Abstract
We report two autopsy cases of severe fever with thrombocytopenia syndrome (SFTS) with a high fatality rate in aged Japanese patients. Both cases were caused by a tick-bite. The pathognomonic histological feature was necrotizing lymphadenitis of systemic lymphoid tissue with SFTS viruses and SFTSV-RNA copies. Marked fungal infections were also observed in the lungs of both patients. Since cellular immune function may be suppressed in SFTS patients, physicians should be aware of possible fungal infections.
Keywords: autopsy cases, fungal infection, necrotizing lymphadenitis, SFTS virus-nucleoprotein antigen, SFTS
Severe fever with thrombocytopenia syndrome (SFTS) is a recently identified viral infectious disease in China that is caused by a novel bunyavirus, which is classified into the genus phlebovirus in the family Bunyaviridae, SFTS virus (SFTSV).1 SFTS occurs mainly in the spring and summer and is transmitted by tick bite. The virus can also infect people by contact with bodily fluids from SFTS patients.2–4 The disease is characterized by a sudden onset of fever, thrombocytopenia, hemorrhagic tendency, and gastrointestinal symptoms. Multi-organ dysfunction is also observed, and the mortality rate ranges from 12% to 30%.1 Cases of SFTS in Japanese patients with a higher fatality rate (55%) are very similar to the severe cases reported in Chinese patients.5 Phylogenetic analyses have indicated that some SFTSVs isolated from Japanese patients formed a genotype independent of those of Chinese patients. There is only one previous report of an autopsy case with SFTS,5 but there are no published reports describing the pathological findings of fungal infection. Another tick-borne phlebovirus, Heartland virus (HRTV), was recently discovered in the USA.6 HRTV, which is phylogenetically associated with SFTSV, causes severe febrile illness with thrombocytopenia, leukopenia, and elevated liver enzymes.6
We report the autopsy findings of two SFTS patients who died due to severe fungal infection. Since SFTS patients have a tendency for immunodeficiency,7–9 we discuss the need for awareness of possible fungal infections in these patients.
Clinical Summary
Case 1
An 83-year-old Japanese female, who lived in the Kagoshima prefecture of Japan, had a tick bite in the left inguinal area in April, 2013, and 6 days later she had a sudden onset of a mild fever with general fatigue and appetite loss. On day 5 after onset, she suffered from remittent fever (around 38 degrees) with systemic muscle pain, and laboratory test revealed leukopenia, thrombocytopenia, and elevated AST, and CPK (Table 1). She was admitted to Kanoya Medical Center and was treated with intravenous minocycline, but there was no improvement in either her clinical symptoms or laboratory data.
Table 1.
Laboratory data of case 1
| Case 1 | Reference range, adult | 5 days after onset | 8 days after onset | 12 days after onset |
|---|---|---|---|---|
| Hematocrit (%) | 35.0–48.0 | 37.3 | 40.9 | 28.5 |
| Hemoglobin (g/dl) | 12.0–16.0 | 12.9 | 14.3 | 9.8 |
| White-cell count (/μL) | 4500–8500 | 2200 | 5460 | 13180 |
| Platelet count (×104/μL) | 13.0–32.0 | 5.6 | 4.1 | 5.0 |
| Lymphocyte count (/μL) | 1000–4000 | 315 | ||
| Neutrophil count (/μL) | 1000–7500 | 1232 | ||
| Aspartate aminotransferase (IU/L) | 13–33 | 679 | 3210 | 323 |
| Alanine aminotransferase (IU/L) | 6–30 | 290 | 1073 | 282 |
| Lactate dehydrogenase (IU/L) | 119–229 | 972 | 2736 | 648 |
| Creatine phosphokinase (IU/L) | 45–163 | 4414 | 5926 | 304 |
| Creatinine (mg/dl) | 0.4-0.7 | 1.4 | 1.42 | 2.94 |
| C-reactive protein (mg/dl) | <0.3 | 0.4 | 0.52 | 4.94 |
| Prothrombin time (%) | 70–120 | 61.4 | 100 | |
| Activated partial thromboplastin time (seconds) | 26.1–35.6 | 64.1 | 35.1 | |
| Fibrinogen (mg/dl) | 150–450 | 142 | 407 | |
| Antithrombin III (mg/dl) | 80–120 | 124 | ||
| Fibrin/fibrinogen degradation products (μg/ml) | <5.0 | 14.0 | 4.7 | |
| D dimer (μg/ml) | <1.0 | 5.4 | 0.8 | |
| (1-3)-β-D glucan (pg/ml) | <3.8 | 261.7 |
On day 8 after onset, she had marked oral hemorrhage. The laboratory test data indicated thrombocytopenia, hemorrhagic tendency, marked liver damage, and disseminated intravascular coagulation (Table 1). A contrast CT scan showed enlargement of the left inguinal lymph node. A bone marrow aspirate showed an increase in hemophagocytes (Fig. 1a). She was treated with methylpredonisolone (1400 mg equivalent to prednisolone), anti-thrombin III, and recombinant thrombomodulin and given preventive therapy with ampicillin/sulbactam and micafungin. Her clinical features suggested SFTS, which was confirmed by RT-PCR of her blood at the National Institute of Infectious Diseases (NIID) in Japan.
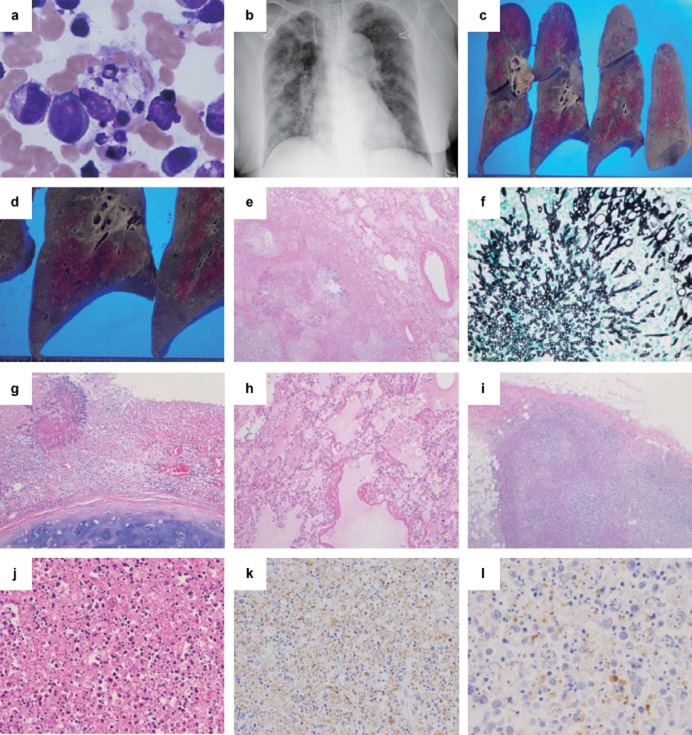
Figure 1.
Clinical images and pathological findings of Case 1. (a) Bone marrow finding and (b) chest X-ray image, (c,d) gross findings in the lungs, (e,g–j) hematoxylin and eosin staining, (f) Grocott staining, and (k,l) immunohistochemistry (IHC) using anti-SFTSV-NP antibody. (a) In the bone aspirate, many histiocytes show hemophagocytosis (×400). (b) A chest X-ray reveals a bilateral infiltrative shadow without consolidation. The cut surface of the right lung shows (c) many dispersed white nodular legions, (d) mainly in the lower lobe. (e) In the lung, there is necrotizing inflammation (×40) and (f)Aspergillus infection in the nodular lesions (Grocott staining ×400). (g) A tracheal ulcer with Aspergillus was also noted (×100). (h) Hyaline membrane formation indicating secondary diffuse alveolar damage is seen (×100). (i,j) In the left inguinal lymph node, the basic architecture of the lymph node is replaced by massive necrosis with infiltration of lymphocytes, histiocytes, some atypical lymphoid cells, and a significant amount of nuclear debris, but no neutrophils are observed (i, ×40; j, ×200). (k) In IHC of the lymph node, SFTSV-NP-positive cells are found (×100), and (l) positive staining for the SFTSV-NP antigen is detected in the cytoplasm of atypical lymphoid cells (×400).
On day 12 after onset when she was admitted to our hospital, she was afebrile, and her hemodynamics were approaching stable levels with use of low-dose dopamine. However, her oxygenation status had not recovered with the use of 80% fractional inspired O2. A chest X-ray revealed a bilateral infiltrative shadow (Fig. 1b). Laboratory tests on admission are shown in Table 1. (1-3)-β-D glucan was markedly increased (Table 1), and her blood culture was positive for pseudomonas aeruginosa. Despite administration of meropenem, amikacin, and amphotericin B, she died on day 14 after onset.
Case 2
An 88-year-old Japanese male, who lived in the Kagoshima prefecture of Japan, had a tick bite in the anterior neck in August, 2013, and 2 days later he had a remittent fever (around 38 degrees). He was treated with minocycline tablets, but there was no improvement in his clinical symptoms, and he developed anorexia and diarrhea. On day 2 after onset, he had lymph node swelling in the anterior neck and livedo reticularis-like skin rashes on both legs. Laboratory tests revealed leukopenia, thrombocytopenia, and liver damage (Table 2). The clinical features suggested SFTS, which was confirmed by RT-PCR using his blood at the NIID. Since a bone marrow aspirate showed an increase of hemophagocytes, he was given prednisolone (900 mg equivalent to prednisolone).
Table 2.
Laboratory data of case 2
| Case 2 | Reference range, adult | 2 days after onset | 5 days after onset | 9 days after onset | 11 days after onset |
|---|---|---|---|---|---|
| Hematocrit (%) | 35.0–48.0 | 38.9 | 31.7 | 33.5 | 34.5 |
| Hemoglobin (g/dl) | 12.0–16.0 | 13.7 | 11.4 | 11.7 | 12.4 |
| White-cell count (/μL) | 4500–8500 | 1000 | 1050 | 5230 | 6240 |
| Platelet count (×104/μL) | 13.0–32.0 | 5.7 | 1.4 | 7.8 | 12.0 |
| Lymphocyte count (/μL) | 1000–4000 | 347 | |||
| Neutrophil count (/μL) | 1000–7500 | 535 | |||
| Aspartate aminotransferase (IU/L) | 13–33 | 144 | 218 | 2162 | 5897 |
| Alanine aminotransferase (IU/L) | 6–30 | 64 | 75 | 370 | 544 |
| Lactate dehydrogenase (IU/L) | 119–229 | 350 | 634 | 6765 | 10018 |
| Creatine phosphokinase (IU/L) | 45–163 | 424 | 1401 | 930 | 795 |
| Blood urea nitrogen (mg/dl) | 8.0–22.0 | 25 | 16.5 | 47.5 | 20.0 |
| Creatinine (mg/dl) | 0.4-0.7 | 0.88 | 0.74 | 3.20 | 1.19 |
| C-reactive protein (mg/dl) | <0.3 | 0.1 | 0.07 | 1.74 | 0.82 |
| Prothrombin time (%) | 70–120 | 73.0 | 84 | 66 | |
| Activated partial thromboplastin time (seconds) | 26.1–35.6 | 48.7 | 66.4 | 126.0 | |
| Fibrinogen (mg/dl) | 150–450 | 137 | 113 | <70 | |
| Antithrombin III (mg/dl) | 80–120 | 75.0 | 67 | 95 | 73 |
| Fibrin/fibrinogen degradation products (μg/ml) | <5.0 | 5.1 | 5.1 | 13.0 | 8.5 |
| D dimer (μg/ml) | <1.0 | 3.1 | 2.0 | 6.6 | 3.6 |
On day 5 after onset when he was admitted to our hospital, he was not fully conscious and had respiratory failure. A non-contrast whole body CT scan indicated acute hepatitis, but no intracranial or lung lesions were observed. Laboratory tests indicated a worsening of thrombocytopenia, liver damage, and a coagulation abnormality (Table 2). On day 9 after onset, renal failure and a circulatory disturbance developed, and the respiratory failure also worsened. A chest X-ray revealed a bilateral infiltrative shadow (Fig. 2a). The thrombocytopenia peaked, but a marked increase in AST, ALT, and LDH indicated significant liver damage (Table 2). A blood culture was negative for both bacteria and fungi. He died 12 days after onset of the fever.
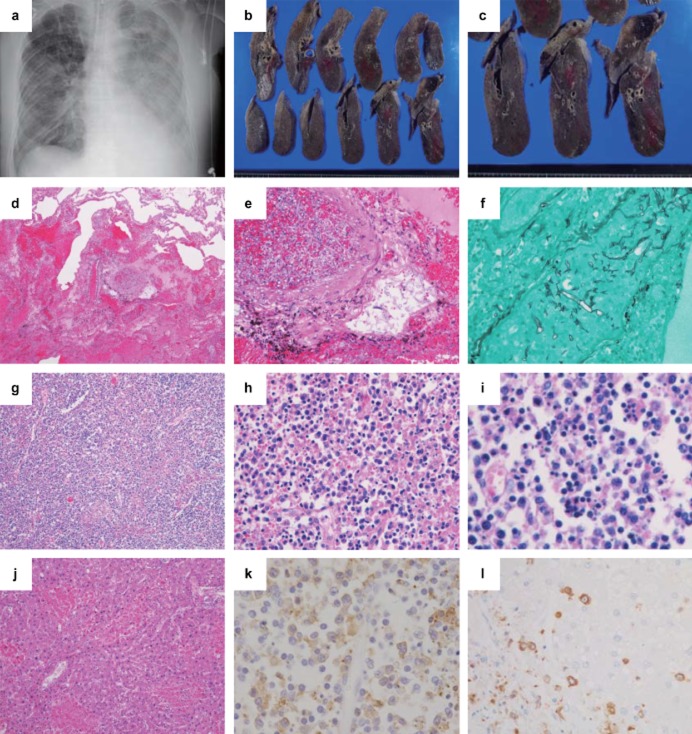
Figure 2.
Clinical images and pathological findings of case 2. (a) Chest X-ray image, (b,c) gross findings in the lungs, (d,e,g–j) hematoxylin and eosin staining, (f) Grocott staining, and (k,l) immunohistochemistry (IHC) using anti-SFTSV-NP antibody. (a) A chest X-ray reveals a bilateral infiltrative shadow without consolidation. (b,c) The cut surface of the right lung shows foci of pulmonary hemorrhage and infarction. (d) Diffuse hemorrhagic infarction (×40) and (e,f) angio-invasion of Mucor (e, ×200; f, Grocott staining ×400) are seen in the lung. (g,h) Necrotizing lymphadenitis is present in the lymph node around the abdominal aorta (g, ×40; h, ×400), and (i) hemophagocytosis is also observed (i, ×400). (j) The liver shows lobular necroses and mild portal fibrosis (×100). (k) IHC of the lymph node (×400) shows numerous SFTSV-NP-positive cells and positive signals for the SFTSV-NP antigen in the cytoplasm of atypical lymphoid cells. (l) IHC of the liver shows SFTSV-positive cells, but hepatocytes were negative for the SFTSV-NP antigen (×100).
Pathological Findings
Immnohistochemistry (IHC) was performed as previously described.5 Rabbit anti-SFTSV-nucleoprotein (NP) serum and peroxidase-labeled polymer-conjugated anti-rabbit immunoglobulin (En Vision/HRP, Dako, Glostrup, Denmark) were used.5
SFTSV RNA was extracted from paraffin-embedded tissue sections using a Pure Link FFPE RNA isolation kit (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA) at the NIID. The SFTSV copy number was determined by performing quantitative real-time RT-PCR on the tissue extracts as previously described.5,10 The amount of human β-actin mRNA in the RNA extracted from each section was also determined and used as an internal reference for normalization.10
The study was conducted in accordance with the guiding principles of the Declaration of Helsinki. Informed written consent was obtained from the family of each patient.
Case 1
At the autopsy, subcutaneous hemorrhages were observed at the neck, bilateral inguinal regions, and limbs. The lungs were heavy (Lt.: 904g, Rt.: 1284g) with many white nodular lesions (Fig. 1c,d) showing necrotizing inflammation with Aspergillus infection, and many bronchi and blood vessels were invaded by Aspergillus (Fig. 1e,f). A tracheal ulcer with Aspergillus was also noted (Fig. 1g). There was focal hyaline membrane formation, indicating diffuse alveolar damage (DAD) (Fig. 1h). Hemophagocytosis was not found in all the sections examined. The left inguinal node near the location of the tick-bite showed necrotizing lymphadenitis with apoptotic cells and necrosis (Fig. 1i). The basic architecture of the lymph node was replaced by massive necrosis with infiltration of lymphocytes, histiocytes, atypical lymphoid cells, and abundant nuclear debris, but without neutrophils (Fig. 1j). The palatine tonsil also showed similar findings. The other lymph nodes did not show necrotizing lymphadenitis. The liver (1204 g) showed single cell necroses and mild periportal lymphocytic infiltration with focal bile stasis. The central nervous system (CNS; 1130 g) showed no pathological changes.
Twenty-seven tissues were analyzed using IHC (Table 3). Granular positive signals for the SFTSV-NP antigen were detected only in the left inguinal node and in the palatine tonsil. Some SFTSV-NP antigen-positive cells were detected only in a left inguinal node (Fig. 1k,l). The SFTSV-NP antigen was negative in all other tissues.
Table 3.
Results of immunohistochemistry (IHC) and RT-PCR for severe fever with thrombocytopenia syndrome virus SFTSV
| Tissue | IHC (anti-SFTSV-NP) | RT-PCR (SFTSV-RNA, copy/cell) | ||
|---|---|---|---|---|
| Case 1 | Case 2 | Case 1 | Case2 | |
| Cerebrum | − | − | ||
| Cerebellum | − | − | ||
| Mid brain | − | − | ||
| Pons | − | − | ||
| Spinal cord | − | − | ||
| Stomach | − | + | − | 9.14 × 101 |
| Colon | + | 4.50 × 101 | ||
| Appendix vermiformis | +++ | 5.72 × 104 | ||
| Pancreas† | − | +++ | − | 8.99 × 104 |
| Spleen | − | ++++ | − | 4.35 × 104 |
| Heart | − | + | − | 1.13 × 103 |
| Liver | − | +++ | − | 1.27 × 104 |
| Left kidney | − | ++ | − | 5.40 × 103 |
| Thyroid gland | − | + | 4.11 × 10−3 | 3.99 × 102 |
| Adrenal gland | − | ++ | − | 6.85 × 103 |
| Uterus | − | − | ||
| Ovary | − | − | ||
| Urinary bladder | − | + | − | 6.58 × 102 |
| Palatine tonsil | + | 3.93 × 101 | ||
| Trachea | − | − | − | 2.43 × 102 |
| Esophagus | − | + | − | 6.70 × 101 |
| Bone marrow | − | − | ||
| Pituitary gland | − | − | ||
| Aorta | + | |||
| Gallbladder | ++ | 6.82 × 102 | ||
| Testis | + | 1.46 × 102 | ||
| Lymph node | ||||
| Mediastinum | − | ++++ | − | 2.66 × 105 |
| Left lung hilum | − | ++++ | − | 1.34 × 105 |
| Right lung hilum | − | − | ||
| Left inguinal region | ++ | 1.14 × 102 | ||
| Right inguinal region | − | 1.59 × 10−2 | ||
| Intraperitoneum | − | 5.74 × 10−3 | ||
| Paraabdominal aorta | ++++ | 1.16 × 105 | ||
Blank spaces indicate that IHC and TR-PCR were not done on those tissues.
The results were graded as follows: −. no positively-stained cells; +, under 10 cells; ++, 10–100 cells; +++, 100–500 cells; ++++, more than 500.
Including para-pancreatic lymph nodes.
Consistent with the IHC, low copies (40–110 copies/cell) of SFTSV-RNA were detected in both the left inguinal node and the palatine tonsil. Very little or no SFTSV-RNA was detected in the other tissues (Table 3).
Case 2
At the autopsy, a tick-bite wound was found in the anterior neck, and the associated cervical lymph nodes were swollen. Subcutaneous hemorrhages in the bilateral forearms, anterior chest, and abdomen were observed. The lungs were heavy (Lt.: 700g, Rt.: 800g) and showed foci of pulmonary hemorrhage and infarction (Fig. 2b,c), with pleural effusions (Lt.: 1100 mL, Rt.: 1200 mL). The bilateral lungs showed marked pulmonary hemorrhage, edema with DAD (Fig. 2d), numerous Mucor infection-forming parenchymal lesions, angio-invasion with thrombosis, and infarction (Fig. 2e,f). Necrotizing lymphadenitis, which was similar to that of the left inguinal node of case 1, with some hemophagocytes was observed in the systemic lymph nodes (Fig. 2g–i). The liver (1012g) showed multiple lobular necroses, mainly in zone 2, and mild portal fibrosis (Fig. 2j). The CNS was not available for analysis.
Eighteen tissues were analyzed using IHC (Table 3). SFTSV-NP antigen-positive atypical lymphoid cells were detected in all the organs examined except for the trachea, and the levels were especially high in both the systemic lymph nodes (Fig. 2k) and spleen. The parenchymal cells of each organ, including hepatocytes (Fig. 2l), were negative for the SFTSV-NP antigen.
Consistent with the IHC, high copies (45–266000 copies/cell) of SFTSV-RNA were detected in all of the samples, including the systemic lymph nodes and spleen (Table 3).
Discussion
The main pathological finding of SFTSV infection was necrotizing lymphadenitis with both numerous apoptotic cells and nuclear debris. However, there was a big difference in the locations of the necrotizing lymphadenitis in our cases. Case 1 had necrotizing lymphadenitis positive for the SFTSV-NP antigen only in the left inguinal lymph node and palatine tonsil. In contrast, Case 2 had necrotizing lymphadenitis in the systemic lymph nodes, and numerous SFTSV-NP antigen-positive cells were present in all examined organs. There may be a large difference in the viral infection load between our two cases i.e. significantly higher SFTSV amounts in Case 2 than that in Case 1. We hypothesize that the discrepancy between the number of SFTSV-NP-positive cells and the SFTSV-RNA levels may be due to the influence of the SFTSV-infected cells in the peripheral blood. There were no pathological changes, SFTSV-NP-positive cells, or SFTSV-RNA detected in the CNS of Case 1.
A retrospective study of 115 hospitalized SFTS patients found that 33.7% of the patients had abnormalities suggestive of pneumonia in either the chest X-ray or CT.11 Our cases showed pulmonary mycosis that was not detected previously. Particularly in Case 1, the aggressive pulmonary aspergillosis exacerbated the clinical course resulting in death, although the thrombocytopenia and the other organ pathologies were improving. SFTS patients are somewhat immunodeficient9 or have damage to their immune system with lower levels of CD3+ and CD4+ T lymphocytes,7,8 which may be due to a viral-associated hemophagocytic syndrome, playing an important role in disease progression, disease severity, and clinical outcome. Also in our cases, the total number of the lymphocytes decreased (Tables 2). These findings suggest that physicians should be aware of potential fungal infection in SFTS patients. In both of our cases, the patients had received corticosteroids for the hemophagocytosis before admission to our hospital. Some lethal SFTS cases have reported a history of early treatment with dexamethasone, which acts to repress immune functions.2 Since use of corticosteroids for SFTS patients is controversial,9,12 a prospective study is needed to evaluate this.
The liver plays a central role in the pathogenesis of SFTSV infection.1,5 In both of our patients, elevation of liver damage markers (AST, ALT, and LDH) was observed, as found in a majority of the patients.11 Especially in Case 2, marked increases of AST, ALT, and LDH were observed at the time of death, and histologically the liver showed multiple lobular necroses. Viruses belong to the family Bunyaviridae, the Crimean-Congo hemorrhagic fever virus, and the Rift Valley fever virus show extensive hepatocellular necroses with necrotic hepatocytes infected with the viruses.13,14 In SFTS, however, hepatocytes were not infected with SFTSV, although numerous SFTSV-NP antigen-positive cells were observed in the liver. Therefore, it suggests that the liver damage in SFTS may be due to a secondary pathological process (e.g. shock status, hypercytokinemia, and hemophagocytosis) rather than due to a direct disturbance by SFTSV, but the pathogenesis is unknown.
In conclusion, the pathognomonic histological features in SFTS patients are necrotizing lymphadenitis in the systemic lymphoid tissue centered on the local lymph node close to the tick-bite region. Since the cellular immune function may be suppressed in SFTS patients, physicians should be aware of potential fungal infections.
Acknowledgments
We thank Ms. Yukari Nishimura, Ms. Sayuri Yoshimura, Ms. Chieko Baba, and Mr. Kei Matsuo for their technical assistance.
Disclosure: The authors declare no relationships with, or financial interest in any commercial companies pertaining to this article.
Ethics statement: The study was conducted in accordance with the guiding principles of the Declaration of Helsinki. Informed written consent was obtained from the family of each patient.
References
- 1.Yu XJ, Liang MF, Zhang SY, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gai Z, Liang M, Zhang Y, et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome bunyavirus through blood contact. Clin Infect Dis. 2012;54:249–252. doi: 10.1093/cid/cir776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Li Q, Hu W, et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis. 2012;12:156–160. doi: 10.1089/vbz.2011.0758. [DOI] [PubMed] [Google Scholar]
- 4.Tang X, Wu W, Wang H, et al. Human-to-human transmission of severe fever with thrombocytopenia syndrome bunyavirus through contact with infectious blood. J Infect Dis. 2013;207:736–739. doi: 10.1093/infdis/jis748. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, Maeda K, Suzuki T, et al. The first identification and retrospective study of severe Fever with thrombocytopenia syndrome in Japan. J Infect Dis. 2013;209:816–827. doi: 10.1093/infdis/jit603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savage HM, Godsey MS, Jr, Lambert A, et al. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg. 2013;89:445–452. doi: 10.4269/ajtmh.13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Hu Y, Niyonsaba A, et al. Detection and evaluation of immunofunction of patients with severe fever with thrombocytopenia syndrome. Clin Exp Med. 2013 doi: 10.1007/s10238-013-0259-0. doi: 10.1007/s10238-013-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weng Y, Chen N, Han Y, Xing Y, Li J. Clinical and laboratory characteristics of severe fever with thrombocytopenia syndrome in Chinese patients. Braz J Infect Dis. 2013;18:88–91. doi: 10.1016/j.bjid.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang YZ, He YW, Dai YA, et al. Hemorrhagic fever caused by a novel Bunyavirus in China: Pathogenesis and correlates of fatal outcome. Clin Infect Dis. 2012;54:527–533. doi: 10.1093/cid/cir804. [DOI] [PubMed] [Google Scholar]
- 10.Kuramochi H, Hayashi K, Uchida K, et al. Vascular endothelial growth factor messenger RNA expression level is preserved in liver metastases compared with corresponding primary colorectal cancer. Clin Cancer Res. 2006;12:29–33. doi: 10.1158/1078-0432.CCR-05-1275. [DOI] [PubMed] [Google Scholar]
- 11.Deng B, Zhou B, Zhang S, et al. Clinical features and factors associated with severity and fatality among patients with severe fever with thrombocytopenia syndrome bunyavirus infection in Northeast China. PLoS ONE. 2013;8:e80802. doi: 10.1371/journal.pone.0080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng B, Zhang S, Geng Y, et al. Cytokine and chemokine levels in patients with severe fever with thrombocytopenia syndrome virus. PLoS ONE. 2012;7:e41365. doi: 10.1371/journal.pone.0041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitehouse CA. Crimean-Congo hemorrhagic fever. Antiviral Res. 2004;64:145–160. doi: 10.1016/j.antiviral.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Shieh WJ, Paddock CD, Lederman E, et al. Pathologic studies on suspect animal and human cases of Rift Valley fever from an outbreak in Eastern Africa, 2006–2007. Am J Trop Med Hyg. 2010;83:38–42. doi: 10.4269/ajtmh.2010.09-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]