Abstract
Introduction: Periodontal diseases are considered as some of the most common reasons of teeth loss, which occur due to the aggregation of microbial plaque and other precipitations on the dental surfaces. In this study, the scaling effect using manual tools, ultrasonic machine and Erbium-Doped Yttrium Aluminum Garnet (Er:YAG)laser on the connection of the human gums connective tissue cells on the root surface of the teeth suffering from severe periodontitis will be compared.
Methods: After removal of the big precipitations with manual tools, Er:YAG laser light emission of Photona machine is used with respect to the following characteristics: wavelength: 2940μm, each pulse: 100mJ, frequency: 10 pulse/sec, optic fiber with cross section 0.5x1.65mm, fiber tip angle with root surface: 15-20 degrees with non-contact mode, 1.5mm farther than the root surface and pulse duration 230 very short. The gingival fibroblast cellular was incubated as a sample of the human gums connective tissue cells under 37C. These cells were departed from the culture medium after the cellular reproduction in the third passage.On the 3rd day after incubation, the gingival fibroblast cells morphology was studied by Scanning Electron Microscopy (SEM).
Results: The results of SEM images in the present study indicated the spread fibroblast cells with philopodia were found in all of 5 groups; untreated healthy group (control), untreated group suffering from periodontitis, the scaling effect using manual tools (Scaled Gracey), ultrasonic machine and Er:YAG laser. There is a meaningful difference among the three treatment groups (P<0.001) in the numbers of the fibroblast cells, while all the four treated groups had a meaningful difference with the positive control group (P < 0.001).
Conclusion: The present study indicated that although various dental surfaces cleaning methods may be different in other aspects, but are similar concerning the fibroblasts morphology. Also in addition to power, laser emission time may also be effective in the cells morphology results.
Keywords: Er-YAG laser, periodontitis, rate, survival
Introduction
Periodontal diseases are considered as some of the most common reasons of teeth loss, which occur due to the aggregation of microbial plaque and other precipitations on the dental surfaces1. Until such precipitations remain on the surface of the root together with the contaminated cement, periodontal diseases may not be successfully cured. On the other hand, the most important target in the treatment of the periodontal diseases is also reconstruction of the lost connection and reestablishment of the adhesiveness of the tissue to the bare surface of the teeth. In order to do so, the surface of the contaminated and unhealthy root shall be prepared in a way that it may be ready to accept new connection, while such phase is performed either mechanically or chemically. Mechanical preparation includes removal of germ and microbial precipitations from the surface of the root (scaling), and planning of the same, which are performed using manual germ removers or ultrasonic machines, while chemical preparation is referred to the application of certain materials such as citric acid; tetracycline or EDTA. Ultrasonic tools have been used for years as a valuable complement besides manual tools to scale the dental surfaces. It has been found that ultrasonic tools remain coarser and present higher level of superficial damages than the manual tools; additionally, applying tools in some of the patients is prohibited and results in certain problems and effects. Therefore, it seems that in case of continuance of such state, several problems may occur for the patients requiring periodontal treatments and dental loss may increase in the same2 , 3. The initiation of exclusive laser for dentistry, have made the periodical Neodymium-Doped Yttrium Aluminium Garnet (Nd:YAG) laser available for the surgery of the interior oral soft tissue by myers4.
Considering the defects of the common methods, studies on certain types of laser, e.g. Nd:YAG and Erbium-Doped Yttrium Aluminum Garnet (Er:YAG) dates back to decades ago, to perform various types of scaling and planning of the root surfaces. Among these lasers, high wavelength absorption of Er:YAG laser by water molecules makes this laser suitable to perform periodontal treatment and specially scaling and planning of the root surfaces5. However, no statistics concerning the manner of performance of this laser in the improvement of the periodontal treatments and also the previous studies have mainly failed in presenting a general conclusion, and the results of the various scholars regarding the laser efficiency in the root surface cleaning are not similar considering the extensiveness of the laser types6.
Increased efficiency of the common mechanical debridement methods and the option to establish new transmission level with the modern tools may affect in the decreasing the severity of extensiveness of the periodontal disease, and also decrease the need for the expensive, complex and specialty treatments of the chewing system reconstruction. Most of the patients are highly tended to apply non-surgery methods and in case of increasing the success chance of these treatments, more patients may be included in the treatment and the toothless development resulted from periodontitis, laser emission time may also be effective in the cells morphology results.
In this study, the scaling effect using manual tools, ultrasonic machine and Er:YAG laser on the connection of the human gums connective tissue cells on the root surface of the teeth suffering from severe periodontitis will be compared.
Methods
Single-rooted extracted teeth suffering from acute periodontitis, which were considered to be pulled in the general treatment plan of the patient were chosen. The respective factors to choose the tooth included the following:
The depth of the clinical probe roughly 5mm or more bigger than in both distal and mesial surfaces, loss of clinical connection surface around 8mm or more in both mesial and distal surfaces, progressive second or third degree clearance, lack of cavity or obvious structural changes on the tooth root surface, lack of receiving previous periodontal treatments on the tooth root within the past 12 months and lack of breakage or tangible anatomic abnormalities, e.g. root superficial grooves.
Teeth were placed into four groups of no cure, treatment by manual tools, ultrasonic machines and Er:YAG laser together with the manual method. Meanwhile, a group of the pulled out healthy teeth were placed due to orthodontia reasons to increase the reliability.
Dental plates were prepared through removal of the crown and apex of the teeth; one-third middle of the root was cut longitudinally on the Bucco- Lingual surface and rectangular blocks with roughly sizes of 2x5x6mm were prepared.
The samples were kept with tissue forceps and scaling was made by using light and hollow courts manual tools Nos. 7,8 of Nordent Company (USA) and scaling by ultrasound machine by Vector made by Bietigheim-Bissingen Company (Germany). Both of these treatment methods continued until reaching a smooth surface which could be felt under the hand of the physician. All such treatments were performed on all the samples by one single person.
Regarding the laser-treated group, after removal of the big precipitations with manual tools, Er:YAG laser light emission of Photona machine is used with respect to the following characteristics: wavelength: 2940μm, each pulse: 100mJ, frequency: 10 pulse/sec, optic fiber with cross section 0.5x1.65mm, fiber tip angle with root surface: 15-20 degrees with non-contact mode, 1.5mm farther than the root surface and pulse duration 230 very short.
Samples Preparation
After the aforementioned phases, all the blocks were washed twice using distilled water for 30 seconds, and the whole of the slices were put into the Fetal Bovin Serum (FBS) solution to be prepared for the cellular culture step.
The gingival fibroblast cellular rank existed in the Iranian Section of Pasteur Institute cellular bank and was incubated as a sample of the human gums connective tissue cells under 37C and in moisturized atmosphere (95% weather, 5% Carbon Dioxide (CO2)), while the culture medium included: Dulbeccos Modified Eagle Medium (DMEM)*, 100u/ml Penicillin, 100mg/ ml Streptomycin, 100 streptomycin, 2mm l-Glutamine 10% FBS. These cells were departed from the culture medium after the cellular reproduction in the third passage from the cultured cells by taking benefit from Trypcin-EDTA sterilized solution and suspended inside the medium environment containing 10% FBS, 1% streptomycin, 1% penicillin, and placed for cellular culture. The slices were placed inside cellular culture mediums and the gums fibroblasts were placed under 80.000 cellular density on root slices.
On the 3rd day after incubation, the gingival fibroblast cells morphology was studied by Scanning Electron Microscopy (SEM). Preparation of the samples for the SEM was performed in the following manner: first of all, the samples were fixed in glutaraldehyde 2.5% for 2 hours in the room temperature and under hood. Then they were washed by taking benefit from 0.1m phosphate buffer three times per hour and placed into the fridge after each time of washing. After that, the samples were dehydrated in various levels of ethanol (50, 70, 85, 100) once and 30 minutes for each time and were finally dehydrated and dried in 100 degrees alcohol for 24 hours in room temperature and under hood. After that, the samples were embedded on the forged aluminum by taking benefit from silver adhesive (Mounting) and coated by a tight film were subject to SEM (Vega-Tescan, Tescan USA Inc, UBA) and the photos were recorded in digital mode.
Findings
The results of SEM images in the present study indicated the spread fibroblast cells with philopodia were found in all of 5 groups; untreated healthy group (control), untreated group suffering from periodontitis, the scaling effect using manual tools (Scaled Gracey), ultrasonic machine and Er:YAG laser (Figure 1-5). There is a meaningful difference among the three treatment groups (P<0.001) in the numbers of the fibroblast cells, while all the four treated groups had a meaningful difference with the positive control group (P < 0.001). (Table 1)
Figure 1.
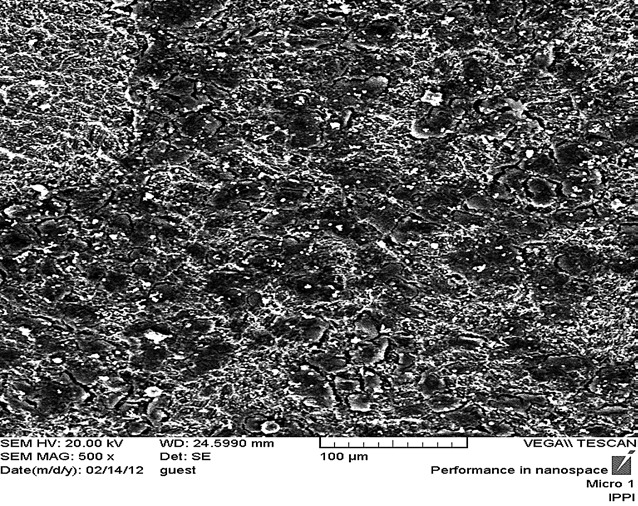
Scanning electron microscopy image from fibroblast cells in ultrasonic group with magnification 500.
Figure 4.

Scanning electron microscopy image from fibroblast cells in manual tool group with magnification 500.
Table 1. The study of difference between groups based on comparison of individual groups with the others and their p value.
| group | number of cells/μm2 | |||||
| means | standard deviations | P value | 95% Confidence Interval | |||
| Lower Bound | Upper bound | |||||
| control- |
ultrasonic + manual Er:YAG control+ manual |
-3.41 | 8.80 | .996 | -27.9 | 19.39 |
| 17.42 | 8.80 | .288 | -7.2 | 32.23 | ||
| -78.21 | 8.0 | .000 | -192.91 | -43.50 | ||
| 3.24 | 8.80 | 0999 | -21.56 | 27.84 | ||
| ultrasonic |
control- + manual+ control |
3.51 | 8.80 | .996 | -23.44 | 26.41 |
| 24.63 | 8.80 | .144 | -4.46 | 55.34 | ||
| -74.60 | 8.80 | .000 | -96.73 | -53.25 | ||
| 6.36 | 8.80 | 0975 | -15.36 | 32.06 | ||
| + manual Er:YAG |
Control- controlultrasonic control+ manual |
-19.43 | 8.80 | 285 | -0.23/44 | 8.21 |
| -20.64 | 8.80 | .144 | -45.34 | 4.06 | ||
| -85.66 | 8.80 | .000 | -123.36 | -73.92 | ||
| -16.27 | 8.80 | 0420 | -38.99 | 9.61 | ||
| Control+ |
Control- controlultrasonic + manual Er:YAG manual |
79.51 | 8.80 | .000 | 54.50 | 122.94 |
| 77.0 | 8.80 | .000 | 50.29 | 93.60 | ||
| 98.69 | 8.80 | .000 | 70.91 | 110.84 | ||
| 80.75 | 8.80 | .000 | 57.65 | 108.05 | ||
| manual |
control – ultrasonic + manual Er:YAG control+ |
-3.15 | 8.844 | .999 | -28.85 | 32.56 |
| 5.556 | 8.806 | .983 | -30.09 | 18.37 | ||
| 1558 | 8.804 | .420 | -9.49 | 35.91 | ||
| -80.39 | 8.804 | .000 | -165.06 | -4.65 | ||
Figure 2.
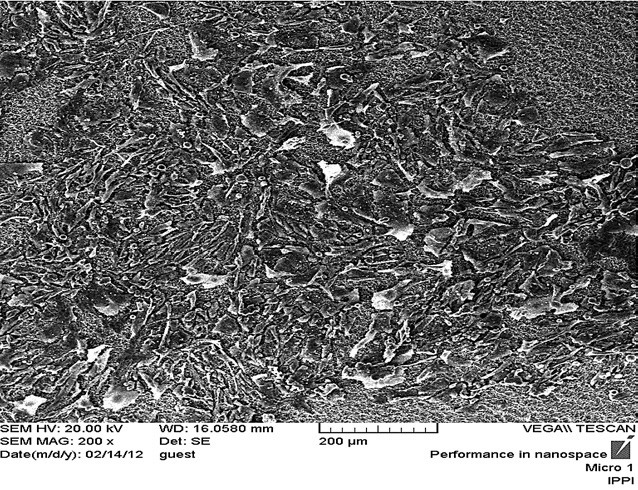
Scanning electron microscopy image from fibroblast cells in unhealthy group with magnification 500.
Figure 3.
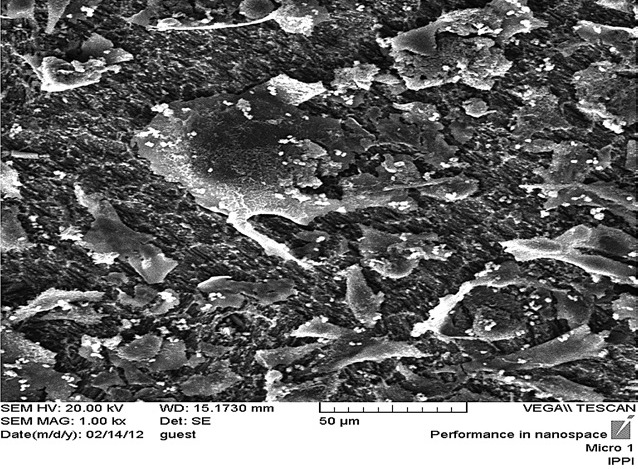
Scanning electron microscopy image from fibroblast cells in irradiated laser group with magnification 1000.
Figure 4.
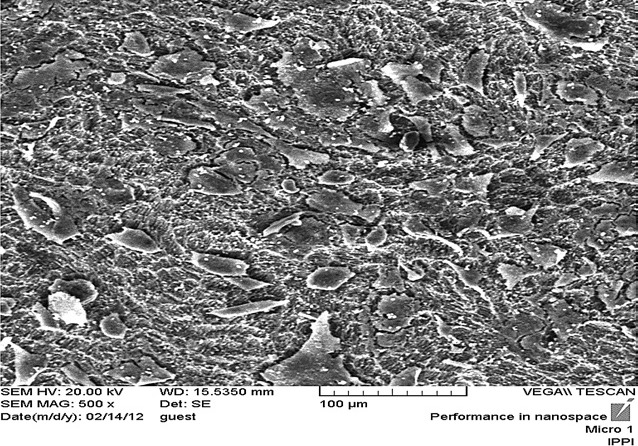
Scanning electron microscopy image from fibroblast cells in healthy (control) group with magnification 200.
Discussion
The present study indicated that although various dental surfaces cleaning methods may be different in other aspects, but are similar concerning the fibroblasts morphology.
Belal et al7 evaluated the effects of Er:YAG laser emission together with the human platelet-derived growth factor-BB recombinant in the biologic compatibility of the roots suffering from periodontal diseases through periodontal ligament fibroblast connection. In this study, the effect of Er:YAG laser together with the growth factor were tested in 5 healthy and 15 unhealthy teeth suffering from periodontal diseases in four groups by electron microscope. In the Er:YAG laser group emission was performed solely and with a 10 mj/pulse energy and 60 Hz in three directions of horizontal (87.4s), vertical (47.5s) and tilted (43.2s), while in combined group, the laser emission was performed with a 10 mj/pulse energy and 60 Hz together with the application of platted growth factor for 50 ng/ml within 5 minutes. They found that the Er:YAG laser emission whether solely or together with rhPDGF-BB may be addressed as a suitable periodontal treatment to prepare the root surfaces. Although the combined treatment had relatively better effects in this field. Using enough number of samples in the groups, taking various criteria of entering into the study for simulation, using standard culturing methods were all of those issues similar to this study. Similar lasers were used in both studies; however, they were differences concerning the laser specifications and incubation time. Meanwhile, whereas the main role in the non-surgical treatment methods is with the gums fibroblast cells, which result in new connective tissue, using the PDL fibroblasts, which is naturally different to the gums fibroblast is not really logical. Maruyama et al6 study may be in conformity to the findings resulted from this study, and verify the same. They studied the effects of mechanical and chemical preparation on the root cementum of 104 premolar and molar teeth after being exposed to the Er:YAG laser emission. 16 cementum healthy plates in the control group and 88 cementum plates were placed in the emission groups (Er:YAG laser emission (L)), emission (laser + tetracycline (L+ TP)), (laser + Burnish with tetracycline (L+TB)), emission (laser with EDTA gel (L + EP)), emission (laser with burnish load with EDTA gel (L+ EB)), emission (laser with normal saline solution (L+MP)). Laser emission was performed with 30 mj/pulse energy and 30 Hz with contact angle of 30 degrees for 45 seconds. Then the samples were evaluated using electron microscope, the histological observations and adhesiveness evaluation were made using periodontal ligament fibroblast. Laser emission resulted in generation of the tight layer (thickness: 5.7μ) together with a superficial micro structure on the cementum surface, while such structure was the result of laser emission in fragile and removable mode through chemical and mechanical treatments. They found out that: in cellular adhesiveness evaluation, the L+TB group (17.3 +/- 3.5 cells) had higher number of adhesive cells in all the groups, while the sole laser emission exposed samples had the lowest number of cells (4.7 +/- 1.2), which are attributed to the tendency of the micron structure features of cementum surfaces after Er:YAG laser emission in prevention of the PDL cells primary adhesiveness. However, mechanical and chemical preparation of the root through removal of tiny structure on the surfaces and barer collagen fibers result in an improvement and increase in the laser emission exposed root cementum biologic compatibility. Also in this study, Er:YAG laser is merely applied in a certain energy and the effects of various energies in results are not clear. Meanwhile, in this study, the dental surface cleaning suffering from periodontal disease using laser failed to make any certain advantage concerning fibroblast adhesiveness with respect to other treatment methods.
In a study, Schwartz et al8 studied the numbers of the PDL fibroblast cells on the root surfaces of the teeth suffering from severe periodontitis in 4 groups. Some 40 single-rooted teeth were treated in 4 groups under Er:YAG laser emission with 160 mj/pulse, 10 Hz energy using vector ultrasound machine, manual scaling and treatment-free. According to the results of the study, the numbers of the fibroblast cells in the aforementioned groups were estimated to be 111+/-27, 75+/-25, 41+/-17 and 25+/-1 per square millimeter. It was shown in this study that root surface treatment with erbium laser and vector machine with respect to the manual tools or treatment-free result in a more considerable increase in the number of the cells adhered to the same. Using the standard methods to study the results together with comparing the findings to the control group may increase the study’s creditability, while such issue is similar to this study. However, the studied samples were different; for instance, in another study made by the same scholar in 2009, laser is considered effective in certain samples whose envelope depth are 1-4mm, while no meaningful difference is found between laser and other methods.
Mizutani et al9 compared the results of the periodontal tissues regeneration after flap surgery following Er:YAG laser emission and normal method. In order to do so, periodontitis disease was generated in the premolar region in both sides in 6 dogs and treated by taking benefit from the periodontal flap surgery method. Degranulation process and debridement process and debridement of the root in the tooth region was performed with Er:YAG laser or court. After that, the histological and histiometric studies were made. The study results indicated that degranulation and debridement of the root have been effectively made after Er:YAG laser emission and in addition to the lack of observing any type of heat damages, also its speed was estimated to be higher in comparison to the court method. Meanwhile, histologically speaking, the volume of the recently formed bone was meaningfully more in the laser group in comparison to the court method. However, both of these groups indicated similar values concerning formation of cementum and connective tissue adhesiveness. It was concluded in this study that Er:YAG laser emission in the periodontal flap surgery has the required effectiveness and safety and had the ability to be used in the treatment with the purposes of formation of the new bones. This study has been performed on the animals and the teeth studied regions (dental furcation) were different to our study and we know that in studying the teeth, each of the dental sites enjoys a certain importance. Meanwhile, short-term laser emission was studied and its longterm effects have not been evaluated. The important point is that the root surface preparation effect or even bone pores with laser not only affect the fibroblast and adhesiveness and regeneration of the soft tissue, but also affects the tissue generating cells.
Romanos et al10 studied the osteoblasts adhesiveness after laser emission by taking benefit from the SMA method. In order to do so, 4 types of titanium discs with 1.5cm dimensions and 2mm diameter were prepared and coated using hydroxyl apatite. Discs were classified based on the superficial mode and type of the laser emission to three groups of exposed to CO2 laser emission (energy 11.600 nm), exposed to Erbium, Chromium doped Yttrium Scandium Gallium Garnet (Er-Cr: YSGG) laser emission (energy 2.780mm), and group three of samples which did not receive any emission as the control group. CO2 laser machine was applied with tip diameter of 1.5mn, energy output of 4-6W and 20 Hz frequency and Erbium, Chromium doped Yttrium Scandium Gallium Garnet (Er-Cr: YSGG) laser with 1.25W energy and weather to water ratio of 42:41. Of a cellular rank with osteoblastic phenotype (Sarcoma osteogenic-2 (Saos-2) human osteosarcoma) was used for the cellular adhesiveness tests. The results of the study indicated that the emission-free control samples have low cellular densities and cellular morphology on the surfaces of these discs has often been spread with philopodid. However, the surfaces of the Er,Cr:YSGG or CO2laser emission exposed discs had higher cellular densities in comparison to the control group, which may be the result of the cleaning effects of laser in the discs superficial layers. Using SEM analysis in the various magnifications and applying standard culture medium were similar to our study. However, they were different concerning the type of laser and studied cell; in our study gums fibroblast cell was used, while in this study it is the osteoblast cell.
In Hakki et al11 study, the effects of Er,Cr:YSGG laser emission and the manual tools on the adhesiveness of the PDL periodontal ligament fibroblasts on the surface of the dental root were studied, where 24 single-rooted teeth suffering from periodontitis and 6 healthy molar teeth pulled out due to the orthodontia reasons (control group) were studied. Totally 45 slices were placed in 5 groups: 1. Untreated healthy group (control); 2. Untreated group suffering from periodontitis (control); 3. Manual scaling group (Scaled Gracey); 4. Er,Cr:YSGG laser emission (150 mj), 1.5W, 10Hz, 65% weather and 55% water were applied with short pulse of 140ms. 5. Er,Cr:YSGG laser emission (1.5W, 10HZ) with 400ms long pulse. Slices were placed in the cellular culture medium and the fibroblasts adhesiveness was observed on the 3rd day by using electron microscope and also the level of bromide tetrazolium was performed based on the MTT test of 5th day to determine the level of fibroblasts cellular survival rate. They found out that based on the MTT test, the group treated with laser showed considerable cellular density, while in the manual group (Gracey), a low cellular density in comparison to the control group was seen (P < 0.05). Meanwhile, laser treatment group with short pulse had higher PDL fibroblast cellular adhesiveness in comparison to the manual and even the laser II and healthy groups. Meanwhile, in this study in order to examine the cellular survival rate the MTT test was used, which is similar to this study; however, the difference in the laser type may be considered as the reason of difference in the results. Also, dental slices were autoclaved prior to the cellular culture, which may cause dental superficial changes and change the results. Therefore, in this study the method of washing the samples with distilled water and keeping in FBS was used.
In order to study the various clinical effects of the parameters of Er:YAG laser and carbon dioxide (CO2) and diode on the titanium surfaces polished, sandblasted or acid, Stubinger et al12 performed a study. In this study, each of the discs was exposed to emission on 6 circular regions with 6mm diameter by a similar laser for 10s.
They understood that using Er:YAG laser is merely safe when its emission on the implement is not higher than 300 or 500 mj, while this study may be documented concerning the Er:YAG laser application. In the aforementioned study, high Er:YAG lase power together with the 10 seconds of time have been used, while in this study power of 100mj with 40s of time were applied. It may be expressed that in addition to the power, laser emission time may also be effective in the cells morphology results.
Conclusion
The present study indicated that although various dental surfaces cleaning methods may be different in other aspects, but are similar concerning the fibroblasts morphology. Also in addition to power, laser emission time may also be effective in the cells morphology results.
Please cite this article as follows:
Foroutan T, Amid R, Karimi MR. A Comparison of Manual Tools, Ultrasonic and Erbium-Doped Yttrium Aluminum Garnet (Er:YAG) Laser on the Debridement Effect of the Surface of the Root of Teeth Suffering from Periodontitis J Lasers Med Sci 2013; 4(4):199-205
References
- 1. Newman MG, Takei HH, Carranza FA. Carranza’s clinical periodontology. 11th ed. Philadelphia: WB Saunders Co. 2011.
- 2.Drisko CL. Scaling and root planning without overinstrumentation: hand versus power-driven scalers. Curr Opin Periodontol. 1993;3:78–88. [PubMed] [Google Scholar]
- 3.Ashimoto A, Chen C, Bakker J, Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbial Immunol. 1996;11(4):266–73. doi: 10.1111/j.1399-302x.1996.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 4. Amid R, Movahedi M. Application of laser in clinical dentistry. 1st edition, Tehran, Shayan Nemoudar Press, 2006.
- 5.Frentzen M, Koort HJ. Lasers in dentistry: new possibilities with advancing laser technology. Int Dent J. 1990;40(6):323–32. [PubMed] [Google Scholar]
- 6.Maruyama H, Aoki A, Sasaki KM, Takasaki AA, Iwasaki K, Ichinose SH. et al. The effect of chemical and/or mechanical conditioning on the Er:YAG laser-treated root cementum: analysis of surface morphology and periodontal ligament fibroblast attachment. Laser Surg Med. 2008;40(3):211–22. doi: 10.1002/lsm.20609. [DOI] [PubMed] [Google Scholar]
- 7.Belal MH, Watanabe H, Ichinose S, Ishikawa I. Effect of Er:YAG laser combined with rhPDGF-BB on attachment of cultured firbroblasts to periodontally involved root surfaces. J Periodontol. 2007;78:1329–41. doi: 10.1902/jop.2007.060440. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz F, Aoki A, Sculean A, George T, Scherbaum W, Becker J. In vivo effects of an Er:YAG laser, an ultrasonic system and scaling and root planning on the biocompatibility of periodontally diseased and surfaces in cultures of human PDL fibroblasts. Lasers Surg Med. 2003;33(2):140–7. doi: 10.1002/lsm.10201. [DOI] [PubMed] [Google Scholar]
- 9.Mizutani K, Aoki A, Takasaki AA, Kinshita A, Hayashi C, Oda SH. et al. Periodontal tissue healing following flap surgery using an Er:YAG laser in dogs. Lasers Surg Med. 2006;38(4):314–24. doi: 10.1002/lsm.20299. [DOI] [PubMed] [Google Scholar]
- 10.Romanos G, Crespi R, Barone A, Covani U. Osteoblast attachment on titanium disks after laser irradiation. Int J Oral Maxillofac Implants. 2006;21(3):232–6. [PubMed] [Google Scholar]
- 11.Hakki SS, Korkusuz P, Berk G, Dundar N, Saglam M, Bozkurt B. et al. Comparison of Er,Cr: YGG Laser and Hand Instrumentation on the Attachment of periodontal Ligament Fibroblasts to Periodontally Diseased Root Surfaces: An In Vitro Study. J Periodontol. 2010;81(8):1216–25. doi: 10.1902/jop.2010.090715. [DOI] [PubMed] [Google Scholar]
- 12.Stubinger S, Etter C, Miskiewick M, Homann F, Saldamli B, Wieland M, Sader R. Surface alteration of polished and sandblasted and acid-etched titanium implants after Er:YAG, Carbone Dioxide, And Diode laser irradiation. Int J Oral Maxillofac Implants. 2010;25:104–11. [PubMed] [Google Scholar]


