Abstract
Mantle cell lymphoma (MCL) is an aggressive B cell lymphoma, where survival has been remarkably improved by use of protocols including high dose cytarabine, rituximab and autologous stem cell transplantation, such as the Nordic MCL2/3 protocols. In 2008, a MCL international prognostic index (MIPI) was created to enable stratification of the clinical diverse MCL patients into three risk groups. So far, use of the MIPI in clinical routine has been limited, as it has been shown that it inadequately separates low and intermediate risk group patients. To improve outcome and minimize treatment-related morbidity, additional parameters need to be evaluated to enable risk-adapted treatment selection. We have investigated the individual prognostic role of the MIPI and molecular markers including SOX11, TP53 (p53), MKI67 (Ki-67) and CCND1 (cyclin D1). Furthermore, we explored the possibility of creating an improved prognostic tool by combining the MIPI with information on molecular markers. SOX11 was shown to significantly add prognostic information to the MIPI, but in multivariate analysis TP53 was the only significant independent molecular marker. Based on these findings, we propose that TP53 and SOX11 should routinely be assessed and that a combined TP53/MIPI score may be used to guide treatment decisions.
Keywords: lymphoid malignancies, molecular diagnostics, prognostic factors
Mantle cell lymphoma (MCL) is an aggressive B cell lymphoma defined by cyclin D1 (CCND1) overexpression (Oka et al, 1994) and, until recently, a short median survival of 3–5 years (Weisenburger & Armitage, 1996; Herrmann et al, 2009). However, more recent treatment protocols, including high dose cytarabine, rituximab and autologous stem cell transplantation (ASCT), have achieved long-term remission in subgroups of patients (Geisler et al, 2008; Romaguera et al, 2010). A follow-up of the Nordic MCL2 trial showed a median survival of more than 10 years, a significant improvement in survival compared to previously used regimens (Geisler et al, 2012). However, to further improve survival, prognostic markers are needed to identify patients with poorer outcome, to enable the delivery of alternative treatments for these patients.
The Mantle Cell Lymphoma International Prognostic Index (MIPI) has recently been designed to stratify MCL patients into risk groups (low, intermediate or high risk) based on clinical prognostic factors, such as age, performance, leucocyte count and lactate dehydrogenase (LDH) level (Hoster et al, 2008). MKI67 (Ki-67) expression may also be included in the MIPI to account for proliferation; this combined score is referred to as the biological MIPI (MIPI-B). The MIPI index is based on similar parameters to those used in the International Prognostic Index (IPI) and the follicular lymphoma IPI (FLIPI) (Hoster et al, 2008). However, MIPI predicted survival significantly better than the IPI, as shown in the Nordic MCL2 study of 158 patients treated with intensive immunochemotherapy followed by high-dose chemotherapy and ASCT (Geisler et al, 2010). The MIPI could clearly identify high-risk patients and separate those from intermediate and low risk patients, however patients with low and intermediate MIPI scores were poorly segregated (Geisler et al, 2010). In addition to MIPI, molecular markers, such as TP53 mutational status has been shown to have prognostic value (Louie et al, 1995; Bernard et al, 2001; Stefancikova et al, 2010; Nygren et al, 2012; Slotta-Huspenina et al, 2012). However, despite the correlation of TP53 mutational status and strong TP53 (p53) staining in immunohistochemistry (IHC) (Stefancikova et al, 2010), enabling routine analysis, TP53 status is today not used in clinical routine.
In addition to CCND1, we defined SOX11 as a diagnostic antigen in MCL (Ek et al, 2008), which was widely confirmed by others (Wang et al, 2008; Mozos et al, 2009; Fernandez et al, 2010; Royo et al, 2012; Salaverria et al, 2013), and recent studies emphasized the importance of SOX11 in identifying CCND1-negative MCLs and preventing suboptimal treatment (Salaverria et al, 2013). However, previous studies investigating the prognostic role of SOX11 have shown conflicting results (Wang et al, 2008; Fernandez et al, 2010; Navarro et al, 2012; Nygren et al, 2012). This might be explained by the lack of (i) international guidelines to separate MCL into treatment groups depending on clinical behavior (indolent versus classical MCL), (ii) use of heterogeneously-treated patients as a basis of SOX11 prognostic analysis and (iii) potential cross reactivity of the polyclonal reagents used (Nordstrom et al, 2012).
In this study, we used a novel, well characterized monoclonal SOX11 antibody (Nordstrom et al, 2012) to assess the clinical significance of SOX11 in the combined Nordic MCL2/3 cohort of patients. SOX11 was expressed in most patients (95%) at different levels and could be categorized into a dichotomized variable, where SOX11high correlated to improved overall survival (OS) and event-free survival (EFS), in agreement with our previous experimental in vitro data (Gustavsson et al, 2010; Conrotto et al, 2011). With the aim to use information on molecular markers, such as SOX11, TP53, MKI67 and CCND1 that routinely can be assessed using IHC, we optimized a combined molecular/MIPI score. SOX11 could be used to improve the MIPI but, in multivariate analysis, TP53 was the only independent molecular marker that was able to improve the prognostic value of MIPI. We thus propose that SOX11 and TP53 can be used to guide treatment decisions, preferably in combination with the well-established MIPI.
Methods
Patients, cohorts and treatment protocols
Material from patients included in the Nordic Lymphoma Group MCL2 and MCL3 trials, at hospitals in Sweden, Denmark, Norway or Finland was collected. In the Nordic MCL2 trial protocol, patients were treated with first-line intensive immunochemotherapy, followed by high-dose chemotherapy and ASCT (Geisler et al, 2010). The Nordic MCL3 protocol was identical, except for the addition of ibritumumab tiuxetan to patients in partial remission or complete remission unconfirmed (CRu) after induction chemotherapy. The inclusion criteria were (i) MCL stage II-IV, (ii) previously untreated, (iii) CCND1 positivity or presence of t(11;14) and (iv) age 18–65 years. Enrolled patients had a median age at diagnosis of 57 years (range 37–65 years). All tumours had been classified regarding histological subtype prior to tissue microarray (TMA) construction. The separate clinicopathological characteristics for the MCL2 and MCL3 cohorts have been described elsewhere (Geisler et al, 2008; Kolstad et al, 2012), and relevant data for the available patients from the combined cohort, hereafter referred to as the Nordic MCL2/3 cohort, is presented in Table I. Data for the full MCL2/3 cohort is shown in Table SI. The study was approved by the ethic committees in Sweden, Denmark, Norway and Finland. In total, 127 cases were available from the combined MCL2 (n = 58) and MCL3 (n = 69) trials.
Table 1.
Patient characteristics of the MCL2/3 cohort.
| Parameter | n (%) |
|---|---|
| Male | 83 (74) |
| Stage IV | 95 (85) |
| MIPIlow | 60 (54) |
| MIPIintermediate | 28 (25) |
| MIPIhigh | 23 (21) |
| Blastoid variant of MCL | 26 (23) |
| Common variant of MCL | 86 (77) |
| TP53weak | 78 (70) |
| TP53intermediate | 5 (4) |
| TP53strong | 10 (9) |
| TP53not available | 19 (17) |
| MKI67 (0–9%) | 12 (11) |
| MKI67 (10–29%) | 47 (42) |
| MKI67 (30–100%) | 38 (34) |
| MKI67 (not available) | 15 (13) |
TMA construction
TMAs were constructed according to a method previously described (Kononen et al, 1998). Representative tumour areas were chosen from haemotoxylin and eosin-stained sections from paraffin blocks and duplicate cores with a diameter of 1 mm tissue were transferred to a recipient block using an automated device (ATA-27; Beecher Instruments, Sun Prairie, WI, USA).
IHC and scoring
Immunohistochemistry was performed on 2-μm sections that were dried, deparaffinized, rehydrated and microwave-treated as previously described (Nordstrom et al, 2012). The sections were stained for SOX11 (SOX11-C1 developed in-house as previously described (Nordstrom et al, 2012), CCND1 (ab M3635; Dako, Glostrup, Denmark) or TP53 (sc-126; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). All samples were routinely processed and embedded in paraffin for tissue conservation. Antigen retrieval was performed using the PT-LINK system (Dako) at pH 9. All sections were analysed using a Nikon ECLIPSE 80i microscope (Nikon Instrument Inc., Melville, NY, USA) at a magnification of 20× (Plan Fluor 20× DIC M N2, Nikon) with a numerical aperture of 0·5. Images were captured using a Nikon DS-U2/L2 USB (Nikon) camera and NIS Elements br 3·10 (Nikon) as acquisition software. For each antigen (CCND1, TP53 and SOX11), the fraction of positive nuclei was scored and samples divided into groups as follows; (i) negative, (ii) weak, (iii) intermediate (strong nuclear staining in <30% of cells) and (iv) strong (nuclear staining in ≥30% of cells) staining. For SOX11, negative, weak and intermediate cases are referred to as SOX11low, while strong cases are referred to as SOX11high when the dichotomized variable is used. Among the collected 127 cases, tissues from 120 patients were evaluable for SOX11 and CCND1 staining, while 93 cases were evaluable for TP53 staining. MKI67 scoring was available since previously (Geisler et al, 2010).
Statistical analysis
Among the collected 127 cases, clinicopathological information was available for 112 cases. OS was defined as time from study entry to death or last follow-up. EFS was defined as time from study entry to the date of last follow-up or failure resulting from any of the following events: death from any cause, nonresponse to induction treatment, lymphoma relapse or progression, any toxic event that prohibited treatment according to protocol, failure to harvest stem cells from peripheral blood or bone marrow, failure to engraft, or patient refusal to undergo ASCT, as previously described (Geisler et al, 2008). Both OS and EFS were estimated according to the Kaplan-Meier method and the log-rank test was used to compare survival between different groups. To compare clinicopathological and biological differences between SOX11low and SOX11high cases, χ2-test and linear-by-linear association were used. Cox regression using both uni and multivariate models were used to investigate the significance of SOX11, TP53, MKI67, CCND1, morphology and MIPI in relation to OS and EFS. The statistical analysis was performed using either ibm spss statistics Version 20 (IBM, Armonk, NY, USA) or matlab (matlab and Statistics Toolbox Release 2012b, The MathWorks, Inc., Natick, MA, USA).
SOX11/MIPI and TP53/MIPI
Multivariate Cox regression on all of the MIPI components (age, white blood cell [WBC] count, LDH and Eastern Cooperative Oncology Group performance score [ECOG]) gave slightly different coefficients compared to original MIPI (see Table SII). However, to be able to compare with previous studies, MIPI was kept constant in further analyses.
The original MIPI was calculated for each patient (Hoster et al, 2008), in brief: MIPI = [0·03535 × age (years)] + 0·6978 (if ECOG > 1) + [1·367 × log10(LDH/ULN)] + [0·9393 × log10(WBC count)], where ULN is defined as the upper limit of mormal. The high, intermediate and low subgroups were defined according to standard guidelines (Hoster et al, 2008). A score <5·7 indicates low-risk, 5·7–6·2 indicates intermediate risk, and a score >6·2 indicates high risk disease. Similarly, the biological MIPI was calculated as: MIPI-B = [0·03535 × age (years)] + 0·6978 (if ECOG > 1) + [1·367 × log10(LDH/ULN)] + [0·9393 × log10(WBC count)] + [0·02142 × MKI67(%)]. The high, intermediate and low subgroups were defined according to standard guidelines (Hoster et al, 2008). A score <5·7 indicates low-risk, 5·7–6·5 indicates intermediate risk, and a score >6·5 indicates high risk disease. Using Cox regression (Cox, 1972), the statistical value of adding information on molecular markers, SOX11, TP53, MKI67 and CCND1, to the MIPI was assessed. The optimal regression coefficient (maximizing likelihood of observed events) for each significant molecular marker was also calculated. The combined indices were calculated using Cox-regression coefficients. Expressed in terms of hazard ratios (HRs), each parameter (X) was scaled against the MIPI, log HR(X)/log HR(MIPI).
Results
In this study, we have investigated SOX11 in relation to prognostic use and clinicopathological and biological parameters in the Nordic MCL2/3 cohort. We further explored the prognostic use of MIPI and the well-known molecular markers TP53, MKI67 and CCND1. The potential of SOX11, TP53, MKI67 and CCND1 to add prognostic value to the MIPI score was assessed and optimized with the aim of establishing a combined molecular marker/MIPI score as a tool for treatment decisions.
IHC analysis of SOX11, CCND1 and TP53
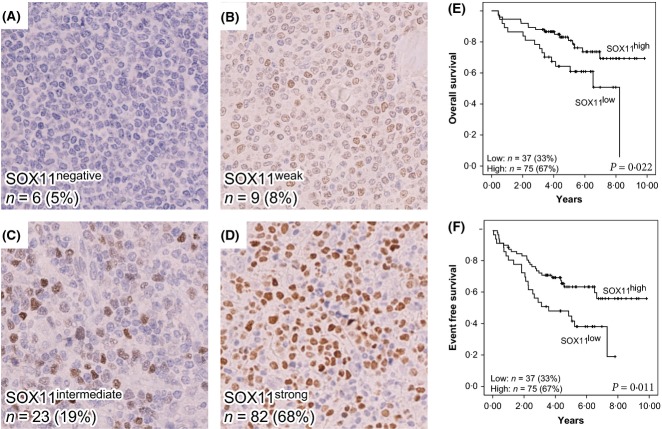
The expression of SOX11, CCND1 and TP53 was evaluated using IHC. Representative stainings and information on the frequency and number of cases for each marker are shown in Figs1 and 2.
Fig 1.
Representative immunostaining of SOX11 and correlation to overall survival and event-free survival. The immunohistochemistry panel shows representative figures of (A) negative SOX11 staining, detected in 5% of cases, (B) weak SOX11 staining, detected in 8% of cases, (C) intermediate SOX11 staining, detected in 19% of cases and (D) strong SOX11staining, detected in 68% of cases. Cases were grouped into a dichotomized variable depending on SOX11 expression. Strong cases are referred to as SOX11high and negative, weak or intermediate cases are referred to as SOX11low. Survival analysis using Kaplan-Meier's method comparing SOX11high (D) versus SOX11low (A-C) shows a positive correlation between SOX11 and (E) overall survival and (F) event-free survival with P-values of 0·022 and 0·001, respectively, as determined by the log rank test.
Fig 2.
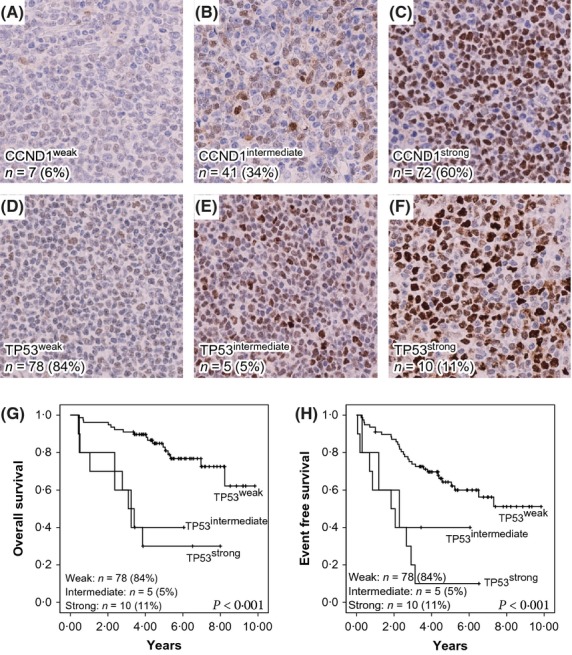
Representative immunostainings of CCND1 and TP53 and correlation of TP53 to overall survival and event-free survival. (A) Weak CCND1 staining was detected in 6% of cases, (B) intermediate CCND1 staining was detected in 34% of cases, and (C) strong CCND1 staining was detected in 60% of cases. (D) Weak TP53 staining was detected in 84% of cases, (E) intermediate TP53 staining was detected in 5% of cases and (F) strong TP53 was detected in 11% of cases. Survival analysis using Kaplan-Meier's method comparing p53weak (F), p53intermediate (E) and p53strong (F) shows a negative correlation between TP53 and (G) overall survival and (H) event-free survival with P-values <0·001 in both cases, as determined by the log rank test.
Among the evaluable 120 cases, 68% (n = 82) showed strong SOX11 staining in a large fraction of cells while 27% (n = 32) showed a weak or intermediate staining (refers to Groups 2 and 3). Only 5% (n = 6) of cases were SOX11-negative. Thus overall, 95% of cases were SOX11-positive, in accordance with previous studies (Ek et al, 2008; Wang et al, 2008; Mozos et al, 2009; Nygren et al, 2012). In all subsequent correlation analyses, a dichotomized variable combining SOX11 nuclear fraction and intensity was used. These two groups are referred to as SOX11high (includes SOX11strong cases) and SOX11low (includes SOX11negative/weak/intermediate cases). This optimal grouping of the SOX11 cases in relation to survival was assessed by Cox univariate analysis (see Table SIII).
In accordance with the Nordic MCL2/3 inclusion criteria, all cases were CCND1-positive. Among these, 60% and 34% of patients showed strong or intermediate CCND1 staining, respectively, and only 6% showed weak staining (Fig2A–C). In non-selected cohorts, 6–15% of MCL were negative for CCND1 (Yatabe et al, 2000; Rosenwald et al, 2003). Out of the 127 cases in the cohort, 93 were evaluable for TP53 expression, of which 15% showed strong or intermediate TP53 staining (Fig2E, F). Of major interest, all strong TP53 cases were found among the SOX11low subgroup (Fig1A–C, Table II), indicating that these may harbour an increased frequency of TP53 mutations and potentially other genetic aberrations, as previously suggested (Stefancikova et al, 2010; Navarro et al, 2012).
Table 2.
Clinical, pathological and biological features of the SOX11low compared to SOX11high subgroups in the MCL2/3 cohort.
| Clinical and pathologic features | SOX11low n = 37 (32%) | SOX11high n = 77 (68%) | P-value* |
|---|---|---|---|
| Median age (years) | 54 (41–65) | 58 (37–65) | 0·108 |
| Age >60 years | 10/37 (27%) | 21/75 (28%) | 0·914 |
| Male sex | 27/37 (73%) | 56/75 (75%) | 0·848 |
| Blastoid morphology | 15/37 (41%) | 11/75 (15%) | 0·002 |
| CCND1weak | 4/37 (11%) | 3/75 (4%) | |
| CCND1intermediate | 18/37 (49%) | 21/75 (28%) | 0·006 |
| CCND1strong | 15/37 (41%) | 51/75 (68%) | |
| TP53strong | 10/35 (29%) | 0/58 (0%) | ≤0·001 |
| MIPIlow | 18/37 (49%) | 42/74 (57%) | |
| MIPIintermediate | 9/37 (24%) | 19/74 (26%) | 0·276 |
| MIPIhigh | 10/37 (27 %) | 13/74 (17%) | |
| MKI67 (30–100%) | 20/35 (57%) | 18/62 (29%) | 0·006 |
Statistical significant P-values are shown in bold.
Survival in relation to SOX11 and TP53 expression
The median OS and EFS for the cases used and available from the combined Nordic MCL2/3 cohort were 9·0 and 7·0 years, respectively. SOX11high identified a large subgroup of patients (67%) with both favourable 5- and 10-year OS (81%, 69%) and EFS (64%, 56%). Similarly, TP53weak identified a subgroup of patients (84%) with favourable 5- and 10-year OS (83%, 62%) and EFS (64%, 51%; Fig2G, H).
Correlation between SOX11 and established clinicopathological and biological parameters
The dichotomized variable for SOX11 was also used to investigate the correlation between SOX11 and established clinicopathological and biological parameters (Table II). A positive correlation between SOX11 and CCND1 (P = 0·006) was seen, in contrast to previous data, where SOX11 expression was found to be independent of the t(11;14) translocation (Chen et al, 2010). Blastoid morphology (P < 0·002), TP53 (P < 0·001) and MKI67 (P = 0·006) showed a negative correlation to SOX11, indicating that SOX11high may identify patients with lower proliferation, non-blastoid morphology and functional TP53.
Prognostic significance of SOX11, TP53, MKI67, CCND1, blastoid morphology and MIPI
To determine the prognostic significance of relevant molecular and clinicopathological parameters, Cox univariate analyses were performed. SOX11 expression positively correlated to OS and EFS (P = 0·025 and 0·013; Table III). MIPI and TP53 correlated negatively to both OS (<0·001) and EFS (<0·001), while histology and MKI67 showed negative correlation to OS (P = 0·001 and P = 0·02) but showed no significant correlation to EFS. CCND1 showed no significant correlation to either OS or EFS.
Table 3.
Cox univariate analysis of SOX11, TP53, MKI67, CCND1, blastoid morphology and MIPI in relation to overall and event-free survival in the MCL2/3 cohort.
| Patients (n) | HR | 95% CI | P-value* | |
|---|---|---|---|---|
| Overall survival | ||||
| Common morphology | 86 | 1·0 | ||
| Blastoid morphology | 26 | 3·2 | 1·6–6·5 | 0·001 |
| CCND1weak | 7 | 1·0 | ||
| CCND1intermediate | 39 | 3·3 | 0·4–25·3 | 0·248 |
| CCND1strong | 66 | 2·5 | 0·3–18·8 | 0·373 |
| MKI67 (continuous) | 97 | 1·0 | 1·0–1·0 | 0·02 |
| MIPI (continuous) | 111 | 3·1 | 2·0–4·7 | <0·001 |
| TP53weak | 78 | 1·0 | ||
| TP53intermediate | 5 | 5·1 | 1·5–17·9 | 0·010 |
| TP53strong | 10 | 5·7 | 2·3–14·1 | <0·001 |
| SOX11high | 75 | 1·0 | ||
| SOX11low | 37 | 2·2 | 1·1–4·3 | 0·025 |
| Event-free survival | ||||
| Common morphology | 86 | 1·0 | ||
| Blastoid morphology | 26 | 1·6 | 0·9–3·0 | 0·118 |
| CCND1weak | 7 | 1·0 | ||
| CCND1intermediate | 39 | 2·0 | 0·4–8·4 | 0·370 |
| CCND1strong | 66 | 2·1 | 0·5–8·8 | 0·312 |
| MKI67 | 97 | 1·0 | 1·0–1·0 | 0·086 |
| MIPI (continuous) | 111 | 2·3 | 1·5–3·5 | <0·001 |
| P53weak | 78 | 1·0 | ||
| P53intermediate | 5 | 2·6 | 0·8–8·4 | 0·123 |
| P53strong | 10 | 4·8 | 2·2–10·3 | <0·001 |
| SOX11high | 75 | 1·0 | ||
| SOX11low | 37 | 2·0 | 1·2–3·6 | 0·013 |
HR, hazard ratio; 95% CI, 95% confidence interval; MIPI, Mantle cell lymphoma International Prognostic Index.
Statistical significant P-values are shown in bold.
TP53 adds independent prognostic significance to the MIPI
As previously discussed, patients with low and intermediate MIPI are poorly separated based on survival in the Nordic MCL2/3 cohort (Fig3A, B). It has previously been suggested that MKI67 may add prognostic value to MIPI (Hoster et al, 2008) but the MIPI-B also failed to separate the low and intermediate risk groups in this combined cohort (Fig3C, D). The multimodality of the cohort was investigated by Gaussian Mixture Model analysis, optimizing maximum likelihood and evaluating with the Akaike Information Criterion (Akaike, 1974). This analysis confirmed that the cohort is bimodal in MIPI, with a transition point at 5·92 (where both risk groups are equally probable). However, to be able to compare the novel proposed indices with previous studies of MIPI, the low, intermediate and high risk groups, and the sizes thereof, were kept constant when visualizing the indices using Kaplan Meier.
Fig 3.
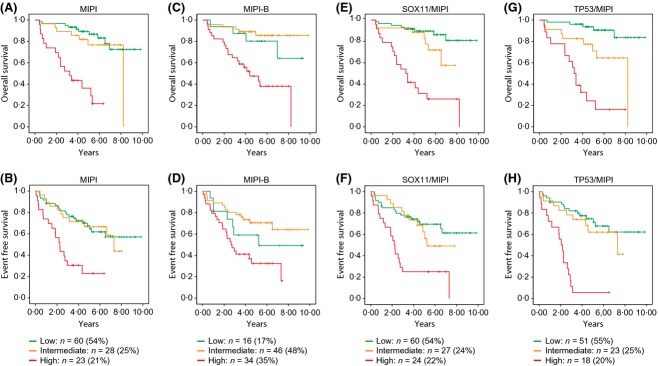
Kaplan-Meier's estimate of overall survival and event-free survival in relation to MIPI, MIPI-B, SOX11/MIPI and TP53/MIPI. (A) OS and (B) EFS analysis using the Kaplan-Meier method reveal a poor separation between MIPIlow and MIPIintermediate groups. When applying the proposed biological MIPI (MIPI-B) both (C) OS and (D) EFS analysis show inverted low and intermediate survival curves. The combined SOX11/MIPI improves separation between low and intermediate risk groups (although, P > 0·05) in relation to (E) OS and (F) EFS. The combined TP53/MIPI show significant (P = 0·006) separation between low and intermediate risk groups in relation to (G) OS and identifies a high risk group with decreased (H) EFS. OS, overall survival; EFS, event-free survival; MIPI, Mantle cell lymphoma International Prognostic Index; MIPI-B, biological MIPI.
To assess the ability of SOX11 to add information to MIPI, these were analysed together in a multivariate analysis where MIPI was used as a continuous variable. It was shown that SOX11 significantly improved the MIPI and that an optimal score should be calculated as MIPI-0·72[if SOX11high] for OS and MIPI-0·92[if SOX11high] for EFS respectively (see Table IV for HR values). When divided into the standard low, intermediate and high risk groups for visualization using Kaplan Meier, the adjusted scores were 3·94–5·23 for the low risk group, 5·27–5·77 for the intermediate risk group and 5·8–7·77 for the high risk group using the combined SOX11/MIPI, optimized for OS. Similarly, when optimized for EFS, the adjusted scores for the three risk groups were 3·73–5·13 for the low risk group, 5·17–5·75 for the intermediate risk group and 5·77–7·77 for the high risk group. Using the combined SOX11/MIPI, survival analysis showed the improved separation of low, intermediate and high risk groups for OS and EFS, although the separation of low and intermediate risk groups was still not statistically significant (see Table IV and Fig3E, F). Cox regression was used to further evaluate the potential of SOX11 to add prognostic value to MIPI in relation to known molecular markers including TP53, MKI67 and/or CCND1 (see Methods). When using all these parameters in a multivariate analysis, only TP53 was able to independently add prognostic information to MIPI (see Table SIV). The optimal scaled factors were 1·47 and 1·65 for OS and EFS, respectively (see Table SIV and Table V). Thus, the TP53-adjusted MIPI was calculated as: MIPI + 1·47 [if p53strong] for OS and MIPI + 1·65 [if p53strong] for EFS. TP53 was also assessed together with the individual MIPI parameters, which slightly changed the indices for OS and EFS (see Table SV). The adjusted scores for the different risk groups were 4·66–5·71 for the low risk group, 5·73–6·67 for the intermediate risk group and 6·76–8·84 for the high risk group when optimized in relation to OS. When optimized for EFS, the adjusted risk group scores were 4·66–5·71 for the low risk group, 5·73–6·67 for the intermediate risk group and 6·86–8·94 for the high risk group. Using the combined TP53/MIPI, survival analysis showed the improved separation of low and intermediate risk groups for OS (P = 0·006; see Table V and Fig3G, H). Furthermore, the 5-year EFS for the combined TP53/MIPI identified a high risk group with lower EFS (6%) compared to MIPI (23%) and TP53 (10%) as stand-alone biomarkers. Thus, by combining MIPI with information on TP53, improved prognostic information was achieved.
Table 4.
Cox multivariate analysis of SOX11 and MIPI in relation to overall and event free survival in the MCL2/3 cohort.
| Patients (n) | HR | 95% CI | P-value* | |
|---|---|---|---|---|
| Overall survival | ||||
| SOX11high | 74 | 1·0 | ||
| SOX11low | 37 | 2·3 | 1·2–4·6 | 0·017 |
| MIPI (continuous) | 111 | 3·2 | 2·1–4·9 | <0·001 |
| Event-free survival | ||||
| SOX11high | 74 | 1·0 | ||
| SOX11low | 37 | 2·2 | 1·3–4·0 | 0·005 |
| MIPI (continuous) | 111 | 2·4 | 1·6–3·6 | <0·001 |
HR, hazard ratio; 95% CI, 95% confidence interval; MIPI, Mantle cell lymphoma International Prognostic Index.
Statistical significant P-values are shown in bold.
Table 5.
Cox multivariate analysis of TP53 and MIPI in relation to overall and event free survival in the MCL2/3 cohort.
| Patients (n) | HR | 95% CI | P-value* | |
|---|---|---|---|---|
| Overall survival | ||||
| TP53weak/intermediate | 82 | 1·0 | ||
| TP53strong | 10 | 6·4 | 2·6–16·1 | <0·001 |
| MIPI (continuous) | 92 | 3·6 | 2·3–5·6 | <0·001 |
| Event-free survival | ||||
| TP53weak/intermediate | 82 | 1·0 | ||
| TP53strong | 10 | 6·1 | 2·8–13·4 | <0·001 |
| MIPI (continuous) | 92 | 3·0 | 1·9–4·6 | <0·001 |
HR, hazard ratio; 95% CI, 95% confidence interval; MIPI, Mantle cell lymphoma International Prognostic Index.
Statistical significant P-values are shown in bold.
TP53 is not routinely assessed, and as TP53 data was missing for a number of cases, the multivariate analysis was also performed with only MIPI, SOX11, MKI67 and CCND1. In this analysis, SOX11 and MKI67 were the only molecular markers that independently added prognostic value to the MIPI in relation to EFS and OS, respectively (see Table SVI). The optimal scaled factor was 0·012 (OS) for MKI67, which is similar to the previously established MIPI-B, calculated as MIPI + 0·021[MKI67%] (Hoster et al, 2008).
Discussion
The treatment of MCL is ever-changing and recent improvements of clinical protocols have had a pronounced effect on patient outcome (Geisler et al, 2008; Romaguera et al, 2010; Delarue et al, 2013). In younger patients (<65 years), the introduction of autologous stem cell transplantation, high dose cytarabine, and rituximab has clearly improved PFS and OS (Delarue et al, 2013). Even more recently, it was shown that an inhibitor of Bruton′s tyrosine kinase (BTK), ibrutinib, induces a response rate of >70% in relapsed and refractory MCL as a single agent (Wang et al, 2013). Combinatory studies with ibrutinib are still lacking, but the initial results may indicate a shift from chemotherapy-based approach to therapies targeting the underlying biological mechanisms of disease in MCL.
Prognostic factors, such as the MIPI and proliferation rate, are part of the routine work-up for patients with MCL, but are still rarely used for treatment decisions, as recently discussed by the European MCL network (Dreyling et al, 2013). Even in recent studies of MCL, MIPI is not used to assess differences in response to treatment (Delarue et al, 2013). It is evident that molecular subtype, MIPI (Hoster et al, 2008) and other biological factors need to be tested as potential companion biomarkers to enable individualized treatment selection among the plethora of current treatment strategies. To be clinically useful, these markers need to be robust and easily scored in routine IHC analysis.
It is well established that aberrations of TP53 is associated with aggressive behavior (Louie et al, 1995), the blastoid subtype (Bernard et al, 2001) and high proliferation (Slotta-Huspenina et al, 2012). Recent studies have shown a prognostic value of mutational status of TP53 but also correlation to TP53 levels by IHC (Stefancikova et al, 2010), and thus able to be included in routine assessments. In a recent population-based series, this was used to show the prognostic value of TP53 IHC status (Nygren et al, 2012).
Another important biomarker in MCL is SOX11, which during recent years has been identified as a diagnostic (Ek et al, 2008; Wang et al, 2008; Mozos et al, 2009; Fernandez et al, 2010; Royo et al, 2012; Salaverria et al, 2013) and prognostic antigen (Wang et al, 2008; Fernandez et al, 2010; Navarro et al, 2012; Nygren et al, 2012). The prognostic significance of SOX11 has so far only been assessed in population-based cohorts, and thus the potential of using SOX11 as a tool for treatment selection has not been evaluable. In this study, we showed that SOX11 correlates with favourable survival among MCL patients treated according to the Nordic MCL2/3 protocols. However, the molecular mechanism of SOX11 in MCL is still not fully elucidated. It is very likely that SOX11 contributes to the tumour development of classical MCLs, as recently presented by Vegliante et al (2013) who showed that SOX11 regulated PAX5 expression and blocked terminal B-cell differentiation. However, in relation to treatment response among aggressive MCLs, a high level of SOX11 is beneficial as shown here, in agreement with our previous molecular studies (Gustavsson et al, 2010; Conrotto et al, 2011).
Previous studies correlating the absence or presence of nuclear SOX11 to survival in MCL have shown conflicting results. A positive correlation between SOX11 and improved survival was reported in two studies with cohorts of 53 and 186 MCL patients, respectively (Wang et al, 2008; Nygren et al, 2012). In contrast, absence of SOX11 correlated to better survival in two other series (Fernandez et al, 2010; Navarro et al, 2012). Of note, in the studies correlating SOX11 negativity to better survival, the SOX11-negative cases were classified as an indolent form of MCL characterized by more frequent non-nodal presentation, hypermutated IGHV and less genomic complexity, which represents a distinct clinical subgroup of MCL (Navarro et al, 2012; Royo et al, 2012). It can be argued that these cases might even be classified as a different disease, as their clinical course is very different from that of the classical, more aggressive MCL. No indolent cases were included in the present Nordic MCL2/3 cohort. In the study reported by Nygren et al (2012), in which a positive correlation between SOX11 staining and survival was seen, 69% of the SOX11-negative cases showed strong TP53 staining. It has been argued that these cases might harbour a TP53 mutation associated with a more rapid clinical evolution, compared to the SOX11-negative cases with wild type TP53 that has a stable disease and long survival (Navarro et al, 2012). Also in our study the TP53 strong cases were found within the SOX11low subgroup, but future investigations of the TP53 mutational status need to be performed to verify this potential negative correlation to SOX11. We here show that TP53 status, as assessed by IHC, is associated with inferior survival. In addition to strong TP53 expression, an increased fraction of cases with blastoid morphology (Bernard et al, 2001) and high MKI67 was found within the SOX11low subgroup, in agreement with a more aggressive clinical course for these patients.
It has previously been suggested that the proliferation index (fraction of MKI67-positive cells) may add prognostic value to MIPI, referred to as biological MIPI (MIPI-B) (Hoster et al, 2008). We here explored the potential of a range of molecular markers, including TP53, SOX11, MKI67 and/or CCND1, to add prognostic value to MIPI for patients treated with the Nordic MCL2/3 protocol. Although SOX11 significantly added prognostic value to MIPI, TP53 was the only molecular marker that remained significant in multivariate analysis. The combined TP53/MIPI was able to separate low and intermediate risk groups in relation to OS and identified a high risk group of patients, with poor EFS, in need of alternative treatment. When TP53 was omitted, SOX11 was the only independent molecular marker that could add prognostic value to MIPI in relation to EFS and may thus be used for patient stratification when data on TP53 is missing.
In summary, we have used the homogenously treated Nordic MCL2/3 cohort to demonstrate the prognostic significance of SOX11, using a novel monoclonal antibody. A quantitative assessment showed that OS and EFS were superior for SOX11high compared to SOX11low patients, and that a large group of patients (81%) with long-term response (5-year EFS) to the Nordic MCL2/3 protocol was identified. Of note, p53strong cases were only found among the SOX11low subgroup, indicating that these may constitute cases with more complex genetic aberrations, consistent with a shorter survival. In multivariate analysis, TP53 was the only molecular marker that significantly added prognostic value to the MIPI. Based on these findings, we propose that SOX11 and TP53 should routinely be assessed by IHC and that a combined TP53/MIPI score may be used for treatment decisions in MCL.
Acknowledgments
Kristina Lövgren is acknowledged for all her help and support with IHC stainings. The study was supported by the Lund Institute of Technology (LTH), Cancerfonden (2010/584), Vetenskapsrådet (K2012-99X-22004-01-3) Crafoord foundation, BioCARE – a strategic program for Cancer Research at Lund and Gothenburg Universities and CREATE Health.
Authorship and disclosures
LN developed the antibody, performed the statistical analyses and took active part in the writing of the manuscript. PE performed all Cox regression analysis in relation to the combined MIPI indexes. SS evaluated IHC stainings and wrote parts of the manuscript. KG, AK, RR, MLK, CG, ER, CS, AL, JD and ME were involved in the collection of material and clinicopathological parameters. MJ took active part in the design of the study, interpretation of the data and writing of the manuscript. SE was responsible for the design of the study, interpretation of data and writing of the manuscript. All authors read and approved the final manuscript. A patent has previously been filed on the diagnostic, prognostic and therapeutic use of SOX11 in B cell lymphomas.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Patient characteristics of the full MCL2/3 cohort compared to available and used cohort.
Table SII. Iterative Cox-regression of individual MIPI components where the least significant parameter is removed in each step until only statistical significant parameters remain.
Table SIII. Univariate analysis to determine most significant cut-off point for SOX11 in relation to survival.
Table SIV. Iterative Cox-regression including TP53, SOX11, MKI67 and CCND1 where the least significant parameter is removed in each step until only statistical significant parameters remain.
Table SV. Final parameters after Cox-regression of all individual MIPI components and TP53.
Table SVI. Iterative Cox-regression SOX11, MKI67 and CCND1 where the least significant parameter is removed in each step until only statistical significant parameters remain.
References
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- Bernard M, Gressin R, Lefrere F, Drenou B, Branger B, Caulet-Maugendre S, Tass P, Brousse N, Valensi F, Milpied N, Voilat L, Sadoun A, Ghandour C, Hunault M, Leloup R, Mannone L, Hermine O. Lamy T. Blastic variant of mantle cell lymphoma: a rare but highly aggressive subtype. Leukemia. 2001;15:1785–1791. doi: 10.1038/sj.leu.2402272. [DOI] [PubMed] [Google Scholar]
- Chen YH, Gao J, Fan G. Peterson LC. Nuclear expression of sox11 is highly associated with mantle cell lymphoma but is independent of t(11;14)(q13;q32) in non-mantle cell B-cell neoplasms. Modern Pathology. 2010;23:105–112. doi: 10.1038/modpathol.2009.140. [DOI] [PubMed] [Google Scholar]
- Conrotto P, Andreasson U, Kuci V, Borrebaeck CA. Ek S. Knock-down of SOX11 induces autotaxin-dependent increase in proliferation in vitro and more aggressive tumors in vivo. Molecular Oncology. 2011;5:527–537. doi: 10.1016/j.molonc.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society Series B: Methodological. 1972;34:187–220. [Google Scholar]
- Delarue R, Haioun C, Ribrag V, Brice P, Delmer A, Tilly H, Salles G, Van Hoof A, Casasnovas O, Brousse N, Lefrere F, Hermine O Groupe d'Etude des Lymphomes de l'Adulte. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d'Etude des Lymphomes de l'Adulte. Blood. 2013;121:48–53. doi: 10.1182/blood-2011-09-370320. [DOI] [PubMed] [Google Scholar]
- Dreyling M, Kluin-Nelemans HC, Bea S, Klapper W, Vogt N, Delfau-Larue MH, Hutter G, Cheah C, Chiappella A, Cortelazzo S, Pott C, Hess G, Visco C, Vitolo U, Klener P, Aurer I, Unterhalt M, Ribrag V, Hoster E, Hermine O European Mantle Cell Lymphoma Network. Update on the molecular pathogenesis and clinical treatment of mantle cell lymphoma: report of the 11th annual conference of the European Mantle Cell Lymphoma Network. Leukaemia & Lymphoma. 2013;54:699–707. doi: 10.3109/10428194.2012.733882. [DOI] [PubMed] [Google Scholar]
- Ek S, Dictor M, Jerkeman M, Jirstrom K. Borrebaeck CA. Nuclear expression of the non B-cell lineage Sox11 transcription factor identifies mantle cell lymphoma. Blood. 2008;111:800–805. doi: 10.1182/blood-2007-06-093401. [DOI] [PubMed] [Google Scholar]
- Fernandez V, Salamero O, Espinet B, Sole F, Royo C, Navarro A, Camacho F, Bea S, Hartmann E, Amador V, Hernandez L, Agostinelli C, Sargent RL, Rozman M, Aymerich M, Colomer D, Villamor N, Swerdlow SH, Pileri SA, Bosch F, Piris MA, Montserrat E, Ott G, Rosenwald A, Lopez-Guillermo A, Jares P, Serrano S. Campo E. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Research. 2010;70:1408–1418. doi: 10.1158/0008-5472.CAN-09-3419. [DOI] [PubMed] [Google Scholar]
- Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, Eriksson M, Nordstrom M, Kimby E, Boesen AM, Kuittinen O, Lauritzsen GF, Nilsson-Ehle H, Ralfkiaer E, Akerman M, Ehinger M, Sundstrom C, Langholm R, Delabie J, Karjalainen-Lindsberg ML, Brown P, Elonen E Nordic Lymphoma Group. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler CH, Kolstad A, Laurell A, Raty R, Jerkeman M, Eriksson M, Nordstrom M, Kimby E, Boesen AM, Nilsson-Ehle H, Kuittinen O, Lauritzsen GF, Ralfkiaer E, Ehinger M, Sundstrom C, Delabie J, Karjalainen-Lindsberg ML, Brown P, Elonen E Nordic Lymphoma Group. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT) Blood. 2010;115:1530–1533. doi: 10.1182/blood-2009-08-236570. [DOI] [PubMed] [Google Scholar]
- Geisler CH, Kolstad A, Laurell A, Jerkeman M, Raty R, Andersen NS, Pedersen LB, Eriksson M, Nordstrom M, Kimby E, Bentzen H, Kuittinen O, Lauritzsen GF, Nilsson-Ehle H, Ralfkiaer E, Ehinger M, Sundstrom C, Delabie J, Karjalainen-Lindsberg ML, Brown P. Elonen E. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. British Journal of Haematology. 2012;158:355–362. doi: 10.1111/j.1365-2141.2012.09174.x. [DOI] [PubMed] [Google Scholar]
- Gustavsson E, Sernbo S, Andersson E, Brennan DJ, Dictor M, Jerkeman M, Borrebaeck CA. Ek S. SOX11 expression correlates to promoter methylation and regulates tumor growth in hematopoietic malignancies. Molecular Cancer. 2010;9:187. doi: 10.1186/1476-4598-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann A, Hoster E, Zwingers T, Brittinger G, Engelhard M, Meusers P, Reiser M, Forstpointner R, Metzner B, Peter N, Wormann B, Trumper L, Pfreundschuh M, Einsele H, Hiddemann W, Unterhalt M. Dreyling M. Improvement of overall survival in advanced stage mantle cell lymphoma. Journal of Clinical Oncology. 2009;27:511–518. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- Hoster E, Dreyling M, Klapper W, Gisselbrecht C, Van Hoof A, Kluin-Nelemans HC, Pfreundschuh M, Reiser M, Metzner B, Einsele H, Peter N, Jung W, Wormann B, Ludwig WD, Duhrsen U, Eimermacher H, Wandt H, Hasford J, Hiddemann W, Unterhalt M German Low Grade Lymphoma Study Group and European Mantle Cell Lymphoma Network. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- Kolstad A, Laurell A, Jerkeman M, Gronbaek K, Elonen E, Raty R, Pedersen LB, Loft A, Bogsrud TV, Nordstrom M, Hansen PB, Fagerli U-M, Nilsson-Ehle H, Lauritzsen GF, Lehmann AK, Sundstrom C, Karjalainen-Lindsberg M-L, Ralfkiaer E, Ehinger M, Delabie J, Bentzen H, Schildt J, Kostova-Aherdan K, Frederiksen H, De Nully Brown P. Geisler CH. Nordic MCL3 Study: Zevalin combined with high-dose chemotherapy followed by autologous stem cell support as late intensification for mantle cell lymphoma (MCL) patients < 66 years not in CR after induction chemoimmunotherapy: no benefit of Zevalin. Blood (ASH Annual Meeting Abstracts) 2012;120:747. [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G. Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nature Medicine. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- Louie DC, Offit K, Jaslow R, Parsa NZ, Murty VV, Schluger A. Chaganti RS. p53 overexpression as a marker of poor prognosis in mantle cell lymphomas with t(11;14)(q13;q32) Blood. 1995;86:2892–2899. [PubMed] [Google Scholar]
- Mozos A, Royo C, Hartmann E, De Jong D, Baro C, Valera A, Fu K, Weisenburger DD, Delabie J, Chuang SS, Jaffe ES, Ruiz-Marcellan C, Dave S, Rimsza L, Braziel R, Gascoyne RD, Sole F, Lopez-Guillermo A, Colomer D, Staudt LM, Rosenwald A, Ott G, Jares P. Campo E. SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1-negative subtype. Haematologica. 2009;94:1555–1562. doi: 10.3324/haematol.2009.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Clot G, Royo C, Jares P, Hadzidimitriou A, Agathangelidis A, Bikos V, Darzentas N, Papadaki T, Salaverria I, Pinyol M, Puig X, Palomero J, Vegliante MC, Amador V, Martinez-Trillos A, Stefancikova L, Wiestner A, Wilson W, Pott C, Calasanz MJ, Trim N, Erber W, Sander B, Ott G, Rosenwald A, Colomer D, Gine E, Siebert R, Lopez-Guillermo A, Stamatopoulos K, Bea S. Campo E. Molecular subsets of mantle cell lymphoma defined by the IGHV mutational status and SOX11 expression have distinct biologic and clinical features. Cancer Research. 2012;72:5307–5316. doi: 10.1158/0008-5472.CAN-12-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom L, Andreasson U, Jerkeman M, Dictor M, Borrebaeck C. Ek S. Expanded clinical and experimental use of SOX11 - using a monoclonal antibody. BMC Cancer. 2012;12:269. doi: 10.1186/1471-2407-12-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren L, Baumgartner Wennerholm S, Klimkowska M, Christensson B, Kimby E. Sander B. Prognostic role of SOX11 in a population-based cohort of mantle cell lymphoma. Blood. 2012;119:4215–4223. doi: 10.1182/blood-2011-12-400580. [DOI] [PubMed] [Google Scholar]
- Oka K, Ohno T, Kita K, Yamaguchi M, Takakura N, Nishii K, Miwa H. Shirakawa S. PRAD1 gene over-expression in mantle-cell lymphoma but not in other low-grade B-cell lymphomas, including extranodal lymphoma. British Journal of Haematology. 1994;86:786–791. doi: 10.1111/j.1365-2141.1994.tb04830.x. [DOI] [PubMed] [Google Scholar]
- Romaguera JE, Fayad LE, Feng L, Hartig K, Weaver P, Rodriguez MA, Hagemeister FB, Pro B, McLaughlin P, Younes A, Samaniego F, Goy A, Cabanillas F, Kantarjian H, Kwak L. Wang M. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. British Journal of Haematology. 2010;150:200–208. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- Rosenwald A, Wright G, Wiestner A, Chan WC, Connors JM, Campo E, Gascoyne RD, Grogan TM, Muller-Hermelink HK, Smeland EB, Chiorazzi M, Giltnane JM, Hurt EM, Zhao H, Averett L, Henrickson S, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Montserrat E, Bosch F, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Fisher RI, Miller TP, LeBlanc M, Ott G, Kvaloy S, Holte H, Delabie J. Staudt LM. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3:185–197. doi: 10.1016/s1535-6108(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Royo C, Navarro A, Clot G, Salaverria I, Gine E, Jares P, Colomer D, Wiestner A, Wilson WH, Vegliante MC, Fernandez V, Hartmann EM, Trim N, Erber WN, Swerdlow SH, Klapper W, Dyer MJ, Vargas-Pabon M, Ott G, Rosenwald A, Siebert R, Lopez-Guillermo A, Campo E. Bea S. Non-nodal type of mantle cell lymphoma is a specific biological and clinical subgroup of the disease. Leukemia. 2012;26:1895–1898. doi: 10.1038/leu.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaverria I, Royo C, Carvajal-Cuenca A, Clot G, Navarro A, Valera A, Song JY, Woroniecka R, Rymkiewicz G, Klapper W, Hartmann EM, Sujobert P, Wlodarska I, Ferry JA, Gaulard P, Ott G, Rosenwald A, Lopez-Guillermo A, Quintanilla-Martinez L, Harris NL, Jaffe ES, Siebert R, Campo E. Bea S. CCND2 rearrangements are the most frequent genetic events in Cyclin D1-negative mantle cell lymphoma. Blood. 2013;121:1394–1402. doi: 10.1182/blood-2012-08-452284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotta-Huspenina J, Koch I, De Leval L, Keller G, Klier M, Bink K, Kremer M, Raffeld M, Fend F. Quintanilla-Martinez L. The impact of cyclin D1 mRNA isoforms, morphology and p53 in mantle cell lymphoma: p53 alterations and blastoid morphology are strong predictors of a high proliferation index. Haematologica. 2012;97:1422–1430. doi: 10.3324/haematol.2011.055715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefancikova L, Moulis M, Fabian P, Ravcukova B, Vasova I, Muzik J, Malcikova J, Falkova I, Slovackova J. Smardova J. Loss of the p53 tumor suppressor activity is associated with negative prognosis of mantle cell lymphoma. International Journal of Oncology. 2010;36:699–706. doi: 10.3892/ijo_00000545. [DOI] [PubMed] [Google Scholar]
- Vegliante MC, Palomero J, Perez-Galan P, Roue G, Castellano G, Navarro A, Clot G, Moros A, Suarez-Cisneros H, Bea S, Hernandez L, Enjuanes A, Jares P, Villamor N, Colomer D, Martin-Subero JI, Campo E. Amador V. SOX11 regulates PAX5 expression and blocks terminal B-cell differentiation in aggressive mantle cell lymphoma. Blood. 2013;121:2175–2185. doi: 10.1182/blood-2012-06-438937. [DOI] [PubMed] [Google Scholar]
- Wang X, Asplund AC, Porwit A, Flygare J, Smith CI, Christensson B. Sander B. The subcellular Sox11 distribution pattern identifies subsets of mantle cell lymphoma: correlation to overall survival. British Journal of Haematology. 2008;143:248–252. doi: 10.1111/j.1365-2141.2008.07329.x. [DOI] [PubMed] [Google Scholar]
- Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, Barrientos JC, Chmielowska E, Radford J, Stilgenbauer S, Dreyling M, Jedrzejczak WW, Johnson P, Spurgeon SE, Li L, Zhang L, Newberry K, Ou Z, Cheng N, Fang B, McGreivy J, Clow F, Buggy JJ, Chang BY, Beaupre DM, Kunkel LA. Blum KA. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. New England Journal of Medicine. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenburger DD. Armitage JO. Mantle cell lymphoma– an entity comes of age. Blood. 1996;87:4483–4494. [PubMed] [Google Scholar]
- Yatabe Y, Suzuki R, Tobinai K, Matsuno Y, Ichinohasama R, Okamoto M, Yamaguchi M, Tamaru J, Uike N, Hashimoto Y, Morishima Y, Suchi T, Seto M. Nakamura S. Significance of cyclin D1 overexpression for the diagnosis of mantle cell lymphoma: a clinicopathologic comparison of cyclin D1-positive MCL and cyclin D1-negative MCL-like B-cell lymphoma. Blood. 2000;95:2253–2261. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient characteristics of the full MCL2/3 cohort compared to available and used cohort.
Table SII. Iterative Cox-regression of individual MIPI components where the least significant parameter is removed in each step until only statistical significant parameters remain.
Table SIII. Univariate analysis to determine most significant cut-off point for SOX11 in relation to survival.
Table SIV. Iterative Cox-regression including TP53, SOX11, MKI67 and CCND1 where the least significant parameter is removed in each step until only statistical significant parameters remain.
Table SV. Final parameters after Cox-regression of all individual MIPI components and TP53.
Table SVI. Iterative Cox-regression SOX11, MKI67 and CCND1 where the least significant parameter is removed in each step until only statistical significant parameters remain.