Abstract
Objective
To evaluate the effect of golimumab on physical function, health-related quality of life (HRQOL), and productivity in psoriatic arthritis (PsA).
Methods
GO-REVEAL was a multicenter, randomized, placebo-controlled study. Adult patients with active PsA (n = 405) received golimumab (50 or 100 mg) or placebo every 4 weeks, with early escape at week 16 (placebo → 50 mg, 50 → 100 mg) or placebo crossover to golimumab 50 mg at week 24. Patient-reported outcomes included physical function (Health Assessment Questionnaire [HAQ] disability index [DI] score), HRQOL (36-item Short Form health survey [SF-36] mental component summary [MCS] and physical component summary [PCS] scores), and productivity (home/school/work). Clinical response was assessed using the 28-joint Disease Activity Score using the C-reactive protein level (DAS28-CRP) and the Psoriasis Area and Severity Index (PASI) score for arthritis and skin symptoms, respectively.
Results
At week 24, golimumab-treated patients had significant mean improvements in HAQ DI (0.36), SF-36 (PCS 7.83, MCS 3.84), and productivity (2.24) scores compared with placebo (−0.01, 0.67, −0.60, and 0.08, respectively; P <0.001 for all). Also, greater proportions of golimumab- than placebo-treated patients had clinically meaningful improvements in HAQ DI (≥0.30) and SF-36 PCS and MCS (≥5) scores at week 24 (P <0.05). Also at week 24, improvements in DAS28-CRP scores were significantly but moderately correlated with improvements in HAQ DI, SF-36 PCS, and productivity scores. Correlations between these patient-reported outcomes and improvements in PASI, enthesitis, and dactylitis scores were very weak. Improvements in HAQ DI, SF-36, and productivity scores were similar among all groups by week 52 and week 104 when including placebo → golimumab crossover patients.
Conclusion
Golimumab-treated patients had significant improvements in physical function, HRQOL, and productivity through week 24; these improvements correlated with clinical improvement in signs and symptoms of peripheral arthritis and were sustained through 2 years.
INTRODUCTION
Psoriatic arthritis (PsA) is a chronic, debilitating, inflammatory immune-mediated disease of the skin and joints. Patients with PsA may experience significant disability resulting from emotional distress associated with psoriatic skin lesions, as well as arthritis-related joint pain and physical limitations (1). Patients with PsA have exhibited degrees of impaired physical function and health-related quality of life (HRQOL) similar to patients with rheumatoid arthritis (RA) (2,3). Patients with PsA, who tend to be younger and are more commonly male, also have constraints on productivity similar to or worse than those observed in patients with RA (2–6).
Previous evaluations of tumor necrosis factor α (TNFα) antagonists have demonstrated the effectiveness of these agents in ameliorating disease burden (7). In a recent review of relevant head-to-head clinical trials comparing either adalimumab, etanercept, golimumab, or infliximab with placebo, these agents were determined to provide similar improvements in functional capacity and HRQOL. These treatment effects generally reached levels defined as minimum clinically important differences (8,9).
Golimumab is a human anti-TNF monoclonal antibody that binds with high affinity and specificity to soluble and transmembrane TNF and has demonstrated efficacy in RA (10), PsA (11), and ankylosing spondylitis (12). We previously reported on the efficacy and safety of golimumab in patients with PsA through week 104 of the GO-REVEAL trial, a phase III, multicenter, randomized, double-blind, placebo-controlled trial, the results of which indicated that subcutaneously administered golimumab therapy (50 or 100 mg every 4 weeks) yielded significant and sustained improvements in the signs and symptoms of active PsA, including associated skin disease (11,13,14). Here we report findings related to patient HRQOL and productivity through week 104 of the GO-REVEAL trial.
Significance & Innovations.
Six months of golimumab therapy yielded significant and clinically meaningful improvements in physical function, health-related quality of life, and productivity in patients with active psoriatic arthritis (PsA) despite conventional therapy.
In golimumab-treated PsA patients, improvements in physical function, health-related quality of life, and productivity at week 24 were significantly but moderately correlated with improvements in disease activity as assessed by the 28-joint Disease Activity Score; improvements were maintained through 2 years of golimumab therapy.
PATIENTS AND METHODS
Patients
Patient eligibility criteria for the GO-REVEAL trial have been previously detailed (11). Briefly, eligible patients had active PsA despite therapy with disease-modifying antiinflammatory drugs or nonsteroidal antiinflammatory drugs (NSAIDs). Active PsA was defined as ≥3 swollen and ≥3 tender joints and a qualifying plaque psoriasis lesion, i.e., ≥2 cm in diameter. Previous use of anti-TNF agents, rituximab, natalizumab, or cytotoxic agents was prohibited. Continuation of stable doses of methotrexate, NSAIDs, and/or corticosteroids (prednisone ≤10 mg/day) was permitted. Institutional review board or ethics committee approval and patient written informed consent were obtained prior to the study procedures.
Study design
The study design of this phase III, multicenter, double-blind, placebo-controlled trial has been reported previously (11). Briefly, 405 patients were randomized to receive subcutaneous injections of placebo, golimumab 50 mg, or golimumab 100 mg at baseline and every 4 weeks thereafter. Randomization was stratified by investigational site and baseline methotrexate use (yes/no). At week 16, patients with <10% improvement from baseline in both swollen and tender joint counts entered blinded early escape, with dose adjustment from placebo to golimumab 50 mg or from golimumab 50 mg to 100 mg. Patients in the golimumab 100 mg group who met the early escape criteria continued with blinded golimumab 100 mg. At week 24, all patients in the placebo group who were still receiving placebo crossed over to golimumab 50 mg. After the week 52 database lock, one-time golimumab dose adjustments (50→100 mg or 100→50 mg) were permitted at the investigator's discretion.
Study end points
Physical function was assessed using the Health Assessment Questionnaire (HAQ) disability index (DI) (15), with clinically meaningful change defined as a decrease (improvement) of ≥0.30 (16,17). HRQOL was assessed using the physical component summary (PCS) and mental component summary (MCS) scores of the 36-item Short Form health survey (SF-36) (18). In post hoc analyses of these quality of life variables, a clinically meaningful change was defined as an improvement of ≥5 points (19). The impact of PsA on daily productivity at work, school, or home in the previous 4 weeks was self-reported on a visual analog scale (VAS) ranging from 0–10 cm, where 0 = productivity not affected at all and 10 = productivity affected very much.
Among patients with ≥3% of body surface area with psoriasis skin involvement at baseline, response of psoriasis symptoms was assessed using ≥75% improvement in Psoriasis Area and Severity Index (PASI75) (20) response criteria. Arthritis disease activity was assessed using the American College of Rheumatology criteria for ≥20% improvement (ACR20) (21) and also with the 28-joint Disease Activity Score using the C-reactive protein level (DAS28-CRP) (22). In post hoc analyses, the proportions of patients with a DAS28-CRP score <2.6 were determined (23).
Dactylitis was scored in each of 20 fingers and toes from 0–3, where 0 = no dactylitis and 3 = severe dactylitis (range 0–60). Entheseal tenderness was scored for 15 sites (where 0 = absent and 1 = present, range 0–15) using the PsA-modified (left and right insertion of plantar fascia added) Maastricht Ankylosing Spondylitis Enthesitis Score (24). For both enthesitis and dactylitis, a score ≥1 denoted presence.
Statistical methods
As prespecified in the study analytical plan, treatment group differences at weeks 16 and 24 were assessed with a 2-sided analysis of variance on the van der Waerden normal scores test for continuous variables or the Cochran-Mantel-Haenszel test for categorical variables. All tests were conducted with patients' baseline methotrexate usage (yes/no) as a factor in the model and at a significance level of 0.05. Week 16 and week 24 data were analyzed using the intent-to-treat method, so that analyses were based on randomized treatment. Although patients who discontinued treatment were encouraged to return for scheduled visits, any missing data were replaced using the last observation carried forward (LOCF) method. For patients in the placebo or golimumab 50 mg group who entered early escape, the last observation before their dose adjustment at week 16 was used for week 24 analyses (LOCF method). Patients in the 100 mg group were not eligible to receive a dose adjustment according to the protocol; therefore, no adjustment of week 24 data was made for patients who met the early escape criteria. For all week 52 and week 104 analyses, a completer analysis utilizing observed data was employed.
In post hoc analyses, Spearman's correlation analysis was performed to evaluate the relationships between improvements from baseline to week 24 in DAS28-CRP, enthesitis, dactylitis, and PASI scores and improvements from baseline to week 24 in HAQ DI score, SF-36 PCS and MCS scores, and the impact of disease on productivity. Also, the proportion of patients with normal HAQ DI (≤0.5) and SF-36 PCS (≥50) and MCS (≥50) scores and the percent improvement in the impact of disease on productivity at both weeks 52 and 104 were summarized, with patients stratified by achievement (or not) of a DAS28-CRP score <2.6. Odds ratios (ORs) for patients achieving normal HAQ DI or SF-36 summary scores among patients who achieved the DAS28-CRP criterion were estimated using logistic regression models that were adjusted for age, sex, and baseline HAQ DI or SF-36 summary scores.
RESULTS
Patient disposition and characteristics at baseline
Four hundred five patients were randomized and received the study agent; 25 patients discontinued treatment through week 24 (11), 47 discontinued through week 52 (13), and 70 discontinued through week 104 (14). Baseline characteristics indicated active disease (mean DAS28-CRP scores of 4.9–5.0 and mean CRP levels of 1.3–1.4 mg/dl) and a moderate impact of disease on productivity (mean scores of 5.2–5.6 on a 10-cm VAS). No relevant differences in baseline patient characteristics were observed among the randomized treatment groups (Table1).
Table 1.
Baseline patient characteristics*
| Placebo (n = 113) | Golimumab | |||
|---|---|---|---|---|
| 50 mg (n = 146) | 100 mg (n = 146) | Combined (n = 292) | ||
| Men, no. (%) | 69 (61) | 89 (61) | 86 (59) | 175 (60) |
| White, no. (%) | 110 (97) | 141 (97) | 142 (97) | 283 (97) |
| Age, years | 47.0 ± 10.6 | 45.7 ± 10.7 | 48.2 ± 10.9 | 47.0 ± 10.9 |
| PsA duration, years | 7.6 ± 7.9 | 7.2 ± 6.8 | 7.7 ± 7.8 | 7.5 ± 7.3 |
| No. of swollen joints (range 0–66) | 13.4 ± 9.8 | 14.1 ± 11.4 | 12.0 ± 8.5 | 13.0 ± 10.1 |
| No. of tender joints (range 0–68) | 21.9 ± 14.7 | 24.0 ± 17.1 | 22.5 ± 15.7 | 23.3 ± 16.4 |
| CRP level, mg/dl | 1.3 ± 1.6 | 1.3 ± 1.6 | 1.4 ± 1.8 | 1.3 ± 1.7 |
| HAQ DI score (range 0–3) | 1.03 ± 0.55 | 0.98 ± 0.65 | 1.05 ± 0.62 | 1.02 ± 0.64 |
| SF-36 PCS score (range 0–100) | 31.9 ± 9.3 | 33.0 ± 10.7 | 32.8 ± 8.9 | 32.9 ± 9.8 |
| SF-36 MCS score (range 0–100) | 47.6 ± 10.7 | 45.4 ± 12.2 | 45.0 ± 11.7 | 45.2 ± 12.0 |
| DAS28-CRP score | 4.9 ± 1.0 | 5.0 ± 1.1 | 4.9 ± 1.1 | 4.9 ± 1.1 |
| PASI score (range 0–72)† | 8.4 ± 7.4 | 9.8 ± 8.6 | 11.1 ± 9.5 | 10.4 ± 9.1 |
| Patients with fingers/toes with dactylitis, no. (%) | 38 (34) | 50 (34) | 49 (34) | 99 (34) |
| Patients with enthesitis, no. (%)‡ | 88 (78) | 109 (75) | 115 (79) | 224 (77) |
| Productivity score (0–10-cm VAS) | 5.3 ± 2.8 | 5.2 ± 2.9 | 5.6 ± 2.6 | 5.4 ± 2.8 |
Values are the mean ± SD unless otherwise indicated. PsA = psoriatic arthritis; CRP = C-reactive protein; HAQ = Health Assessment Questionnaire; DI = disability index; SF-36 = 36-item Short Form health survey; PCS = physical component summary; MCS = mental component summary; DAS28-CRP = 28-joint Disease Activity Score using the CRP level; PASI = Psoriasis Area and Severity Index; VAS = visual analog scale.
In patients with ≥3% of body surface area with psoriasis skin involvement.
As determined using the PSA-modified Maastricht Ankylosing Spondylitis Enthesitis Score index.
Physical function, HRQOL, and productivity through week 24
Mean ± SD improvements from baseline to week 14 in the HAQ DI score were significantly greater in the golimumab 50 mg (0.31 ± 0.50) and 100 mg (0.38 ± 0.51) groups when compared with the placebo group (0.04 ± 0.44; P <0.001 for both comparisons), and these differences were maintained at week 24 (Table2). Similarly, improvements in SF-36 PCS and MCS scores at weeks 14 and 24 were significantly greater in both golimumab groups than in the placebo group (Table2). Additionally, significantly greater proportions of patients in the golimumab groups achieved clinically meaningful improvements in HAQ DI and SF-36 PCS scores at weeks 14 and 24 (Figure 1).
Table 2.
Improvements from baseline to weeks 14, 24, 52, and 104 in physical function, health-related quality of life, and impact of disease on productivity*
| Placebo (n = 113) | Golimumab | |||
|---|---|---|---|---|
| 50 mg (n = 146) | 100 mg (n = 146) | Combined (n = 292) | ||
| HAQ DI score | ||||
| Week 14 | 0.04 ± 0.44 | 0.31 ± 0.50† | 0.38 ± 0.51† | 0.35 ± 0.50† |
| Week 24‡ | −0.01 ± 0.49 | 0.33 ± 0.55† | 0.39 ± 0.50† | 0.36 ± 0.53† |
| Week 52§ | 0.37 ± 0.56 | 0.41 ± 0.53 | 0.43 ± 0.53 | 0.42 ± 0.53 |
| Week 104§ | 0.36 ± 0.58 | 0.43 ± 0.56 | 0.45 ± 0.55 | 0.44 ± 0.55 |
| SF-36 PCS score | ||||
| Week 14 | 0.63 ± 7.68 | 6.53 ± 8.88† | 7.85 ± 9.55† | 7.19 ± 9.23† |
| Week 24‡ | 0.67 ± 8.72 | 7.42 ± 9.17† | 8.22 ± 9.64† | 7.83 ± 9.41† |
| Week 52§ | 8.25 ± 10.50 | 9.87 ± 9.51 | 9.19 ± 10.29 | 9.53 ± 9.90 |
| Week 104§ | 8.76 ± 11.41 | 8.70 ± 9.56 | 8.50 ± 10.45 | 8.60 ± 10.00 |
| SF-36 MCS score | ||||
| Week 14 | 0.40 ± 11.39 | 2.79 ± 10.27¶ | 3.56 ± 12.06¶ | 3.18 ± 11.20¶ |
| Week 24‡ | −0.60 ± 12.13 | 3.37 ± 10.55# | 4.29 ± 11.03# | 3.84 ± 10.79† |
| Week 52§ | 3.69 ± 11.23 | 3.95 ± 11.73 | 4.84 ± 11.60 | 4.40 ± 11.65 |
| Week 104§ | 2.99 ± 11.08 | 4.71 ± 11.35 | 4.69 ± 12.21 | 4.70 ± 11.77 |
| Impact of disease on productivity | ||||
| Week 16‡ | 0.01 ± 2.56 | 1.69 ± 2.77† | 2.43 ± 2.98† | 2.06 ± 2.90† |
| Week 24‡ | 0.08 ± 2.62 | 1.86 ± 2.74† | 2.63 ± 2.99† | 2.24 ± 2.89† |
| Week 52§ | 2.81 ± 3.23 | 2.58 ± 2.65 | 2.88 ± 3.06 | 2.74 ± 2.87 |
| Week 104§ | 2.80 ± 3.20 | 2.50 ± 2.83 | 3.12 ± 3.11 | 2.82 ± 2.99 |
Values are the mean ± SD. HAQ = Health Assessment Questionnaire; DI = disability index; SF-36 = 36-item Short Form health survey; PCS = physical component summary; MCS = mental component summary.
P <0.001 vs. placebo.
Week 16 and week 24 data were analyzed using the intent-to-treat method; the last observation carried forward method was employed to impute missing data. For patients in the placebo or golimumab 50 mg group who entered early escape, the last observation before their dose adjustment at week 16 was used for week 24 analyses. Patients in the 100 mg group were not eligible to receive a dose adjustment according to the protocol; therefore, no adjustment of week 24 data was made for patients who met the early escape criteria.
For all week 52 and week 104 analyses, a completer analysis utilizing observed data was employed.
P <0.05 vs. placebo.
P <0.01 vs. placebo.
Figure 1.
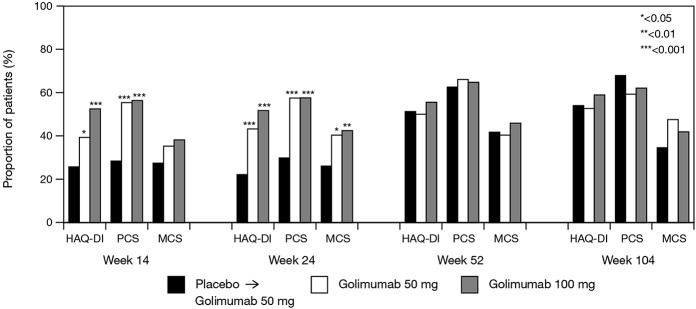
Proportions of patients achieving a clinically meaningful change from baseline in Health Assessment Questionnaire (HAQ) disability index (DI; ≥0.30) and 36-item Short Form health survey physical component summary (PCS; ≥5) and mental component summary (MCS; ≥5) scores at weeks 14, 24, 52, and 104. At weeks 52 and 104, all patients initially randomized to placebo were receiving golimumab (50 or 100 mg), and some patients initially randomized to golimumab 50 mg were receiving golimumab 100 mg.
The impact of disease on productivity was also significantly decreased in both the golimumab 50 and 100 mg groups versus the placebo group at weeks 16 and 24 (Table2). Among all patients who received golimumab, the mean ± SD impact of disease on productivity lessened by>2 points, while there was little change from baseline in the placebo group at both week 16 (2.06 ± 2.90 versus 0.01 ± 2.56; P <0.001) and week 24 (2.24 ± 2.89 versus 0.08 ± 2.62; P <0.001) (Table2).
Among all patients, improvements from baseline to week 24 in DAS28-CRP scores were significantly and moderately correlated with improvements in HAQ DI and SF-36 PCS scores and the impact of disease on productivity. In addition, patients with more severe disease at baseline, as assessed by DAS28-CRP or PASI scores, demonstrated greater improvement in these patient-reported outcomes. Improvement in enthesitis and dactylitis also correlated with improvements in HAQ DI and SF-36 PCS scores, as well as with improvements in the impact of disease on productivity. In all cases, however, the correlations were relatively weak, with correlation coefficients <0.3 (data not shown).
Overall, patients achieving an ACR20 and/or PASI75 response had greater improvement in productivity and the SF-36 PCS and MCS scores versus patients who did not achieve such response(s). The observed improvements in work productivity and SF-36 PCS scores were more closely related to ACR20 response, whereas improvement in the SF-36 MCS score was more closely related to improvement in both arthritis and skin symptoms of PsA (Figure 2).
Figure 2.
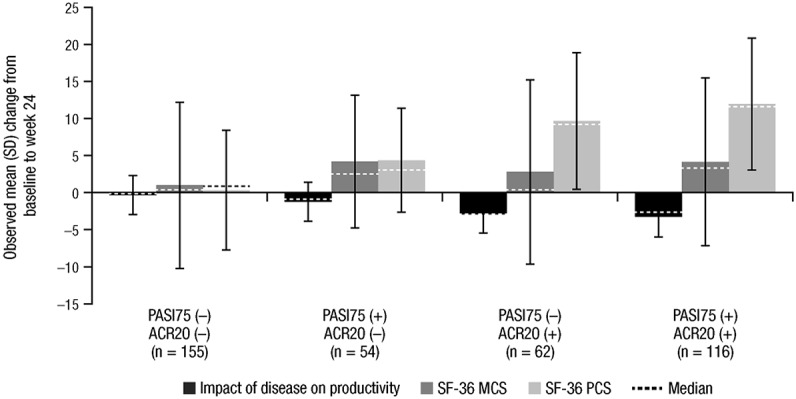
Relationships between improvements in physical component summary (PCS) and mental component summary (MCS) scores of the 36-item Short Form health survey (SF-36) and the impact of disease on productivity and achievement (+) or nonachievement (−) of an American College of Rheumatology ≥20% improvement criteria (ACR20) and Psoriasis Area and Severity Index ≥75% improvement (PASI75) response criteria at week 24.
Physical function, HRQOL, and productivity through week 104
Early improvements in physical function and HRQOL, as well as the lessened impact of disease on productivity, were maintained and/or enhanced through week 104 among patients in the golimumab groups (Table2). In addition, patients who were randomized to placebo and crossed over to golimumab 50 mg at week 16 (early escape) or week 24 (crossover) achieved mean improvements in HAQ DI, SF-36 PCS, SF-36 MCS, and impact of disease on productivity scores at weeks 52 and 104 that were similar to those observed in patients who started golimumab therapy at week 0 (Table2). Likewise, the proportions of patients with clinically meaningful improvements in HAQ DI and SF-36 PCS and MCS scores were generally similar among the 3 treatment groups at weeks 52 and 104, although patients who received placebo followed by golimumab appeared to experience less improvement in the SF-36 MCS score at week 104 than patients who received golimumab at the study outset (Figure 1).
At week 52, after adjusting for age, sex, and HAQ DI or SF-36 PCS or MCS scores at baseline, and when compared with patients who did not achieve a DAS28-CRP score <2.6, greater proportions of patients who achieved a DAS28-CRP score <2.6 also had normal physical function, i.e., a HAQ DI score ≤0.5 (78.8% versus 30.0%; OR 6.8, P <0.0001), and normal (≥50) PCS (55.6% versus 12.6%; OR 8.4, P <0.0001) and MCS (68.8% versus 52.1%; OR 2.0, P <0.01) scores. Comparable findings were observed when these analyses were repeated with week 104 data (Table3). Likewise, productivity was improved to a greater extent in patients who achieved a DAS28-CRP score <2.6 versus those who did not at both week 52 (median improvement of 82.5% versus 33.3%; P <0.001) and week 104 (median improvement of 82.1% versus 29.4%; P <0.001).
Table 3.
Physical function and health-related quality of life by achievement of a DAS28-CRP score <2.6 in all treatment groups combined*
| Week 52 | Week 104 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | No | Yes | OR† | P | N | No | Yes | OR† | P | |
| HAQ DI score ≤0.5 | 348 | 57 (30.0) | 126 (78.8) | 6.8 | <0.0001 | 328 | 55 (34.2) | 127 (75.1) | 4.9 | <0.0001 |
| SF-36 PCS score ≥50 | 347 | 24 (12.6) | 89 (55.6) | 8.4 | <0.0001 | 328 | 11 (6.8) | 79 (46.7) | 9.9 | <0.0001 |
| SF-36 MCS score ≥50 | 347 | 99 (52.1) | 110 (68.8) | 2.0 | <0.01 | 328 | 81 (50.0) | 120 (71.0) | 2.5 | <0.001 |
Values are the number (percentage) unless otherwise indicated. DAS28-CRP = 28-joint Disease Activity Score using the C-reactive protein level; OR = odds ratio; HAQ = Health Assessment Questionnaire; DI = disability index; SF-36 = 36-item Short Form health survey; PCS = physical component summary; MCS = mental component summary.
ORs were estimated based on the logistic regression model by adjusting for age, sex, and baseline value (HAQ DI or SF-36 PCS or MCS scores).
DISCUSSION
In the GO-REVEAL trial, a phase III, multicenter, randomized, placebo-controlled study, the safety and efficacy of subcutaneous golimumab (50 or 100 mg) was assessed in 405 adult patients with PsA. Golimumab-treated patients with PsA had significant improvements in measures of joint and skin disease versus placebo (11), and treatment benefits were maintained through week 104 (13,14). The golimumab safety profile was acceptable during this same time period and was similar to that of other anti-TNF agents (11,13,14). Owing to the substantial impact that PsA can have on patient physical function and HRQOL (2,3), which in turn affect work ability (productivity), the GO-REVEAL trial also included measurements of patient physical function, HRQOL, and productivity. In addition to providing further evidence of the clinical efficacy of golimumab treatment, such assessments provide information on whether this efficacy is truly “meaningful” from patient and societal perspectives.
At baseline, patients enrolled in the GO-REVEAL trial had SF-36 PCS and MCS scores that were lower than those in the general population of the US, indicating impairment in both the physical and psychosocial aspects of HRQOL. The effects of golimumab on physical function as measured by the HAQ DI, HRQOL as measured by the SF-36 PCS and MCS scores, and the impact of disease on productivity appear to be substantial through week 104 of the GO-REVEAL trial. Through week 24 (the placebo-controlled period), patients treated with golimumab 50 or 100 mg had significantly greater improvements in physical function, HRQOL, and the impact of disease on productivity when compared with patients who received placebo; these improvements with golimumab treatment are generally comparable in magnitude to those observed with infliximab therapy for PsA (25). Improvements at week 24 were maintained in the golimumab groups at weeks 52 and 104, at which time improvements in physical function, HRQOL, and productivity were similar among the 3 treatment groups, including patients randomized to placebo treatment who had a 4- to 6-month delay in initiating golimumab.
At week 24, improvements in arthritis symptoms (per the DAS28-CRP score) were significantly correlated with improvements in physical function and the physical component of HRQOL, and a decrease in the impact of disease on productivity. Overall, patients achieving an ACR20 and/or PASI75 response exhibited greater improvement in productivity and in the SF-36 PCS and MCS scores than did patients not achieving an ACR20 or PASI75 response. Although improvements in work productivity and the SF-36 PCS score more closely paralleled an ACR20 response, improvement in the SF-36 MCS score was more in line with achievement of both an ACR20 and PASI75 response, which would not be unexpected given the additional psychosocial burden that accompanies the skin component of PsA (26). Compared with improvement in the SF-36 PCS score, improvement in the SF-36 MCS score was numerically smaller. These findings may be related to the greater impairment in physical function (SF-36 PCS) than mental function (SF-36 MCS) at baseline (means of 31.9–33.0 versus 45.0–47.6), which is consistent with findings generally observed in inflammatory rheumatic diseases (26), and would make it difficult to discern improvements over time. Another possibility is that changes in psychologically-based measures, such as the SF-36 MCS score, may lag behind changes observed in the physical aspects of PsA. Although greater improvements from baseline to week 24 in enthesitis and dactylitis scores were observed in golimumab- versus placebo-treated patients (data not shown), these improvements exhibited only weak correlations with physical function, HRQOL, and work productivity. The reasons for the weak correlations are not entirely clear, but may relate to some extent to the methods currently used to assess these areas of involvement. At present, there is an ongoing debate concerning the optimal means by which dactylitis and enthesitis should be assessed. At the time this study was designed, the analyses chosen and included in this study represented the consensus of those involved in the study design. However, it is possible that more sensitive methods of evaluation (e.g., ultrasound examination) may allow more detailed quantification of change; therefore, these outcomes might correlate more strongly with outcomes such as HRQOL, physical function, and productivity.
Furthermore, greater proportions of patients who achieved a DAS28-CRP score <2.6 at week 52 and week 104 achieved normal physical function and normal HRQOL when compared with patients who did not. Likewise, productivity was improved to a greater extent at both week 52 and week 104 in patients who achieved a DAS28-CRP score <2.6 at these time points.
Assessment of productivity in patients and the impact of treatment is an important and practical way to measure disability from individual and societal perspectives. We evaluated the impact of PsA on daily productivity at work, school, or home in the previous 4 weeks using a self-reported 10-cm VAS. Although this specific instrument has not undergone rigorous measurement property testing, it has been used in previously conducted clinical trials of biologic agents in ankylosing spondylitis (27) and PsA (28), and has been determined to be significantly correlated with physical function and disease activity, as well as sensitive to change in physical function and disease activity (27). In addition, the use of a similar tool, i.e., a 0–100 VAS, where 0 = not affected at all and 100 = completely affected, in measuring the impact of RA on work performance has demonstrated acceptable reliability and validity (29). Therefore, it is likely that the significant and sustained improvement we observed in patient productivity with golimumab therapy will result in decreased indirect costs over the long term. Although our evaluations extended through week 104 of the trial, findings are limited by the lack of a placebo control group beyond week 24.
In conclusion, monthly subcutaneous injections of golimumab 50 or 100 mg significantly improved physical function and HRQOL and lessened the impact of disease on productivity in patients with active PsA. These findings indicate that treatment with golimumab significantly reduces the overall disease burden placed on patients with PsA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Kavanaugh had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Kavanaugh, McInnes, Gladman, Beutler, Mack, Tandon, Han, Mease.
Acquisition of data. Kavanaugh, Gladman, Beutler, Mease.
Analysis and interpretation of data. Kavanaugh, McInnes, Krueger, Gladman, Gathany, Mack, Han, Mease.
ROLE OF THE STUDY SPONSOR
Janssen Research & Development employees serving as authors on this paper had a role in the study design, data collection, data analysis, and writing of the manuscript, as well as approval of the content of the submitted manuscript. Publication of this article, however, was not contingent on the approval of Janssen Research & Development or Merck/Schering-Plough. All authors were required to follow the International Committee of Medical Journal Editors criteria that included approving the final content of the manuscript.
Acknowledgments
The authors would like to thank the patients, investigators, and study personnel who made the GO-REVEAL study possible. We acknowledge Michelle Perate, MS, Rebecca Clemente, PhD, and Mary Whitman, PhD, of Janssen Services, LLC, for their assistance in preparing the manuscript, and Dr. Julie Zrubek, a former employee of Janssen who is currently an employee of GlaxoSmithKline, for assistance with the GO-REVEAL trial.
REFERENCES
- 1.Kimball AB, Jackson JM, Sobell JM, Boh EE, Grekin S, Yu EB, et al. Reductions in healthcare resource utilization in psoriatic arthritis patients receiving etanercept therapy: results from the EDUCATE trial. J Drugs Dermatol. 2007;6:299–306. [PubMed] [Google Scholar]
- 2.Husted JA, Gladman DD, Farewell VT, Cook RJ. Health-related quality of life of patients with psoriatic arthritis: a comparison with patients with rheumatoid arthritis. Arthritis Rheum. 2001;45:151–8. doi: 10.1002/1529-0131(200104)45:2<151::AID-ANR168>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 3.Sokoll KB, Helliwell PS. Comparison of disability and quality of life in rheumatoid and psoriatic arthritis. J Rheumatol. 2001;28:1842–6. [PubMed] [Google Scholar]
- 4.Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27:864–72. doi: 10.1002/art.1780270805. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe F, Hawley DJ. The longterm outcomes of rheumatoid arthritis. Work disability: a prospective 18 year study of 823 patients. J Rheumatol. 1998;25:2108–17. [PubMed] [Google Scholar]
- 6.Yelin E, Henke C, Epstein W. The work dynamics of the person with rheumatoid arthritis. Arthritis Rheum. 1987;30:507–12. doi: 10.1002/art.1780300504. [DOI] [PubMed] [Google Scholar]
- 7.Strand V, Sharp V, Koenig AS, Park G, Shi Y, Wang B, et al. Comparison of health-related quality of life in rheumatoid arthritis, psoriatic arthritis and psoriasis and effects of etanercept treatment. Ann Rheum Dis. 2012;71:1143–50. doi: 10.1136/annrheumdis-2011-200387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donahue KE, Jonas D, Hansen RA, Roubey R, Jonas B, Lux LJ, et al. Drug therapy for psoriatic arthritis in adults: update of a 2007 report. Rockville (MD): Agency for Healthcare Research and Quality; 2012. [PubMed] [Google Scholar]
- 9.Emery P, Gabay C, Kraan M, Gomez-Reino J. Evidence-based review of biologic markers as indicators of disease progression and remission in rheumatoid arthritis. Rheumatol Int. 2007;27:793–806. doi: 10.1007/s00296-007-0357-y. [DOI] [PubMed] [Google Scholar]
- 10.Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, et al. Golimumab, a human antibody to tumour necrosis factor α given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD study. Ann Rheum Dis. 2009;68:789–96. doi: 10.1136/ard.2008.099010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, et al. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four–week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009;60:976–86. doi: 10.1002/art.24403. [DOI] [PubMed] [Google Scholar]
- 12.Inman RD, Davis JC, Jr, van der Heijde D, Diekman L, Sieper J, Kim SI, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008;58:3402–12. doi: 10.1002/art.23969. [DOI] [PubMed] [Google Scholar]
- 13.Kavanaugh A, van der Heijde D, McInnes IB, Mease P, Krueger GG, Gladman DD, et al. Golimumab in psoriatic arthritis: one-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum. 2012;64:2504–17. doi: 10.1002/art.34436. [DOI] [PubMed] [Google Scholar]
- 14.Kavanaugh A, McInnes IB, Mease PJ, Krueger GG, Gladman DD, van der Heijde D, et al. Clinical efficacy, radiographic, and safety findings through 2 years of golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of the randomized, placebo-controlled, GO-REVEAL study. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-202035. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 16.Mease PJ, Antoni CE, Gladman DD, Taylor WJ. Psoriatic arthritis assessment tools in clinical trials. Ann Rheum Dis. 2005;64(Suppl):ii49–54. doi: 10.1136/ard.2004.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mease PJ, Woolley JN, Bitman B, Wang BC, Globe DR, Singh A. Minimally important difference of Health Assessment Questionnaire in psoriatic arthritis: relating thresholds of improvement in functional ability to patient-rated importance and satisfaction. J Rheumatol. 2011;38:2461–5. doi: 10.3899/jrheum.110546. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Sherbourne CD. The MOS 36-item Short-Form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 19.Strand SV, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Manag Care. 2008;14:234–54. [PubMed] [Google Scholar]
- 20.Fredriksson T, Pettersson U. Severe psoriasis: oral therapy with a new retinoid. Dermatologica. 1978;157:238–44. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 21.Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–35. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- 22.Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 23.Castrejon I, Ortiz AM, Toledano E, Castaneda S, Garcia-Vadillo A, Patino E, et al. Estimated cutoff points for the 28-joint Disease Activity Score based on C-reactive protein in a longitudinal register of early arthritis. J Rheumatol. 2010;37:1439–43. doi: 10.3899/jrheum.091333. [DOI] [PubMed] [Google Scholar]
- 24.Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A, Landewe R, van der Tempel H, Mielants H, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis. 2003;62:127–32. doi: 10.1136/ard.62.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kavanaugh A, Antoni C, Krueger GG, Yan S, Bala M, Dooley LT, et al. Infliximab improves health related quality of life and physical function in patients with psoriatic arthritis. Ann Rheum Dis. 2006;65:471–7. doi: 10.1136/ard.2005.040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes. 2009;7:25. doi: 10.1186/1477-7525-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van der Heijde D, Han C, DeVlam K, Burmester G, van den Bosch F, Williamson P, et al. Infliximab improves productivity and reduces workday loss in patients with ankylosing spondylitis: results from a randomized, placebo-controlled trial. Arthritis Rheum. 2006;55:569–74. doi: 10.1002/art.22097. [DOI] [PubMed] [Google Scholar]
- 28.Kavanaugh A, Antoni C, Mease P, Gladman D, Yan S, Bala M, et al. Effect of infliximab therapy on employment, time lost from work, and productivity in patients with psoriatic arthritis. J Rheumatol. 2006;33:2254–9. [PubMed] [Google Scholar]
- 29.Revicki D, Roy S, Kimel M, Thompson C, Cifaldi M. Validation of the work performance visual analog scale: measuring work and household productivity of patients with early, aggressive rheumatoid arthritis. Arthritis Rheum. 2010;62(Suppl):S37. [abstract] [Google Scholar]


