The animals were classified into three groups: only AAB reinserted (group 1, control), and AAB and ErhBMP-2 inserted using an ACS (group 2) or FG (group 3) as a carrier. Animals were sacrificed six or 12 weeks after surgery. Specimens were evaluated using radiology and histology.
Results:
Micro-computed tomography images showed the best bony union in group 2 at six and 12 weeks after operation. Quantitative analysis showed all indices except trabecular thickness were the highest in group 2 and the lowest in group 1 at twelve weeks. Histologic results showed the greatest bony union between AAB and radial bone at twelve weeks, indicating the highest degree of engraftment.
Conclusion:
ErhBMP-2 increases bony healing when applied on AAB graft sites. In addition, the ACS was reconfirmed as a useful delivery system for ErhBMP-2.
Keywords: Bone morphogenetic protein 2, Autogenous autoclaved bone, Critical bone defect, Rabbit radius, BMP carrier
Introduction
The most widely used method for reconstruction of large bone defects is a vascularized free bone graft; although it requires longer and more involved. Vascularized free bone grafts can cause donor site impediment and carry a high risk of failing to find vessels for microanastomosis in patients who have received radiation therapy. In addition, osseous flaps have esthetic and functional disadvantages due to morphological differences from recipient bone. To overcome these limitations, reconstruction using removed autogenous bone after autoclaving is employed as an alternative method for segmental defects[1]. This method is advantageous because antigenicity is no longer a problem and the anatomical form of the bone defect is reproducible. However, autoclaved autogenous bone (AAB) has some limitations. It is only osteoconductive, not osteoinductive[2]. Segmental bone defects have been reconstructed with AAB in orthopedics for nearly a century; however, AAB acts as a tolerated foreign body, gradually getting resorbed and sometimes causing infection or nonunion[3–6]. There have been attempts in recent years to increase the engraftment rate of AAB by incorporating proteins such as recombinant human fibro-blast growth factor-2[7]. However, incorporation of recombinant human bone morphogenetic protein (rhBMP)-2 has not been attempted.
BMP-2 is involved in the process of differentiation of osteoblasts and chondroblasts from mesenchymal stem cells, suggesting that BMP-2 induces adult bone formation and chondrogenesis, consequently increasing the recovery rate from skeletal defects and reducing infection rates[7–10]. However, the high production cost of rhBMPs from Chinese hamster ovary cells has limited commercial use. After several attempts using prokaryotic expression systems, researchers found rhBMPs isolated from Escherichia coli (ErhBMPs) were 98% pure, adequate for bone regeneration in vivo, and producible in commercial quantities at low prices. Furthermore, their effects on bone formation have been validated in rat calvarial and fibular defect models[11–13].
This osteoinductive protein could increase the engraftment rate of AAB, which acts as a scaffold to maintain initial strength in segmental defects. The clinical efficacy of rhBMPs may depend on the carrier system used to ensure an effective delivery of adequate protein concentrations to the desired site. Two different delivery systems are available: absorbable collagen sponge (ACS) and fibrin glue (FG). Therefore, this study aims to validate the effect of ErhBMP-2, delivered with the ACS or FG, on engraftment of AAB in a critical-sized, segmental bone defect model of the rabbit radius.
Materials and Methods
1. Surgical procedure
Twelve adolescent female New Zealand white rabbits weighing 3.5 to 4.5 kg were used. All steps of the experiment were approved by the Institutional Animal Care and Use Committee of the Department of Laboratory Animal Medicine, Medical Research Center, Yonsei University College of Medicine (2010–0264). Radial injuries were created on 24 sites in the 12 animals.
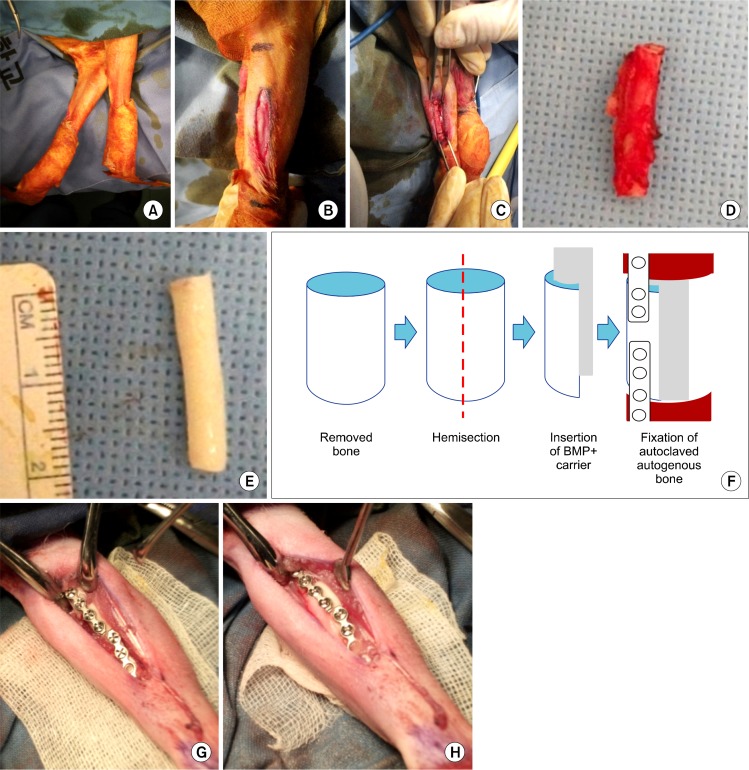
The animal was placed in a true lateral position on the surgery table and the foreleg shaved after orotracheal intubation (Fig. 1A). The leg was scrubbed with Betadine, and local anesthesia with lidocaine (1.8 mL containing 1:100,000 epinephrine) was injected. A longitudinal skin incision of 3 cm and supraperiosteal dissection were performed (Fig. 1B). Segmental bone (2 cm in size) was harvested with a soft tissue cuff at bilateral radii (Fig. 1C, 1D). The osteotomies were created with a thin fissure bur (#701). Resected bone fragments were cut in half longitudinally, then part of the bone marrow and all soft tissues covering the bone were removed (Fig. 1E, 1F). The remaining cortical bone was autoclaved at 123°C, 0.2 MPa for 10 minutes. The prepared AAB was then replanted and fixed with two microplates and eight screws (Biomaterials America Inc., New York, NY, USA). After copious saline irrigation, the deep fascial layer was reapproximated as an envelope around the AAB with 3-0 PolysorbTM (Covidien, Mansfield, MA, USA) followed by subcutaneous layer sutures using the same material and skin sutures using 4-0 DermalonTM (Covidien). Antibiotic treatment was maintained for five days after the operation and the stitches removed after 10 days.
Fig. 1.
Experimental procedure for the AAB graft on the rabbit radius. (A) Reinsertion of the resected segments after autoclaving, then internal fixation with the screws and plate. (B) Insertion of ErhBMP-2 along with ACS carrier material. (C) Segmental resection of the radial bone. (D) Radius removed with soft tissue cuff. (E) Radius after removal of soft tissue, including periosteum. (F) Schematic drawing of longitudinal hemisection and bone marrow removal. (G) Preparation and drape of foreleg. (H) Exposure of periosteum of radius. AAB, autoclaved autogenous bone; ErhBMP, Escherichia coli-derived recombinant human bone morphogenetic protein; ACS, absorbable collagen sponge.
The animals were divided into three groups, with eight sites allotted to each group.
Group 1 (control): Only AAB was replanted and fixed (Table 1, Fig. 1G).
Group 2: AAB was replanted with 50 μg ErhBMP-2 at a concentration of 1.0 mg/mL (BioAlpha Inc., Seongnam, Korea), incorporated in a 2×4×20 mm-sized ACS (Teruplug®, Terumo Co., Tokyo, Japan) (Fig. 1H).
Group 3: AAB was replanted with 50 μg ErhBMP-2 at a concentration of 1.0 mg/mL (BioAlpha Inc.), incorporated in FG (Tisseel®; Baxter, Wien, Austria).
Table 1.
Classification of groups to validate effects of ErhBMP-2 and carrier systems
| Scaffold | ErhBMP-2 | Delivery system | |
|---|---|---|---|
| Group 1 (control) | AAB | Not used | Not used |
| Group 2 | AAB | 50 μg | Absorbable collagen sponge (Teruplug®) |
| Group 3 | AAB | 50 μg | Fibrin glue (Tisseel®) |
ErhBMP, Escherichia coli-derived recombinant human bone morphogenetic protein; AAB, autoclaved autogenous bone.
2. Evaluation of the results
Animals were sacrificed at weeks six and 12 after surgery. Plain x-ray images of both radius injury sites were compared. Three-dimensional images were obtained using micro-computed tomography (Micro-CT, Skyscan 1076; SKYSCAN, Konitch, Belgium), and the bone fraction volume (BV/TV), trabecular thickness (TbTh), trabecular number (TbN), and trabecular separation (TbSp) were measured using analysis software (CTAn, SKYSCAN) at the proximal and distal ends of the AAB with a region of interest of 100 μm (Fig. 2). The amount of bony union at both ends of AAB was observed in coronal sections of the Micro-CT.
Fig. 2.
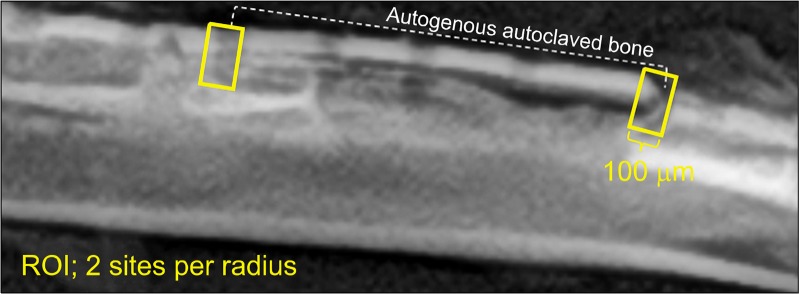
Schematic drawing of ROI. Quantitative analyses: BV/TV, TbTh, TbN, and TbSp, were measured using micro-computed tomography within this ROI at each proximal end of the autoclaved autogenous bone. ROI, region of interest; BV, bone volume; TV, total volume; Tb, trabecular; Th, thickness; N, number; Sp, separation.
Resected tissue was fixed at 4°C in 4% paraformalde-hyde/0.01 M phosphate-buffered saline (pH 7.4) for 24 hours, and demineralized at room temperature with formic acid-sodium formate. After embedding in paraffin, the tissue was cut in the direction of the long axis of the radius at a thickness of 7 μm, stained with hematoxylin and eosin, and observed using optical microscopy. Slides were scanned by digital virtual microscope (BX51; Olympus®, Tokyo, Japan) and analyzed with the master program. For quantitative analysis, the perimeter of each entire graft was measured at low magnification, with the presence of fusion being judged by observing osteoid, including the osteocyte and reversal line at high magnification (Fig. 3). The proportion of united perimeter within the AAB perimeter was measured to calculate the degree of engraftment. Subjects whose graft shapes were unclear on the slide were excluded from measurement.
Fig. 3.
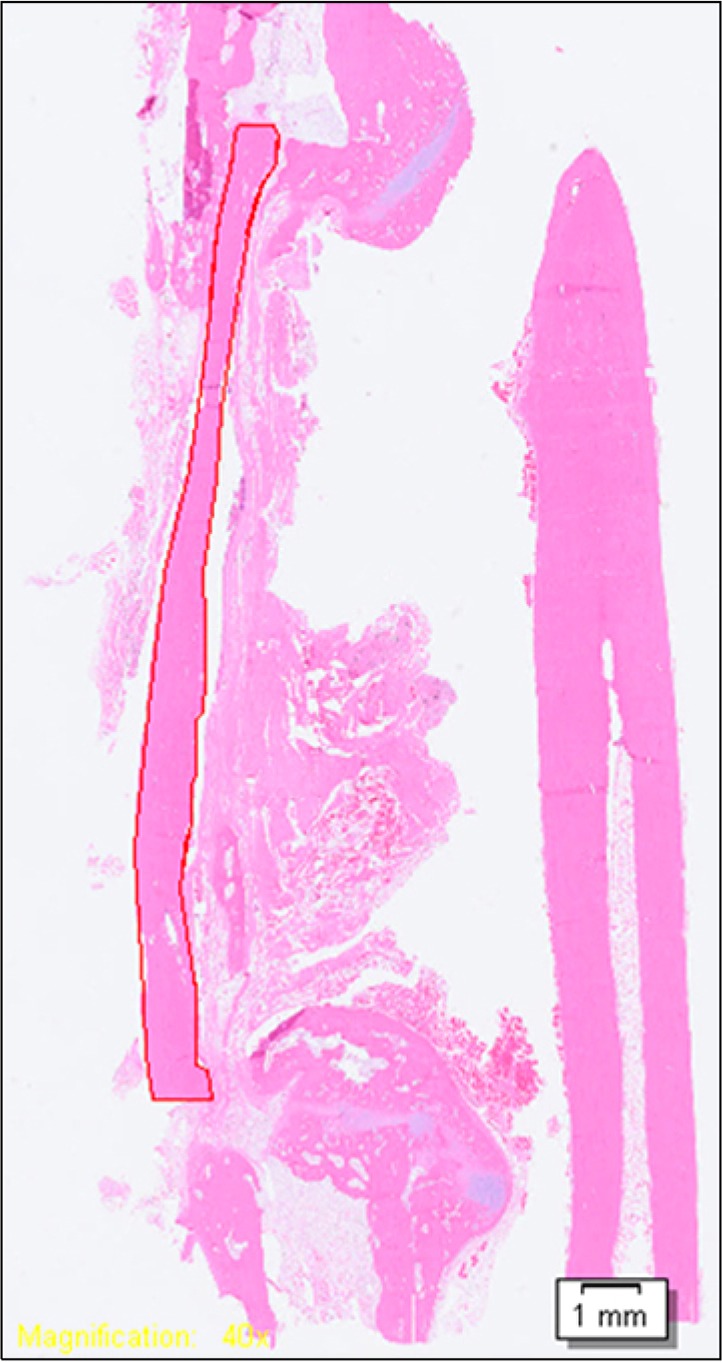
Method for calculating the degree of engraftment. Red line represents the autoclaved autogenous bone perimeter, within which we measured the proportion of perimeter showing osteoid, osteoblasts, and the reversal line at higher magnification (H&E, ×40).
Quantitative data were reported as the mean±standard deviation. Statistical analysis was performed using a one-way analysis of variance (one-way ANOVA) with statistical significance evaluated at P <0.05 and using the PASW Statistics ver. 18.0 (IBM Co., Armonk, NY, USA).
Results
1. Radiographic evaluation and findings
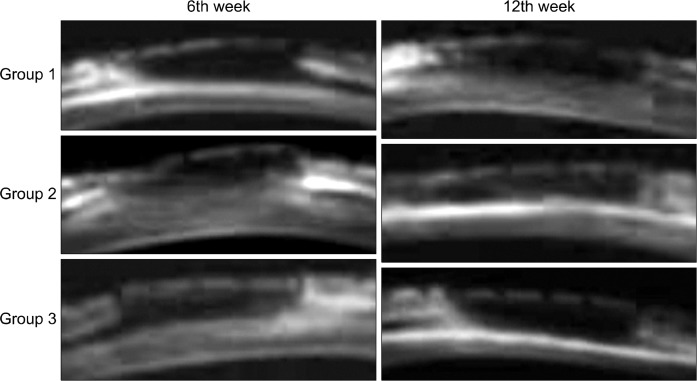
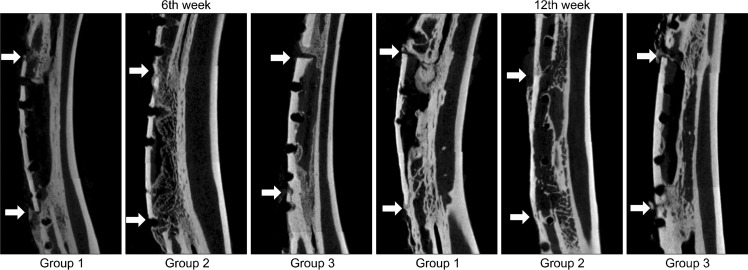
In plain radiography, AABs in the control group united with the radius at only one site, either the proximal or distal cut ends, six weeks after operation. Both graft sites united with the radius in group 2, whereas neither site did in group 3. Twelve weeks after surgery, new bone formation was observed on both AAB sites in the control group and group 3, but complete bony fusion in both ends of AAB was observed only in group 2 (Fig. 4). At 6 weeks, the BT/TV, TbTh, and TbSp were highest in group 1, lower in group 2, and even lower in group 3. TbN was highest in group 2, lower in group 1, and even lower in group 3. All twelfth week indices were highest in group 2, followed by 3 and 1, except for TbTh (Table 2). Coronal images of Micro-CT showed newly formed trabecular bone united with the AAB and radius in group 2, while AAB was surrounded by little osteoid without trabecular pattern in groups 1 and 3 at week 6. In groups 1 and 3, one of the two graft ends regained fusion between the AAB and cortex of radius, whereas in group 2 both sides of the cut ends regained fusion with the trabecular bone at 12 weeks (Fig. 5).
Fig. 4.
Plain radiographic findings after graft of AAB. Six weeks after operation, discontinuity was seen in groups 1 and 3; whereas, 12 weeks after operation, continuity between the AAB and radius was seen in all groups. Only AAB reinserted (group 1), and AAB and ErhBMP-2 inserted using an ACS (group 2) or FG (group 3) as a carrier. AAB, autoclaved auto-genous bone; ErhBMP-2, Escherichia coli-derived recombinant human bone morphogenetic protein-2; ACS, absorbable collagen sponge; FG, fibrin glue.
Table 2.
Quantitative results for the 12 rabbits used for the reconstruction of segmental defects of the radius
| BV/TV | TbTh (μm) | TbN (1/mm) | TbSp (μm) | |
|---|---|---|---|---|
| 6th week | ||||
| Group 1 | 10.74±2.36 | 243.14±17.76 | 0.54±0.29 | 276.68±3.40 |
| Group 2 | 10.56±1.23 | 210.39±19.95 | 0.64±0.24 | 271.73±4.94 |
| Group 3 | 8.31±0.84 | 209.74±27.27 | 0.46±0.18 | 258.44±29.60 |
| P-value | 0.09 | 0.01* | 0.33 | 0.12 |
| 12th week | ||||
| Group 1 | 8.96±2.31 | 263.85±11.97 | 0.29±0.25 | 258.85±60.54 |
| Group 2 | 10.33±0.83 | 257.12±8.81 | 0.54±0.07 | 278.84±1.05 |
| Group 3 | 9.38±2.33 | 247.09±9.45 | 0.51±0.20 | 278.32±1.40 |
| P-value | 0.48 | 0.01* | 0.03* | 0.44 |
Values are presented as mean±standard deviation. Only AAB reinserted (group 1), and AAB and ErhBMP-2 inserted using an ACS (group 2) or FG (group 3) as a carrier.
BV, bone volume; TV, total volume; Tb, trabecular; Th, thickness; N, number; Sp, separation; AAB, autoclaved autogenous bone; ErhBMP-2, Escherichia coli-derived recombinant human bone morphogenetic protein-2; ACS, absorbable collagen sponge; FG, fibrin glue.
P<0.05.
Fig. 5.
Micro-computed tomography findings for each group at six and 12 weeks. White arrows represent both ends of autoclaved autogenous bone. Only AAB reinserted (group 1), and AAB and ErhBMP-2 inserted using an ACS (group 2) or FG (group 3) as a carrier. AAB, autoclaved autogenous bone; ErhBMP-2, Escherichia coli-derived recombinant human bone morphogenetic protein-2; ACS, absorbable collagen sponge; FG, fibrin glue.
2. Histological evaluation and findings
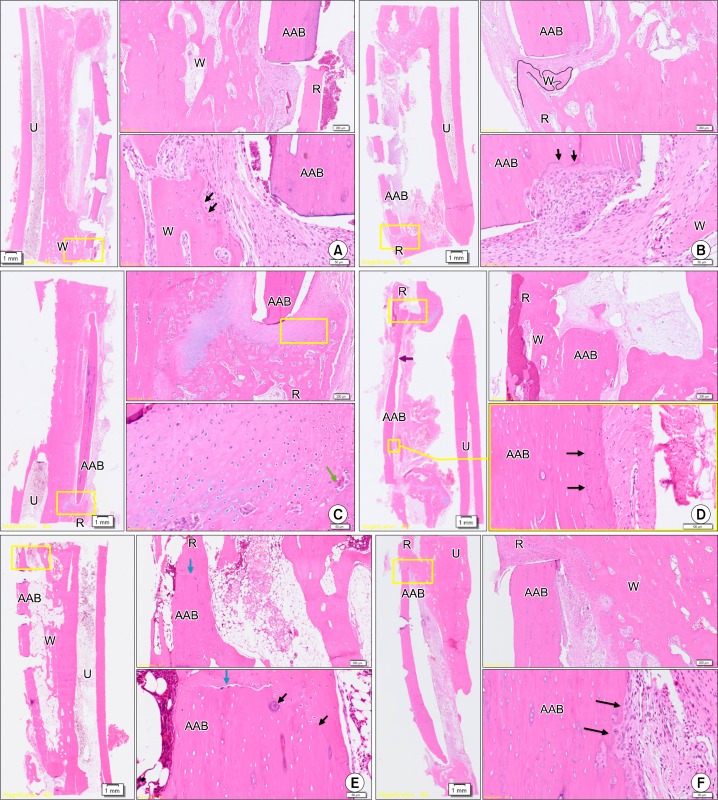
Although woven bone originated from the adjacent ulnar bone and a reversal line, suggesting bony remodeling, was observed six weeks after surgery in groups 1 and 3 (Fig. 6A, 6B), the new bone did not unite with the AAB. High magnification revealed extensive cartilage formation around the AAB, active cell division of chondroblasts, and replacement of cartilage by osteoid in group 2 (Fig. 6C). Some osteoclasts and osteoblasts were observed, suggestive of bony remodeling. Twelve weeks after surgery in group 1 (Fig. 6D), woven bone originated from a single end of the radial bone and restored some continuity with the AAB. Sparse new bone formation and a reversal line suggested proper engraftment of AAB. In group 2 (Fig. 6E), woven bone originated from the adjacent cutting ends of the radius and showed complete fusion with AAB. At low magnification, AAB had completely restored continuity with the radial bone marrow. Moreover, high magnification revealed reversal lines and osteocytes inside the woven bone, suggesting that active bony remodeling was in progress. In group 3 (Fig. 6F), there was some evidence suggesting bony remodeling around AAB, similar to the group 1 results, although not as extensive as in group 2. The proportion of bony union area was highest in group 2 at the 12th week but lowest in group 3, which used FG as the carrier material. Engraftment degree was measurable in three specimens of group 1 and group 2 at the sixth week and group 2 and group 3 at the twelfth week. However, only two specimens were able to be measured for engraftment degree in group 3 at the sixth week and group 1 at the 12th week. The AAB engraftment was highest in group 2 at both six and 12 weeks and lowest in group 3 at 12 weeks (Table 3).
Fig. 6.
Histologic assessment of each animal at six and 12 weeks (A∼C: six weeks after surgery, D∼F: twelve weeks after surgery). (A) Group 1, six weeks after surgery. Although woven bone originated from the adjacent ulnar bone and a reversal line, suggesting bony remodeling, was observed, the new bone did not unite with the AAB. (B) Group 3, six weeks after surgery. Although woven bone originated from the adjacent ulnar bone and a reversal line which suggests bony remodeling was observed, the new bone did not unite with the AAB. (C) Group 2, six weeks after surgery. High magnification revealed extensive cartilage formation around the AAB, active cell division of chondroblasts, and replacement of cartilage by osteoid. (D) Group 1, twelve weeks after surgery. Some osteoclasts and osteoblasts were also observed, suggestive of bony remodeling, and woven bone originated from a single end of the radial bone and restored some continuity with the AAB. Sparse new bone formation and a reversal line suggested proper engraftment of AAB. (E) Group 2, 12 weeks after surgery. Woven bone originated from the adjacent cutting ends of the radius and showed complete fusion with AAB. At low magnification, AAB had completely restored continuity with the radial bone marrow. Moreover, high magnification revealed reversal lines and osteocytes inside the woven bone, suggesting that active bony remodeling was in progress. (F) Group 3, twelve weeks after surgery. There was some evidence suggesting bony remodeling around AAB, similar to the group 1 results, although not as extensive as in group 2 (H&E; left: ×40, right: ×100/×400). Boxes with yellow line in left slides (×40) were magnified in right slides (×100/×400) to be determined whether complete bony fusion with osteoblast, osteoclast, and osteoid was done. U, ulnar bone; R, radial bone; AAB, autoclaved autogenous bone; W, woven bone; black arrows, reversal line; blue arrows, end of AAB; purple arrows, resorption of AAB; green arrow, osteoclast.
Table 3.
Degree of engraftment of the autoclaved autogenous bone for each animal by group
| 6th week (%) | 12th week (%) | |
|---|---|---|
| Group 1 | 0.00 | 37.50 |
| 0.00 | 19.90 | |
| 29.01 | ||
| Group 2 | 3.16 | 57.68 |
| 43.44 | 69.79 | |
| 60.26 | 87.00 | |
| Group 3 | 0.00 | 0.93 |
| 0.89 | 2.05 | |
| ND | 8.11 |
Only subjects whose entire graft shapes were clear on the slide were measured. One subject was excluded from group1 and group 2 at 6th week, and from group 2, and group 3 at 12th week. Two subjects were excluded in group 3 at 6th week, and from group 1 at 12th week. Engraftment degree: proportion of united perimeter within the entire AAB perimeter. Perimeters of the entire graft were measured at low magnification, with the presence or absence of fusion being judged by observing osteoid, including the osteocyte and reversal line at high magnification. Only AAB reinserted (group 1), and AAB and ErhBMP-2 inserted using an ACS (group 2) or FG (group 3) as a carrier.
ND, no data; AAB, autoclaved autogenous bone; ErhBMP-2, Escherichia coli-derived recombinant human bone morphogenetic protein-2; ACS, absorbable collagen sponge; FG, fibrin glue.
Discussion
The results of this study suggest that ErBMP-2 is remarkably effective for engraftment of AAB when used in conjunction with an ACS as carrier material, but rarely effective with FG. We used the radius as a segmental defect model. The radius was selected as a rabbit long bone model in this experiment for the following reasons: it is smaller than the ulnar bone, bears less load, and is easily accessible. A radial bone defect of critical size (20 mm) was formed in the rabbit, as in previous studies[14–16]. We followed the surgical protocols of Huber et al.[17]. The conventional method of quantitative analysis could not be applied in this model because of great differences in AAB volumes and screw-based defects, producing too much variability in volume and position for quantification. Thus it was necessary to use a new method of measuring the proportion of bony union in this study. However, not all subjects could be included in this quantitative analysis because longitudinal cutting of some radial bones failed to show the entire graft bone cross-section. Eight subjects whose graft shapes were unclear on the slide were excluded from measurement. Although some measurement errors arose due to screw fixation defects using this method, the intra-group differences among the proportions of bony fusion were no greater than the inter-group differences measured (0% to 87%). Only one subject in group 2 showed a relatively low proportion of bony fusion (3.16%) six weeks after surgery (Fig. 6B), although active chondrogenesis was observed around the AAB despite a low degree of engraftment. This suggests that regenerated bone may unite with the AAB afterward. This study’s method of assessing the proportion of bony union using the perimeter of graft may be useful in future quantitative analyses of AAB graft results. Graft bone union can be achieved in three ways: new bone deposition on the periosteum, at the endosteal surface of the adjacent host bone, and osteogenesis from the graft bone[18]. The authors removed the soft tissue cuff when resecting the segmental bone to make an environment similar to periosteum removal due to an invading tumor. Heat-treatment at 100°C destroys bone collagen, thus eliminating osteoconductive activity[6]. Therefore, heat-treated autogenous bone is united mainly by new bone deposition on the endosteal surface of the adjacent host bone when the periosteum is lost, leading to delayed healing with a high risk of engraftment failure. Autoclaving is a useful method for preventing local recurrence of malignant bone tumors[19].
Zellin[7] reported that revitalization of replanted autoclaved grafts was significantly affected by local delivery of recombinant human fibroblast growth factor-2. In this study, 50 μg of ErhBMP-2 was used, based on reports that a rhBMP-2 dosage exceeding 30 μg did not affect the amount of bone formation in segmental long bone defects of rabbits, although varying dosages have been used depending on the experimental models and type of BMPs[15]. Typically rhBMP-2 diffuses rapidly and this supraphysiological concentration may result in some complications[20–25]. Adequate delivery systems, which enable localized and controlled release of protein, are under investigation, although most are produced with complex methods. ACS and FG are the most popular and inexpensive delivery systems for rhBMP-2, although they have disadvantages[13,26–32].
Rigid fixation is not recommended in the rabbit radius defect model because it is unnecessary for bony union and can cause delayed healing due to occlusion of the bone marrow. The authors fixed the AAB rigidly using metal plates and screws to create an environment similar to the segmental bony defect, which should induce early migration and adhesion of osteoblasts[17]. The small dimensions of the radial bone and thin cortical bone made it difficult to fix the AAB with plates and screws, causing some fractures and loss of cortical bone while screwing. As a carrier, FG prevents bone ingrowth by controlling BMP diffusion and BMP stimulated bone growth[33]. In this experiment, the ErhBMP-2 may have been partially lost when the fibrin block was transferred to the defect site. Conversely, injection of a mixture of FG and rhBMP-2 promotes healing in a bone-tendon injury[34]. FG could serve as a carrier material in future systems where ErhBMP-2 dissolves properly and elutes without loss.
Although AAB incorporating ErhBMP-2 was expected to recover mechanical strength soon after reconstruction because it reproduces the original anatomy and minimizes bony defects in cutting ends, mechanical testing of AAB could not be conducted in this study. The radius cannot be separated from the ulnar bone due to their proximity in the rabbit. However, part of the woven bone derived from ulnar bone would be lost in the process of separation, possibly rendering the subsequent histologic assessment inaccurate. Future mechanical strength tests of AAB after bony reconstruction should include comparisons with experiments that did not use AAB in the reconstruction. Further research is also necessary to examine the effectiveness of AAB in maxillofacial reconstructions, such as the mandible, under controlled trials.
Conclusion
The results of this study suggest ErBMP-2 is remarkably effective for engraftment of AAB when used in conjunction with the ACS as a carrier material, but is rarely effective with FG. When comparing bone formation in control and experimental groups, more new bone was observed in the control group six weeks after surgery, while by the twelfth week it became highly noticeable in group 2. In terms of replanted and adjacent bone, group 2 showed the best continuity compared to groups 1 and 3 at the end of the observation period, suggesting that ErhBMP-2 increases bony healing when applied on an AAB graft site. In addition, we reconfirmed the effectiveness of the ACS as a delivery system for ErhBMP-2.
Acknowledgments
This study was supported by a faculty research grant from Yonsei University College of Dentistry (6-2011-0040).
References
- 1.Lee JG, Lee EW. Healing process of the reimplanted autoclaved autogenous mandible in adult dogs. J Korean Assoc Oral Maxillofac Surg. 1993;19:514–32. [Google Scholar]
- 2.Urist MR, Silverman BF, Büring K, Dubuc FL, Rosenberg JM. The bone induction principle. Clin Orthop Relat Res. 1967;53:243–83. [PubMed] [Google Scholar]
- 3.Harding RL. Replantation of the mandible in cancer surgery. Plast Reconstr Surg (1946) 1957;19:373–83. doi: 10.1097/00006534-195705000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Harding RL. Replantation of the mandible in cancer surgery. Plast Reconstr Surg. 1971;48:586–7. doi: 10.1097/00006534-195705000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hamaker RC. Irradiation autogenous mandibular grafts in primary reconstructions. Laryngoscope. 1981;91:1031–51. doi: 10.1288/00005537-198107000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Shin S, Yano H, Fukunaga T, et al. Biomechanical properties of heat-treated bone grafts. Arch Orthop Trauma Surg. 2005;125:1–5. doi: 10.1007/s00402-004-0746-6. [DOI] [PubMed] [Google Scholar]
- 7.Zellin G. Growth factors and bone regeneration. Implications of barrier membranes. Swed Dent J Suppl. 1998;129:7–65. [PubMed] [Google Scholar]
- 8.Lee SJ. Cytokine delivery and tissue engineering. Yonsei Med J. 2000;41:704–19. doi: 10.3349/ymj.2000.41.6.704. [DOI] [PubMed] [Google Scholar]
- 9.Garrison KR, Shemilt I, Donell S, et al. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev. 2010;(6):CD006950. doi: 10.1002/14651858.CD006950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JB, Kim TW, Ryu SH, et al. The Use of Recombinant Human Bone Morphogenic Protein-2 (rhBMP-2) in Treatment for Cysts of the Oral and Maxillofacial Regions. J Korean Assoc Maxillofac Plast Reconstr Surg. 2014;36:25–9. [Google Scholar]
- 11.Kübler NR, Reuther JF, Faller G, Kirchner T, Ruppert R, Sebald W. Inductive properties of recombinant human BMP-2 produced in a bacterial expression system. Int J Oral Maxillofac Surg. 1998;27:305–9. doi: 10.1016/s0901-5027(05)80621-6. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Kim CS, Choi KH, et al. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli. Biomaterials. 2010;31:3512–9. doi: 10.1016/j.biomaterials.2010.01.075. [DOI] [PubMed] [Google Scholar]
- 13.Nam JW. Comparison of autoclaved autogenous bone and fibrin glue as BMP carriers for bone regeneration in a critical sized segmental defect in the rat fibula. [dissertation] Seoul: Yonsei University; 2010. p. 67. [Google Scholar]
- 14.Bostrom M, Lane JM, Tomin E, et al. Use of bone morphogenetic protein-2 in the rabbit ulnar nonunion model. Clin Orthop Relat Res. 1996;(327):272–82. doi: 10.1097/00003086-199606000-00034. [DOI] [PubMed] [Google Scholar]
- 15.Zegzula HD, Buck DC, Brekke J, Wozney JM, Hollinger JO. Bone formation with use of rhBMP-2 (recombinant human bone morphogenetic protein-2) J Bone Joint Surg Am. 1997;79:1778–90. doi: 10.2106/00004623-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, Takahashi Y, Tabata Y. Enhanced bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2006;12:1305–11. doi: 10.1089/ten.2006.12.1305. [DOI] [PubMed] [Google Scholar]
- 17.Huber F, Belyaev O, Huber C, Meeder P. A standard surgical protocol for a rabbit ulnar osteotomy model. Scand J Lab Anim Sci. 2006;33:89–95. [Google Scholar]
- 18.Gallie WE. Discussion on bone-grafting: (Abstract) Proc R Soc Med. 1919;12:22–3. doi: 10.1177/003591571901201904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Böhm P, Springfeld R, Springer H. Re-implantation of autoclaved bone segments in musculoskeletal tumor surgery. Clinical experience in 9 patients followed for 1.1–8.4 years and review of the literature. Arch Orthop Trauma Surg. 1998;118:57–65. doi: 10.1007/s004020050312. [DOI] [PubMed] [Google Scholar]
- 20.Takaoka K, Nakahara H, Yoshikawa H, Masuhara K, Tsuda T, Ono K. Ectopic bone induction on and in porous hydroxyapatite combined with collagen and bone morphogenetic protein. Clin Orthop Relat Res. 1988;(234):250–4. [PubMed] [Google Scholar]
- 21.Nakahara H, Takaoka K, Koezuka M, Sugamoto K, Tsuda T, Ono K. Periosteal bone formation elicited by partially purified bone morphogenetic protein. Clin Orthop Relat Res. 1989;(239):299–305. [PubMed] [Google Scholar]
- 22.Winn SR, Uludag H, Hollinger JO. Carrier systems for bone morphogenetic proteins. Clin Orthop Relat Res. 1999;(367):S95–106. doi: 10.1097/00003086-199910001-00010. [DOI] [PubMed] [Google Scholar]
- 23.Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007;77:626–31. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- 24.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613–29. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Robinson Y, Heyde CE, Tschöke SK, Mont MA, Seyler TM, Ulrich SD. Evidence supporting the use of bone morphogenetic proteins for spinal fusion surgery. Expert Rev Med Devices. 2008;5:75–84. doi: 10.1586/17434440.5.1.75. [DOI] [PubMed] [Google Scholar]
- 26.Nevins M, Kirker-Head C, Nevins M, Wozney JA, Palmer R, Graham D. Bone formation in the goat maxillary sinus induced by absorbable collagen sponge implants impregnated with recombinant human bone morphogenetic protein-2. Int J Periodontics Restorative Dent. 1996;16:8–19. [PubMed] [Google Scholar]
- 27.Boyne PJ, Marx RE, Nevins M, et al. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent. 1997;17:11–25. [PubMed] [Google Scholar]
- 28.Kirker-Head CA, Nevins M, Palmer R, Nevins ML, Schelling SH. A new animal model for maxillary sinus floor augmentation: evaluation parameters. Int J Oral Maxillofac Implants. 1997;12:403–11. [PubMed] [Google Scholar]
- 29.Hollinger JO, Schmitt JM, Buck DC, et al. Recombinant human bone morphogenetic protein-2 and collagen for bone regeneration. J Biomed Mater Res. 1998;43:356–64. doi: 10.1002/(sici)1097-4636(199824)43:4<356::aid-jbm3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 30.McKay WF, Peckham SM, Marotta JS, editors. The science of RhBMP-2. St Louise, MA: Quality Medical Publishing Inc; 2006. [Google Scholar]
- 31.Herford AS, Boyne PJ. Reconstruction of mandibular continuity defects with bone morphogenetic protein-2 (rhBMP-2) J Oral Maxillofac Surg. 2008;66:616–24. doi: 10.1016/j.joms.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Burkus JK, Gornet MF, Glassman SD, et al. Blood serum antibody analysis and long-term follow-up of patients treated with recombinant human bone morphogenetic protein-2 in the lumbar spine. Spine (Phila Pa 1976) 2011;36:2158–67. doi: 10.1097/BRS.0b013e3182059a8c. [DOI] [PubMed] [Google Scholar]
- 33.Kim SJ, Kim MR, Oh JS, Han I, Shin SW. Effects of polycaprolactone-tricalcium phosphate, recombinant human bone morphogenetic protein-2 and dog mesenchymal stem cells on bone formation: pilot study in dogs. Yonsei Med J. 2009;50:825–31. doi: 10.3349/ymj.2009.50.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HJ, Kang SW, Lim HC, et al. The role of transforming growth factor-beta and bone morphogenetic protein with fibrin glue in healing of bone-tendon junction injury. Connect Tissue Res. 2007;48:309–15. doi: 10.1080/03008200701692610. [DOI] [PubMed] [Google Scholar]