Abstract
Although initial rituximab-containing chemotherapies achieve high response rates, indolent B-cell non-Hodgkin lymphoma (B-NHL), such as follicular lymphoma (FL), is still incurable. Therefore, new effective agents with novel mechanisms are anticipated. In this multicentre phase II study, patients with relapsed/refractory indolent B-NHL and mantle cell lymphoma (MCL) received vorinostat 200 mg twice daily for 14 consecutive days in a 21-d cycle until disease progression or unacceptable toxicity occurred. The primary endpoint was overall response rate (ORR) in FL patients and safety and tolerability in all patients. Secondary endpoints included progression-free survival (PFS). Fifty-six eligible patients were enrolled; 50 patients (39 with FL, seven with other B-NHL, and four with MCL) were evaluable for ORR, and 40 patients had received rituximab-containing prior chemotherapeutic regimens. For the 39 patients with FL, the ORR was 49% [95% confidence interval (CI): 32·4, 65·2] and the median PFS was 20 months (95% CI: 11·2, 29·7). Major toxicities were manageable grade 3/4 thrombocytopenia and neutropenia. Vorinostat offers sustained antitumour activity in patients with relapsed or refractory FL with an acceptable safety profile. Further investigation of vorinostat for clinical efficacy is warranted.
Keywords: vorinostat, indolent B-cell non-Hodgkin lymphoma, follicular lymphoma, phase II trial, HAT mutation
Indolent B-cell non-Hodgkin lymphoma (B-NHL) comprises a heterogeneous group of lymphoid malignancies with varying degrees of aggressiveness (Swerdlow et al, 2008). Follicular lymphoma (FL), the most common form of the indolent B-NHL, is slow-growing and incurable in most patients. It can transform into a more aggressive histological type in some patients (Relander et al, 2010). Mantle cell lymphoma (MCL), which represents 3–10% of NHL, is more aggressive, with a median survival in the range of 3–5 years (Swerdlow et al, 2008). Rituximab in combination with cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) chemotherapy is one of the most frequently applied first-line treatments for patients with FL and achieves a high response rate (Tobinai et al, 2010; Watanabe et al, 2011). However, most patients eventually relapse and become chemorefractory, which leads to poor outcomes in the relapsed setting, though recently developed agents, including fludarabine, bendamustine and ibritumomab tiuxetan, have demonstrated improved therapeutic outcomes (Witzig et al, 2002; Tobinai et al, 2006; Kahl et al, 2010; Ohmachi et al, 2010; Rummel et al, 2013). Therefore, new effective agents with novel mechanisms of action are needed to treat relapsed/refractory indolent B-NHL.
Histone deacetylase (HDAC) inhibitors, such as vorinostat (suberoylanilide hydroxamic acid; SAHA), are thought to exhibit anti-tumour activity by up-regulating tumour-suppressive genes, promoting cell differentiation and inducing cell apoptosis. In addition, several non-histone proteins [e.g. tubulin (TUBB), Hsp90 (HSP90AA1) and p53 (TP53)] are reversibly acetylated on lysine residues and undergo hyperacetylation following exposure to vorinostat (Warrener et al, 2003; Glaser et al, 2004; Bali et al, 2005; Carlisi et al, 2008). Vorinostat is an oral HDAC inhibitor approved for the treatment of refractory cutaneous T-cell lymphoma (CTCL; Olsen et al, 2007). In a phase I study in Japanese patients with relapsed or refractory malignant lymphoma, including FL and MCL, vorinostat (200 mg twice daily) was well-tolerated, and responses were observed in three of four treated patients with FL and one of two treated patients with MCL (Watanabe et al, 2010). Similarly, a single-arm, phase II study of vorinostat in the United States reported a 47% overall response rate (ORR) in 17 patients with relapsed/refractory FL and a 22% ORR in nine patients with marginal zone B-cell lymphoma (MZL) among the 35 eligible patients with relapsed/refractory indolent B-NHL and MCL dosed at 200 mg twice daily for 14 consecutive days in a 21-d cycle (Kirschbaum et al, 2011). The median progression-free survival (PFS) in the study was 15·6 months in patients with relapsed/refractory FL treated with vorinostat (Kirschbaum et al, 2011); however, the small sample size limited the interpretation of these results.
Recently, two groups reported that frequent somatic mutations in the CREBBP and EP300 genes encoding histone acetyltransferases (HATs) were present in primary tumours and acquired at relapse in several different subtypes of B-cell lymphomas (Morin et al, 2011; Pasqualucci et al, 2011). Forty-one percent of patients with FL and 39% of patients with diffuse large B-cell lymphoma (DLBCL) exhibited somatic mutations and/or genomic deletions in the CREBBP and EP300 genes, as well as decreased levels of acetyltransferase activity. Therefore, it is reasonable to hypothesize that mutation-induced loss of acetylation of histones and other transcription factors caused an imbalance between acetylation and deacetylation in those patients, which partly accounted for the development of lymphomas resulting from deregulated gene expression and cell proliferation. We therefore conducted a multicentre phase II study of vorinostat in patients with relapsed/refractory FL, other subtypes of indolent B-NHL and MCL to assess the efficacy and safety. Additionally, we explored the CREBBP and EP300 mutations in tumour tissues obtained from patients in the phase II study.
Patients and methods
Patient selection
Patients were eligible if they were 20–74 years of age and had histopathologically confirmed FL, MCL or other histological subtypes of indolent B-NHL, including small lymphocytic lymphoma (SLL), lymphoplasmacytic lymphoma, splenic MZL, extranodal MZL of mucosa-associated lymphoid tissue (MALT) type, or nodal MZL, diagnosed based on the 2008 World Health Organization classification (Swerdlow et al, 2008). Patients must have undergone ≥1 but ≤4 prior chemotherapeutic regimens, not including steroids alone or local radiation. Eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status score ≤2, recurrent or progressive disease after the most recent therapy, the presence of a two-dimensional measurable nodal lesion or ex-nodal lesion [>1·5 cm in greatest transverse diameter by computerized tomography (CT) scan], and life expectancy >4 months. Patients had to have adequate haematological [absolute neutrophil count (ANC) ≥1·5 × 109/l, platelet count ≥100 × 109/l], hepatic (bilirubin ≤1·5 × the upper limit of the normal range) and renal function. Patients were excluded if they had received chemotherapy within 4 weeks, antibody therapy or radioimmunotherapy within 3 months, radiotherapy within 4 weeks, had not recovered from adverse events (AEs) from prior therapy, had received previous treatment with other HDAC inhibitors, had received allogeneic or autologous stem cell transplantation within 6 months, were pregnant, had central nervous system involvement, active systemic infection including hepatitis B, C or human immunodeficiency virus (HIV), severe hepatic dysfunction or other active malignancies.
The trial (Protocol 103) was approved by the institutional review board at each centre and complied with Good Clinical Practice guidelines, the Declaration of Helsinki and local laws. All patients provided written informed consent.
Treatment plan
This open-label, single arm phase II study was conducted at 18 centres. Enrolled patients received oral vorinostat 200 mg twice daily for 14 consecutive days followed by a 7-d break on a 21-d cycle. If patients had an ANC ≥0·5 × 109/l, but <1·0 × 109/l (grade 3) or a platelet count of ≥50 × 109/l but <75 × 109/l (grade 2) at the start of a cycle, the dose was decreased by 100 mg daily from their previous dose. If stepwise dose reduction did not improve the ANC or platelet count, the patients were excluded from the study. If the ANC and platelet counts recovered to below grade 1 or the pre-dosing counts at the end of the cycle, the dose reduction was reversed. Treatment was withheld from patients who developed grade 3 or higher non-haematological toxicity or haematological toxicity of ANC <0·5 × 109/l (grade 4), platelet count of <50 × 109/l with blood transfusion (grade 3) or a platelet count of <25 × 109/l (grade 4). Patients were restarted at a daily dose that was 100 mg less than their previous dose when the toxicity resolved to below grade 1 non-haematological toxicity or to an ANC >1·0 × 109/l and a platelet count of >75 × 109/l. Therapy was terminated if toxicity persisted or recurred in spite of two dose reductions; otherwise, treatment was continued until unacceptable AEs, progression of primary disease, protocol violation, pregnancy, patient's request or investigator's judgment.
Patient evaluation and study design
Laboratory tests, history, physical examination and evaluation of AEs and therapeutic efficacy were conducted during study visits. Radiological assessment by CT scan was performed at baseline and on Day 21 every three cycles to assess anti-tumour efficacy. Once patients achieved complete response (CR), anti-tumour efficacy was assessed every five cycles.
The primary endpoint was ORR according to the criteria for relapsed/refractory FL patients (Cheson et al, 1999), as well as the safety and tolerability in indolent B-NHL and MCL. To confirm the histopathological diagnosis, an Independent Central Pathological Committee (ICPC) was formed. When 12 months had elapsed from enrollment of the last patient, the ORR data was cut off. ORR was defined as the proportion (%) of patients with CR, complete response/unconfirmed (CRu) or partial response (PR) as best response through the study period. Secondary objectives were PFS and overall survival (OS) in relapsed/refractory FL patients. An Independent Radiology Review Committee was formed to assess the CT imaging and confirm adjudicated anti-tumour efficacy through central review meetings.
Safety was assessed by the incidence of AEs and drug-related AEs, body weight, vital signs, electrocardiogram (every three cycles) and laboratory tests. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). The incidence of ≥grade 3 drug-related AEs, leucopenia, neutropenia, lymphopenia, infections accompanied by a normal ANC or grade 1/2 neutropenia, febrile neutropenia and infections accompanied by grade 3/4 neutropenia, were assessed for all patients. The Efficacy and Safety Evaluation Committee evaluated the safety and efficacy results of the central efficacy assessment by the Independent Radiology Review Committee.
Statistical analysis
Referring to the phase II study design of fludarabine (Tobinai et al, 2006), vorinostat was considered effective in patients with FL if the ORR was ≥25%. The ORR was examined with the exact test for binomial proportion (α = 0·025, one-sided) and the sample size was estimated based on the ORR of the FL patients. Assuming a true ORR of 50% with vorinostat and <25% without vorinostat, 39 patients provided a 90% power to demonstrate an ORR >25%. With 39 patients with FL, the primary objective for an ORR would have been met if ≥16 patients demonstrated an objective response. Survival curves were plotted using the Kaplan–Meier product-limit method.
Mutation analysis of CREBBP/EP300
The formalin-fixed paraffin-embedded (FFPE) materials were obtained from the archives of each study site provided from 33 patients participating in the phase II study. Genomic DNA was extracted from the FFPE samples using the QIAamp DNA FFPE Tissue kit (QIAGEN Inc., Valencia, CA, USA). Sequences for all annotated exons and flanking introns of CREBBP and EP300 were obtained from the NCBI 37.3 Genome database (mRNA accession numbers: NM_004380.2 and NM_001429.3, respectively). Coding sequences were obtained using polymerase chain reaction (PCR) and Sanger sequencing on genomic DNA. Primer extension sequencing was performed by GENEWIZ, Inc. (South Plainfield, NJ, USA). The sequencing data were analysed to detect any mutations compared to the genomic DNA reference sequence. Synonymous mutations and previously reported polymorphisms (Human dbSNP Database at NCBI, Build 137) were excluded.
Results
Patient characteristics
Between February 2009 and February 2010, 56 patients from 18 centres in Japan, Korea, Taiwan and Hong Kong were enrolled in this study. Demographics and patient characteristics are listed in Table I. Of these 56 patients, six were diagnosed with non-pre-specified diseases by the ICPC (two patients with a mixture of FL and DLBCL, two with DLBCL, one with high-grade nodal MZL and one with malignant lymphoma, unclassified) were excluded from the full analysis set (FAS) population for efficacy analysis. The histologies of the 50 FAS patients were FL in 39 patients, including a patient with FL grade 3b according to the protocol definition because no diffuse area was recognized by the ICPC, other indolent B-NHL in seven patients [four patients with extranodal MZL of MALT type, two with small B-cell lymphoma not otherwise specified (NOS), and one with SLL], and MCL in four patients. Patients with FL (n = 39) were categorized according to the Follicular Lymphoma International Prognostic Index (FLIPI) low (n = 16, 41%), intermediate (n = 15, 39%) and high (n = 8, 21%) risks. The median age of the 56 patients at the start of the protocol treatment was 60 years (range, 33–75 years). The median number of prior therapies was 2 (range, 1–4). Most of the patients (30 patients with FL, six with the other indolent B-NHL, and four with MCL) had received rituximab-containing prior chemotherapeutic regimens. Other prior regimens included alkylating agents (n = 7), purine analogs (n = 5), and radioimmunotherapy (n = 3) (Table I).
Table I.
Patient characteristics.
| Characteristics | FL (n = 39) | Indolent B-NHL (non-FL)* (n = 7) | MCL (n = 4) | Others† (n = 6) | Total (n = 56) |
|---|---|---|---|---|---|
| Median age, years (range) | 60 (33–73) | 61 (44–70) | 72 (60–75) | 55 (46–70) | 60 (33–75) |
| ECOG performance status, n (%) | |||||
| 0 | 31 (80) | 6 (86) | 4 (100) | 4 (67) | 45 (80) |
| 1 | 8 (21) | 1 (14) | 0 (0) | 2 (33) | 11 (20) |
| Number of prior therapies | |||||
| Median (range) | 1 (1–4) | 3 (1–4) | 3 (3–4) | 2 (1–2) | 2 (1–4) |
| Prior therapy, n (%) | |||||
| R-chemotherapy | 30 (77) | 6 (86) | 4 (100) | 6 (100) | 46 (82) |
| Alkylating agent | 3 (8) | 1 (14) | 3 (75) | 0 (0) | 7 (13) |
| Purine analog | 2 (5) | 3 (43) | 0 (0) | 0 (0) | 5 (9) |
| Radioimmunotherapy | 2 (5) | 0 (0) | 0 (0) | 1 (17) | 3 (5) |
| Other | 3 (8) | 2 (30) | 1 (25) | 0 (0) | 6 (11) |
| FLIPI, n (%) | |||||
| Low risk | 16 (41) | N.A. | N.A. | N.A. | 16 (29) |
| Intermediate risk | 15 (39) | N.A. | N.A. | N.A. | 15 (27) |
| High risk | 8 (21) | N.A. | N.A. | N.A. | 8 (14) |
| FL grade, n (%) | |||||
| 1 | 25 (64) | N.A. | N.A. | N.A. | 25 (45) |
| 2 | 13 (33) | N.A. | N.A. | N.A. | 13 (23) |
| 3b‡ | 1 (3) | N.A. | N.A. | N.A. | 1 (2) |
FL, follicular lymphoma; B-NHL, B-cell non-Hodgkin lymphoma; MCL, mantle cell lymphoma; ECOG, Eastern Cooperative Oncology Group; R, rituximab; FLIPI, Follicular Lymphoma International Prognostic Index; R-chemotherapy, rituximab-containing chemotherapy; N.A., Not applicable.
Indolent B-NHL (non-FL) included four patients with extranodal marginal zone B-cell lymphoma (MZL) of mucosa-associated lymphoid tissue type, two patients with small B-cell lymphoma not otherwise specified, and one patient with small lymphocytic lymphoma.
Others included following diagnoses; diffuse large B-cell lymphoma (DLBCL; two patients), malignant lymphoma unclassified, follicular lymphoma (FL) grade 3a (50%) with DLBCL (50%), FL grade 3b (40%) with DLBCL (60%), and high grade nodal MZL.
A patient with FL grade 3b was enrolled according to the protocol definition because no diffuse area was recognized in the specimen by the Independent Central Pathological Committee.
Treatment
The median duration of the protocol treatment was 8 months, with a maximum of 26+ months. The median ratio of the planned dosage (200 mg twice daily, orally, for 14 consecutive days in a 21-d cycle) was 86% for the 56 patients. As of the data cut-off date (February 2011), 18 patients continued to receive treatment and 38 patients had discontinued treatment due to disease progression (n = 25), drug-related AEs (n = 9) or withdrawal of consent (n = 4). An additional data cut-off (February 2012) was made for the PFS and OS evaluation.
Efficacy
At the data cut-off date (1 year after the last patient enrolled), a 49% ORR [95% confidence interval (CI): 32·4, 65·2] was observed among 39 assessable patients with relapsed/refractory FL (Table II), which included four patients with CR (10%), three with CRu (8%), and 12 with PR (31%). Tumour size was reduced in 92% (36/39) of FL patients, and tumour shrinkage occurred not only in the patients with CREBBP/EP300 mutations, but also in those without the mutations (Fig1). The relationship between clinical efficacy and CREBBP/EP300 mutation statuses is described in more detail in the following CREBBP and EP300 mutation analysis section. At the additional data cut-off for PFS and OS evaluation (2 years after the last patient enrolled), the best response of one patient with FL was upgraded from stable disease (SD) to PR, the median PFS for patients with FL was 20 months (95% CI: 11·2, 29·7) (Table II, Fig2A), and the median PFS for patients with FL who responded was 26 months. The FL patients with low, intermediate or high FLIPI scores achieved a median PFS of 20 months, 20 months or 13 months, respectively (Table II, Fig2B). During the trial and follow-up period, two of three patients with FL refractory to rituximab obtained CRu and PR, and a 43% ORR (3/7) was observed in patients with relapsed/refractory other indolent B-NHL (two patients with small B-cell lymphoma NOS and one with extranodal MZL of MALT type) including one CR in a patient with small B-cell lymphoma NOS. None of the assessable MCL patients responded. Within the 18 patients who continued vorinostat treatment, three patients (two patients with FL and one with small B-cell lymphoma NOS) had CR and two patients with FL had CRu. Approximately 81% of the intention to treat (ITT) population (56 patients) remained alive at the additional data cut-off (February 2012) (Table II), and the median OS had not yet been reached (Fig2C).
Table II.
Efficacy results.
| ORR, % (95% CI)* | Median PFS, month (95% CI)† | Survival, % ‡ | |
|---|---|---|---|
| FL (n = 39) | 49 (32·4, 65·2) | 20 (11·2, 29·7) | 86 |
| FLIPI low risk (n = 16) | 44 (19·8, 70·1) | 20 (8·5, 29·9) | 94 |
| FLIPI intermediate risk (n = 15) | 53 (26·6, 78·7) | 20 (7·5, N.E.) | 87 |
| FLIPI high risk (n = 8) | 50 (15·7, 84·3) | 12 (2·0, 30·1) | 75 |
| Indolent B-NHL (non-FL) § (n = 7) | 43 (9·9, 81·6) | 10 (4·1, N.E.) | 86 |
| MCL (n = 4) | 0 (0·0, 60·2) | N.E. | 50 |
| All FAS population (n = 50) | 44 (30·0, 58·7) | 18 (10·4, 25·8) | 84 |
| All ITT population (n = 56) | N.A. | N.A. | 81 |
ORR, overall response rate; CI, confidence interval; PFS, progression-free survival; FL, follicular lymphoma; FLIPI, Follicular Lymphoma International Prognostic Index; B-NHL, B-cell non-Hodgkin lymphoma; MCL, mantle cell lymphoma; FAS, full analysis set; ITT, intention to treat; N.E., not estimable; N.A., not applicable.
ORR was analysed by the data 1 year after the last patient enrolled.
Median PFS was analysed by the data 2 years after the last patient enrolled.
Survival rate was analysed at the data 2 years after the last patient enrolled.
Indolent B-NHL (non-FL) included four patients with extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type, two patients with small B-cell lymphoma not otherwise specified, and one patient with small lymphocytic lymphoma.
Fig 1.
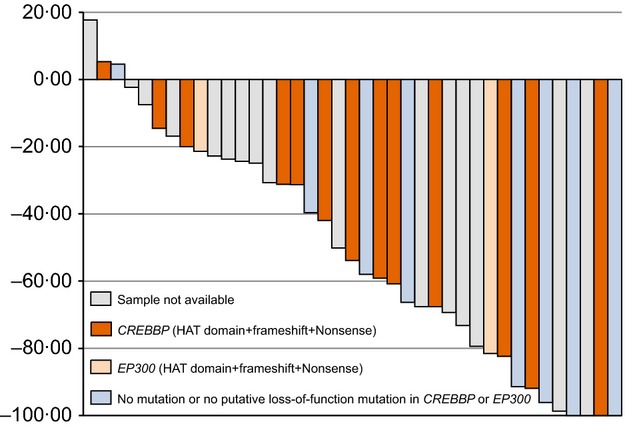
Waterfall plot showing percent change in tumour size for all assessable follicular lymphoma (FL) patients at the time of best response and results of histone acetyltransferase (HAT) mutation analysis.
Fig 2.
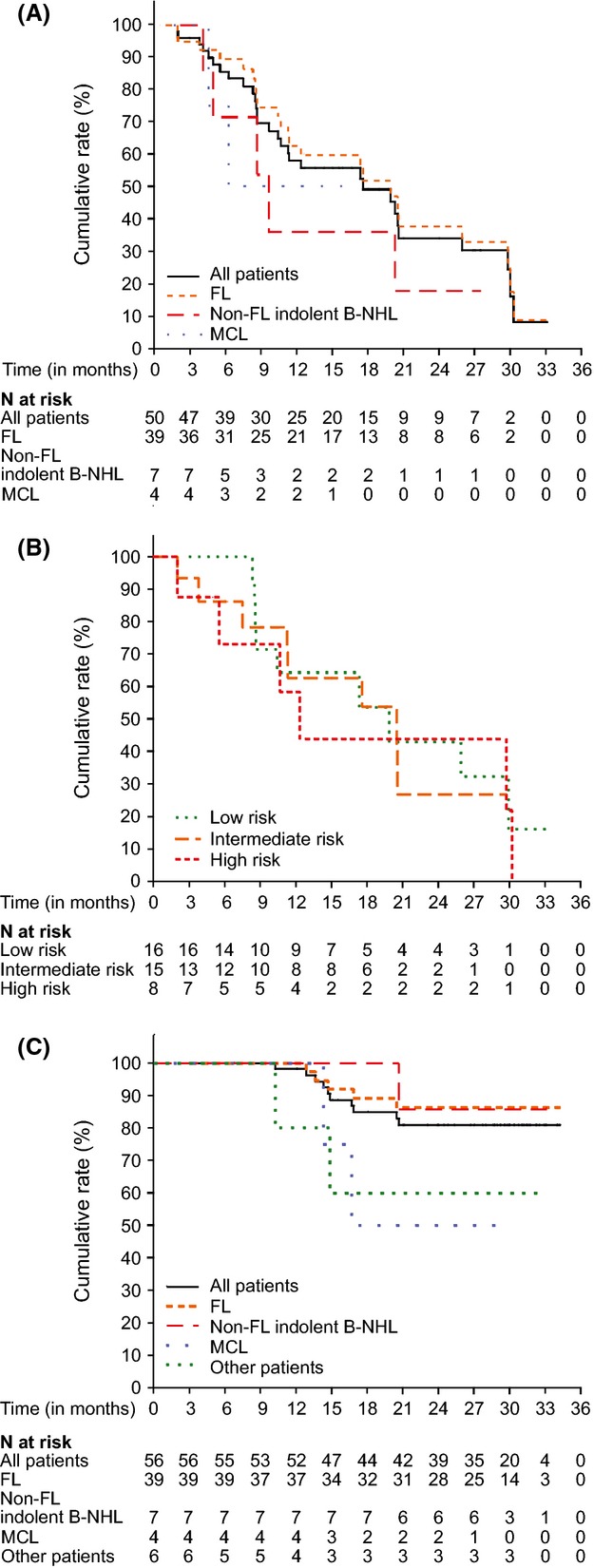
Kaplan–Meier estimates of progression-free survival (PFS) or overall survival (OS). (A) PFS by disease types for all assessable patients (n = 50), patients with FL (n = 39), other (non-FL) indolent B-NHL (n = 7) and MCL (n = 4). PFS was defined as the time from allocation to the first documented disease progression or death due to any cause, whichever occurred first. (B) Correlation between Follicular Lymphoma International Prognostic Index (FLIPI) scores and outcomes for PFS of the patients with FL. PFS for the patients at low (n = 16), intermediate (n = 15), or high (n = 8) risk according to FLIPI were calculated. (C) OS by disease types for all enrolled patients (n = 56), patients with FL, other (non-FL) indolent B-NHL and MCL. FL, follicular lymphoma; B-NHL, B-cell non-Hodgkin lymphoma; MCL, mantle cell lymphoma.
Safety
Forty-five patients (80%) experienced grade 3/4 AEs. The median time to onset was 15 d (range: 2–497 d). The most common drug-related AEs were thrombocytopenia, diarrhoea, neutropenia, decreased appetite, nausea, leucopenia and fatigue (Table III). Thrombocytopenia and neutropenia were the most frequently observed grade 3 or 4 AEs (Table III). All patients who developed grade 3 or 4 thrombocytopenia or neutropenia recovered after dose reduction, interruption or discontinuation of vorinostat and adequate supportive measures. The incidences of drug-related grade 3 leucopenia and lymphopenia were 13% each, and a grade 3 infection was observed in one patient with herpes zoster. No grade 4 infection was reported. There were no treatment-related deaths during this trial.
Table III.
Drug-related adverse events.
| Preferred term | Total, n = 56 | ||
|---|---|---|---|
| All grades n (%) | Grade 3 n (%) | Grade 4 n (%) | |
| Haematological | |||
| Thrombocytopenia | 52 (93) | 13 (23) | 14 (25) |
| Neutropenia | 38 (68) | 20 (36) | 3 (5) |
| Leucopenia | 31 (55) | 7 (13) | 0 (0) |
| Lymphopenia | 19 (34) | 7 (13) | 0 (0) |
| Anaemia | 19 (34) | 2 (4) | 0 (0) |
| Non-haematological | |||
| Diarrhoea | 38 (68) | 3 (5) | 0 (0) |
| Decreased appetite | 35 (63) | 4 (7) | 0 (0) |
| Nausea | 34 (61) | 0 (0) | 0 (0) |
| Fatigue | 29 (52) | 1 (2) | 0 (0) |
| Weight decrease | 13 (23) | 1 (2) | 0 (0) |
| Hyperglycaemia | 12 (21) | 2 (4) | 0 (0) |
CREBBP and EP300 mutation analysis
Of the 33 tissue samples obtained for mutation analysis, the genomic DNA extraction or PCR failed in five samples, and sequencing succeeded in 28 samples (23 samples of FL and five of other B-NHL) (Table IV). For CREBBP, 19 of the 28 samples had a total of 34 mutations and 17 samples had putative loss-of-function mutations for HAT activity (total 22 mutations: 17 in the HAT domain, three frame shift and two nonsense). For EP300, six samples had a total of 14 mutations, and two samples had putative loss-of-function mutations (two mutations in the HAT domain) (Fig3). Of the 23 samples from FL patients, the putative loss-of-function mutations of CREBBP and EP300 were found in 13 and two samples, respectively. Of the 13 patients with CREBBP putative loss-of-function mutations, the ORR was 54% (one CRu, six PR and six SD) and median PFS was 11 months.
Table IV.
Mutation analysis results.
| FL (n = 23) | Indolent B-NHL (non-FL)* (n = 3) | Others† (n = 2) | Total (n = 28) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| CREBBP | ||||
| Mutation | 15 (65) | 2 (67) | 2 (100) | 19 (68) |
| HAT domain | 10 (43) | 2 (67) | 2 (100) | 14 (50) |
| HAT domain + Frameshift + Nonsense | 13 (57) | 2 (67) | 2 (100) | 17 (61) |
| No mutation | 8 (35) | 1 (33) | 0 (0) | 9 (32) |
| EP300 | ||||
| Mutation | 5 (22) | 1 (33) | 0 (0) | 6 (21) |
| HAT domain | 2 (9) | 0 (0) | 0 (0) | 2 (7) |
| HAT domain + Frameshift + Nonsense | 2 (9) | 0 (0) | 0 (0) | 2 (7) |
| No mutation | 18 (78) | 2 (67) | 2 (100) | 22 (79) |
Samples were not collected from patients with mantle cell lymphoma.
FL, follicular lymphoma; B-NHL, B-cell non-Hodgkin lymphoma; HAT, histone acetyltransferase.
Indolent B-NHL (non-FL) included a patient with extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue type, and two patients with small B-cell lymphoma not otherwise specified.
Others included a patient with diffuse large B-cell lymphoma (DLBCL), and a patient with FL grade 3b (40%) with DLBCL (60%).
Fig 3.
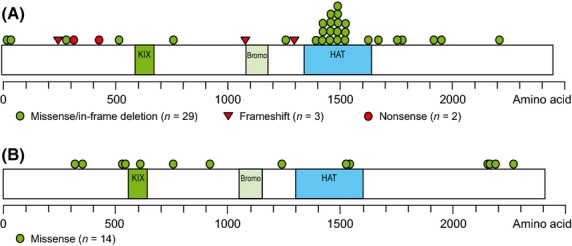
CREBBP and EP300 mutations in patients with FL and other B-NHL. (A) Schematic diagram of the CREBBP protein and (B) the EP300 protein. FL, follicular lymphoma; B-NHL, B-cell non-Hodgkin lymphoma; KIX, CREB-binding domain; bromo: bromodomain; HAT, histone acetyltransferase domain.
For the two FL patients with putative loss-of-function mutations in EP300, the objective responses were CR and SD, for which the PFS times were 16 and 21 months, respectively. For the six FL samples without mutations in either CREBBP or EP300, ORR and median PFS were 67% (two CRu, two PR and two SD) and 21 months, respectively.
Discussion
We conducted an open-label, single-arm phase II study of vorinostat in patients with relapsed/refractory indolent B-NHL and MCL. The ORR and median PFS values in relapsed/refractory FL (49% and 20 months respectively, n = 39) and the ORR in other indolent B-NHL (43%, n = 7) in this study are consistent with the data of a previous phase II trial of vorinostat, which reported ORR and median PFS in relapsed/refractory FL patients (n = 17) of 47% and 15·6 months, and a 22% ORR in other indolent B-NHL patients (n = 9) (Kirschbaum et al, 2011), which suggests there is no evidence of any ethnic difference for efficacy between Japanese and Caucasian people. Although our study had a similar single-arm design, it had a larger sample size. Our study also showed the potential efficacy of vorinostat, such as a median PFS of 26 months, in responsive FL patients and the fact that 18 of 56 patients continued vorinostat, including three CR and two CRu patients.
The main vorinostat-related toxicities were thrombocytopenia, neutropenia, diarrhoea and decreased appetite, which appeared to be reversible and manageable. The incidences of treatment-related grade 3/4 leucopenia and lymphopenia were both 13%. In the phase II studies of purine analogs (cladribine and fludarabine) in patients with relapsed indolent B-NHL, higher frequencies of drug-related haematological toxicities were reported (Ogura et al, 2004; Tobinai et al, 2006), including reduction of white blood cells, which may increase the risk of infectious disease. Of note, grade 3 infections in 10 patients (19%) were reported in the phase II study of fludarabine (Tobinai et al, 2006). In contrast, in this trial, drug-related grade 3 infection was observed in one patient (2%) with herpes zoster, and no grade 4 infection was reported.
CREBBP or EP300 mutations were identified in the HAT domain and other regions in which the mutations were predicted to cause HAT inactivation in FL patients consistent with previous reports. However, we could not confirm the correlation between the mutations and clinical response, possibly due to the retrospective approach and small number of samples. MEF2B mutation is another target, but we did not analyse this because of the sample limitation. Additionally, given that HDAC inhibitors have several mechanisms of action, such as transcriptional/non-transcriptional effects, actions other than transcriptional modulation may also be candidates for predicting clinical efficacy. Further investigations are needed to find factors that predict the clinical efficacy of vorinostat, such as a prospective mutation analysis of CREBBP and EP300 in randomized clinical trials.
In conclusion, this phase II study demonstrated the promising efficacy and safety of vorinostat in patients with relapsed or refractory FL. As this was a single-arm study with limited data to interpret, a comparative study to verify the efficacy of vorinostat is warranted. Given the less toxic profile of vorinostat, this drug could possibly be combined with rituximab or other molecular targeted drugs or could be used for maintenance after rituximab-containing chemotherapy in FL patients. Further clinical studies are warranted to elucidate the drug efficacy of vorinostat.
Acknowledgments
We thank all of the patients who participated in this study and the investigators from all of the following study sites: Drs. K. Meguro (Sendai Medical Centre), K. Usuki (NTT Medical Centre Tokyo), N. Takayama (Kyorin University Hospital), N. Usui (The Jikei University Daisan Hospital), M. Taniwaki (University Hospital, Kyoto Prefectural University of Medicine), S. Okamura (Kyushu Medical Centre), K. Tsukasaki (National Cancer Centre Hospital East), Y. Imaizumi (Nagasaki University Hospital) and H. J. Kang (Korea Cancer Centre Hospital); the Independent Central Pathological Committee: Drs. T. Yoshino (Okayama University) and C. Kim (Seoul National University Hospital); the Independent Radiology Review Committee: Drs. C.-M. Tiu (Taipei Veteran General Hospital) and U. Tateishi (Yokohama City University); and the Efficacy and Safety Evaluation Committee: Drs. T. Hotta (National Cancer Centre), Takayo Suzuki (Shiga Medical Centre for Adults) and K. Ohnishi (Hamamatsu University School of Medicine). The authors thank K. Matsuda of MSD K.K. and Dr. M. E. Hanson of Merck Sharp & Dohme, Corp. for editorial assistance. This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, N.J., U.S.A. M. Ogura designed the research study, performed the research, analysed the data and wrote the paper. K. Ando and K. Ishizawa analysed the data, performed the research and revised the paper critically. Tatsuya Suzuki, S. Y. Oh, K. Itoh, Kazuhito Yamamoto, W. Y. Au and H. F. Tien performed the research and revised the paper critically. Y. Matsuno and T. Terauchi contributed essential tools and revised the paper critically. Keiko Yamamoto, M. Mori and Y. Tanaka performed the research and wrote the paper. T. Shimamoto designed the research study, performed the research and wrote the paper. K. Tobinai and W. S. Kim designed the research study, performed the research, revised the paper critically and analysed the data. All authors approved the manuscript and submission.
Competing interests
M. Ogura has consultant or advisory relationships (Merck/MSD, Symbio, Zenyaku, Kyowakirin, Meijiseika Pharma) and receives research funding (Pfizer, GSK, Eisai, Chugai/Roche, Zenyaku, Symbio, Biomedics, AstraZeneca). K. Tobinai has consultant or advisory relationships (Merck/MSD, Symbio, Spectrum) and receives research funding (Pfizer, GSK, Eisai, Chugai/Roche, Symbio, Merck/MSD, Zenyaku, Biomedics). Keiko Yamamoto, M. Mori, Y. Tanaka and T. Shimamoto are employees of MSD K.K. All remaining authors have declared no conflicts of interest.
References
- Bali P, Pranpat M, Swaby R, Fiskus W, Yamaguchi H, Balasis M, Rocha K, Wang HG, Richon V. Bhalla K. Activity of suberoylanilide hydroxamic acid against human breast cancer cells with amplification of Her-2. Clinical Cancer Research. 2005;11:6382–6389. doi: 10.1158/1078-0432.CCR-05-0344. [DOI] [PubMed] [Google Scholar]
- Carlisi D, Vassallo B, Lauricella M, Emanuele S, D'Anneo A, Di Leonardo E, Di Fazio P, Vento R. Tesoriere G. Histone deacetylase inhibitors induce in human hepatoma HepG2 cells acetylation of p53 and histones in correlation with apoptotic effects. International Journal of Oncology. 2008;32:177–184. doi: 10.3892/ijo.32.1.177. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R. Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. Journal of Clinical Oncology. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- Glaser KB, Li J, Pease LJ, Staver MJ, Marcotte PA, Guo J, Frey RR, Garland RB, Heyman HR, Wada CK, Vasudevan A, Michaelides MR, Davidsen SK. Curtin ML. Differential protein acetylation induced by novel histone deacetylase inhibitors. Biochemical and Biophysical Research Communications. 2004;325:683–690. doi: 10.1016/j.bbrc.2004.10.082. [DOI] [PubMed] [Google Scholar]
- Kahl BS, Bartlett NL, Leonard JP, Chen L, Ganjoo K, Williams ME, Czuczman MS, Robinson KS, Joyce R, van der Jagt RH. Cheson BD. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study. Cancer. 2010;116:106–114. doi: 10.1002/cncr.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum M, Frankel P, Popplewell L, Zain J, Delioukina M, Pullarkat V, Matsuoka D, Pulone B, Rotter AJ, Espinoza-Delgado I, Nademanee A, Forman SJ, Gandara D. Newman E. Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin's lymphoma and mantle cell lymphoma. Journal of Clinical Oncology. 2011;29:1198–1203. doi: 10.1200/JCO.2010.32.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, Johnson NA, Severson TM, Chiu R, Field M, Jackman S, Krzywinski M, Scott DW, Trinh DL, Tamura-Wells J, Li S, Firme MR, Rogic S, Griffith M, Chan S, Yakovenko O, Meyer IM, Zhao EY, Smailus D, Moksa M, Chittaranjan S, Rimsza L, Brooks-Wilson A, Spinelli JJ, Ben-Neriah S, Meissner B, Woolcock B, Boyle M, McDonald H, Tam A, Zhao Y, Delaney A, Zeng T, Tse K, Butterfield Y, Birol I, Holt R, Schein J, Horsman DE, Moore R, Jones SJ, Connors JM, Hirst M, Gascoyne RD. Marra MA. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura M, Morishima Y, Kobayashi Y, Uike N, Sugai S, Chou T, Kasai M, Miura I, Murayama T, Matsuno Y, Nakamura S, Mori S, Ohashi Y, Tobinai K Cladribine Study Group. Durable response but prolonged cytopenia after cladribine treatment in relapsed patients with indolent non-Hodgkin's lymphomas: results of a Japanese phase II study. International Journal of Hematology. 2004;80:267–277. doi: 10.1532/ijh97.04077. [DOI] [PubMed] [Google Scholar]
- Ohmachi K, Ando K, Ogura M, Uchida T, Itoh K, Kubota N, Ishizawa K, Yamamoto J, Watanabe T, Uike N, Choi I, Terui Y, Usuki K, Nagai H, Uoshima N, Tobinai K Japanese Bendamustine Lymphoma Study Group. Multicenter phase II study of bendamustine for relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Cancer Science. 2010;101:2059–2064. doi: 10.1111/j.1349-7006.2010.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, Frankel SR, Chen C, Ricker JL, Arduino JM. Duvic M. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. Journal of Clinical Oncology. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- Pasqualucci L, Dominguez-Sola D, Chiarenza A, Fabbri G, Grunn A, Trifonov V, Kasper LH, Lerach S, Tang H, Ma J, Rossi D, Chadburn A, Murty VV, Mullighan CG, Gaidano G, Rabadan R, Brindle PK. Dalla-Favera R. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471:189–195. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relander T, Johnson NA, Farinha P, Connors JM, Sehn LH. Gascoyne RD. Prognostic factors in follicular lymphoma. Journal of Clinical Oncology. 2010;28:2902–2913. doi: 10.1200/JCO.2009.26.1693. [DOI] [PubMed] [Google Scholar]
- Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M, Balser C, Kaiser U, Weidmann E, Dürk H, Ballo H, Stauch M, Roller F, Barth J, Hoelzer D, Hinke A, Brugger W Study group indolent Lymphomas (StiL) Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. Vardman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. 4th edn. [Google Scholar]
- Tobinai K, Watanabe T, Ogura M, Morishima Y, Ogawa Y, Ishizawa K, Minami H, Utsunomiya A, Taniwaki M, Terauchi T, Nawano S, Matsusako M, Matsuno Y, Nakamura S, Mori S, Ohashi Y, Hayashi M, Seriu T. Hotta T. Phase II study of oral fludarabine phosphate in relapsed indolent B-Cell non-Hodgkin's lymphoma. Journal of Clinical Oncology. 2006;24:174–180. doi: 10.1200/JCO.2005.03.9313. [DOI] [PubMed] [Google Scholar]
- Tobinai K, Ogura M, Itoh K, Kinoshita T, Hotta T, Watanabe T, Morishima Y, Igarashi T, Terauchi T, Ohashi Y All Collaborators of the IDEC-C2B8 Study Group in Japan. Randomized phase II study of concurrent and sequential combinations of rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) chemotherapy in untreated indolent B-cell non-Hodgkin lymphoma: 7-year follow-up results. Cancer Science. 2010;101:2579–2585. doi: 10.1111/j.1349-7006.2010.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrener R, Beamish H, Burgess A, Waterhouse NJ, Giles N, Fairlie D. Gabrielli B. Tumor cell-selective cytotoxicity by targeting cell cycle checkpoints. FASEB Journal. 2003;17:1550–1552. doi: 10.1096/fj.02-1003fje. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kato H, Kobayashi Y, Yamasaki S, Morita-Hoshi Y, Yokoyama H, Morishima Y, Ricker JL, Otsuki T, Miyagi-Maesima A, Matsuno Y. Tobinai K. Potential efficacy of the oral histone deacetylase inhibitor vorinostat in a phase I trial in follicular and mantle cell lymphoma. Cancer Science. 2010;101:196–200. doi: 10.1111/j.1349-7006.2009.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Tobinai K, Shibata T, Tsukasaki K, Morishima Y, Maseki N, Kinoshita T, Suzuki T, Yamaguchi M, Ando K, Ogura M, Taniwaki M, Uike N, Takeuchi K, Nawano S, Terauchi T. Hotta T. Phase II/III study of R-CHOP-21 versus R-CHOP-14 for untreated indolent B-cell non-Hodgkin's lymphoma: JCOG 0203 trial. Journal of Clinical Oncology. 2011;29:3990–3998. doi: 10.1200/JCO.2011.34.8508. [DOI] [PubMed] [Google Scholar]
- Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, Pohlman BL, Bartlett NL, Wiseman GA, Padre N, Grillo-López AJ, Multani P. White CA. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. Journal of Clinical Oncology. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]


