Abstract
The goal of this study was to determine whether the input-output characteristics of the zona incerta (ZI) are appropriate for it to serve as a conduit for cortical control over saccade-related activity in the superior colliculus. The study utilized the neuronal tracers wheat germ agglutinin-horseradish peroxidase (WGA-HRP) and biotinylated dextran amine (BDA) in the cat. Injections of WGA-HRP into primary somatosensory cortex (SI) revealed sparse, widespread nontopographic projections throughout ZI. In addition, region-specific areas of more intense termination were present in ventral ZI, although strict topography was not observed. In comparison, the frontal eye fields (FEF) also projected sparsely throughout ZI, but terminated more heavily, medially, along the border between the two sublaminae. Furthermore, retrogradely labeled incertocortical neurons were observed in both experiments. The relationship of these two cortical projections to incertotectal cells was also directly examined by retrogradely labeling incertotectal cells with WGA-HRP in animals that had also received cortical BDA injections. Labeled axonal arbors from both SI and FEF had thin, sparsely branched axons with numerous en passant boutons. They formed numerous close associations with the somata and dendrites of WGA-HRP-labeled incertotectal cells. In summary, these results indicate that both sensory and motor cortical inputs to ZI display similar morphologies and distributions. In addition, both display close associations with incertotectal cells, suggesting direct synaptic contact. From these data, we conclude that inputs from somatosensory and FEF cortex both play a role in controlling gaze-related activity in the superior colliculus by way of the inhibitory incertotectal projection.
Keywords: zona incerta, oculomotor, superior colliculus, thalamus
The zona incerta (ZI) has recently become the subject of increased scientific study. A consistent feature of these investigations is the designation of a dorsal sublamina (dZI) and ventral sublamina (vZI), which differ in their patterns of connection [rat: Watanabe and Kawana (1982); cat: May et al. (1997); monkey: Ma et al. (1992)]. The dZI projects to the brainstem tegmentum, pretectum, parafascicular nucleus, and cortex [cat: May et al. (1997); rat: Watanabe and Kawana (1982), Lin et al. (1997), and Power and Mitrofanis (1999a)]. In contrast, the cells of origin for the incertotectal projection are primarily found in the vZI [cat: Ficalora and Mize (1989); May et al. (1997); monkey: May (2006); rat: Wantanabe and Kawana (1982), Romanowski et al. (1985)]. The predominant transmitter used by the cells in both these sublaminae is GABA. For example, both the incertotectal projection from vZI [rat: Kim et al. (1992) and Nicolelis et al. (1992); cat: Ficalora and Mize (1989)] and the incertocortical projection from dZI [rat: Lin et al. (1990) and Nicolelis et al. (1992)] are GABAergic. This makes the incertotectal pathway a long-range inhibitory influence over the superior colliculus (SC), which parallels the GABAergic nigrotectal projection from pars reticulata [rat: Redgrave et al. (1992); cat: Graybiel (1978), Harting et al. (1988), and Ficarola and Mize (1989); squirrel: May and Hall (1984)].
In an early myelin study of ZI, Rioch (1929) suggested that one of the functions of the ZI is to serve as a relay station interconnecting cortical areas with motor centers, such as the tectum. Indeed, the ZI projects to the intermediate gray layer (SGI) of the SC [rat: Ricardo (1981) and Wantanabe and Kawana (1982); cat: May et al. (1997)]. This SC layer contains multisensory output cells, which project by way of the predorsal bundle to contralateral brainstem regions controlling saccadic eye movements [cat: Cowie and Holstege (1992); macaque: Harting (1977) and May and Porter (1992); rat: Dean et al. (1988) and Bickford and Hall (1989); squirrel: May and Hall (1984)]. Furthermore, incertotectal terminals are found in close association with cells projecting in the predorsal bundle of rats, suggesting direct synaptic contact (Kim et al., 1992). It is precisely this subpopulation of SC output neurons that exhibit saccade-related activity [cat: Grantyn and Grantyn (1982), Moschovakis and Karabelas (1985), and Munoz and Guitton (1991); monkey: Wurtz and Goldberg (1972), Sparks and Mays (1981), and Moschovakis et al. (1988)]. Thus, the ZI is well positioned to play a role in the guidance of saccades initiated by the SC.
In view of the possibility that the incertotectal projection plays a role in controlling gaze, it is of interest to determine what types of input modulate the activity of these incertotectal neurons. Several of the projections described as ending primarily in vZI are somatosensory in nature. Inputs from somatosensory cortex, the trigeminal nuclei, and the dorsal column nuclei terminate in this sublamina in the rat (Nicolelis et al., 1992; Mitrofanis and Mikuletic, 1999; Wang et al., 2004). These same somatosensory structures also provide inputs directly to the SC, where they help determine the pattern of saccade-related activity [monkey: Groh and Sparks (1996); rat: Redgrave et al. (1996)]. Thus, the ascending somatosensory inputs to the SC from the trigeminal nuclei and spinal cord, as well as the descending ones from somatosensory cortex, may converge with an inhibitory somatosensory feed-forward path that includes ZI [rat: Killackey and Erzurumlu (1981), Huerta et al. (1983), Cadusseau and Roger (1985), and Rhoades et al. (1989); cat: Huerta et al. (1981) and Meredith and Clemo (1989); macaque: Wiberg et al. (1987)]. However, at present, most of the evidence concerning somatosensory inputs to ZI comes from experiments conducted in the rodent model, where the somatosensory system provides the dominant modality for controlling behavior (Nicolelis et al., 1992; Redgrave et al., 1996; Shaw and Mitrofanis, 2002). It remains to be determined whether somatosensory inputs to ZI are as prominent in species where the visual system dominates. It also has not been specifically demonstrated that these somatosensory inputs directly contact incertotectal neurons.
An alternative model for incertotectal function must also be considered. It may be that the ZI acts primarily as an inhibitory feed-forward path for descending cortical motor output, a variation on the proposal of Rioch (1929). In this case, it would be expected that the frontal eye fields (FEF), one of the most important cortical inputs to SGI, should provide a prominent input to incertotectal cells. In support of this hypothesis, there is some evidence of a FEF input to ZI, but the cellular and sublaminar targets of this input have not been specified [cat: Miyashita and Tamai (1989); monkey: Huerta et al. (1986); rat: Mitofanis and Mikuletic (1999)].
In the experiments detailed here, we have attempted to examine these possibilities by better establishing the pattern of cortical input to ZI, and specifically to incertotectal neurons. These experiments have been undertaken in the cat in order to determine the extent of cortical input to ZI in a species more reliant on visual cues. Anterograde tracers were used to investigate the sublaminar distribution of the primary somatosensory cortex (SI) and FEF projections to ZI. Dual-tracer techniques were employed to determine whether each of these cortical inputs directly contacts incertotectal cells. The analysis of the cat ZI is also of interest because earlier work suggests that in the cat, unlike the rat, the dZI and vZI extend dorsolaterally, medial to the reticular thalamic nucleus. This portion of ZI has been designated as the dorsolateral extension (eZI) for the sake of convenience (May et al., 1997). It was of interest to see whether cortical inputs terminate within eZI. Finally, it has been reported that the dZI provides a widespread projection to layer I of cerebral cortex in rodents (Nicolelis et al., 1992; Lin et al., 1997). In analyzing our data, we sought evidence of this projection in the cat. A portion of this work has been presented previously in abstract form (Perkins et al., 2002).
MATERIALS AND METHODS
Fourteen adult cats (Felix domesticus) of both sexes were used in this study. All experiments were performed in accordance with National Institutes of Health guidelines for animal care and use under protocols approved by the Animal Care and Use Committee of the University of Mississippi Medical Center. Two experimental approaches were employed in this study (see Fig. 1 for details of animal usage). In the first set of experiments, wheatgerm agglutinin-horseradish peroxidase (WGA-HRP) was injected either into the region of cortex containing the primary somatosensory representation or the portion of the medial frontal lobe containing the frontal eye fields. This was done in order to anterogradely label corticoincertal axon terminals and to retrogradely label incertocortical cells. In the second series of experiments, a dual-tracer technique was employed. Biotinylated dextran amine (BDA) was injected into either SI cortex or the FEF to anterogradely label corticoincertal terminals. In these same animals, WGA-HRP was injected into the SC to retrogradely label incertotectal neurons.
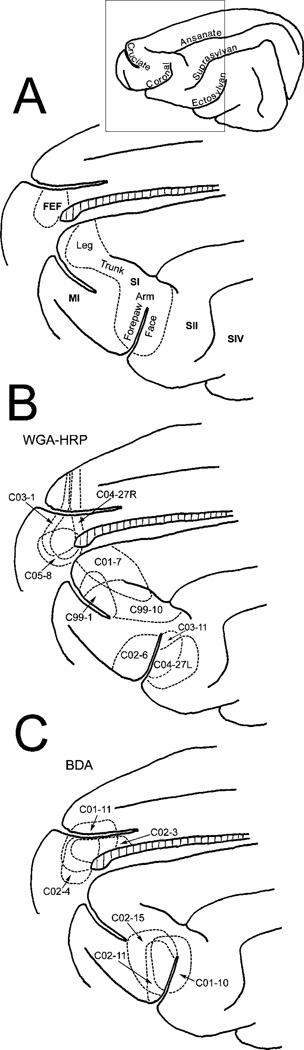
Fig. 1.
Cat cortical regions and summary of WGA-HRP and BDA injections. A shows the representation of the skin surface in SI and the approximate location of the FEF. B shows the location of WGA-HRP injections. C shows the location of BDA injections. BDA, biotinylated dextran amine; BP, brachium pontis; dZI, dorsal sublamina of zona incerta; EML, external medullary lamina; eZI, dorsolateral extension of zona incerta; FEF, frontal eye fields; FF, fields of Forel; MI, primary motor cortex; RT, reticular thalamic nucleus; SI, primary somatosensory cortex; SII, secondary somatosensory cortex; SIV, quaternary somatosensory cortex; SNr, substantia nigra pars reticulata; VPL, ventral posterior lateral nucleus of the thalamus; vS, trigeminal sensory nuclei; vZI, ventral sublamina of zona incerta; WGA-HRP, wheat germ agglutinin-conjugated horseradish peroxidase; ZI, zona incerta.
Surgical Procedures
Cats weighing 3–5 kg were anesthetized with sodium pentobarbital (up to 35 mg/kg, i.v.). Anesthesia level was monitored, and a surgical level of anesthesia was maintained, with supplemental doses of the anesthetic as needed. In each cat, either 1.0% WGA-HRP or 10.0% BDA or both were pressure-injected into target structures with a 1.0 µl Hamilton syringe. For WGA-HRP cases, each SI injection ranged from 0.03 to 0.2 µl, with 6–10 injections being placed in the cortex (total = 0.12–2.0 µl). In the case of the FEF, the injection volumes ranged from 0.02 to 0.06 µl, with 1–4 injections (total = 0.06–0.24 µl). For the BDA injections in SI, 6–10 injections of 0.2 µl were made (total = 1.2–2.0 µl), while in the case of the BDA injections of the FEF, 0.4 µl was deposited in 3–4 sites (total = 1.2–1.6 µl). For cortical injections, a unilateral craniotomy was performed and the syringe was visually guided into the cortex using surface landmarks. Portions of SI and the FEF were targeted based on the topography and locations previously defined by physiologic means (Fig. 1A) (Guitton and Mandl, 1978a, 1978b; Felleman et al., 1983). Injection depth was 1.0 mm from the surface in the SI cases, except that depths of 1.5–2.0 mm were used to drive the syringe along the banks of sulci. Depths of 5.0–7.0 mm were used in the injections of the FEF regions in the cruciate and presylan sulci, respectively. In the dual-tracer cases, a BDA injection of cortex was performed first, followed by the surgery for the SC injection 13 days later. In the SC injections, a unilateral craniotomy was performed over the occipital cortex, and medial occipital cortex overlying the superior colliculus was aspirated using the bony tentorium cerebelli as a landmark. The injection syringe was visually guided into the SC. Several injections (2–3) of 0.02 µl were made at a depth of 1.0–1.5 mm from the surface (total = 0.04–0.06 µl) to provide near total filling of the SC. For all surgeries, an analgesic, buprenorphene (Buprenex), was administered (0.01 mg/kg) during the immediate postoperative period. Animals were sacrificed 24–48 hr after the WGA-HRP injection in all cases.
Histological Procedures
Following the survival period, each animal received an overdose of sodium pentobarbital (50 mg/kg, i.p.) and was perfused transcardially. Animals were perfused with a buffered saline prewash followed by a fixative solution of 1% paraformaldehyde and 1.25–1.5% glutaraldehyde in 0.1 M phosphate buffer (PB; pH 7.2). Brains were blocked in the frontal plane, then removed and sectioned at a thickness of 100 µm by using a vibratome. The tissue sections containing WGA-HRP were reacted using a tetramethylbenzidine (TMB) protocol (Chen and May, 2002). Briefly, the sections were placed in multiwell trays and rinsed with 0.1 M (pH 6.0) PB followed by incubation in a 0.005% TMB (Free Base), 0.25% ethanol, and 0.245% ammonium molybdate in a 0.1 M (pH 6.0) PB solution for 20 min. Next, the sections were reacted by the addition of H2O2 solution (0.011% total concentration) and incubated overnight at 4°C with gentle agitation. Sections were then transferred to a stabilizer solution of 5.0% ammonium molybdate for 15 min, followed by multiple rinses in 0.1 M (pH 6.0) PB.
Tissue sections processed for the presence of both WGA-HRP and BDA were reacted as follows. First, the TMB-molybdate steps described above were employed. Next, the sections were incubated in a 5% solution of DAB (Diaminobenzidene HCl), in 0.1 M (pH 7.2) PB, and the reaction was initiated by the addition of H2O2 (0.011%) to produce a brown reaction product in the cells. The tissue was rinsed first in 0.1 M (pH 7.2) PB and then in 0.5% triton X-100 in 0.1 M (pH 7.2) PB. Sections were incubated overnight at 4°C in an Avidin-HRP (Vector Laboratories) solution (1:500) in 0.1 M (pH 7.2) PB containing 0.05% triton X-100. After rinsing with 0.1 M (pH 7.2) PB, they were reacted in a 5% DAB solution of 0.1 M (pH 7.2) PB containing hydrogen peroxide (0.011%) and 0.05% nickel ammonium sulfate and 0.05% cobalt chloride for 10–30 min to produce a black reaction product in the axons. In both the single- and dual-tracer cases, individual sections were mounted on gelatinized slides, air-dried, counterstained in cresyl violet, dehydrated, cleared, and coverslipped.
Data Analysis
The distribution of labeled elements were charted at 32× magnification and individual cells and axons were drawn at 800× magnification by use of an Olympus BH-2 microscope equipped with a drawing tube. Selected areas containing labeled cells and terminals were digitally photographed with a Nikon Eclipse E600 microscope equipped with Nikon Digital DXM1200F and Photometrics Cool-Snap ES cameras using MetaMorph image analysis software. Digitized information from up to 10 Z-axis focal planes was combined into a single plane using the Metamorph “stack arithmetic minimum” function. The digitized images were adjusted in Adobe Photoshop to appear as close as possible to the visualized section.
RESULTS
Location of Cortical Injection Sites
WGA-HRP was injected into several areas of SI cortex in order to determine the laminar distribution of connections within ZI (Fig. 1B). These regions included the representations of the forepaw, face, and leg, as shown in Figure 1A (Felleman et al., 1983). In a separate series of animals, injections of WGA-HRP were made into medial frontal cortex and were centered within the FEF in order to anterogradely label terminals within ZI (Fig. 1B). Retrogradely labeled cells observed in the expected regions of the dorsal thalamus, including the ventral posterior nucleus (SI injections) and medial dorsal nucleus (FEF injections), acted as a guide for the accuracy of the injection sites. In the dual-tracer cases, the animals received collicular injections of WGA-HRP, in addition to cortical injections of BDA that were located in either the forepaw and face SI representation, or in the FEF (Fig. 1C). In each case, labeled axons were observed entering the dorsolateral extension of ZI (eZI) from the internal capsule, rostral and lateral to ZI. A portion of these fibers continued on through ZI and terminated in the thalamus or proceeded into the midbrain tegmentum. Consequently, a portion of what appeared to be terminal label in ZI may actually have been cut fibers of passage. We have striven to discriminate between these two, but this point should be kept in mind.
Corticoincertal Projections From Somatosensory Cortex
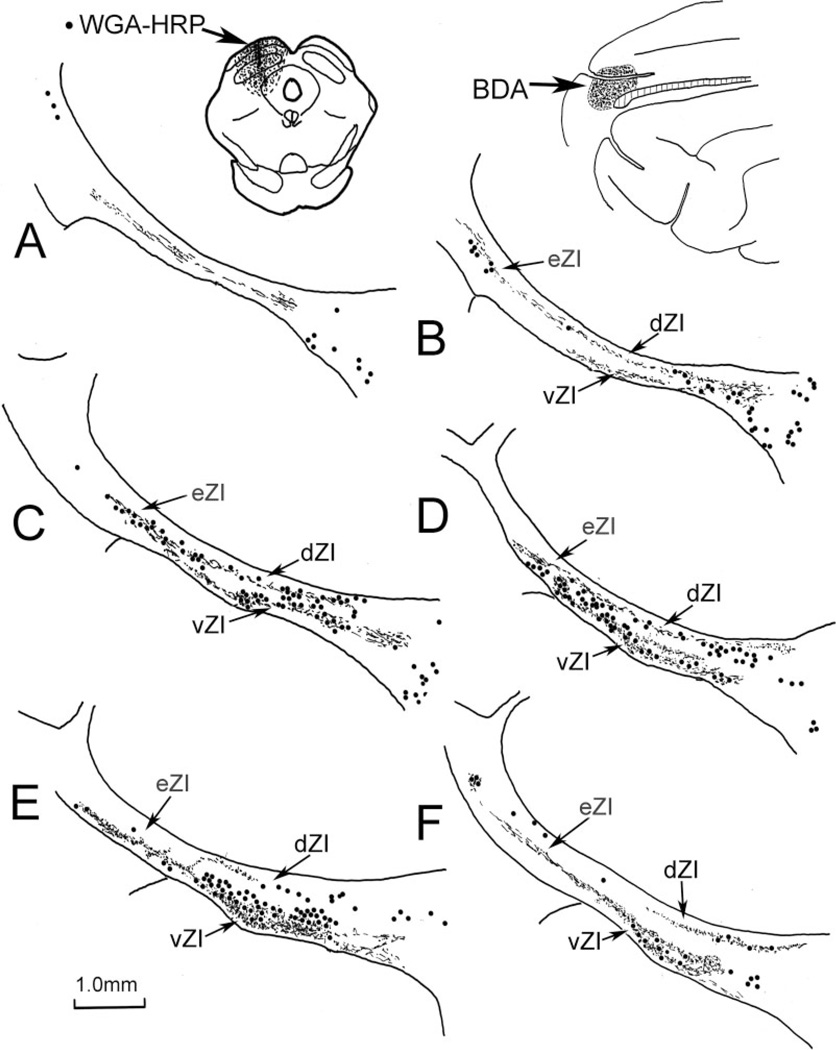
In those cases where WGA-HRP was injected into the SI cortex, labeled axons and sparse terminals were scattered throughout much of the rostrocaudal and mediolateral extent of ZI. These terminals were found in both the dorsal and the ventral sublaminae (dZI and vZI) and within eZI. However, the terminal distribution was not homogeneous. There were areas with no terminal label, as well as regions that exhibited much denser terminal labeling. Chartings from a case where WGA-HRP was injected into the face representation of SI cortex are provided as an example (Fig. 2). In addition to the widespread light distribution of terminals, a dense band of terminals was present in vZI in the middle of the nucleus (Fig. 2C–E). In SI injection cases involving other portions of the body representation, the area of ZI with the most intense terminal fields was found medial or lateral to this region. In addition to labeled terminals, retrogradely labeled neurons were sometimes observed. In the illustrated case, retrogradely labeled cells (dots) were primarily observed in the fields of Forel medial to ZI (Fig. 2D–F), but in other cases, labeled cells were observed in dZI and the external medullary lamina.
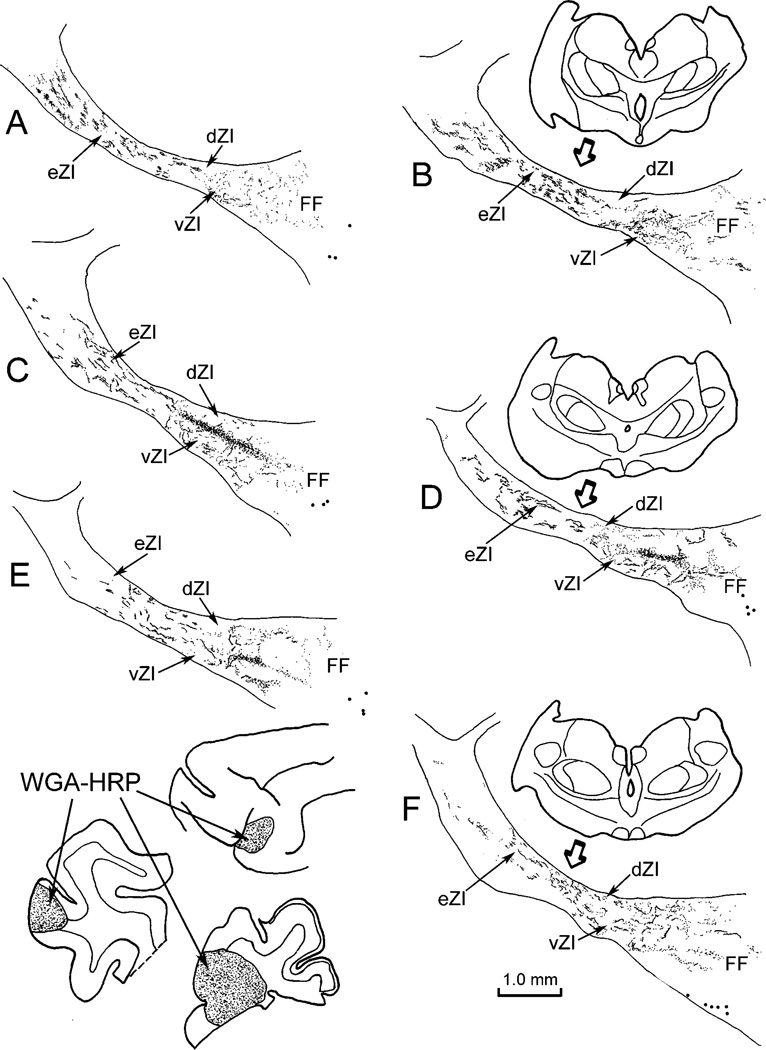
Fig. 2.
Distribution of label in zona incerta following placement of a WGA-HRP injection into the SI face representation (insert lower left). Labeled terminals (stipple) are present throughout ZI, but are densest within vZI in the middle of the nucleus (C–E). Retrogradely labeled neurons (dots) were also present, primarily in the fields of Forel. The illustrated case is designated as C04-27L in Figure 1. In this and the chartings to follow, sections are arranged in rostral-to-caudal order.
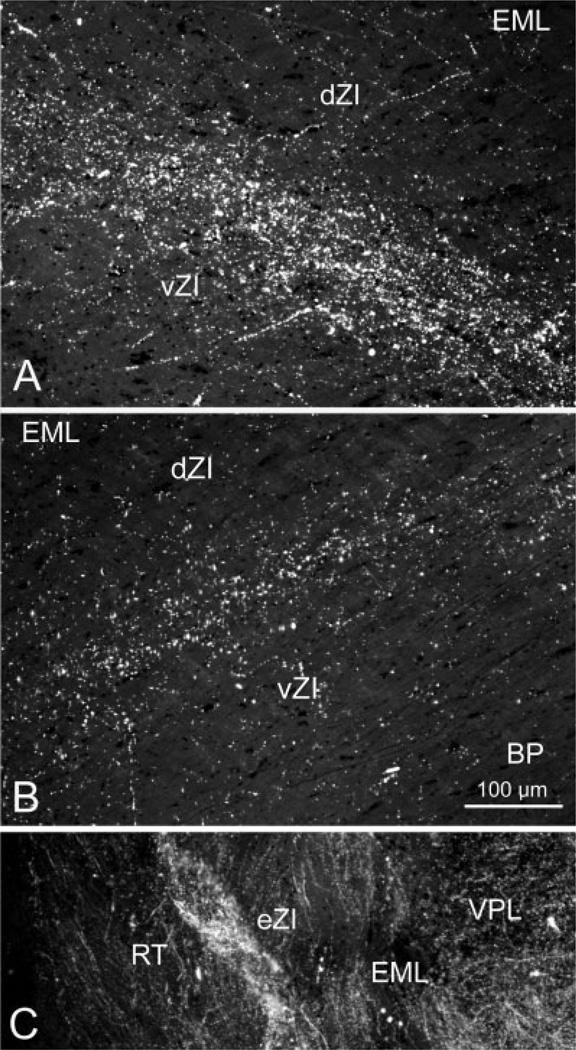
The pattern of labeling seen in these cases is further demonstrated in photomicrographs (Fig. 3A and C). The terminals observed in ZI after a face representation injection produced a band of label in vZI that increased in density as it approached the border between the sublaminae (Fig. 3A). These injections also produced axonal labeling in eZI (Fig. 3C). Within eZI, patches of terminals were evident medial to the thalamic reticular nucleus among bundles of labeled axons.
Fig. 3.
Photomicrographs of corticoincertal terminals labeled from injections of WGA-HRP into cortex, as observed with crossed polarizing filters. A: Labeled terminals located in vZI are densest along the border between the sublaminae following an injection of the SI cortex face representation. B: The pattern of terminal label in ZI forms a band of label located at the border between the sublaminae following a WGA-HRP injection in the FEF. C: Terminal patches were interspersed between bundles of labeled fibers in eZI in this example from an injection of the SI cortex forepaw representation.
Somatosensory Cortex: Dual-Tracer Experiments
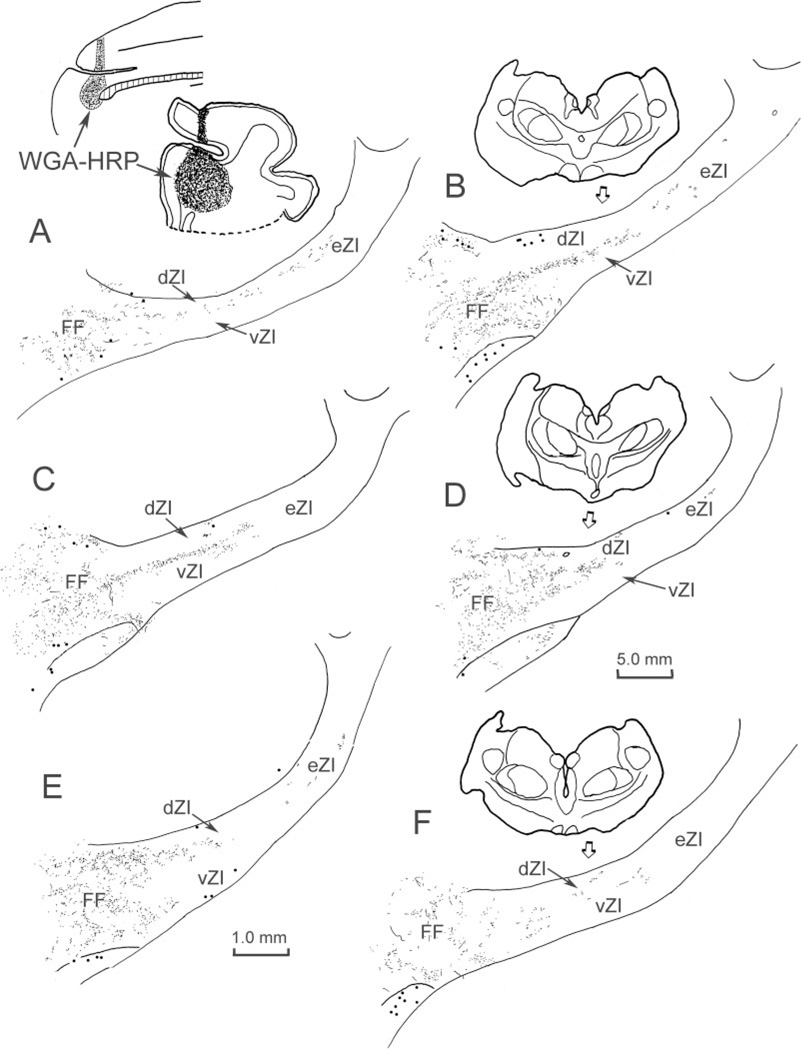
In order to determine whether this projection might directly contact incertotectal neurons, dual-tracer experiments were conducted. Figure 4 provides an example of such a case. Following an injection of WGA-HRP into the superior colliculus, retrogradely labeled neurons (dots) were concentrated at middle levels (Fig. 4B–F) within the rostrocaudal axis of ZI. Most of these labeled incertotectal cells were located in vZI, with the distribution of labeled cells continuing well into the eZI (Fig. 4B–D). Scattered labeled cells in dZI are most likely due to spread of the injection site into the pretectum (May et al., 1997). In this case, the BDA injection spanned the coronal sulcus at its caudal end and so included portions of the face, arm, and forepaw representations of SI cortex (Fig. 1A). The anterogradely labeled terminal field (stipple) was found in both sublaminae of ZI (Fig. 4). The distribution of BDA-labeled corticoincertal terminals primarily overlapped the distribution of WGA-HRP-labeled incertotectal cells in vZI (Fig. 4B–E).
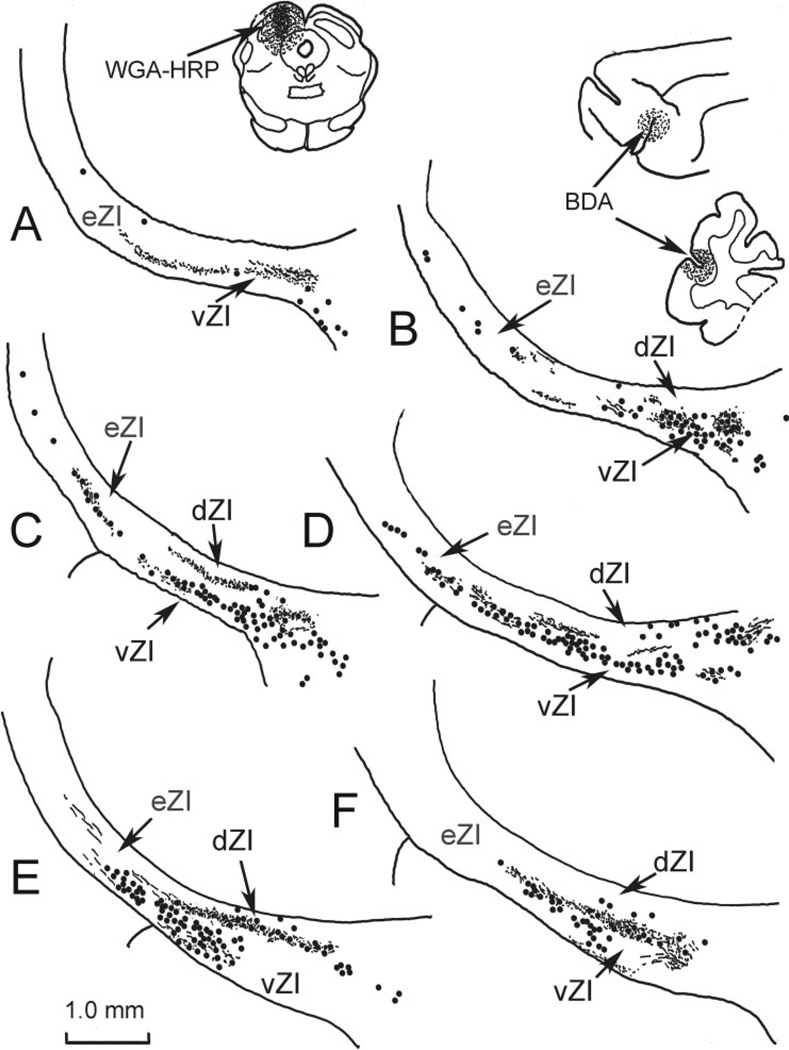
Fig. 4.
Distribution of labeled elements in a dual-tracer experiment in which BDA was injected into face/forepaw representation of SI somatosensory cortex (insert upper right) to anterogradely label corticoincertal terminals (stipple), and WGA-HRP was injected into the SC (insert upper left) to retrogradely label incertotectal cells (dots). Overlap between BDA-labeled corticoincertal terminals (stipple) and HRP-labeled incertotectal cells (dots) was most prominent in vZI (B–E). The illustrated case is designated as case C01–10 in Figure 1.
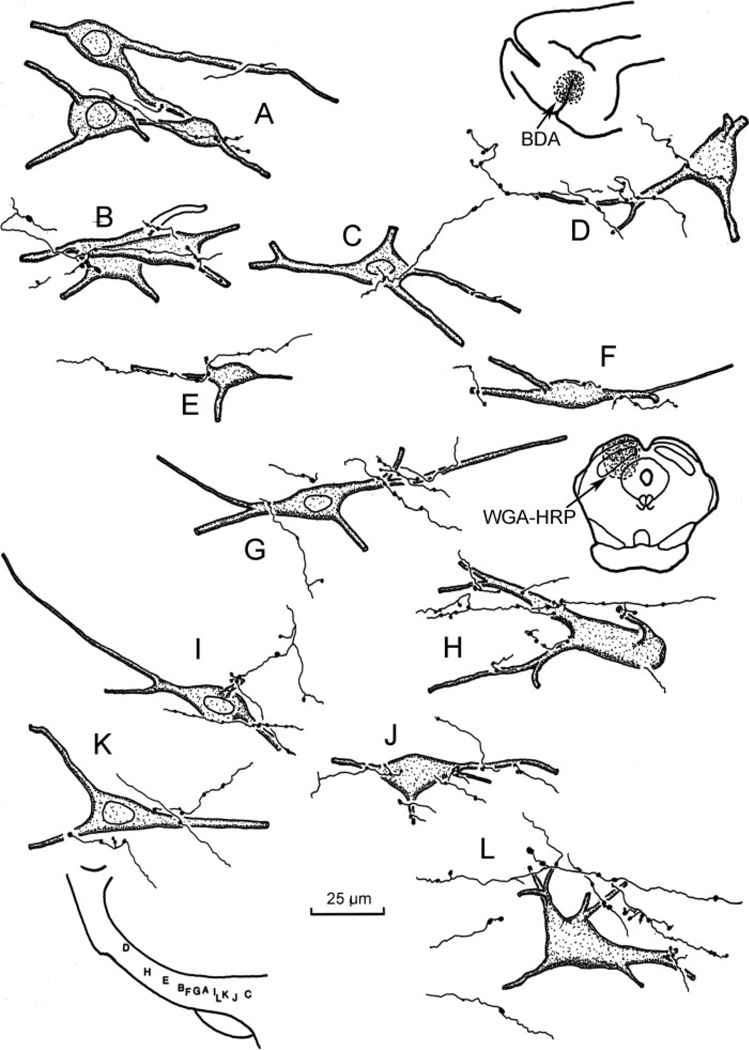
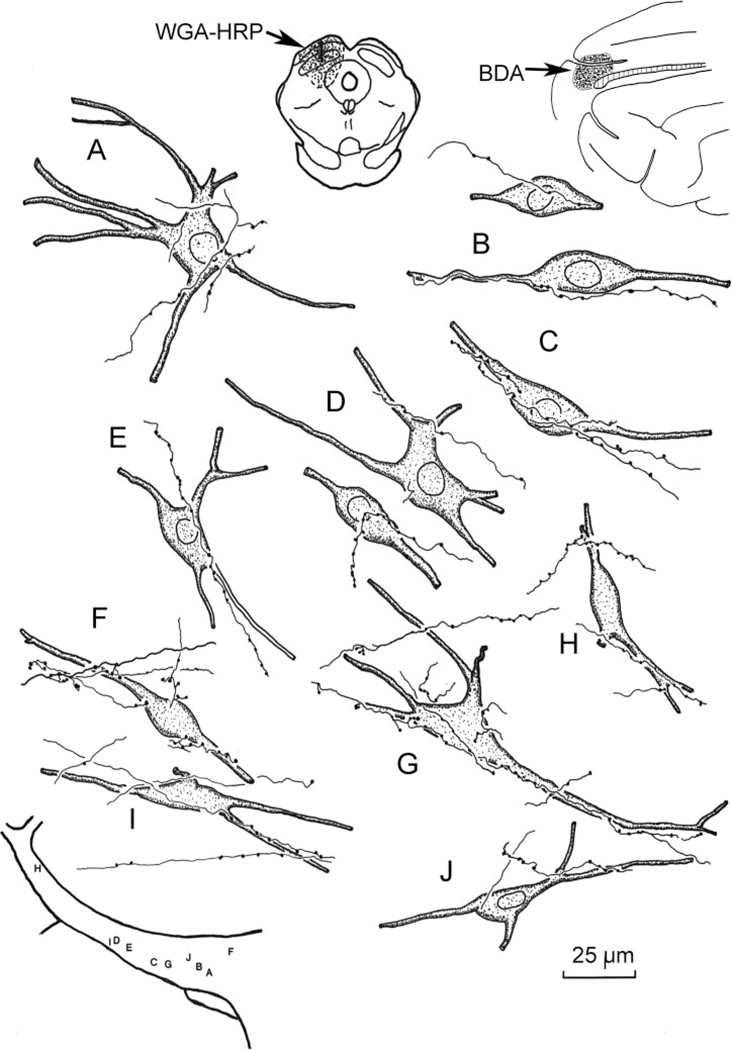
The morphological characteristics of incertotectal neurons and their related corticoincertal arbors are illustrated in Figure 5 and shown in Figure 6A–D. The WGA-HRP-labeled incertotectal neurons have multipolar or spindle-shaped somata ranging in size from 25 to 50 µm (long axis). WGA-HRP staining revealed that these neurons have from two to five primary dendrites, some of which branch very proximally. Most of the labeled dendrites were oriented along the long mediolateral axis of ZI. Following BDA injections of SI cortex, the labeled corticoincertal terminals arbors appear as thin fibers with occasional branches (Fig. 5). These axons are decorated along their lengths with relatively small varicosities. Some axons draped short portions of the dendrites of the retrogradely labeled neurons (Figs. 5D, E, G, and J and 6B and D). Others were seen in close association with somata (Figs. 5 B–F and I–K and 6C). However, corticoincertal fibers were not exclusively directed at an individual cell or cell cluster, as they passed on through ZI making contacts (Figs. 5 D, H, and L and 6A). The vast majority of close associations between the BDA-labeled corticoincertal terminals and the WGA-HRP-labeled neurons were seen in vZI. Close associations of this type were found throughout the mediolateral axis of ZI, and also within eZI (Figs. 5D and H and 6D).
Fig. 5.
High-magnification illustration showing the spatial relationship between BDA-labeled corticoincertal terminals from an SI cortex injection (insert upper right) and WGA-HRP-labeled neurons within ZI from an injection in the SC (insert middle right). The location of the illustrated elements is shown schematically at the lower left.
Fig. 6.
Photomicrographs showing close associations between brown incertotectal cells, retrogradely labeled with WGA-HRP, and black corticoincertal terminals, anterogradely labeled with BDA, following an injection of either SI cortex (A–D) or the FEF (E–J). Arrowheads indicate close associations indicative of synaptic contact. Multiple Z-axis planes were combined to create these photomicrographs.
Corticoincertal Projections From Frontal Eye Fields
Figure 7 illustrates an example of the cases in which the WGA-HRP injection included the dorsal bank of the presylvian sulcus and the ventral bank of the cruciate sulcus and thus encompassed portions of the cat FEF. Anterogradely labeled terminals were found in both sublaminae of ZI. Only a small amount of terminal label was present in eZI. Instead, the terminal labeling became much more prominent medially and continued into the fields of Forel (Fig. 7). The densest terminal field was found along the border between the sublaminae of ZI (Fig. 7B–D). The nature of this portion of the projection may be further appreciated in the photomicrographs (Fig. 3B). Under crossed polarizer illumination, the WGA-HRP-labeled terminals appear as a band, extending on either side of the border between the sublaminae. Retrogradely labeled incertocortical cells (dots) were seen primarily in dZI (Fig. 7A–E). Similar labeling patterns were observed in the other two cases of this type.
Fig. 7.
Distribution of label in zona incerta following placement of a WGA-HRP injection into the FEF region of medial frontal cortex (insert upper left). Labeled terminals (stipple) are found in both sublaminae and are densest medially, along the border between the sublaminae (A–C). A few retrogradely labeled cells (dots) were also observed, primarily in dZI. The illustrated case is designated C03-1 in Figure 1.
Frontal Eye Fields: Dual-Tracer Experiments
The relationship between the terminals from the FEF and incertotectal cells was examined in dual-tracer experiments. Following placement of BDA into the medial frontal cortex, including the cat FEF, labeled boutons indicative of terminals were distributed in both vZI and dZI (Fig. 8B–F). These terminals extended through the rostrocaudal axis of ZI, and boutons, along with numerous axons, were also present in eZI (Fig. 8B–F). The terminals were most densely distributed in the ventral sublamina (Fig. 8D–F), but a second narrow band of label was present in dZI (particularly Fig. 8F). Thus, the terminal field, while more extensive medially, was not as restricted in the BDA case as in the WGA-HRP cases with FEF injections (Fig. 7). This is most likely due to the fact that the BDA injections covered a wider area, including cingulate cortex, and that terminals and axons could be more easily distinguished in the BDA material. The WGA-HRP-labeled incertotectal cells were, as expected, located primarily in vZI in this case. Occasional WGA-HRP-labeled cells were seen in dZI, but these were most likely due to spread of the tracer into the pretectum or periaqueductal gray. Once again, the distribution of the cortical terminals overlapped the distribution of labeled incertotectal cells, primarily within vZI.
Fig. 8.
Dual-tracer experiment in which BDA was injected into medial frontal cortex including the FEF (insert upper right) to anterogradely label corticoincertal terminals (stipple), and WGA-HRP was injected into the SC (insert upper left) to retrogradely label incertotectal cells (dots). Most of the overlap between anterogradely labeled terminals and retrogradely labeled cells was found in vZI (C–E). Some labeled cells were present in dZI, perhaps due to spread of the WGA-HRP into the pretectum. Case illustrated is designated C01–11 in Fig. 1.
As shown in high-magnification illustrations, the BDA-labeled axon terminal arbors from the FEF were thin, with occasional branches (Fig. 9). The boutons appeared as small beads arranged along these fibers. They were quite similar in morphology to those labeled following SI cortex injections. Close associations were often evident between the BDA-labeled cortical terminals and WGA-HRP-labeled incertotectal neurons within vZI (Fig. 9A–C, E–G, and J) and eZI (Fig. 9 D, H, and I). Examples of both axodendritic and axosomatic relationships were observed. As with the SI fibers, the FEF corticoincertal fibers did not appear to focus on individual cells, but instead extended through considerable portions of ZI. The appearance of close associations between BDA-labeled corticoincertal boutons from the FEF- and WGA-HRP-labeled incertotectal neurons in vZI is further displayed in the photomicrographs of Figure 6E–I. Individual axons often ran along the soma and proximal dendrites of these cells, contributing several boutons (Fig. 6F–I). As shown in Figure 6E, they were not confined to contacting any one target cell. Close associations between terminals and incertotectal cells located in eZI were also present (Fig. 6J).
Fig. 9.
High-magnification illustrations showing the relationship between BDA-labeled corticoincertal terminals from an FEF injection (insert upper right) and WGA-HRP-labeled incertotectal neurons labeled from SC injections (insert upper left). The locations of the illustrated elements within ZI are shown schematically at the lower left. Case illustrated is designated CO2–3 in Fig. 1.
Incertocortical Neurons
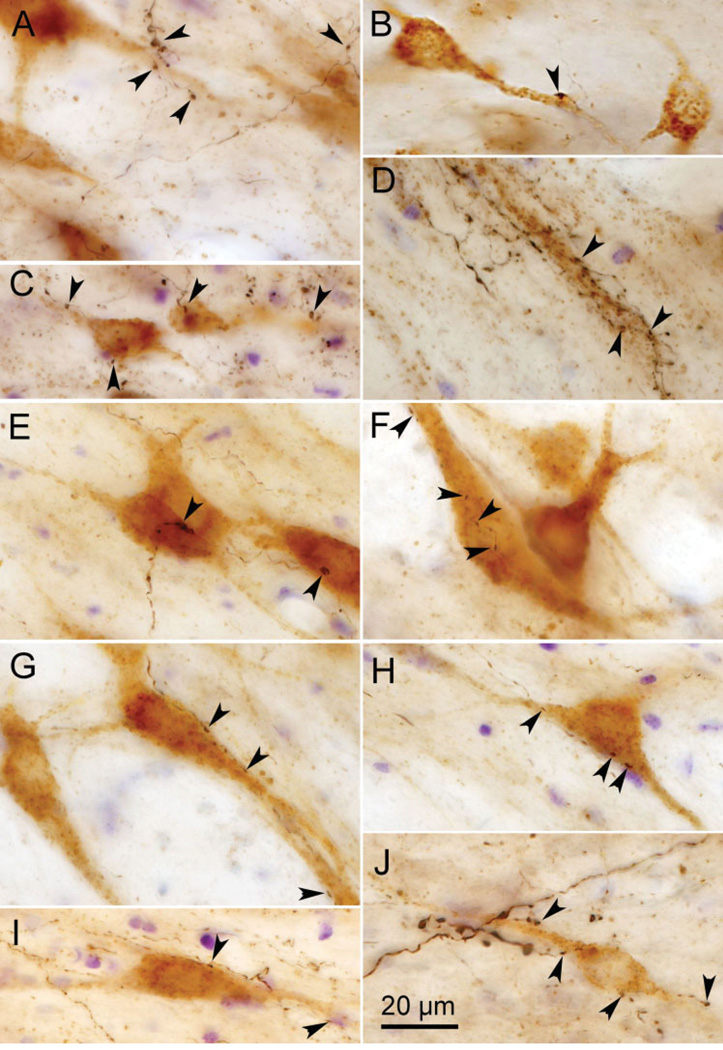
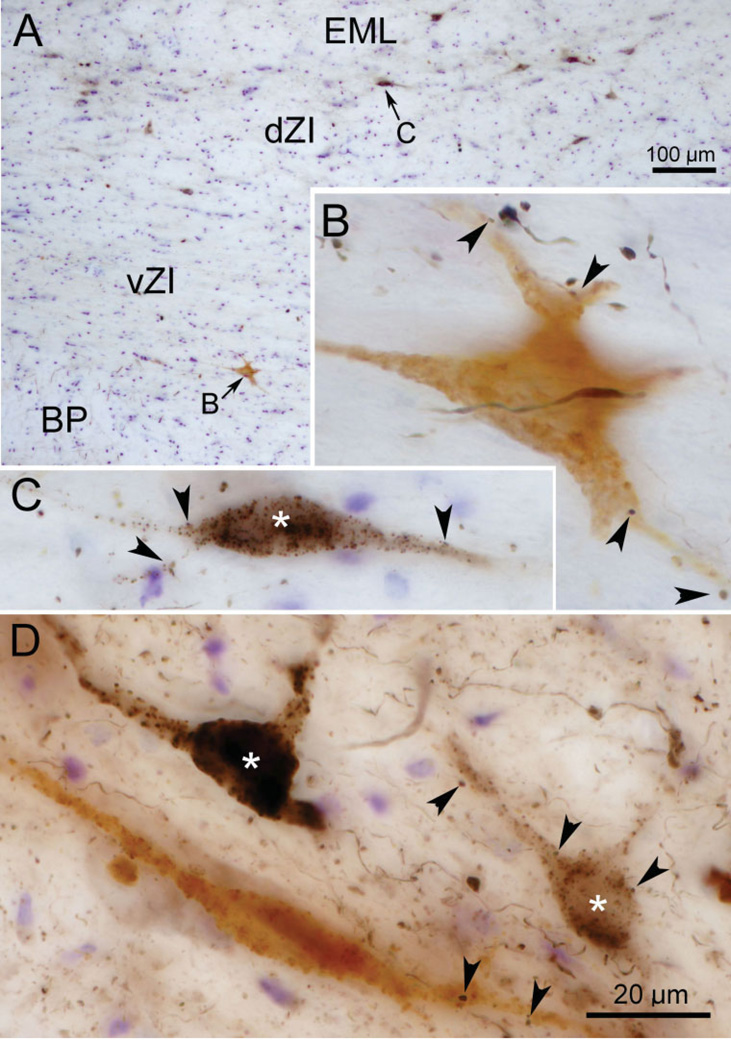
Retrogradely labeled incertocortical were observed in the dZI and the adjacent external medullary lamina (EML) in most cases with cortical injections of tracer. Their number varied between cases, perhaps due to the degree of involvement of their layer I cortex target (Lin et al., 1997). In the dual-tracer experiments, a few incertocortical neurons were retrogradely labeled with BDA, in addition to the BDA-labeled corticoincertal terminals and the incertotectal neurons, which were retrogradely labeled with WGA-HRP. The two populations of retrogradely labeled cells were easily distinguished due to chromagen color, as shown in examples taken from an FEF injection case (Fig. 10A). The BDA-labeled cells (asterisk) were stained black and were primarily found dorsally, in dZI and EML, while HRP-labeled neurons were light brown in color and were primarily found ventrally, in vZI (Fig. 10A–C). BDA-labeled corticoincertal boutons were observed in close association with both incertotectal (Fig. 10B and D) and incertocortical (Fig. 10C and D) cell populations (arrowheads). These could be distinguished from the light brown tectoincertal terminals by their black color and the more complete staining of intervening axon segments produced by the BDA present in cortical fibers.
Fig. 10.
Photomicrographs showing retrogradely labeled (black) incertocortical neurons (asterisk) from a BDA injection of FEF and retrogradely labeled incertotectal neurons (light brown) from a WGA-HRP injection of the SC. B and C are higher-magnification images from A and show incertocortical and incertotectal neurons, respectively. D is an additional example with two incertocortical neurons (asterisk) and one incertotectal neuron. Arrowheads in B–D indicate close associations between corticoincertal axonal boutons (black), which are labeled anterogradely with BDA, and the two types of retrogradely labeled neuron. Multiple Z-axis planes were combined to create these photomicrographs.
DISCUSSION
This study represents the first detailed examination of the distribution of cortical inputs to the zona incerta in the cat. The results show that WGA-HRP injections into primary somatosensory cortex yield anterogradely labeled terminals distributed throughout much of ZI, but with the most intense fields in vZI. This finding is further supported from cases that employed BDA as the anterograde tracer. Examination of the topography of the corticoincertal projection revealed that the sharp boundaries and a clear progression of input, indicative of a strict topographic projection, were not evident. Comparison of the SI corticoincertal projection to that of the FEF showed that the FEF also projects throughout ZI and contributes to both sublaminae, but it terminates more heavily medially and is densest along the border between the sublaminae. More significantly, the relationship of these two cortical projections with respect to incertotectal cells was examined. Our data reveal that both SI and FEF terminals display areas of overlap with the distribution of retrogradely labeled incertotectal cells, which are primarily located in vZI. Moreover, the terminal arbors from these two cortical sources formed numerous close associations with the somata and dendrites of incertotectal cells, indicative of direct synaptic contact. It will be interesting to see whether other cortical regions also supply the ZI, and incertotectal cells in particular, with converging inputs. Finally, retrogradely labeled neurons were observed in dZI and the adjacent EML following cortical injections, indicating there are reciprocal connections between ZI and both SI and FEF cortex in the cat.
Technical Limitations
Studies involving neuronal tracers have inherent limitations with regard to the placement of the tracer in the desired target, tracer spread, and uptake by fibers of passage. In this study, fiber-of-passage problems were minimal due to the anatomy of the targets, which have few such passing fibers. Specifically, while involvement of the cortical white matter may have labeled fibers from more rostral regions, e.g., motor cortex, we saw few labeled cells in motor cortex, suggesting that there was little contamination by this process. Both SI cortical and collicular injections were done under direct visualization of the target, improving the chances for correct placement of the injection site. In the case of the SI injections, in some cases there was spread of tracer into the adjacent secondary somatosensory or motor cortices. Injections of the buried FEF cortex were more problematic. Invariably, some tracer spread into adjacent regions of cingulate and motor cortex. Tracer spread was also an issue for the SC injections, so the borders of the site were verified histologically. There was, in some cases, limited spread of tracer into the pretectum, the underlying midbrain reticular formation, or the periaqueductal gray. This may explain why labeled cells were sometime present in dZI (May et al., 1997). In analyzing the pattern of labeling in ZI, it is difficult to distinguish between terminals and cut axons passing orthogonal to the section. It may be that the scattered, sparse terminals noted in ZI represent such axons. However, we have utilized two different methods of anterograde label to confirm the existence of cortical input to ZI and feel that the presence of bouton-like enlargements in BDA-labeled axons are highly indicative of synaptic terminals.
Organization of Cat Zona Incerta
While a variety of approaches have been taken to defining the organization of the ZI, most recent studies in the rat subdivide the nucleus into four sectors—dorsal, ventral, caudal, and rostral—based on cytoarchitecture and connections (for review, see Mitrofanis, 2005). In the cat, we have subdivided the main body of ZI into a dorsal and ventral sublamina, which appear to be comparable to those of the rat. In fact, these subdivisions in the cat are based on the similar appearance and patterns of connectivity to the rat’s dorsal and ventral sublaminae, as described previously and shown in the present study (May et al., 1997). Rostral and caudal subdivisions may also exist within the cat ZI, but this aspect has not received enough study to allow such a determination. Although similar in many aspects, the shape of the cat ZI differs from that of the rat due to the presence of a dorsolateral extension (eZI). This region was initially noted because in the cat, the populations of incertotectal and incertopretectal neurons continue dorsolaterally without a break, so that they lie internal to the thalamic reticular nucleus (May et al., 1997). The present study lends further credence to this extension of the sublaminae by demonstrating that corticoincertal projections, particularly those from SI cortex, also terminate within eZI.
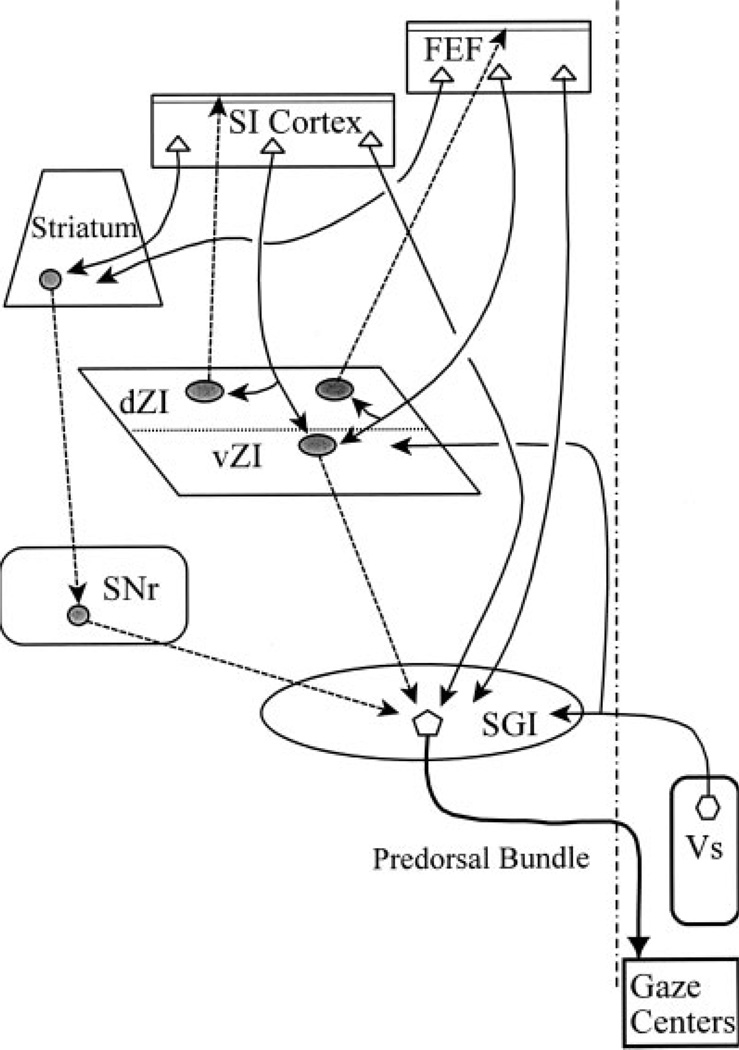
The two main sublaminae within ZI differ in terms of their projection patterns (see summary diagram, Fig. 11). Cells in vZI are known to project to the SC, nucleus of Darkschewitsch, and the thalamic posterior and lateral posterior nuclei [cat: May et al. (1997) and Onodera and Hicks (1998); rat: Watanabe and Kawana (1982), Roger and Cadusseau (1985), Romanowski et al. (1985), and Power and Mitofanis (1999a)]. The projections of dZI have mainly been described in the rat. They include projections to the parafascicular nucleus and the pontomedullary tegmentum (Watanabe and Kawana, 1982; Romanowski et al., 1985; Power and Mitofanis, 1999b). The dZI is also the source of a GABAergic projection to layer I of cerebral cortex (Lin et al., 1990; Nicholelis et al., 1992). The present study has extended these findings by demonstrating that this incertocortical pathway is also present in the cat, and that it includes projections to both SI and the FEF. In this case, the cells of origin for this incertocortical projection are located in both dZI and the adjacent EML. In addition, we have demonstrated that there is a degree of reciprocity in the projection, as retrogradely labeled incertocortical cells occasionally display close associations with anterogradely labeled corticoincertal terminal boutons (Fig. 10). In rat, this projection has been demonstrated to terminate in layer I, suggesting it acts as a diffuse modulatory input to cortex, not a driving one (Lin et al., 1990, 1997).
Fig. 11.
Schematic wiring diagram of input-output relationships of the zona incerta in the cat. Inhibitory projections are indicated by shaded cell bodies and dashed lines.
Somatosensory Inputs to Zona Incerta
The cat ZI is targeted by both descending and ascending somatosensory inputs (Fig. 11). An early study by Jones and Powell (1968) revealed that somatosensory cortex sends a descending projection to the ZI of the cat. With respect to ascending projections, dorsal column nuclei projections to ZI are denser in the center of vZI (Boivie, 1971; Berkley and Hand, 1978) and dense terminal labeling is also found in ZI following spinal cord injections (Craig and Burton, 1985). Both these terminal fields for the body representation have a location similar to the one we observed following SI face injections, supporting the assertion that there is little somatosensory topography within ZI. With respect to ascending head projections, only a few labeled axons are scattered in ZI following injections of the caudal spinal trigeminal nucleus (Berkley, 1980; Craig, 2003). Unfortunately, no descriptions of inputs to ZI from more rostral levels of the cat trigeminal nucleus are currently available. Nevertheless, it appears that somatosensory inputs target the cat ZI. The projections are more robust in the ventral sublamina, but are certainly not confined to it. In fact, we found that the densest label was often located at the boundary between the sublaminae, where the inputs may have access to cells in dZI as well.
The somatosensory inputs to the rat ZI have received more attention. The corticoincertal cells in rat SI lie in layer V, and their projections terminate as bands in the dorsal and ventral sublaminae, respectively, with the vZI projection more extensive (Nicolelis et al., 1992; Mitrofanis and Mikuletic, 1999; Veinante et al., 2000). In the cat, we observed that injections of SI also produced terminal label in both sublaminae, but a distinct band was only observed in vZI. Of special note is the robust projection of the trigeminal sensory nuclei to rat ZI neurons. The principal trigeminal nucleus and both the oralis and the interpolaris subdivision of the spinal trigeminal nucleus provide an intense projection to the ventral subdivision of ZI, with a more modest contribution to the dorsal subdivision (Smith, 1973; Veinante and Deschênes, 1999; Shaw and Mitrofanis, 2001; De Chazeron et al., 2004; Wang et al., 2004; Wang, 2005). Thus, in the rat, the ZI receives extensive ascending and descending somatosensory inputs, which are dominated by input from the face.
The only detailed physiological investigation of somatosensory responses in ZI has also been undertaken in the rat (Nicolelis et al., 1992). This study found that dZI was dominated by the whisker representation, but the units had wide receptive fields, low spontaneous activity, and vague topographic organization. In contrast, vZI units displayed high levels of spontaneous activity. They had smaller fields, albeit still larger than those found in the ventral posterior nucleus. In general, the body representation was displaced ventrolateral to that of the face. In comparison, anatomical investigations of rat somatosensory cortex projections indicate the hindlimb is located laterally, with forelimb and face found successively more medially (Shaw and Mitrofanis, 2002).
The very limited information on somatosensory responses in the cat ZI indicates it contains units with very large fields with indistinct topography (Kruger and Albe-Fessard, 1960). Similarly, the present anatomical study found little evidence for a discrete topographic organization of inputs from different representations within SI cortex. Considering the extensive reach of the dendrites of ZI cells [cat: May et al. (1997); mouse: Iwahori and Mizuno (1980); monkey: Ma et al. (1992)], the lack of restricted point-to-point projection in the corticoincertal projection may not be surprising, as such cells would tend to gather information from large areas of the somatosensory input to ZI. In addition, Power and Mitrofanis (1999b) reported extensive neuronal interconnections within ZI, and similar connections are present in the cat (see Fig. 8B in May et al., 1997). Others have observed evidence for the presence of gap junction-associated proteins in ZI, particularly vZI [rat: Wang et al. (2005); monkey: Ma et al. (1992)]. Both the gap junctions and intranuclear connections may serve as conduits for spreading signals across the ZI. Thus, this nucleus is unlikely to function in a typical point-to-point relay mode, like nuclei within the dorsal thalamus, and may instead serve as a center for general modulation of activity in its targets.
A number of studies in the rat have identified and characterized modality-specific subsectors within ZI. Shaw and Mitrofanis (2002) described a somatosensory subsector in ZI. Specifically, labeled somatosensory projections lay within the middle third of the mediolateral extent of the nucleus. Similar connectional studies indicate the presence of separate auditory and visual subsectors in ZI within the lateral third of the mediolateral extent of ZI (Power et al., 2001; Mitrofanis, 2002). Finally, a motor subsector has been proposed within medial ZI due to input from motor cortex (Mitofanis and Mikuletic, 1999). In the present study, somatosensory cortex injections resulted in nonspecific labeling that was not confined to any specific sector of the cat ZI. However, injections of face cortex did result in dense projections to the main portion of ZI that were roughly comparable in distribution to those observed in the rat somatosensory subsector. The cat ZI does differ from that of the rodent due to the presence of a dorsolateral extension [cat: May et al. (1997); rabbit: Vaccaro and Mitrofanis (1996)]. Patches of dense SI terminations were observed in this region of ZI (Fig. 3C), which is clearly more lateral than the rodent somatosensory subsector. Whether there are regions of the cat ZI that represent visual or auditory subsectors remains to be investigated. However, in the cat, the “motor” projection from the FEF overlies that of the SI face representation, which would appear to argue against the concept of discrete subsectors in the cat.
A major finding of this study is that SI cortex provides direct input to incertotectal neurons (Fig. 3, 4, and 11). The incertotectal pathway terminates in the intermediate gray layer of the SC [cat: May et al. (1997); rat: Kim et al. (1992)]. These layers contain multimodal cells, many of which display somatosensory responses [cat: Clemo and Stein (1991), Meredith et al. (1991, 2000), and Stein et al. (1999)]. These fields are presumably provided both by ascending input and by descending projections from cortex, including SI and the banks of the anterior ectosylvan sulcus, which contains the fourth somatosensory area (SIV) (Killacky and Erzurummlu, 1981; Meredith and Clemo, 1989; Wallace et al., 1993; Wilkinson et al., 1996; Harting et al., 1997). Of these two, the SIV input to SC appears to be more critical to SC multimodal neurons. In one animal (not illustrated), the injection included portions of SIV. It produced similar levels of terminal label in ZI. Thus, it appears that in addition to its direct excitatory drive to multimodal SC cells, the somatosensory cortex also provides an inhibitory input by way of GABAergic vZI incertotectal neurons.
Since vZI also projects to nontectal targets, the corticoincertal projection may have other targets as well. For example, cells in vZI also project to the posterior nucleus of the dorsal thalamus (Power and Mitrofanis, 1999a), where they terminate on the proximal dendrites of thalamocortical neurons (Barthó et al., 2002). This GABAergic incertothalamic projection appears to regulate thalamic function, such that when ZI is active, ascending inputs to the posterior nucleus are suppressed and cortical inputs dominate its activity (Barthó et al., 2002; Tragese and Keller, 2004; Lavallée et al., 2005). It is possible that the incertotectal projection might play a similar role in determining which inputs influence activity in the multisensory neurons of the intermediate gray layer of the SC.
Frontal Eye Field Inputs to Zona Incerta
The frontal eye fields are regions of cortex where stimulation produces saccades, and in which the neurons are active before saccades. They were first described in man and primates (for review, see Lynch and Tian, 2006). Subsequently, electrophysiological methods were used to identify the FEF within the presylvian and cruciate sulci of the cat’s frontal lobe (Schlag and Schlag-Rey, 1970; Guitton and Mandl, 1978a, 1978b). Unfortunately, virtually all other physiological and behavioral studies of the FEF have been carried out in primates, and we must consequently interpret the present findings on the basis of assumed homologies between the cat and primate FEF.
A number of studies have used both physiological and anatomical methods to characterize the projections of the FEF to other parts of the brain [cat: Miyashita and Tamai (1989), Meredith (1999), and McHaffie et al. (2001); monkey: Leichnetz et al. (1981), Huerta et al. (1986), Seagraves and Goldberg (1987), Stanton et al. (1988), Seagraves (1992), and Wurtz et al. (2001)]. Particularly prominent are FEF projections to the SC that terminate primarily on the intermediate gray layer, in which saccade-related neurons reside [cat: Hartwich-Young and Weber (1986) and McHaffie et al. (2001); monkey: Kunzle and Akert (1977), Leichnetz et al. (1981), Huerta et al. (1986), and Stanton et al. (1988)]. While initial ablation studies indicated that both the FEF and SC can act independently of each other (Schiller and Sandell, 1983), more recent studies using acute inactivation of the superior colliculus indicate the FEF projection to the SC is crucial for normal initiation of saccades by the FEF (Wurtz et al., 2001). A few anatomical studies have also noted FEF projections to ZI [cat: Miyashita and Tamai (1989); monkey: Huerta et al. (1986)]. The distribution of FEF corticoincertal terminals as a band located along the border between dZI and vZI, and extending into the fields of Forel, which was observed in the present experiments, is consistent with the pattern of labeling illustrated by Miyashita and Tamai (1989). The present results also show close associations between corticoincertal terminals and incertotectal cells, suggesting direct synaptic contact. Thus, in addition to the direct projection of the FEF on the gaze-related neurons in the intermediate gray layer of the SC, there is an indirect pathway targeting this same layer by way of vZI [cat: May et al. (1997); rat: Kim et al. (1992)].
Within the SC, the frontotectal projection terminates in patches that are in close register with the inputs from the substantia nigra pars reticulata (Illing and Graybiel, 1985), and both terminate on the saccade-related neurons projecting to the brainstem gaze centers [cat: Tokuno and Nakamura (1987) and Harting et al. (1988); rat: Williams and Faull (1988) and Bickford and Hall (1992); squirrel: May and Hall (1984)]. The nigrotectal neurons are GABAergic (Ficalora and Mize, 1989) and inhibit the gaze-related activity of their collicular target cells [cat: Karabelas and Moschovakis (1985); rat: Chevalier et al. (1981)]. As the nigrotectal neurons have high spontaneous activity, which halts before saccades, their role in initiating gaze-related SC activity is generally accomplished through disinhibition [cat: Joseph and Boussaoud (1985); monkey: Hikosaka and Wurtz (1983), Hikosaka et al. (1993), Basso and Wurtz (2002), and Bayer et al. (2004)]. This disinhibition is provided by having two inhibitory neurons in series, such that the cortical pathway through the striatum to the substantia nigra acts to disinhibit collicular gaze neurons (Fig. 11) (Chevalier et al., 1985). In this context, the frontotectal pathway can then be seen as a direct pathway activating gaze-related neurons in the SC, even as the indirect pathway via the striatum and substantia nigra withdraws inhibition from these same neurons (McHaffie et al., 2001).
The present study indicates that a third pathway from the FEF exists, a frontoincertotectal pathway (Fig. 11). In fact, judged by the number of cells involved, the incertotectal and nigrotectal projections derive from equally large populations (Ficalora and Mize, 1989; May et al., 1997). They also both terminate on gaze-related neurons in the intermediate gray layer [rat: Kim et al. (1992); squirrel: May and Hall (1984)]. Finally, the present results show frontotectal terminals in close association with these incertotectal neurons. Thus, there is good evidence for a second indirect pathway between the FEF and the SC. However, as the corticoincertal projection is direct, and presumably excitatory, the influence of activating the frontoincertotectal circuits should be inhibitory, not disinhibitory like the pathway via the basal ganglia (Fig. 11). On the face of it, this seems to suggest that the incertotectal path would work against the action of the direct corticotectal and indirect transbasal ganglia circuits. However, if the projections of individual incertotectal axons are diffuse, as opposed to discretely topographic, the effect of activating this projection could be to increase the distinction between the activated saccade target region of the SC with respect to the portions of the SC that do not represent the target. Alternatively, the incertotectal may be able to control which excitatory inputs have access to the SC motor output cells, a role similar to that posited for the incertal input to the posterior nucleus (Lavallée et al., 2005). Clearly more detailed knowledge of the physiology and specific targets of incertotectal projections is needed to test this possibility. Nevertheless, the broad dendritic fields of cells in vZI (Ma et al., 1992) and the lack of specific movement fields for the saccadic activity of ZI neurons (Ma, 1996) would seem to support a diffuse inhibition model. In view of this possibility, the fact that strokes, which include the FEF, lead to an inability to suppress reflexive glances (Guitton et al., 1985) may be attributable to loss of drive to the inhibitory incertotectal neurons, and not just to disruption of basal ganglia paths.
This study examined two questions. What are the characteristics of the SI cortical and FEF projections to ZI in the cat? Are FEF and SI projections to ZI in a position to influence the gaze circuitry? With respect to the first question, we have shown that both FEF and SI cortices project to ZI. While none of these incertal projections is restricted to a specific ZI sublamina, they are denser in vZI. In addition, these inputs do not have circumscribed projections, but instead display varying degrees of overlap. In view of the evidence for both motor and sensory cortical fields supplying ZI, it seems likely that other areas of cortex converge on ZI as well. With respect to the second question, this study directly demonstrated that the FEF and SI corticoincertal terminals have similar morphologies and that both display close associations with incertotectal cells. The latter suggests that the influences of both the FEF and SI cortex over collicular gaze circuits are mediated, in part, through ZI. In addition, the similarities between the terminal morphology and distribution of these corticoincertal projections suggest the ZI is neither predominantly sensory nor motor in nature. The question still remains: What is the precise function of this nucleus? Experiments done in sheep suggest ZI activity is directly correlated with the internal set of the animal, such that GABA levels in ZI are increased only when the sheep are presented with items they desire (Kendrick and Baldwin, 1986; Kendrick et al., 1991). Perhaps the various cortical inputs to incertotectal cells may allow this projection to suppress gaze responses to nonsalient targets.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of Dr. Yue Wang with some of the surgeries undertaken for these experiments, and Dr. Olga Golanov and Ms. Jennifer Cotton for technical assistance with the experimental procedures and development of the illustrations. Supported by the National Institutes of Health (NS037931 to R.C.-S.L.; EY014263 to P.J.M.).
LITERATURE CITED
- Barthó P, Freund TF, Acsády L. Selective GABAergic innervation of thalamic nuclei from zona incerta. Eur J Neurosci. 2002;16:999–1014. doi: 10.1046/j.1460-9568.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Neuronal activity in substantia nigra pars reticulata during target selection. J Neurosci. 2002;22:1883–1894. doi: 10.1523/JNEUROSCI.22-05-01883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer HM, Handel A, Glimcher PW. Eye position and memory saccade related responses in substantia nigra pars reticulata. Exp Brain Res. 2004;154:428–441. doi: 10.1007/s00221-003-1735-7. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Hand PJ. Efferent projections of the gracile nucleus in the cat. Brain Res. 1978;153:263–283. doi: 10.1016/0006-8993(78)90406-7. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. Spatial relationships between the terminations of somatic sensory and motor pathways in the rostral brainstem of cats and monkeys: I, ascending somatic sensory inputs to lateral diencephalon. J Comp Neurol. 1980;193:283–317. doi: 10.1002/cne.901930119. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Hall WC. Collateral projections of predorsal bundle cells of the superior colliculus in the rat. J Comp Neurol. 1989;283:86–106. doi: 10.1002/cne.902830108. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Hall WC. The nigral projection to predorsal bundle cells of the superior colliculus in the rat. J Comp Neurol. 1992;319:11–33. doi: 10.1002/cne.903190105. [DOI] [PubMed] [Google Scholar]
- Boivie J. The termination in the thalamus and zona incerta of fibers from the dorsal column nuclei (DCN) in the cat: an experimental study with silver impregnation methods. Brain Res. 1971;28:459–490. doi: 10.1016/0006-8993(71)90056-4. [DOI] [PubMed] [Google Scholar]
- Cadusseau J, Roger M. Afferent projections to the superior colliculus in the rat, with special attention to the deep layers. J Hirnforschung. 1985;26:667–681. [PubMed] [Google Scholar]
- Chen BZ, May PJ. Premotor circuits controlling eyelid movements in conjunction with vertical saccades in the cat: I, the rostral interstitial nucleus of the medial longitudinal fasciculus. J Comp Neurol. 2002;450:183–202. doi: 10.1002/cne.10313. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM, Thierry AM, Feger J. The nigro-tectal pathway: an electrophysiological reinvestigation in the rat. Brain Res. 1981;213:253–263. doi: 10.1016/0006-8993(81)90232-8. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Vacher S, Deniau JM, Desban M. Disinhibition as a basic process in the expression of striatal functions: I, the striatonigral influence on tecto-spinal/tecto-diencephalic neurons. Brain Res. 1985;334:215–226. doi: 10.1016/0006-8993(85)90213-6. [DOI] [PubMed] [Google Scholar]
- Clemo HR, Stein BE. Receptive field properties of somatosensory neurons in the cat superior colliculus. J Comp Neurol. 1991;314:534–544. doi: 10.1002/cne.903140310. [DOI] [PubMed] [Google Scholar]
- Cowie RJ, Holstege G. Dorsal mesencephalic projections to pons, medulla, and spinal cord in the cat: limbic and non-limbic components. J Comp Neurol. 1992;319:536–559. doi: 10.1002/cne.903190406. [DOI] [PubMed] [Google Scholar]
- Craig AD, Burton H. The distribution and topographical organization in the thalamus of anterogradely-transported horseradish peroxidase after spinal injections in cat. Exp Brain Res. 1985;58:227–254. doi: 10.1007/BF00235306. [DOI] [PubMed] [Google Scholar]
- Craig AD. Distribution of trigeminothalamic and spinothalamic lamina I terminations in the cat. Somatosens Motor Res. 2003;20:209–222. doi: 10.1080/08990220310001623013. [DOI] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Mitchell IJ. Organization of efferent projections from superior colliculus to brain stem in rat: evidence for functional output channels. Prog Brain Res. 1988;75:27–36. doi: 10.1016/s0079-6123(08)60463-x. [DOI] [PubMed] [Google Scholar]
- De Chazeron I, Raboisson P, Dallel R. Organization of diencephalic projections from the spinal trigeminal nucleus oralis: an anterograde tracing study in the rat. Neuroscience. 2004;127:921–928. doi: 10.1016/j.neuroscience.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Wall JT, Cusick CG, Kaas JH. The representation of the body surface in S-I of cats. J Neurosci. 1983;3:1648–1669. doi: 10.1523/JNEUROSCI.03-08-01648.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficalora AS, Mize RR. The neurons of the substantia nigra and zona incerta which project to the cat superior colliculus are gaba immunoreactive: a double-label study using GABA immunocytochemistry and lectin retrograde transport. Neuroscience. 1989;29:567–581. doi: 10.1016/0306-4522(89)90131-0. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Grantyn R. Axonal patterns and sites of termination of cat superior colliculus neurons projecting in the tecto-bulbo-spinal tract. Exp Brain Res. 1982;46:243–256. doi: 10.1007/BF00237182. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Organization of the nigrotectal connection: an experimental tracer study in the cat. Brain Res. 1978;143:339–348. doi: 10.1016/0006-8993(78)90573-5. [DOI] [PubMed] [Google Scholar]
- Groh JM, Sparks DL. Saccades to somatosensory targets: II, motor convergence in primate superior colliculus. J Neurophysiol. 1996;75:428–438. doi: 10.1152/jn.1996.75.1.428. [DOI] [PubMed] [Google Scholar]
- Guitton D, Mandl G. Frontal “oculomotor” area in alert cat: I, eye movements and neck activity evoked by stimulation. Brain Res. 1978a;149:295–312. doi: 10.1016/0006-8993(78)90477-8. [DOI] [PubMed] [Google Scholar]
- Guitton D, Mandl G. Frontal “oculomotor” area in alert cat: II, unit discharges associated with eye movements and neck muscle activity. Brain Res. 1978b;149:313–327. doi: 10.1016/0006-8993(78)90478-x. [DOI] [PubMed] [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and generating goal directed saccades. Exp Brain Res. 1985;58:455–472. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- Harting JK. Descending pathways from the superior colliculus: an autoradiographic analysis in the rhesus monkey (Macaca mulatta) J Comp Neurol. 1977;173:583–612. doi: 10.1002/cne.901730311. [DOI] [PubMed] [Google Scholar]
- Harting JK, Huerta MF, Hashikawa T, Weber JT, Van Lieshout DP. Neuroanatomical studies of the nigrotectal projection in the cat. J Comp Neurol. 1988;278:615–631. doi: 10.1002/cne.902780412. [DOI] [PubMed] [Google Scholar]
- Harting JK, Feig S, Van Lieshout DP. Cortical somatosensory and trigeminal inputs to the cat superior colliculus: light and electron microscopic analyses. J Comp Neurol. 1997;388:313–326. doi: 10.1002/(sici)1096-9861(19971117)388:2<313::aid-cne9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Hartwich-Young R, Weber JT. The projection of frontal cortical oculomotor areas to the superior colliculus in the domestic cat. J Comp Neurol. 1986;253:342–357. doi: 10.1002/cne.902530305. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata: I, relation of visual and auditory responses to saccades. J Neurophysiol. 1983;49:1230–1300. doi: 10.1152/jn.1983.49.5.1230. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Miyashita N. Effects of caudate nucleus stimulation on substantia nigra cell activity in monkey. Exp Brain Res. 1993;95:457–472. doi: 10.1007/BF00227139. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Frankenfurter AJ, Harting JK. The trigeminocollicular projection in the cat: patch-like endings within the intermediate gray. Brain Res. 1981;211:1–13. doi: 10.1016/0006-8993(81)90063-9. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Frankfurter A, Harting JK. Studies of the principal sensory and spinal trigeminal nuclei of the rat: projections to the superior colliculus, inferior olive, and cerebellum. J Comp Neurol. 1983;220:147–167. doi: 10.1002/cne.902200204. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys: I, subcortical connections. J Comp Neurol. 1986;253:415–439. doi: 10.1002/cne.902530402. [DOI] [PubMed] [Google Scholar]
- Illing J-B, Graybiel AM. Convergence of afferents from frontal cortex and substantia nigra onto acetylcholinesterase-rich patches of the cat’s superior colliculus. Neuroscience. 1985;14:455–482. doi: 10.1016/0306-4522(85)90303-3. [DOI] [PubMed] [Google Scholar]
- Iwahori N, Mizuno N. A golgi study on the zona incerta of the mouse. Anat Embryol. 1980;161:145–158. doi: 10.1007/BF00305341. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TPS. The projections of the somatosensory cortex upon the thalamus in the cat. Brain Res. 1968;10:369–391. doi: 10.1016/0006-8993(68)90206-0. [DOI] [PubMed] [Google Scholar]
- Joseph JP, Boussaoud D. Role of the cat substantia nigra pars reticulata in eye and head movements: I, neural activity. Exp Brain Res. 1985;57:286–296. doi: 10.1007/BF00236534. [DOI] [PubMed] [Google Scholar]
- Karabelas AB, Moschovakis AK. Nigral inhibitory termination of efferent neurons of the superior colliculus: an intracellular horseradish peroxidase study in the cat. J Comp Neurol. 1985;239:309–329. doi: 10.1002/cne.902390305. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Baldwin BA. The activity of neurons in the lateral hypothalamus and zona incerta of sheep responding to the sight or approach of food is modified by learning and satiety and reflects food preference. Brain Res. 1986;375:320–328. doi: 10.1016/0006-8993(86)90752-3. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Hinton MR, Baldwin BA. GABA release in the zona incerta of the sheep in response to the sight and ingestion of food and salt. Brain Res. 1991;550:165–168. doi: 10.1016/0006-8993(91)90423-s. [DOI] [PubMed] [Google Scholar]
- Killackey H, Erzurumlu R. Trigeminal projections to the superior colliculus of the rat. J Comp Neurol. 1981;201:221–242. doi: 10.1002/cne.902010207. [DOI] [PubMed] [Google Scholar]
- Kim U, Gregory E, Hall WC. Pathway from the zona incerta to the superior colliculus in the rat. J Comp Neurol. 1992;321:555–575. doi: 10.1002/cne.903210405. [DOI] [PubMed] [Google Scholar]
- Kruger L, Albe-Fessard D. Distribution of responses to somatic afferent stimuli in the diencephalon of the cat under chloralose anesthesia. Exp Neurol. 1960;2:442–467. doi: 10.1016/0014-4886(60)90027-3. [DOI] [PubMed] [Google Scholar]
- Kunzle H, Akert K. Efferent connections of cortical, area 8 (frontal eye field) in Macaca fascicularis: a reinvestigation using the autoradiographic technique. J Comp Neurol. 1977;173:147–164. doi: 10.1002/cne.901730108. [DOI] [PubMed] [Google Scholar]
- Lavallée P, Urbain N, Defresne C, Bokor H, Acsády L, Deschênes M. Feed-forward inhibitory control of sensory information in higher-order thalamic nuclei. J Neurosci. 2005;25:7489–7498. doi: 10.1523/JNEUROSCI.2301-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichnetz GR, Spencer RF, Hardy SGP, Astruc J. The prefrontal corticotectal projection in the monkey: an anterograde and retrograde horseradish peroxidase study. Neuroscience. 1981;6:1023–1041. doi: 10.1016/0306-4522(81)90068-3. [DOI] [PubMed] [Google Scholar]
- Lin CS, Nicolelis MAL, McLean J, Chapin KJ. A major direct GABAergic pathway from zona incerta to neocortex. Science. 1990;248:1553–1556. doi: 10.1126/science.2360049. [DOI] [PubMed] [Google Scholar]
- Lin RCS, Nicolelis MAL, Chapin JK. Topographic and laminar organizations of the incertocortical pathway in rats. Neuroscience. 1997;81:641–651. doi: 10.1016/s0306-4522(97)00094-8. [DOI] [PubMed] [Google Scholar]
- Lynch JC, Tian JR. Cortico-cortical networks and corticosubcortical loops for higher control of eye movements. In: Büttner-Ennever JA, editor. Neuroanatomy of the oculomotor system. Amsterdam: Elsevier; 2006. pp. 461–502. [Google Scholar]
- Ma TP, Hu X-J, Anavi Y, Rafols JA. Organization of the zona incerta in the macaque: a Nissl and Golgi study. J Comp Neurol. 1992;320:273–290. doi: 10.1002/cne.903200302. [DOI] [PubMed] [Google Scholar]
- Ma TP. Saccade-related omnivectoral pause neurons in the primate zona incerta. Neuroreport. 1996;7:2713–2716. doi: 10.1097/00001756-199611040-00061. [DOI] [PubMed] [Google Scholar]
- May PJ, Hall WC. Relationships between the nigrotectal pathway and the cells of origin of the predorsal bundle. J Comp Neurol. 1984;226:357–376. doi: 10.1002/cne.902260306. [DOI] [PubMed] [Google Scholar]
- May PJ, Porter JD. The laminar distribution of macaque tectobulbar and tectospinal neurons. Visual Neurosci. 1992;8:257–276. doi: 10.1017/s0952523800002911. [DOI] [PubMed] [Google Scholar]
- May PJ, Sun W, Hall WC. Reciprocal connections between the zona incerta and the pretectum and superior colliculus of the cat. Neuroscience. 1997;77:1091–1114. doi: 10.1016/s0306-4522(96)00535-0. [DOI] [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: laminar structure and connections. In: Büttner-Ennever JA, editor. Neuroanatomy of the oculomotor system. Amsterdam: Elsevier; 2006. pp. 321–378. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Thomson CM, Stein BE. Corticotectal and corticostriatal projections from the frontal eye fields of the cat: an anatomical examination using WGA-HRP. Somatosens Motor Res. 2001;18:117–130. doi: 10.1080/135578501012006200. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Clemo HR. Auditory cortical projection from the anterior ectosylvian sulcus (field AES) to the superior colliculus in the cat: an anatomical and electrophysiological study. J Comp Neurol. 1989;289:687–707. doi: 10.1002/cne.902890412. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Clemo HR, Stein BE. Somatotopic component of the multisensory map in the deep laminae of the cat superior colliculus. J Comp Neurol. 1991;312:353–370. doi: 10.1002/cne.903120304. [DOI] [PubMed] [Google Scholar]
- Meredith MA. The frontal eye fields target multisensory neurons in cat superior colliculus. Exp Brain Res. 1999;128:460–470. doi: 10.1007/s002210050869. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Clemo HRH, Dehneri LR. Responses to innocuous, but not noxious, somatosensory stimulation by neurons in the ferret superior colliculus. Somatosens Motor Res. 2000;17:297–308. doi: 10.1080/08990220020001999. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J, Mikuletic L. Organisation of the cortical projection to the zona incerta of the thalamus. J Comp Neurol. 1999;412:173–185. [PubMed] [Google Scholar]
- Mitrofanis J. Evidence for an auditory subsector within zona incerta of rats. Anat Embryol. 2002;205:453–462. doi: 10.1007/s00429-002-0268-3. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J. Some certainty for the “zone of uncertainty”? exploring the function of the zona incerta. Neuroscience. 2005;130:1–15. doi: 10.1016/j.neuroscience.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Miyashita E, Tamai Y. Subcortical connections of frontal “oculomotor” areas in the cat. Brain Res. 1989;502:75–87. doi: 10.1016/0006-8993(89)90463-0. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB. Observations on the somatodendritic morphology and axon trajectory of intracellularly HRP-labeled efferent neurons located in the deeper layers of the superior colliculus of the cat. J Comp Neurol. 1985;239:276–308. doi: 10.1002/cne.902390304. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus: II, morphological identity of presaccadic neurons. J Neurophysiol. 1988;60:263–302. doi: 10.1152/jn.1988.60.1.263. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Guitton D. Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat: II, sustained discharges during motor preparation and fixation. J Neurophysiol. 1991;66:1624–1641. doi: 10.1152/jn.1991.66.5.1624. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Chapin JK, Lin RCS. Somatotopic maps within the zona incerta relay parallel GABAergic somatosensory pathways to the neocortex, superior colliculus, and brainstem. Brain Res. 1992;577:134–141. doi: 10.1016/0006-8993(92)90546-l. [DOI] [PubMed] [Google Scholar]
- Onodera S, Hicks TP. Projections from substantia nigra and zona incerta to the cat’s nucleus of Darkschewitsch. J Comp Neurol. 1998;396:461–482. [PubMed] [Google Scholar]
- Perkins E, Wang Y, Warren S, Lin RCS, May PJ. Cortical projections upon incertotectal neurons in the cat. Soc Neurosci. 2002;28:348. [Google Scholar]
- Power BD, Mitrofanis J. Specificity of projection among cells of the zona incerta. J Neurocytol. 1999a;28:481–493. doi: 10.1023/a:1007005105679. [DOI] [PubMed] [Google Scholar]
- Power BD, Mitrofanis J. Evidence for extensive inter-connections within the zona incerta in rats. Neurosci Lett. 1999b;267:9–12. doi: 10.1016/s0304-3940(99)00313-4. [DOI] [PubMed] [Google Scholar]
- Power BD, Leamey CA, Mitrofanis J. Evidence for a visual subsector within the zona incerta. Visual Neurosci. 2001;18:179–186. doi: 10.1017/s0952523801182027. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Marrow L, Dean P. Topographical organization of the nigrotectal projection in rat: evidence for segregated channels. Neuroscience. 1992;50:571–595. doi: 10.1016/0306-4522(92)90448-b. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Simkins M, McHaffie JG, Stein BE. Nociceptive neurons in the rat superior colliculus: II, effects of lesions to the contralateral descending output pathways on nocifensive behaviours. Exp Brain Res. 1996;109:197–208. doi: 10.1007/BF00231781. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Fish SE, Chiaia NL, Bennett-Clarke C, Mooney RD. Organization of the projections from the trigeminal brainstem complex to the superior colliculus in the rat and hamster: anterograde tracing with Phaseolus vulgaris leucoagglutinin and intra-axonal injection. J Comp Neurol. 1989;289:641–656. doi: 10.1002/cne.902890409. [DOI] [PubMed] [Google Scholar]
- Ricardo JA. Efferent connections of the subthalamic region in the rat: II, the zona incerta. Brain Res. 1981;214:43–60. doi: 10.1016/0006-8993(81)90437-6. [DOI] [PubMed] [Google Scholar]
- Rioch DM. Studies on the diencephalon of Carnivora: II, certain nuclear configurations and fiber connections of the subthalamus and midbrain of the dog and cat. J Comp Neurol. 1929;49:121–153. [Google Scholar]
- Roger M, Cadusseau J. Afferents to the zona incerta in the rat: a combined retrograde and anterograde study. J Comp Neurol. 1985;241:480–492. doi: 10.1002/cne.902410407. [DOI] [PubMed] [Google Scholar]
- Romanowski CAJ, Mitchel IJ, Crossman AR. The organization of the efferent projections of the zona incerta. J Anat. 1985;143:75–95. [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH. Interactions between visually and electrically elicited saccades before and after superior colliculus and frontal eye field ablations in rhesus monkeys. Exp Brain Res. 1983;49:381–392. doi: 10.1007/BF00238780. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Induction of oculomotor responses by electrical stimulation of the prefrontal cortex in the cat. Brain Res. 1970;22:1–13. doi: 10.1016/0006-8993(70)90398-7. [DOI] [PubMed] [Google Scholar]
- Segraves MA, Goldberg ME. Functional properties of corticotectal neurons in the monkey’s frontal eye field. J Neurophysiol. 1987;58:1387–1419. doi: 10.1152/jn.1987.58.6.1387. [DOI] [PubMed] [Google Scholar]
- Segraves MA. Activity of monkey frontal eye field neurons projecting to oculomotor regions of the pons. J Neurophysiol. 1992;68:1967–1985. doi: 10.1152/jn.1992.68.6.1967. [DOI] [PubMed] [Google Scholar]
- Shaw VE, Mitrofanis J. Lamination of spinal cells projecting to the zona incerta of rats. J Neurocytol. 2001;30:695–704. doi: 10.1023/a:1016529817118. [DOI] [PubMed] [Google Scholar]
- Shaw V, Mitrofanis J. Anatomical evidence for somatotopic maps in the zona incerta of rats. Anat Embryol. 2002;206:119–130. doi: 10.1007/s00429-002-0280-7. [DOI] [PubMed] [Google Scholar]
- Smith RL. The ascending fiber projections from the principal sensory trigeminal nucleus in the rat. J Comp Neurol. 1973;148:423–446. doi: 10.1002/cne.901480403. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Mays LE. The role of the monkey superior colliculus in the control of saccadic eye movements: a current prospective. In: Fuchs AF, Becker W, editors. Progressive occulomotor research. Amsterdam: Elsevier; 1981. pp. 137–144. [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the Macaque monkey: I, subcortical pathways and topography of striatal and thalamic terminal fields. J Comp Neurol. 1988;271:473–492. doi: 10.1002/cne.902710402. [DOI] [PubMed] [Google Scholar]
- Stein BE, Wallace MT, Stanford TR. Development of multisensory integration: transforming sensory input into motor output. Ment Retard Dev Disabil Res Rev. 1999;5:72–85. [Google Scholar]
- Tokuno H, Nakamura Y. Organization of the nigrotectospinal pathway in the cat: a light and electron microscopic study. Brain Res. 1987;436:76–84. doi: 10.1016/0006-8993(87)91558-7. [DOI] [PubMed] [Google Scholar]
- Trageser JC, Keller A. Reducing the uncertainty: gating peripheral inputs by zona incerta. J Neurosci. 2004;24:8911–8915. doi: 10.1523/JNEUROSCI.3218-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro TM, Mitrofanis J. Reticular thalamic region in the rabbit: organization of efferents to the superior colliculus. J Comp Neurol. 1996;369:209–219. doi: 10.1002/(SICI)1096-9861(19960527)369:2<209::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Veinante P, Deschênes M. Single- and multi-whisker channels in the ascending projections from the principal trigeminal nucleus in the rat. J Neurosci. 1999;19:5085–5095. doi: 10.1523/JNEUROSCI.19-12-05085.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Lavallée P, Deschênes M. Corticothalamic projections from layer 5 of the vibrissal barrel cortex in the rat. J Comp Neurol. 2000;424:197–204. doi: 10.1002/1096-9861(20000821)424:2<197::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE. Converging influences from visual, auditory, and somatosensory cortices onto output neurons of the superior colliculus. J Neurophysiol. 1993;69:1797–1809. doi: 10.1152/jn.1993.69.6.1797. [DOI] [PubMed] [Google Scholar]
- Wang TY, Haines DE, Chen RF, Simpson KL, Lin RCS. Patterning of axon terminals within rat zona incerta from the spinal trigeminal nucleus: a light microscopic study. Neurosci Abst. 2004;28:348. [Google Scholar]
- Wang Y. PhD dissertation. Jackson, MS: University of Misissippi Medical Center; 2005. Neuroanatomical and neurochemical relationships between the trigeminal nuclear complex and zona incerta. [Google Scholar]
- Wang Y, Kuo C, Lu Y, Simpson K, Lin RC. Expression of connexin 36 immunoreactivity in rodent zona incerta. Neurosci Abst. 2005;29:978. [Google Scholar]
- Watanabe K, Kawana E. The cells of origin of the incertofugal projections to the tectum, thalamus, tegmentum and spinal cord in the rat: a study using the autoradiographic and horseradish peroxidase methods. Neuroscience. 1982;7:2389–2406. doi: 10.1016/0306-4522(82)90203-2. [DOI] [PubMed] [Google Scholar]
- Wiberg M, Westman J, Blomqvist A. Somatosensory projection to the mesencephalon: an anatomical study in the monkey. J Comp Neurol. 1987;264:92–117. doi: 10.1002/cne.902640108. [DOI] [PubMed] [Google Scholar]
- Wilkinson LK, Meredith MA, Stein BE. The role of anterior ectosylvian cortex in cross-modality orientation and approach behavior. Exp Brain Res. 1996;112:1–10. doi: 10.1007/BF00227172. [DOI] [PubMed] [Google Scholar]
- Williams MN, Faull RLM. The nigrotectal projection and tectospinal neurons in the rat: a light and electron microscopic study demonstrating a monosynaptic nigral input to identified tectospinal neurons. Neuroscience. 1988;25:533–562. doi: 10.1016/0306-4522(88)90257-6. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey: III, cells discharging before eye movements. J Neurophysiol. 1972;35:575–586. doi: 10.1152/jn.1972.35.4.575. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Sommer MA, Paré M, Ferraina S. Signal transformations from cerebral cortex to superior colliculus for the generation of saccades. Vision Res. 2001;41:3399–3412. doi: 10.1016/s0042-6989(01)00066-9. [DOI] [PubMed] [Google Scholar]